Abstract
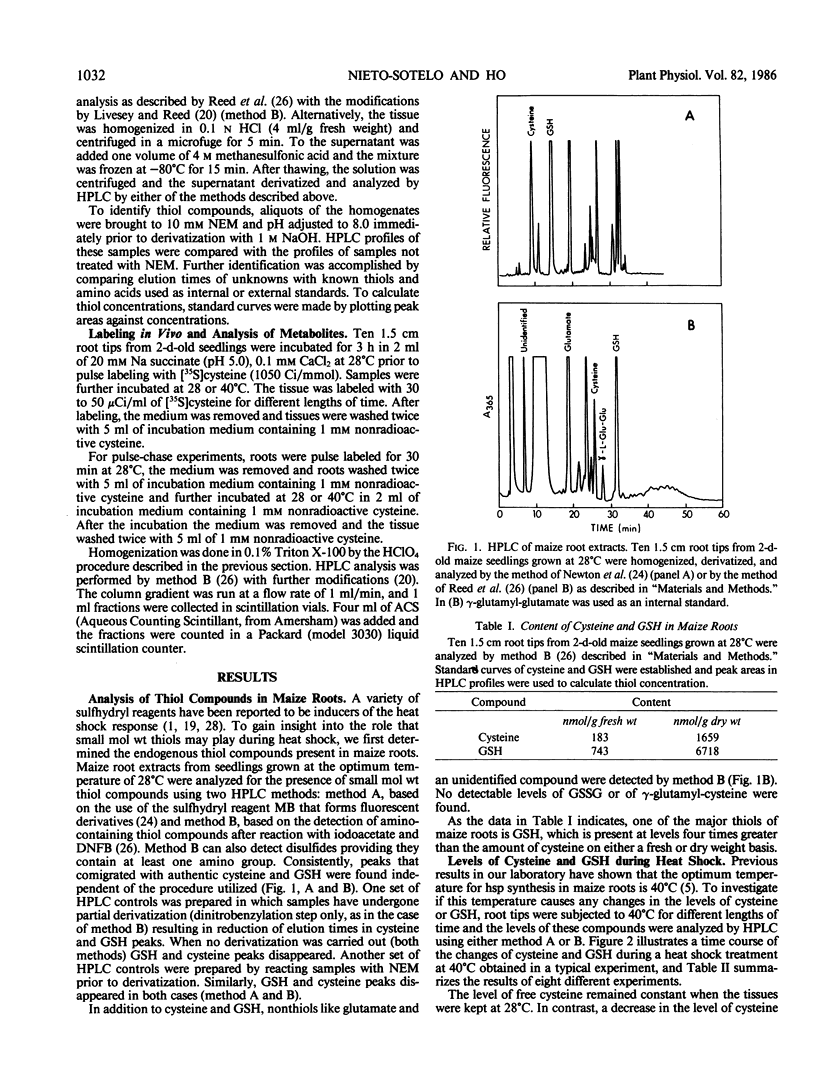
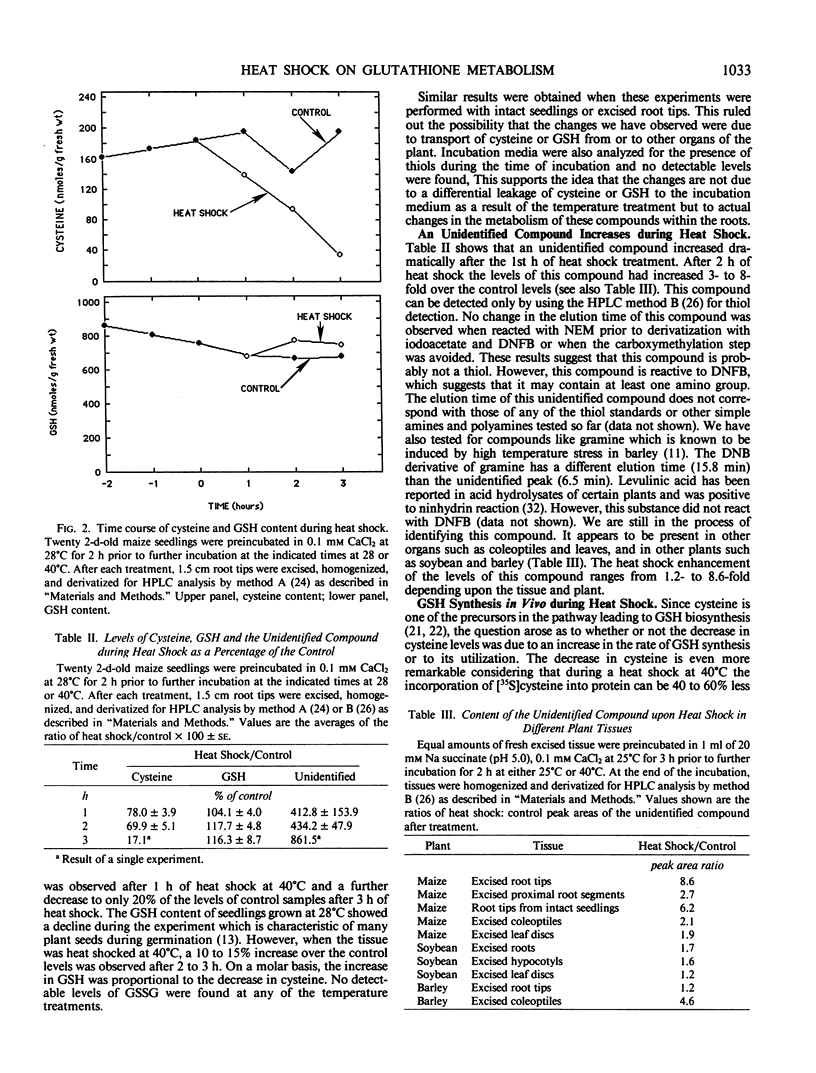
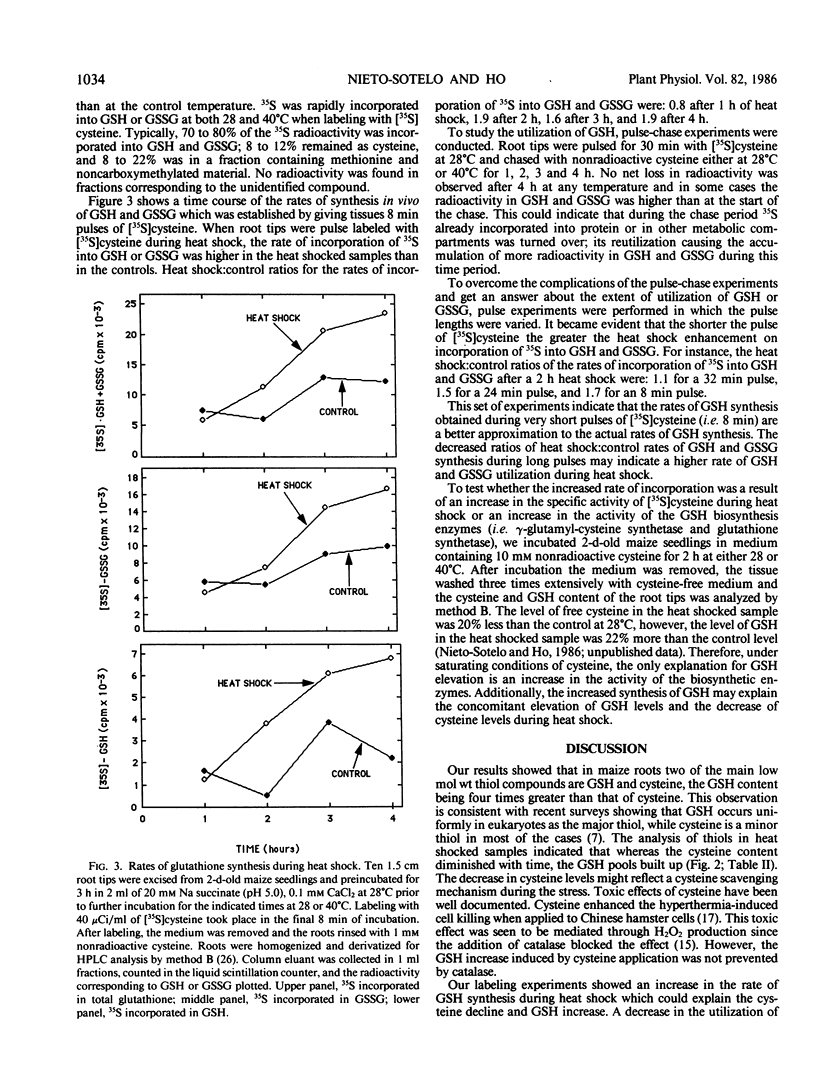
High performance liquid chromatography analyses revealed that glutathione (GSH) and cysteine are two of the major low molecular weight thiol compounds in maize root extracts. Treatment of maize roots to heat shock temperatures of 40°C resulted in a decrease of cysteine levels and an increase of GSH levels. Pulse labeling of maize roots with [35S]cysteine showed that the rate of incorporation of 35S into GSH or glutathione disulfide (GSSG) in heat shocked tissues was twice that in nonheat shocked tissues. In addition, extracts from heat shocked maize, barley, and soybean tissues contained an unidentified low molecular weight compound that increased from 1.2- to 8-fold within 2 hours of heat shock treatment depending on the tissue and plant involved. Our results indicate that during heat shock there is an increase in the activity of the GSH synthetizing capacity in maize root cells. The elevated synthesis of GSH may be related to the cells capacity to cope with heat stress conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Baszczynski C. L., Walden D. B., Atkinson B. G. Regulation of gene expression in corn (Zea Mays L.) by heat shock. Can J Biochem. 1982 May;60(5):569–579. doi: 10.1139/o82-070. [DOI] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. Ubiquitin is a heat shock protein in chicken embryo fibroblasts. Mol Cell Biol. 1985 May;5(5):949–956. doi: 10.1128/mcb.5.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Ho T. H., Hauptmann R. M. Tissue specificity of the heat-shock response in maize. Plant Physiol. 