Abstract
Twenty-nine of 34 (85%) Zn-finger-active compounds at 300 μM or less inhibited the growth of Giardia lamblia. The most active compound, disulfiram (Antabuse), was cidal at 1.23 ± 0.32 μM. In the adult mouse model, significant in vivo activity was demonstrated by increased cure rates and decreased parasite burdens.
Giardia lamblia is a protozoan parasite that inhabits the small intestines of humans and other mammals. It is among the world’s most common disease-causing parasites, and in the United States (3) it is responsible for epidemics of waterborne diarrhea (17) as well as gastrointestinal illness wherever fecal contamination occurs. In addition to its importance as a pathogen, there is considerable interest in its biology because it is among the most primitive eukaryotes (33). The trophozoite, or growing form of the parasite, is completely covered by one of a family of proteins (variant-specific surface proteins [VSPs]) that undergoes surface antigenic variation (1, 22, 23). VSPs are unique surface cysteine-rich proteins with numerous CXXC motifs (11, 23), a conserved carboxyl terminus (20), and one or more Zn-finger motifs which closely resembles the LIM- and RING-finger motifs found in other Zn binding proteins of higher eukaryotes (22, 25, 35). Zn and Fe have been detected in one VSP (19) predominately expressed in GS/H7, an isolate used here (24), but not in another isolate (28). Most interestingly, no other surface-residing Zn-finger protein exists in any other organism.
Zn-finger proteins are essential to normal cellular function and developmental processes (2). Inhibition of microbe-specific Zn-finger protein activity is a novel approach to chemotherapeutic intervention (26, 29). Zn-finger-active chemotherapeutic agents which inhibit replication of human immunodeficiency virus type 1 (HIV-1) have been designed (26–29). These compounds covalently modify the highly conserved Cys(X)2Cys(X)4His(X)4Cys (CCHC) retroviral Zn-finger domains of the HIV-1 nucleocapsid p7 protein (NCp7) (27) and prevent their essential function. Competitive Zn-finger peptides have also been shown to have a modest effect against the influenza virus (15). Because Giardia has abundant surface-located Zn-finger proteins which may be particularly susceptible to Zn-finger-active compounds, a series of compounds (Tables 1 and 2) with known activity toward HIV-1 NCp7 Zn fingers were tested in vitro for their antiparasitic activities, and one of the most active compounds, disulfiram, was also tested for its activity in vivo.
TABLE 1.
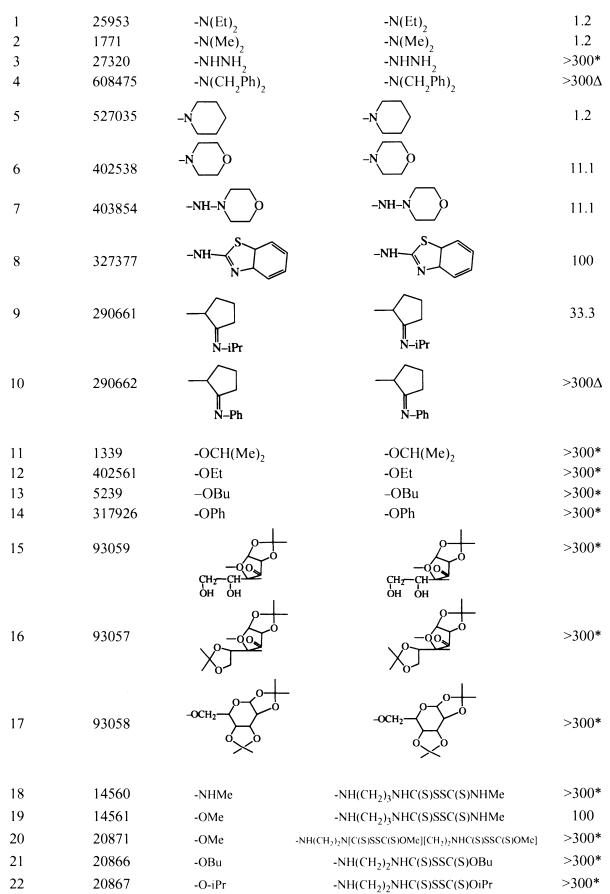
Compound identification, structure, and cidal activity against G. lamblia for thiuram compoundsa
Asterisks indicate that a recognizable degree of inhibition of growth occurred when the compound was used at 300 μM. The delta symbol indicates that the compound at 300 μM had no effect on growth. Compound 1 is disulfira. Me, methyl; Bu, butyl; Et, ethyl; Ph, phenyl; Pr, propyl.
