Abstract
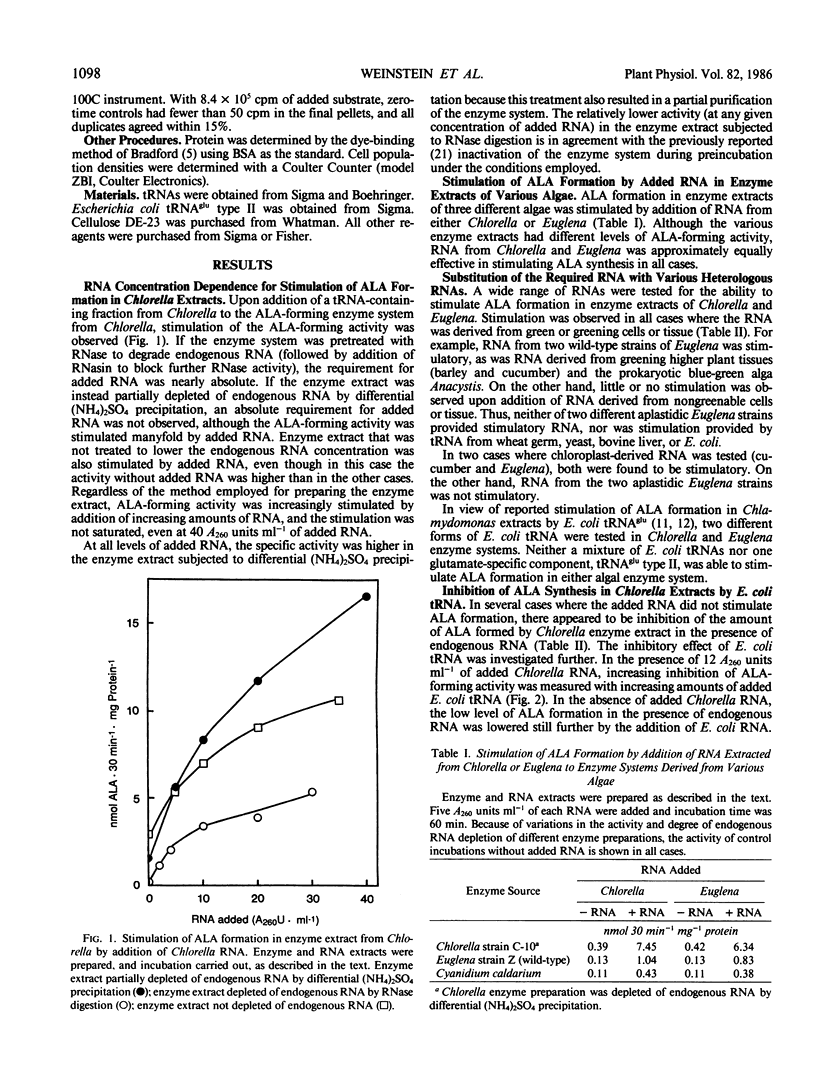
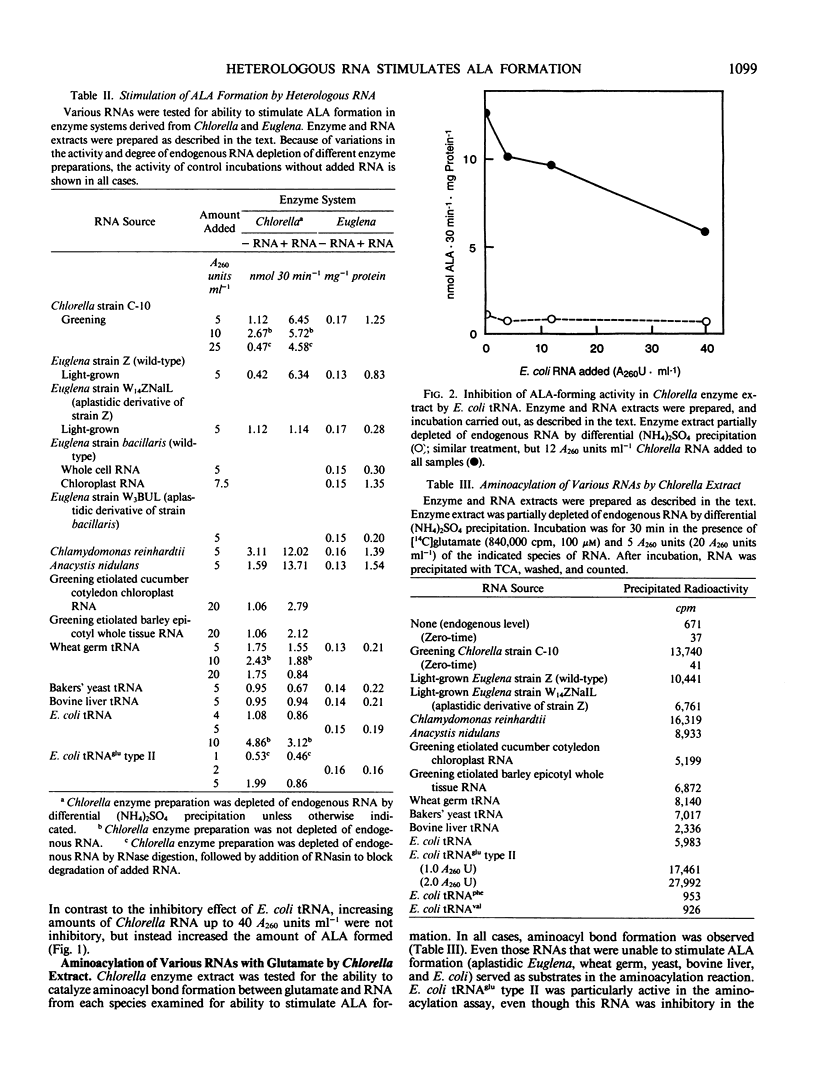
Formation of the chlorophyll and heme precursor δ-aminolevulinic acid (ALA) from glutamate in soluble extracts of Chlorella vulgaris, Euglena gracilis, and Cyanidium caldarium was stimulated by addition of low molecular weight RNA derived from greening algae or plant tissue. Enzyme extracts were prepared for the ALA formation assay by high-speed centrifugation, partial RNA depletion, and gel filtration through Sephadex G-25. RNA was extracted from greening barley epicotyls, greening cucumber cotyledon chloroplasts, and growing cells of Chlorella, Euglena, Chlamydomonas reinhardtii, and Anacystis nidulans, freed of protein, and fractionated on DEAE-cellulose to yield an active component corresponding to the tRNA-containing fraction. RNA from homologous and heterologous species stimulated ALA formation when added to enzyme extracts, and the degree of stimulation was proportional to the amount of RNA added. Algal enzyme extracts were stimulated by algal RNAs interchangeably, with the exception of RNA prepared from aplastidic Euglena, which did not stimulate ALA production. RNA from greening cucumber cotyledon chloroplasts and greening barley epicotyls stimulated ALA formation in algal enzyme incubations. In contrast, tRNA from Escherichia coli, both nonspecific and glutamate-specific, as well as wheat germ, bovine liver, and yeast tRNA, failed to reconstitute ALA formation. Moreover, E. coli tRNA inhibited ALA formation by algal extracts, both in the presence and absence of added algal RNA. Chlorella extracts were capable of catalyzing aminoacyl bond formation between glutamate and both the activity reconstituting and nonreconstituting RNAs, indicating that the inability of some RNAs to stimulate ALA formation was not due to their inability to serve as glutamyl acceptors. The first step in the ALA-forming reaction sequence has been proposed to be activation of glutamate via aminoacyl bond formation with a specific tRNA, analogous to the first step in peptide bond formation. Our results suggest that the RNA that is required for ALA formation may be functionally distinct from the glutamyl-tRNA species involved in protein synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I., Chen N. C. N-Methyl Mesoporphyrin IX Inhibits Phycocyanin, but Not Chlorophyll Synthesis in Cyanidium caldarium. Plant Physiol. 1983 Feb;71(2):263–268. doi: 10.1104/pp.71.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Cornejo J. Enzymatic heme oxygenase activity in soluble extracts of the unicellular red alga, Cyanidium caldarium. Arch Biochem Biophys. 1984 Dec;235(2):371–384. doi: 10.1016/0003-9861(84)90210-8. [DOI] [PubMed] [Google Scholar]