1984 Jun;75(2):431–441. doi: 10.1104/pp.75.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Ho T. H. Heat shock proteins in maize. Plant Physiol. 1983 Feb;71(2):215–222. doi: 10.1104/pp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. L., Malcolm A. W., Meredith M. J. Role of glutathione in cell survival after hyperthermic treatment of Chinese hamster ovary cells. Cancer Res. 1985 Dec;45(12 Pt 1):6308–6313. [PubMed] [Google Scholar]
- Grill E., Winnacker E. L., Zenk M. H. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science. 1985 Nov 8;230(4726):674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Ditz K. M., Singletary G. W., Leland T. J. Gramine Accumulation in Leaves of Barley Grown under High-Temperature Stress. Plant Physiol. 1983 Apr;71(4):896–904. doi: 10.1104/pp.71.4.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Bloch P. L., Van Bogelen R. A., Neidhardt F. C. Multiple forms of lysyl-transfer ribonucleic acid synthetase in Escherichia coli. J Bacteriol. 1981 Apr;146(1):345–351. doi: 10.1128/jb.146.1.345-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issels R. D., Bourier S., Biaglow J. E., Gerweck L. E., Wilmanns W. Temperature-dependent influence of thiols upon glutathione levels in Chinese hamster ovary cells at cytotoxic concentrations. Cancer Res. 1985 Dec;45(12 Pt 1):6219–6224. [PubMed] [Google Scholar]