TABLE 2.
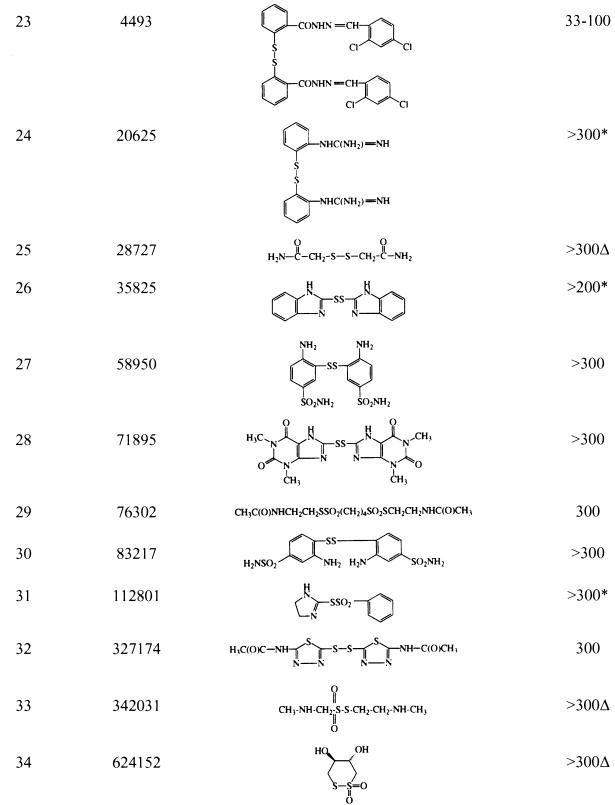
Compound identification, structure, and cidal activity against G. lamblia for nonthiuram compoundsa
See footnote a of Table 1 for definitions of symbols.
Most of the in vitro studies used Giardia isolate WB clone 1267 (WB/1267) (32), but limited assays used isolate GS clone H7 (GS/H7) (24) because this clone was used in vivo. Organisms were maintained as previously described in TYI-S-33 medium with bile and antibiotics (16). Inhibition and cidal activities were determined in 96-well culture plates by methods similar to those reported previously (22). Compounds were supplied at known concentrations in dimethyl sulfoxide (DMSO) by the Drug Synthesis and Chemistry Branch, Development Therapeutics Branch, National Cancer Institute, or, when supplied as dry compound, were dissolved in DMSO at a stock concentration of 100 mM and then added to medium containing various concentrations of cysteine. Typically, 100 to 200 μl of compound in medium was placed in 96-well plates. Depending on the experiment, from 20,000 to 50,000 trophozoites were then added in volumes ranging between 5 to 30 μl. Controls consisted of wells with normal medium, an appropriate concentration of cysteine, and the DMSO solvent, which had no effect on growth at the concentrations used in the study. Plates were incubated anaerobically in sealed bags (22) at 37°C for up to 1 week and were scored visually at various time periods, but the standard period of recording in the present study was at 18 to 20 h. The wells were scored as 0 when no viable organisms were observed, +1/2 when rare motile organisms were present, +1 when a small number of organisms showing movement were present (<20 trophozoites), +2 when moderate growth and adherence of organisms were present, +3 when significant growth that was less than that of untreated controls was present, and +4 when growth equal to that of the control containing the same amount of cysteine was present. Assessment of the cidal effects observed visually at 18 to 20 h was verified in some experiments by quantitative measurement of viable trophozoites after 3 days in culture by a previously described method (22).
