Abstract
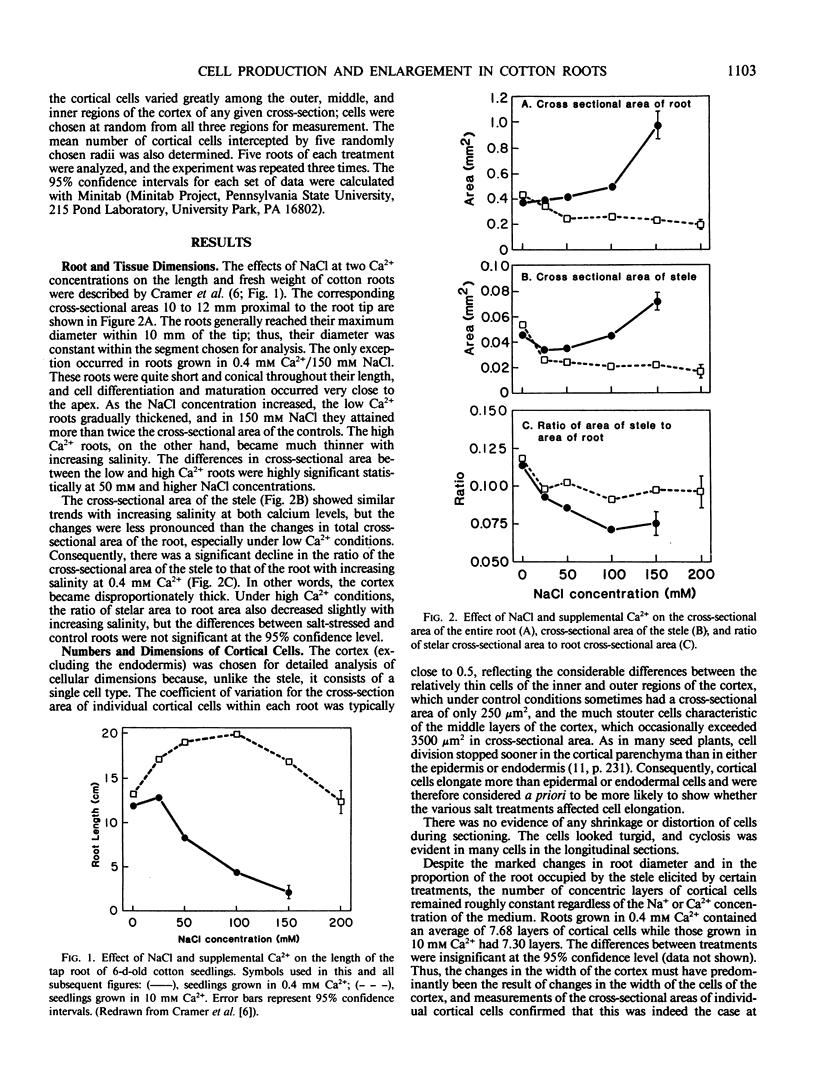
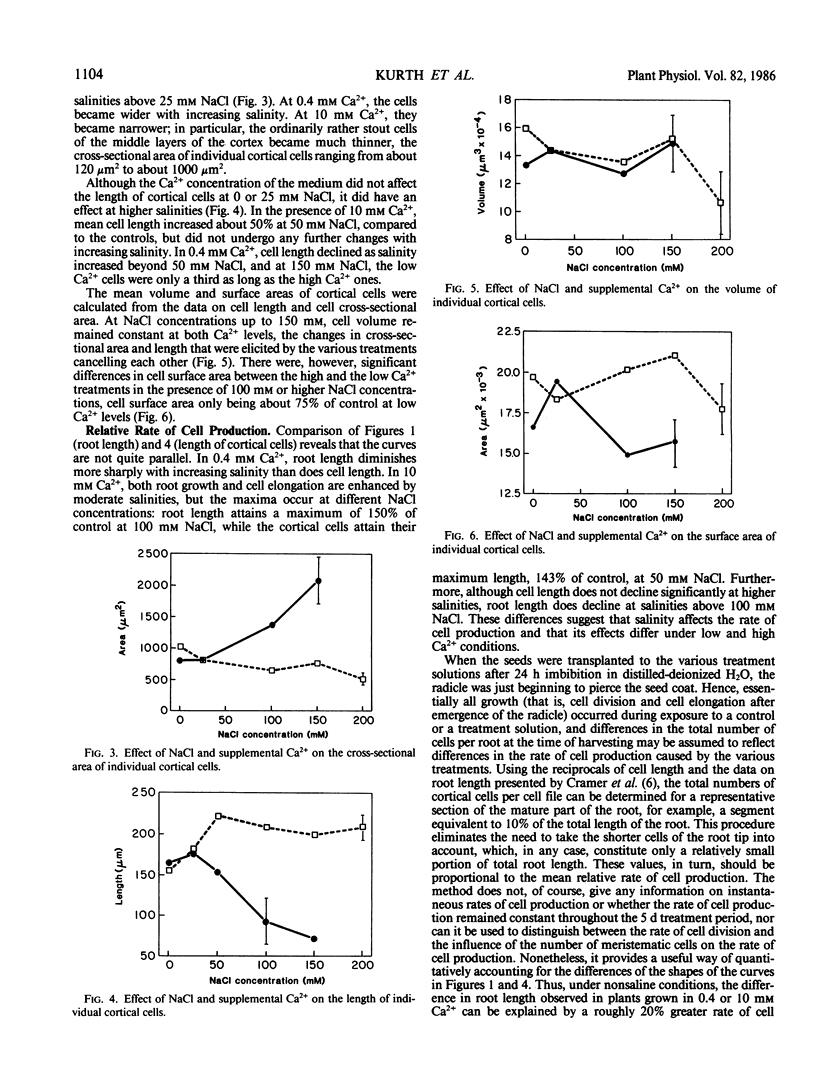
In many crop species, supplemental Ca2+ alleviates the inhibition of growth typical of exposure to salt stress. In hydroponically grown cotton seedlings (Gossypium hirsutum L. cv Acala SJ-2), both length and weight of the primary root were enhanced by moderate salinities (25 to 100 millimolar NaCl) in the presence of 10 millimolar Ca2+, but the roots became thinner. Anatomical analysis showed that the cortical cells of these roots were longer and narrower than those of the control plants, while cortical cells of roots grown at the same salinities but in the presence of only 0.4 millimolar Ca2+ became shorter and more nearly isodiametrical. Cell volume, however, was not affected by salinities up to 200 millimolar NaCl at either 0.4 or 10 millimolar Ca2+. Our observations suggest Ca2+-dependent effects of salinity on the cytoskeleton. The rate of cell production declined with increasing salinity at 0.4 millimolar Ca2+ but at 10 millimolar Ca2+ was not affected by salinities up to 150 millimolar NaCl.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cramer G. R., Läuchli A., Epstein E. Effects of NaCl and CaCl(2) on Ion Activities in Complex Nutrient Solutions and Root Growth of Cotton. Plant Physiol. 1986 Jul;81(3):792–797. doi: 10.1104/pp.81.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer G. R., Läuchli A., Polito V. S. Displacement of ca by na from the plasmalemma of root cells : a primary response to salt stress? Plant Physiol. 1985 Sep;79(1):207–211. doi: 10.1104/pp.79.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham A. C., Walton J. M. Calcium ions and the control of proliferation in normal and cancer cells. Biosci Rep. 1982 Jan;2(1):15–30. doi: 10.1007/BF01142195. [DOI] [PubMed] [Google Scholar]
