Abstract
Epilepsy is one of the most common and oldest neurological disorders, characterized by periodic seizures that affect millions globally. Despite its long history, its pathophysiology is not fully understood. Additionally, the current treatment methods have their limitations. Finding a new alternative is necessary. Activated Protein C (APC) has been proven to have neurological protection in other neurological disorders; however, there is no study that focuses on the role of APC in seizures. We propose that APC's protective effect could be associated with seizures through inflammation and apoptosis regulation. The results demonstrated that APC's pathway proteins are involved in neuroprotection mechanisms in seizure-induced models by acting on certain inflammatory factors, such as NF-κB and apoptosis proteins.
Keywords: Epilepsy, Activated protein C, Inflammation
Graphical abstract
Highlights
-
•
Epilepsy is a neuroinflammation related disorder.
-
•
Activated protein C has anti-inflammation property in brain.
-
•
Activated protein C signal transduction mediates epilepsy pathogenesis.
Nonstandard abbreviations and acronyms
- APC
Activated protein C
- PC
Protein C
- TM
Thrombomodulin
- EPCR
Endothelial protein C receptor
- PAR1
Protease-activated receptor 1
- EEG
Electroencephalography
- i.p.
Intraperitoneal injection
- RIPA
Radioimmunoprecipitation assay
- PVDF
Polyvinylidene fluoride
- TBST
Tris-buffered saline Tween 20
- BSA
Bovine Serum Albumin
- p-NF-kβ
Phosphorylated Nuclear factor kappa B
- NF-kβ
Nuclear factor kappa B
- TNF-ɑ
Tumor Necrosis Factor alpha
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- PFA
Paraformaldehyde
- PBST
Phosphate-buffered saline Triton X-100
- FJC
Fluoro-Jade C
- DAPI
4′,6-diamidino-2-phenylindole
- IBA1
Ionized calcium binding adaptor molecule 1
1. Introduction
Epilepsy is a chronic neurological disorder, characterized by recurrent seizures. It is one of the most common neurological disorders, affecting 50 million people around the world [1,2]. Many underlying conditions, such as infection, metabolism disorder, genetic mutation, neurodegenerative disorders, or brain injury, could cause epilepsy; however, 50% of cases globally are of unknown origin [2,3]. This shows how little this disease is understood and the current gap in knowledge. Once a patient has been diagnosed with epilepsy, the first line of treatment is an anticonvulsant drug to control the seizures. These drugs either increase neuronal inhibition or reduce neuronal excitability by acting on transporter, receptor, and ion channels [4,5]. Even though most patients achieved remission, one-third of patients are still pharmaco-resistant and have drug-refractory epilepsy, where the seizure persists, leading to comorbid disorders [[4], [5], [6], [7]]. Interestingly, epilepsy's drug target does not include inhibition of neuroinflammation or neuronal cell death, even though both are thought to contribute to epileptogenesis [4,[8], [9], [10]]. Thus, there is a critical need to better understand the mechanism of the development of epilepsy and find a new, more effective therapeutic approach targeting inflammation and apoptosis.
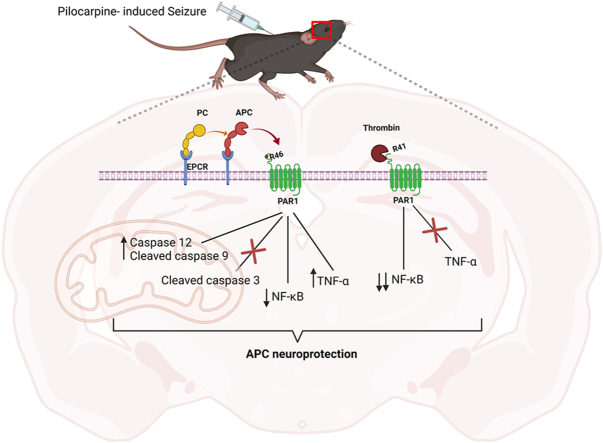
Activated protein C (APC) has been a clinical target for drug discovery and treatment of neurological disorders such as stroke or Alzheimer's disease due to its neuroprotective effect [11,12]. Protein C (PC) pathways play an important role in blood coagulation [13]. The zymogen PC is converted to its active form, APC, through its interaction with the thrombin-thrombomodulin complex (thrombin-TM complex) [[13], [14], [15]]. The amount of APC generated increases when the endothelial protein C receptor (EPCR) presents PC to the thrombin-TM complex [16]. PC-EPCR-thrombin-TM complexes are critical for APC function. However, APC cytoprotective and neuroprotective effects required the activation of protease-activated receptor 1 (PAR1) via noncanonical cleavage at the Arg46 site of the receptor [11,16]. APC and its recombinant variant have been proven to have beneficial effects in the preclinical rodent model of Alzheimer's disease, stroke, sepsis, brain trauma, and multiple sclerosis [[17], [18], [19], [20], [21]]. Whenever APC interacts with PAR1, its neuroprotective effect involves the inhibition of inflammation and apoptosis as well as promoting neurogenesis; however, the mechanism by which this occurs is not fully understood [11,15]. APC inhibits inflammation and apoptosis, hallmarks contribute to epileptogenesis. Here, we sought to study the association of APC in epilepsy pathophysiology using knock-in mice strain carrying point mutations on EPCR and PAR1 receptors, all associated with the APC pathway. We analyze the role of APC through seizure-induced models and aim to provide a mechanism as well as insight into epilepsy pathophysiology and potential new therapeutic targets.