The presence of the usual 11.3 mM cysteine in medium blunted the activities of these compounds. For the most active compounds, cidal activity was decreased from 28- to 250-fold in 11.3 mM cysteine compared to that in 2.8 mM cysteine. In studies with GS/H7 a dose-response inhibition of activity was demonstrated with compound 1, disulfiram (y = 3.183 − 0.032; r = 0.996). The minimal amount of cysteine required to yield +4 growth in control wells varied and was likely due to the differences in the oxidation of the added cysteine and to the amount of cysteine in the serum added to TYI-S-33 medium. Block titrations indicated that 2.8 mM prepared daily and added immediately to the medium gave +4 growth and reproducible cidal levels of drug when the WB isolate was used.
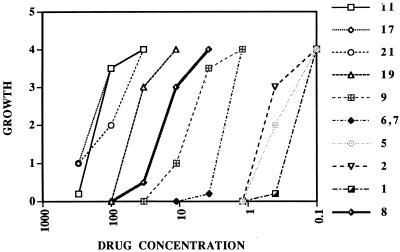
The most active compounds were the thiuram derivatives. Among the 34 compounds tested, 29 of 34 (85%) were cidal or showed some degree of growth inhibition when the compound was used at 300 μM (Tables 1 and 2). Of the 12 nonthiuram compounds screened at 300 μM, four exhibited cidal effects at 300 μM, four caused a reduction of growth, and four did not inhibit growth. On further testing, only compound NSC 4493 was cidal at concentrations of <100 μM. Eleven of 22 thiuram compounds that scored from 0 to +1/2 growth (i.e., effective growth inhibition) when they were used at 300 μM were analyzed further. As shown in Fig. 1, a wide range of activities among these compounds was observed, but disulfiram (NSC 25953; compound 1) was among the most active, yielding a mean total cidal concentration of 1.23 μM. Four different experiments yielded mean cidal levels of 1.17 ± 0.32 μM (standard deviation). Quantitative assessment showed no growth at concentrations of 0.88 μM or higher and a graded increase in organisms with decreasing drug concentration (5), which is consistent with analysis by visual assessment (data not shown). Compound 5 (NSC 527035) showed cidal activity at 1.23 and 1 μM in two separate experiments.
FIG. 1.
Sensitivity of G. lamblia to thiuram compounds. The following compounds have the indicated NSC numbers: 1 (disulfiram), NSC 25953; 2, NSC 1771; 5, NSC 527035; 6, NSC 402538; 7, NSC 403854; 9, NSC 290661; 17, NSC 93058; 19, NSC 14561; and 21, NSC 20866. Growth was scored visually at 18 to 20 h as described in the text. 0 signifies no growth, and +4 signifies growth equal to that in the control wells.
The in vivo efficacy of compound NSC 25953 (disulfiram) against G. lamblia was tested because it was among the most active compounds in vitro, was available in large quantities commercially (Aldrich Chemical Company), and is used for the treatment of alcoholism in humans (8). The commercial drug showed the same activity in vitro as the supplied drug. Since GS/H7 is used in the adult mouse model of G. lamblia infection, the activity of disulfiram was tested against this isolate, and it was found to have sensitivity comparable to that of disulfiram in 2.8 mM cysteine when the sensitivities were compared at the same time. Adult C57B female mice were obtained from Takonic Labs and were housed as described previously (4). The mice were inoculated with 500,000 trophozoites by gavage on day 0, treated on days 3 through 6, and then killed on day 7. Disulfiram or metronidazole was administered by gavage as a fine suspension in 200 μl of water. Small intestines were minced in 10 ml of ice-cold medium, allowed to cool for 30 min, and then warmed to 37°C. The number of motile trophozoites in five random fields at all depths not obscured by intestines were counted at a magnification of ×200. If no organisms were noted, then five counts at a magnification of ×25 were performed. Cure was defined as the failure to detect any trophozoites. All but 1 of 30 untreated control mice were infected. In contrast, cure rates of 40, 40, and 21% were found in the three groups of treated animals, respectively (Table 3). The parasite burden was significantly decreased in all three experiments and in the mice treated with the largest dose of disulfiram (25 mg twice daily for 4 days), an average of 2.03 ± 3.1 trophozoites were found in the treated animals, whereas 78.2 ± 28.6 trophozoites were found in the control group. To confirm this model as a valid measure of chemotherapeutic efficacy for anti-Giardia compounds, the effectiveness of metronidazole, a drug that is known to be active and that is commonly used for the treatment of giardiasis, was evaluated. All of the metronidazole-treated mice were cured, whereas 1 of 10 of the control mice were cured.
