Abstract
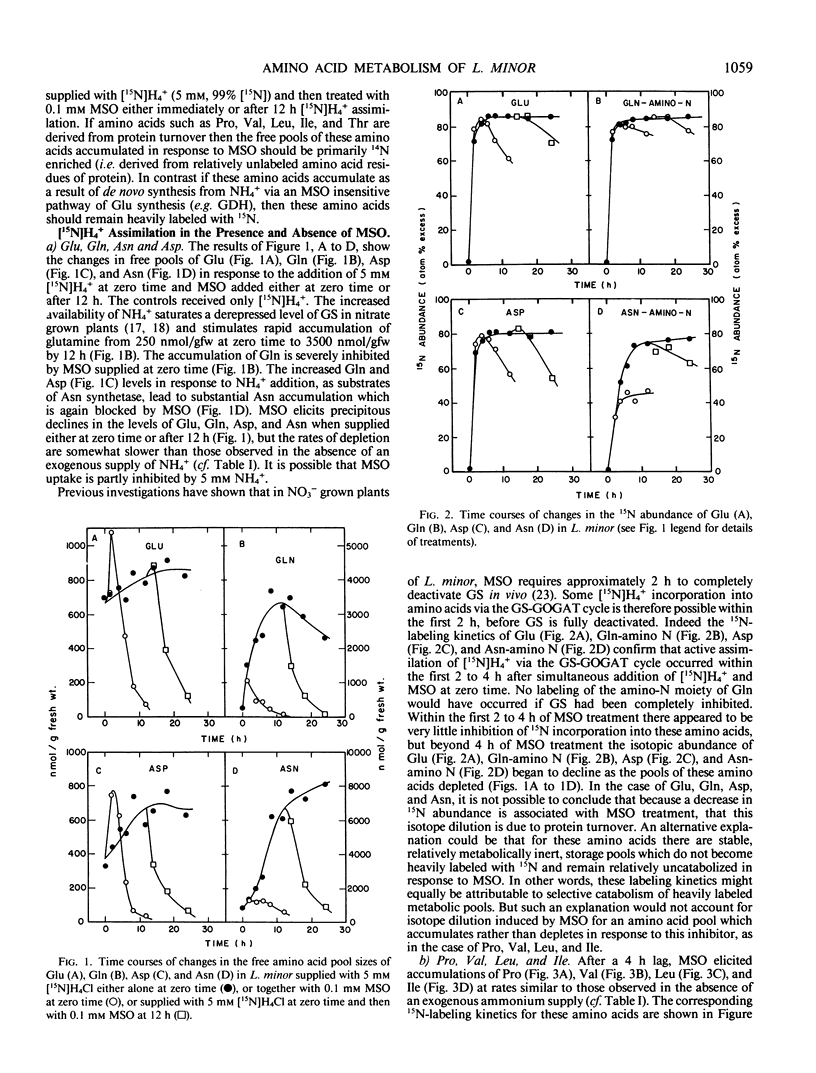
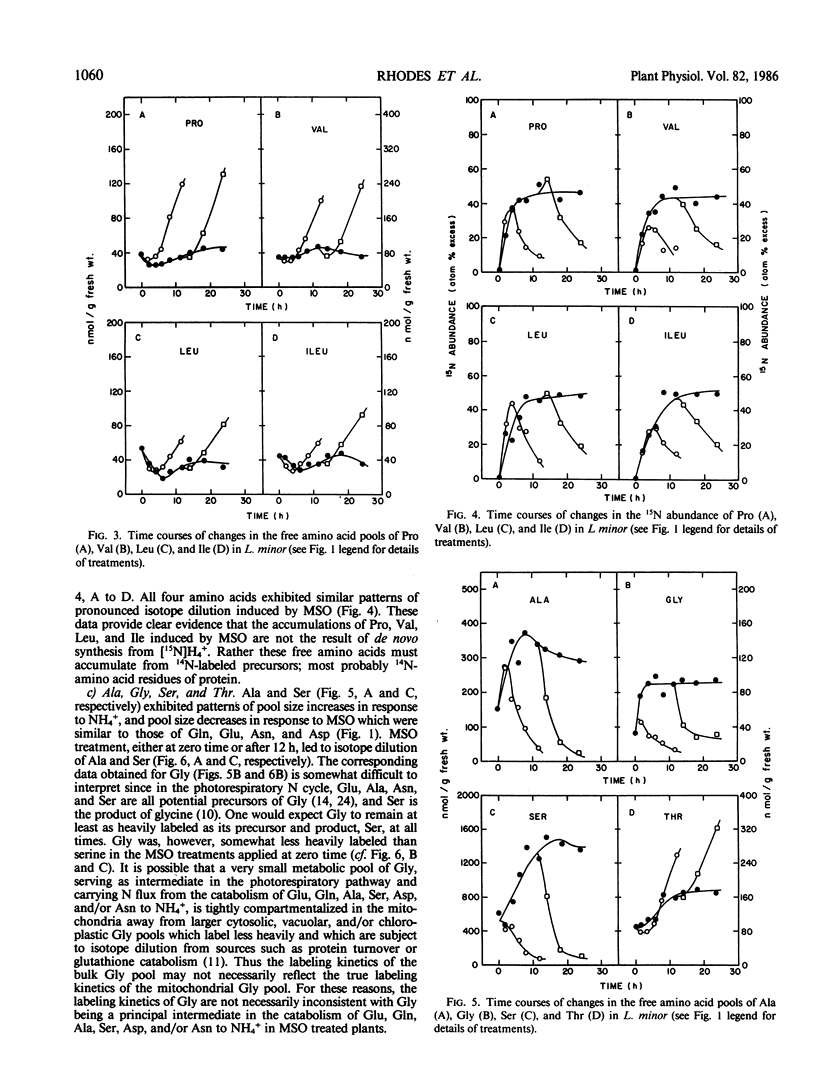
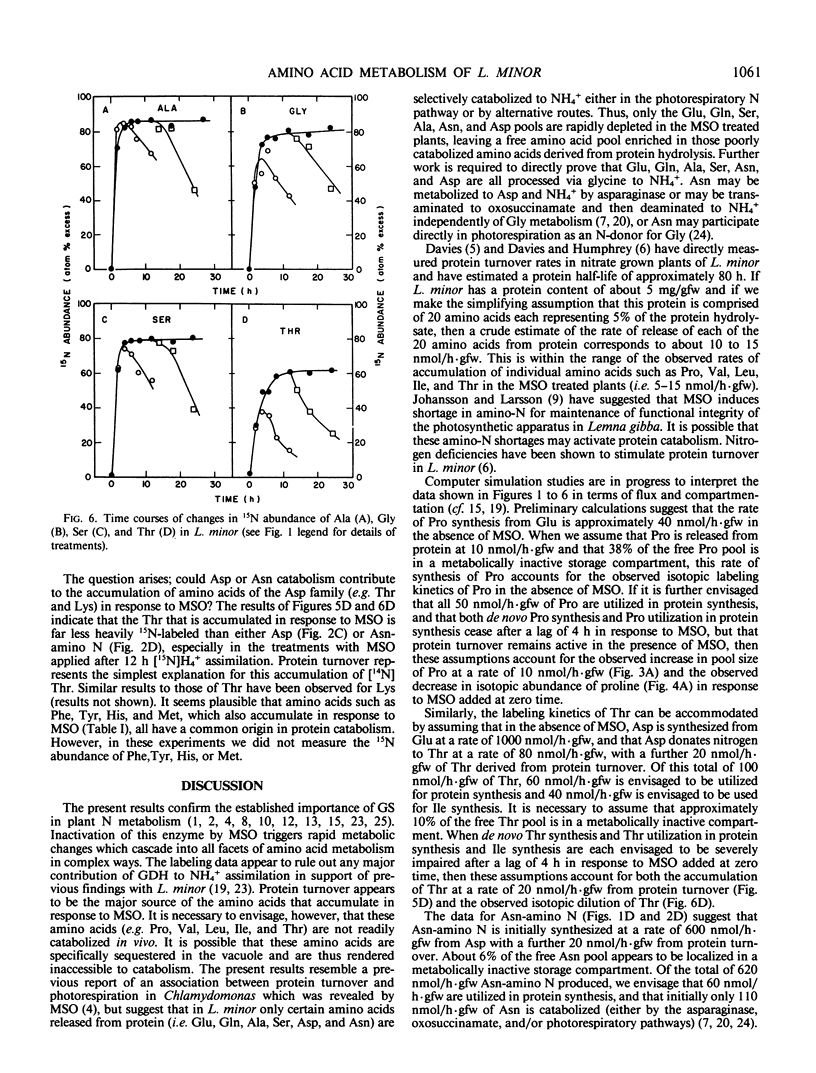
When Lemna minor L. is supplied with the potent inhibitor of glutamine synthetase, methionine sulfoximine, rapid changes in free amino acid levels occur. Glutamine, glutamate, asparagine, aspartate, alanine, and serine levels decline concomitantly with ammonia accumulation. However, not all free amino acid pools deplete in response to this inhibitor. Several free amino acids including proline, valine, leucine, isoleucine, threonine, lysine, phenylalanine, tyrosine, histidine, and methionine exhibit severalfold accumulations within 24 hours of methionine sulfoximine treatment. To investigate whether these latter amino acid accumulations result from de novo synthesis via a methionine sulfoximine insensitive pathway of ammonia assimilation (e.g. glutamate dehydrogenase) or from protein turnover, fronds of Lemna minor were prelabeled with [15N]H4+ prior to supplying the inhibitor. Analyses of the 15N abundance of free amino acids suggest that protein turnover is the major source of these methionine sulfoximine induced amino acid accumulations. Thus, the pools of valine, leucine, isoleucine, proline, and