2. Materials and methods
2.1. Mice genotype
All animal experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee (IACUC) of the University of South Florida approve each protocols prior testing. Several mice were used for experiments. Male and female (12–18 months) C57BL/6J mice supplied by Jackson Laboratory (used as a control group (WT)). Male and female (12–18 months) knock-in EPCRR84A/R84A, which lacks a PC binding site to generate APC and therefore does not have circulating APC. These mice were generated and provided by Pepler, Yu, Dwivedi et al., 2015 from the Department of Medical Sciences, McMaster University [16]. Male and female (12–18 months) PAR1R41Q/R41Q and PAR1R46Q/R46Q knock-in mice. PAR1R41Q/R41Q, due to their mutation, would prevent cleavage at Arg41 by thrombin, and PAR1R46Q/R46Q would inhibit APC cleavage at Arg46. These mice were generated and provided by Sinha, Wang, Zhao et al., 2018 from the Scripps Research Institute [18].
2.2. Open field test
Before each test, mice were taken from their holding room and allowed to acclimate to the testing room for 15 min prior to testing. All behavioral experiments were performed during the light phase during the daytime (8:00 a.m.–12:00 p.m.). Arena was cleaned with 70% ethanol before use and between trials. General locomotor activity and anxiety-like behavior were assessed using the open-field test. Mice were individually placed into a Perspex open field arena (30 cm × 30 cm) and allowed to freely explore for 15 min. The mice were tracked using a computerized tracking system (ANY-Maze, Version 4.99 m), which automatically recorded distance, average speed, time spent in the two zones (outer perimeter; center zone), and time spent freezing.
2.3. Seizure induction, survival, and scoring
All mice were bred on-site. The adult mice were anesthetized with 2% isoflurane. The seizure was induced during the daytime (8:00 a.m.–12:00 p.m.) mostly to control for diurnal variations. First, scopolamine methyl bromide (Sigma Aldrich catalog number; S85002) was injected (0.5 mg/ml) intraperitoneal injection (i.p.) dissolved in a 0.9% saline solution. Then, 30 min later, pilocarpine hydrochloride (Sigma Aldrich; P6503-SG) was injected (300 mg/kg; i.p.) and dissolved in 0.9% saline solution prior. Midazolam (i.p.) was given to attenuate the seizures an hour later. Mice were sacrificed at 1 h, 24 h, 48 h, and 72 h post-seizure. Seizure severity was recorded and scored according to the Racine Scale score [22]. The number of animals that survived the first hour was recorded.
2.4. Electroencephalography (EEG)
Mice were anesthetized (2% isoflurane). The electrodes were surgically implanted in the skull. During the surgery, the body temperature was maintained within the normal range with a heat pad. After an incision, the bregma was located. To place screws on the skull, two craniectomies were performed using a drill. The electrodes from Data Sciences International (DSI) Harvard Bioscience were fixed in place with dental cement. The EEG was recorded using Ponemah software from DSI Harvard Bioscience. Mouse EEG data were analyzed and quantified using NeuroScore software from DSI Harvard Bioscience. Seizures were defined as having a high amplitude (>400 Hz baseline). NeuroScore provides power frequency with Alpha (8–12 Hz), Beta (16–24 Hz), Delta (0.5–4 Hz), Sigma (12–16 Hz), Theta (4–8 Hz), the number of spikes, and spike trains.
2.5. Immunoblotting
The brain cortex and hippocampus were lysed in RIPA buffer (Millipore Sigma; R0278) mixed with phosphatase inhibitor and protease. The supernatants were collected after centrifugation (15000 rpm, 15 min). After normalization, proteins were run on SDS-PAGE gels and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked in TBST (10X Tris-buffered saline (TBS), 0.1% Tween 20) with 5% Bovine Serum Albumin (BSA), and then incubated overnight at 4 °C with the following primary antibodies: anti-p–NF–kβ (Cell Signaling Technology; 3033), anti–NF–kβ (Cell Signaling Technology; 8242S), anti-cleaved caspase 3 (Cell Signaling Technology; 9664S), anti-caspase 12 (Cell Signaling Technology; 2202S), anti-TNF-ɑ (Cell Signaling Technology; 3707), anti-cleaved caspase 9 (Cell Signaling Technology; 9509S), and secondary antibodies. GAPDH (Cell Signaling Technology; 2118L) was used as a standard. Signals were detected with SuperSignal™ West Femto Maximum Sensitivity Substrate (ThermoFisher; 34096). The membranes were visualized using Image Lab (Bio-Rad).