TABLE 3.
In vivo efficacy of disulfiram
| Expt no. | Drug (dosage)a | Treated mice
|
Control mice
|
||
|---|---|---|---|---|---|
| No. cured/total no. (P value) | No. of trophozoites/high-power field (P value) | No. cured/total no. | No. of trophozoites/high-power field | ||
| 1 | Disulfiram (25 mg q.d., 4 days) | 4/10 (0.003) | 2.87 ± 7.85 (0.049) | 0/10 | 6.74 ± 9.30 |
| 2 | Disulfiram (25 mg, q.d., 5 days) | 3/14 (0.082) | 5.23 ± 7.33 (0.011) | 1/10 | 13.16 ± 6.78 |
| Metronidazole (5 mg, q.d., 5 days) | 15/15 | 0 | 1/10 | 13.16 ± 6.78 | |
| 3 | Disulfiram (25 mg, b.i.d., 4 days) | 4/10 (0.003) | 2.03 ± 3.12 (<0.001) | 0/10 | 78.20 ± 28.69 |
q.d., every day; b.i.d., twice a day.
The major findings of the present study are that many Zn-finger-active compounds have activity against G. lamblia in vitro and one of the most active compounds in vitro, disulfiram, showed efficacy in vivo. Additionally, the adult mouse model of infection proved to be a convenient and viable system for testing the efficacies of drugs in vivo.
The compounds tested had various degrees of activity against G. lamblia, and a majority of the most active compounds were thiruram derivatives. Despite the various effects of cysteine on the activities of these compounds, the results were reproducible and relatively consistent. The mode of action of Zn-finger-active compounds and disulfiram (12) against HIV infection is destruction of Zn-finger motifs in NCp7; the mode of action against G. lamblia is unclear. However, there was a strong correlation between the ability of these compounds to eject Zn from the NCp7 protein of HIV-1 and the in vitro activities of the same compounds against G. lamblia (extracted from reference 29). Five of 5 Zn-releasing compounds were cidal at levels of ≤10 μM, while 12 of 12 compounds unable to release Zn effectively were cidal at levels of ≥50 μM and most were cidal at ≥300 μM.
The in vivo activity of disulfiram was clearly demonstrated and was modest compared to the high degree of activity demonstrated in vitro. At the highest dosage, 25 mg twice daily for 4 days, 40% of the mice were cured, whereas 0% of the controls were cured, and the remaining mice had a dramatically decreased parasite burden. Despite the differences in the numbers of parasites in the control mice, which is a variability seen with this animal model, the results of all three experiments were consistent and showed the efficacy of disulfiram. It is likely that the activity of disulfiram would have been greater if the solubility of disulfiram could have been increased to raise the concentration of drug able to affect the parasite and facilitate administration of the drug.
The biological effects of disulfiram and its metabolism are complex and are reviewed elsewhere (5–10). Disulfiram and/or its immediate metabolites have been shown to be active in vitro against a number of organisms, including Plasmodium falciparum (31), Trypanosoma cruzi (18), Trichomonas vaginalis (14), and Entamoeba histolytica (25a); additionally, disulfiram has in vivo efficacy against Trichomonas muris (13) and inhibits Candida albicans in immunosuppressed mice (34). Neither the mode(s) of action of this drug nor the active metabolites that are cidal toward these organisms are known. Of interest, Giardia has a bifunctional alcohol dehydrogenase-coenzyme A-dependent acetaldehyde dehydrogenase which could be a target enzyme (30).
These types of compounds are potentially useful in the treatment of giardiasis. Disulfiram has been given to humans for decades and is relatively safe (8). In addition, newer agents are needed because patients infected with Giardia strains resistant to standard courses of therapy are being more frequently recognized (personal observations).