threonine accumulated in response to the inhibitor in the presence of [15N]H4+, are 14N enriched and are not apparently derived from 15N-labeled precursors. To account for the selective accumulation of amino acids, such as valine, leucine, isoleucine, proline, and threonine, it is necessary to envisage that these free amino acids are relatively poorly catabolized in vivo. The amino acids which deplete in response to methionine sulfoximine (i.e. glutamate, glutamine, alanine, aspartate, asparagine, and serine) are all presumably rapidly catabolized to ammonia, either in the photorespiratory pathway or by alternative routes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies D. D., Humphrey T. J. Amino Acid recycling in relation to protein turnover. Plant Physiol. 1978 Jan;61(1):54–58. doi: 10.1104/pp.61.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Tolbert N. E. Serine: glyoxylate, alanine:glyoxylate, and glutamate:glyoxylate aminotransferase reactions in peroxisomes from spinach leaves. J Biol Chem. 1983 Jun 25;258(12):7631–7638. [PubMed] [Google Scholar]
- Rhodes D., Myers A. C., Jamieson G. Gas Chromatography-Mass Spectrometry of N- Heptafluorobutyryl Isobutyl Esters of Amino Acids in the Analysis of the Kinetics of [N]H(4) Assimilation in Lemna minor L. Plant Physiol. 1981 Nov;68(5):1197–1205. doi: 10.1104/pp.68.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokut T. A., Wolk C. P., Thomas J., Meeks J. C., Shaffer P. W. Initial organic products of assimilation of [N]ammonium and [N]nitrate by tobacco cells cultured on different sources of nitrogen. Plant Physiol. 1978 Aug;62(2):299–304. doi: 10.1104/pp.62.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta T. C., Joy K. W., Ireland R. J. Role of asparagine in the photorespiratory nitrogen metabolism of pea leaves. Plant Physiol. 1985 Jun;78(2):334–337. doi: 10.1104/pp.78.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C., Morot-Gaudry J. F., Summons R. E., Osmond C. B. Evidence for the Glutamine Synthetase/Glutamate Synthase Pathway during the Photorespiratory Nitrogen Cycle in Spinach Leaves. Plant Physiol. 1982 Nov;70(5):1514–1517. doi: 10.1104/pp.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya T., Oaks A., Rhodes D., Matsumoto H. Synthesis of [N]glutamate from [N]h(4) and [N]glycine by mitochondria isolated from pea and corn shoots. Plant Physiol. 1986 Jul;81(3):754–757. doi: 10.1104/pp.81.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]


