Key Clinical Message
This clinical case demonstrates quick resolution of nail psoriasis in a patient treated with risankizumab, highlighting the role of IL‐23 in the pathogenesis of nail psoriasis.
Keywords: nail, nail psoriasis, psoriasis, risankizumab
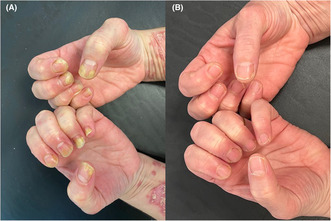
(A) Nail psoriasis in both hands. (B): Week 16 Improvement of Nail Psoriasis (NAPSI: 0).
1. INTRODUCTION
Psoriasis is a chronic inflammatory skin disease, characterized by erythematous and scaly plaques, with possible involvement of scalp, nails, and joints. 1 Nail involvement can affect up to 80% of patients with psoriasis, representing a risk factor for the development of psoriatic arthritis (PsA). 2
2. CASE PRESENTATION
We describe the case of a 47‐year‐old female patient with a 8‐month history of plaque‐type psoriasis with a concomitant nail involvement. At dermatological evaluation, erythematous and scaly patches and plaques were observed on trunk, extremities, and scalp with a PASI score of 36. Moreover, also a nail dystrophy of both hands was observed, with subungual hyperkeratosis, pitting, onycholysis, and discoloration (Nail psoriasis severity index—NAPSI: 55) (Figure 1). Psoriasis had a strong impact of patient's quality of life, affecting her personal and working activities (Dermatology life quality index—DLQI: 20). Patient referred previous treatments with phototherapy (nb UVB) and cyclosporine, both suspended for inefficacy. A biologic treatment was started, with risankizumab 150 mg subcutaneously at Week 0, at Week 4 and then every 12 weeks. At Week 4, a strong clinical improvement was noted, with reduction of cutaneous lesions (PASI 14, DLQI: 4), and mild improvement of nail dystrophy (NAPSI 20). At Week 16, a complete resolution of cutaneous manifestations (PASI 0; DLQI 0) and of nail disease (NAPSI 0) was observed (Figure 1B).
FIGURE 1.
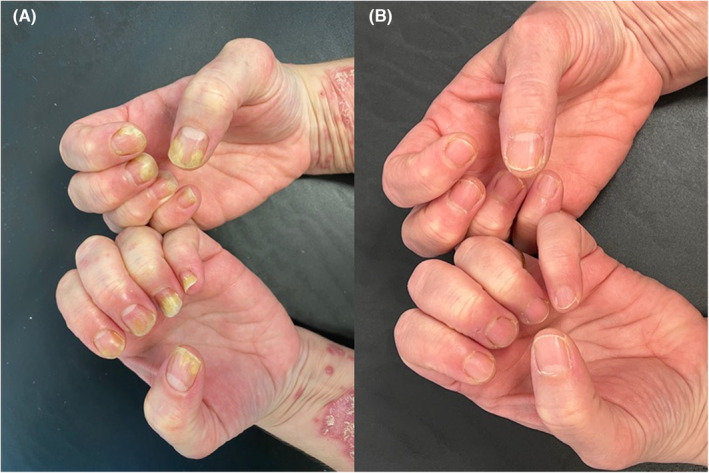
(A) Nail psoriasis in both hands. (B): Week 16 Improvement of Nail Psoriasis (NAPSI: 0).
3. CONCLUSION
Nail psoriasis is frequently associated with severe disease and can be accompanied by pain and functional impairment, with a strong impact on patients' quality of life. 3 It is challenging to treat and is often resistant to common topical and systemic treatments. Risankizumab, a humanized immunoglobulin G1 monoclonal antibody that specifically inhibits interleukin (IL)‐23 binding to p19 subunit, is recommended in patients with moderate‐to‐severe psoriasis. Some studies in the literature showed the efficacy and safety of risankizumab also on nail psoriasis. 4 , 5
We described this case to demonstrate quick resolution of nail psoriasis in a patient treated with risankizumab, highlighting the role of IL‐23 in the pathogenesis of nail psoriasis.
AUTHOR CONTRIBUTIONS
Orsini Diego: Conceptualization; validation; writing – original draft; writing – review and editing. Pacifico Alessia: Supervision; visualization; writing – review and editing. Iacovelli Paolo: Supervision; validation; visualization. Frascione Pasquale: Supervision; validation; visualization. De Simone Paola: Supervision; validation; visualization. Assorgi Chiara: Validation; visualization; writing – original draft; writing – review and editing. Pigliacelli Flavia: Conceptualization; writing – original draft; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
None.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENT
Open access funding provided by BIBLIOSAN.
Orsini D, Pacifico A, Iacovelli P, et al. Rapidly and successful improvement of nail psoriasis with risankizumab. Clin Case Rep. 2023;11:e8035. doi: 10.1002/ccr3.8035
All authors contributed equally to this work.
DATA AVAILABILITY STATEMENT
Additional data supporting the findings of this manuscript are available upon reasonable request to the corresponding author.
REFERENCES
- 1. Nair PA, Badri T. Psoriasis. 2022. StatPearls [Internet]. StatPearls publishing; 2023. [Google Scholar]
- 2. Bardazzi F, Starace M, Bruni F, Magnano M, Piraccini BM, Alessandrini A. Nail psoriasis: an updated review and expert opinion on Available treatments. Including Biologics Acta Derm Venereol. 2019;99(6):516‐523. doi: 10.2340/00015555-3098 [DOI] [PubMed] [Google Scholar]
- 3. Thomas L, Azad J, Takwale A. Management of nail psoriasis. Clin Exp Dermatol. 2021;46(1):3‐8. doi: 10.1111/ced.14314 [DOI] [PubMed] [Google Scholar]
- 4. Kristensen LE, Soliman AM, Papp K, et al. Effects of risankizumab on nail psoriasis in patients with active psoriatic arthritis: results from KEEPsAKE 1. J Eur Acad Dermatol Venereol. 2022;36(5):e389‐e392. [DOI] [PubMed] [Google Scholar]
- 5. AlAjlan A, Qasim R, AlTassan F, et al. A case of nail psoriasis improved with treatment by risankizumab. Oxf Med Case Reports. 2022;2022:397‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional data supporting the findings of this manuscript are available upon reasonable request to the corresponding author.