REFERENCES
- 1.Adam R D, Aggarwal A, Lal A A, de la Cruz V F, McCutchan T, Nash T E. Antigenic variation of a cysteine-rich protein in Giardia lamblia. J Exp Med. 1988;167:109–118. doi: 10.1084/jem.167.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg J M, Shi Y G. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271:1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 3.Bryan R T, Pinner R W, Berkelman R L. Emerging infectious diseases in the United States, improved surveillance, a requisite for prevention. Ann N Y Acad Sci. 1994;740:346–361. doi: 10.1111/j.1749-6632.1994.tb19892.x. [DOI] [PubMed] [Google Scholar]
- 4.Byrd L G, Conrad J T, Nash T E. Giardia lamblia infections in adult mice. Infect Immun. 1994;62:3583–3585. doi: 10.1128/iai.62.8.3583-3585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gessner P K, Gessner T. Disulfiram and its metabolite diethyldithiocarbamate. London, United Kingdom: Chapman & Hall; 1992. pp. 344–345. [Google Scholar]
- 6.Gessner P K, Gessner T. Disulfiram and its metabolite diethyldithiocarbamate. London, United Kingdom: Chapman & Hall; 1992. pp. 137–203. [Google Scholar]
- 7.Gessner P K, Gessner T. Disulfiram and its metabolite diethyldithiocarbamate. London, United Kingdom: Chapman & Hall; 1992. pp. 95–136. [Google Scholar]
- 8.Gessner P K, Gessner T. Disulfiram and its metabolite diethyldithiocarbamate. London, United Kingdom: Chapman & Hall; 1992. pp. 295–305. [Google Scholar]
- 9.Gessner P K, Gessner T. Disulfiram and its metabolite diethyldithiocarbamate. London, United Kingdom: Chapman & Hall; 1992. pp. 7–9. [Google Scholar]
- 10.Gessner P K, Gessner T. Disulfiram and its metabolite diethyldithiocarbamate. London, United Kingdom: Chapman & Hall; 1992. pp. 167–193. [Google Scholar]
- 11.Gillin F D, Hagblom P, Harwood J, Aley S B, Reiner D S, McCaffery M, So M, Guiney D G. Isolation and expression of the gene for a major surface protein of Giardia lamblia. Proc Natl Acad Sci USA. 1990;87:4463–4467. doi: 10.1073/pnas.87.12.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hathout Y, Fabris D, Han M S, Sowder II R C, Hendeson L E, Fenselau C. Characterization of intermediates in the oxidation of zinc fingers in human immunodeficiency virus type 1 nucleocapsid protein P7. Drug Metab Dispos. 1996;24:1395–1400. [PubMed] [Google Scholar]
- 13.Hill D E, Fetter R H. The effect of disulfiram on egg shell formation in adult Trichuris muris. J Parasitol. 1997;83:938–942. [PubMed] [Google Scholar]
- 14.Jeney E, Zsolnai T. Versuche zur chemotherapeutischen Beeinflussung der durch Trichomonas vaginalis hervorgerufenen Infektionen. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1964;193:542–547. [PubMed] [Google Scholar]
- 15.Judd A K, Sanchez A, Bucher D J, Huffman J H, Bailey K, Sidwell R W. In vivo anti-influenza virus activity of a zinc finger peptide. Antimicrob Agents Chemother. 1997;41:687–692. doi: 10.1128/aac.41.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keister D B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- 17.Kramer M H, Herwaldt B L, Craun G F, Calderon R L, Juranek D D. Surveillance for waterborne-disease outbreaks—United States, 1993–1994. Morbid Mortal Weekly Rep. 1996;45:1–33. [PubMed] [Google Scholar]
- 18.Lane J E, Ribeiro R R, Suarez C C, Bogitsh B J, Jones M M, Singh P K, Carter C E. In vitro trypanocidal activity of tetraethylthiuram disulfide and sodium diethylamine-N-carbodithioate on Trypanosoma cruzi. Am J Trop Med Hyg. 1996;55:263–266. doi: 10.4269/ajtmh.1996.55.263. [DOI] [PubMed] [Google Scholar]
- 19.Luján H D, Mowatt M R, Wu J J, Lu Y, Lees A, Chance M R, Nash T E. Purification of a variant-specific surface protein of Giardia lamblia and characterization of its metal-binding properties. J Biol Chem. 1995;270:13807–13813. doi: 10.1074/jbc.270.23.13807. [DOI] [PubMed] [Google Scholar]