- Hepler P. K. Calcium restriction prolongs metaphase in dividing Tradescantia stamen hair cells. J Cell Biol. 1985 May;100(5):1363–1368. doi: 10.1083/jcb.100.5.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Miller M. W., Carstensen E. L., Brayman A. A. The relationship between sensitivity to 60-Hz electric fields and induced transmembrane potentials in plants root cells. Radiat Environ Biophys. 1985;24(4):303–314. doi: 10.1007/BF01210937. [DOI] [PubMed] [Google Scholar]
- Kuzmanoff K. M., Evans M. L. Kinetics of Adaptation to Osmotic Stress in Lentil (Lens culinaris Med.) Roots. Plant Physiol. 1981 Jul;68(1):244–247. doi: 10.1104/pp.68.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahaye P. A., Epstein E. Salt toleration by plants: enhancement with calcium. Science. 1969 Oct 17;166(3903):395–396. doi: 10.1126/science.166.3903.395. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Evans M. L. Polar transport of auxin across gravistimulated roots of maize and its enhancement by calcium. Plant Physiol. 1985;77:824–827. doi: 10.1104/pp.77.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett J. E., Gilbert W. A. Localization of the ca-mediated apparent ion selectivity in the cross sectional volume of soybean roots. Plant Physiol. 1967 Dec;42(12):1658–1664. doi: 10.1104/pp.42.12.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]