- Kapp D. S., Hahn G. M. Thermosensitization by sulfhydryl compounds of exponentially growing Chinese hamster cells. Cancer Res. 1979 Nov;39(11):4630–4635. [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. AppppA, heat-shock stress, and cell oxidation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7496–7500. doi: 10.1073/pnas.80.24.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Roberts J. K., Key J. L. Acquisition of Thermotolerance in Soybean Seedlings : Synthesis and Accumulation of Heat Shock Proteins and their Cellular Localization. Plant Physiol. 1984 Jan;74(1):152–160. doi: 10.1104/pp.74.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey J. C., Reed D. J. Measurement of glutathione-protein mixed disulfides. Int J Radiat Oncol Biol Phys. 1984 Sep;10(9):1507–1510. doi: 10.1016/0360-3016(84)90491-7. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Mitchell J. B., Russo A., Kinsella T. J., Glatstein E. Glutathione elevation during thermotolerance induction and thermosensitization by glutathione depletion. Cancer Res. 1983 Mar;43(3):987–991. [PubMed] [Google Scholar]
- Newton G. L., Dorian R., Fahey R. C. Analysis of biological thiols: derivatization with monobromobimane and separation by reverse-phase high-performance liquid chromatography. Anal Biochem. 1981 Jul 1;114(2):383–387. doi: 10.1016/0003-2697(81)90498-x. [DOI] [PubMed] [Google Scholar]
- Phillips T. A., VanBogelen R. A., Neidhardt F. C. lon gene product of Escherichia coli is a heat-shock protein. J Bacteriol. 1984 Jul;159(1):283–287. doi: 10.1128/jb.159.1.283-287.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfelder M., Horsch A., Schmid H. P. Heat shock increases the synthesis of the poly(A)-binding protein in HeLa cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6884–6888. doi: 10.1073/pnas.82.20.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]