- Beale S. I., Foley T., Dzelzkalns V. delta-Aminolevulinic acid synthase from Euglena gracilis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1666–1669. doi: 10.1073/pnas.78.3.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Damuni Z., Caudwell F. B., Cohen P. Regulation of the aminoacyl-tRNA synthetase complex of rat liver by phosphorylation/dephosphorylation in vitro and in vivo. Eur J Biochem. 1982 Dec;129(1):57–65. doi: 10.1111/j.1432-1033.1982.tb07020.x. [DOI] [PubMed] [Google Scholar]
- EDELMAN M., SCHIFF J. A., EPSTEIN H. T. STUDIES OF CHLOROPLAST DEVELOPMENT IN EUGLENA. XII. TWO TYPES OF SATELLITE DNA. J Mol Biol. 1965 Apr;11:769–774. doi: 10.1016/s0022-2836(65)80034-1. [DOI] [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Huang D. D., Wang W. Y. Chlorophyll biosynthesis in Chlamydomonas starts with the formation of glutamyl-tRNA. J Biol Chem. 1986 Oct 15;261(29):13451–13455. [PubMed] [Google Scholar]
- Huang D. D., Wang W. Y., Gough S. P., Kannangara C. G. delta-Aminolevulinic acid-synthesizing enzymes need an RNA moiety for activity. Science. 1984 Sep 28;225(4669):1482–1484. doi: 10.1126/science.6206568. [DOI] [PubMed] [Google Scholar]
- Pardo A. D., Chereskin B. M., Castelfranco P. A., Franceschi V. R., Wezelman B. E. ATP requirement for mg chelatase in developing chloroplasts. Plant Physiol. 1980 May;65(5):956–960. doi: 10.1104/pp.65.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneegurt M. A., Beale S. I. Biosynthesis of protoheme and heme a from glutamate in maize. Plant Physiol. 1986 Aug;81(4):965–971. doi: 10.1104/pp.81.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Enzymatic conversion of glutamate to delta-aminolevulinate in soluble extracts of the unicellular green alga, Chlorella vulgaris. Arch Biochem Biophys. 1985 Mar;237(2):454–464. doi: 10.1016/0003-9861(85)90299-1. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. RNA is required for enzymatic conversion of glutamate to delta-aminolevulinate by extracts of Chlorella vulgaris. Arch Biochem Biophys. 1985 May 15;239(1):87–93. doi: 10.1016/0003-9861(85)90814-8. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Separate physiological roles and subcellular compartments for two tetrapyrrole biosynthetic pathways in Euglena gracilis. J Biol Chem. 1983 Jun 10;258(11):6799–6807. [PubMed] [Google Scholar]