- 20.Mowatt M R, Aggarwal A, Nash T E. Carboxy-terminal sequence conservation among variant-specific surface proteins of Giardia lamblia. Mol Biochem Parasitol. 1991;49:215–218. doi: 10.1016/0166-6851(91)90065-e. [DOI] [PubMed] [Google Scholar]
- 21.Nash T. Surface antigen variability and variation in Giardia lamblia. Parasitol Today. 1992;8:229–234. doi: 10.1016/0169-4758(92)90119-m. [DOI] [PubMed] [Google Scholar]
- 22.Nash T E, Aggarwal A. Cytotoxicity of monoclonal antibodies to a subset of Giardia isolates. J Immunol. 1986;136:2628–2632. [PubMed] [Google Scholar]
- 23.Nash T E, Aggarwal A, Adam R D, Conrad J T, Merritt J J. Antigenic variation in Giardia lamblia. J Immunol. 1988;141:636–641. [PubMed] [Google Scholar]
- 24.Nash T E, McCutchan T, Keister D, Dame J B, Conrad J D, Gillin F D. Restriction-endonuclease analysis of DNA from 15 Giardia isolates obtained from humans and animals. J Infect Dis. 1985;152:64–73. doi: 10.1093/infdis/152.1.64. [DOI] [PubMed] [Google Scholar]
- 25.Nash T E, Mowatt M R. Variant-specific surface proteins of Giardia lamblia are zinc-binding proteins. Proc Natl Acad Sci USA. 1993;90:5489–5483. doi: 10.1073/pnas.90.12.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Nash, T. E., and T. Miller. Personal communication.
- 26.Rice W G, Baker D C, Schaeffer C A, Graham L, Bu M, Terpening S, Clanton D, Schulz R, Bader J P, Buckheit R W, Jr, Field L, Singh P K, Turpin J A. Inhibition of multiple phases of human immunodeficiency virus type 1 replication by a dithiane compound that attacks the conserved zinc fingers of retroviral nucleocapsid proteins. Antimicrob Agents Chemother. 1997;41:419–426. doi: 10.1128/aac.41.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice W G, et al. Inhibition of HIV infectivity by zinc-ejecting aromatic C-nitroso compounds. Nature. 1993;361:473–475. doi: 10.1038/361473a0. [DOI] [PubMed] [Google Scholar]
- 28.Rice W G, et al. Novel inhibitors of HIV-1 p7NC zinc fingers as candidates for the treatment of AIDS. Science. 1995;270:1194–1197. doi: 10.1126/science.270.5239.1194. [DOI] [PubMed] [Google Scholar]
- 29.Rice W G, et al. Evaluation of selected chemotypes in coupled cellular and molecular target-based screens identifies novel HIV-1 zinc finger inhibitors. J Med Chem. 1996;39:3606–3616. doi: 10.1021/jm960375o. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal B, Mai Z, Caplivski D, Ghosh S, de la Vega H, Graf T, Samuelson J. Evidence for the bacterial origin of genes encoding fermentation enzymes of the amitochondriate protozoan parasite Entamoeba histolytica. J Bacteriol. 1997;179:3736–3745. doi: 10.1128/jb.179.11.3736-3745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheibel L W, Adler A, Trager W. Tetraethylthiuram disulfide (Antabuse) inhibits the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 1979;76:5303–5307. doi: 10.1073/pnas.76.10.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith P D, Gillin F D, Spira W M, Nash T E. Chronic giardiasis: studies on drug sensitivity, toxin production, and host immune response. Gastroenterology. 1982;83:797–803. [PubMed] [Google Scholar]
- 33.Sogin M L, Gunderson J H, Elwood H J, Alonso R A, Peattie D A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989;243:75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- 34.Walker E J, Cannon D J, Reifsteck M E, Hobbs K A, Hardin H F, Jones M M, Skeeles J K. Effects of diethyldithiocarbamate and structural analogs in mice with systemic candidal infections. Res Commun Chem Pathol Pharmacol. 1987;56:253–263. [PubMed] [Google Scholar]
- 35.Zhang Y Y, Aley S B, Stanley S L, Gillin F D. Cysteine-dependent zinc binding by membrane proteins of Giardia lamblia. Infect Immun. 1993;61:520–524. doi: 10.1128/iai.61.2.520-524.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]