2.6. Immunofluorescence
Brain tissues were collected, and the cortex and hippocampus were isolated. The tissues were fixed in 4% paraformaldehyde (PFA) for 24 h, then gradually dehydrated in 10%, 20%, and 30% sucrose for 24 h each. After sectioning, tissues were permeabilized and washed with PBST (0.3% Triton X-100 in phosphate-buffered saline (PBS)). After blocking in 10% donkey serum, tissues were incubated overnight at 4 °C with anti-ionized calcium-binding adaptor molecule 1 (Iba1) (Invitrogen; PA5-27436). The tissues were then washed in PBST and incubated with the conjugated secondary antibody Alexa 594 (Invitrogen; A32740) for 1 h at room temperature. Fluoro-Jade C (FJC) is widely used to stain degenerative mature neurons, including autophagic, necrotic, and apoptosis neurons [23]. To stain for FJC (Biosensis; TR-100), the tissues were then blocked in 0.006% potassium permanganate for 2 min, then washed for 2 min in distilled water. The tissues were then incubated in FJC staining for 10 min and then washed in distilled water. The stained tissues were dry and mounted with a Vectashield® antifade mounting medium with DAPI (Vector Laboratories, H-1200-10) and then observed with a confocal microscope (Olympus FV1200).
2.7. Statistical analysis
Behavioral tests were performed with automated tracking using ANY-Maze, the number spikes, spike trains, and power band were provided by NeuroScores software. The relative intensity of the staining was quantified using NIH Image J software. Racine score and survival were done by the experimenter. Statistical comparisons were made with t-tests, one-way ANOVAs, and two-way ANOVAs using GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA). An alpha set at 0.05 with a P-value <0.05 is considered statistically significant.
3. Results
3.1. Abnormal behavior is reported in APC's related knock-in mouse
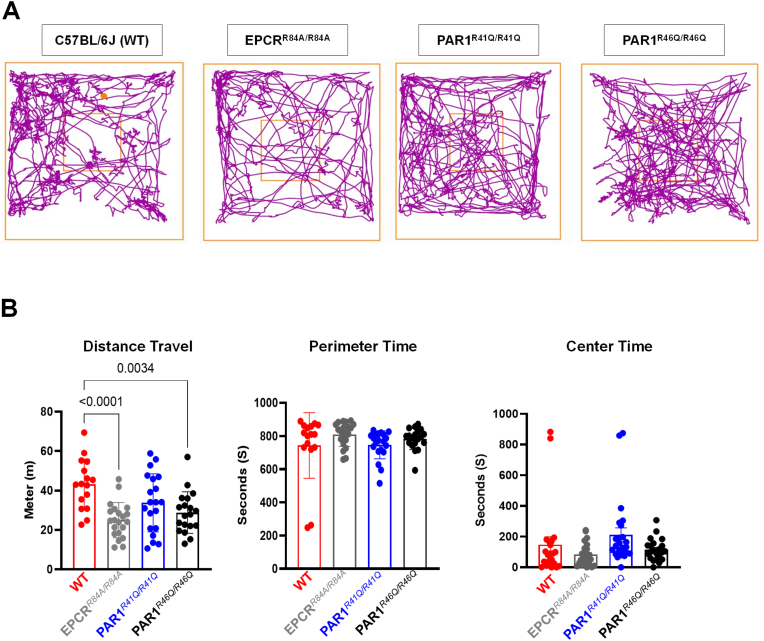
Children diagnosed with epilepsy have a high comorbidity with autism and anxiety [24]. A study has shown Kainic acid-induced status epilepticus mouse models have impaired spatial and anxiety behavior [25]. We hypothesize that EPCRR84A/R84A and PAR1R46Q/R46Q would exhibit more anxiety-like behavior than our control group under normal conditions. These groups would be more likely to develop epileptic seizures. Open-field tests assess locomotor activity and anxiety-like behavior. We recorded the distance traveled and time spent on the perimeter of the arena (Fig. 1). We found a significant decrease in the distance traveled by EPCRR84A/R84A and PAR1R46Q/R46Q compared to WT C57BL/6J (Fig. 1B). However, there was no significant difference in the time spent on the perimeter or the center of the arena among each mouse group (Fig. 1B).
Fig. 1.
Behavioral analysis in an Open Field Test. (A) Illustrative representation of travel pathway (purple) of WT C57BL/6J, EPCRR84A/R84A, PAR1R41Q/R41Q, PAR1R46Q/R46Q of 15 min recording in the open field test arena. The center is indicated by the smaller square and the larger square represents the perimeter. WT C57BL/6J travel a significantly longer distance. (B) Compared to EPCRR84A/R84A, PAR1R46Q/R46Q. The difference in the time spent on the perimeter or the center are not significant. Histograms bars indicate the mean ± SEM; One way ANOVA, post hoc analysis by Tukey's HSD (WT C57BL/6J: n = 16, EPCRR84A/R84A: n = 21, PAR1R41Q/R41Q: n = 20, PAR1R46Q/R46Q: n = 19). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. APC's association to the spontaneity and severity of pilocarpine-induced seizures
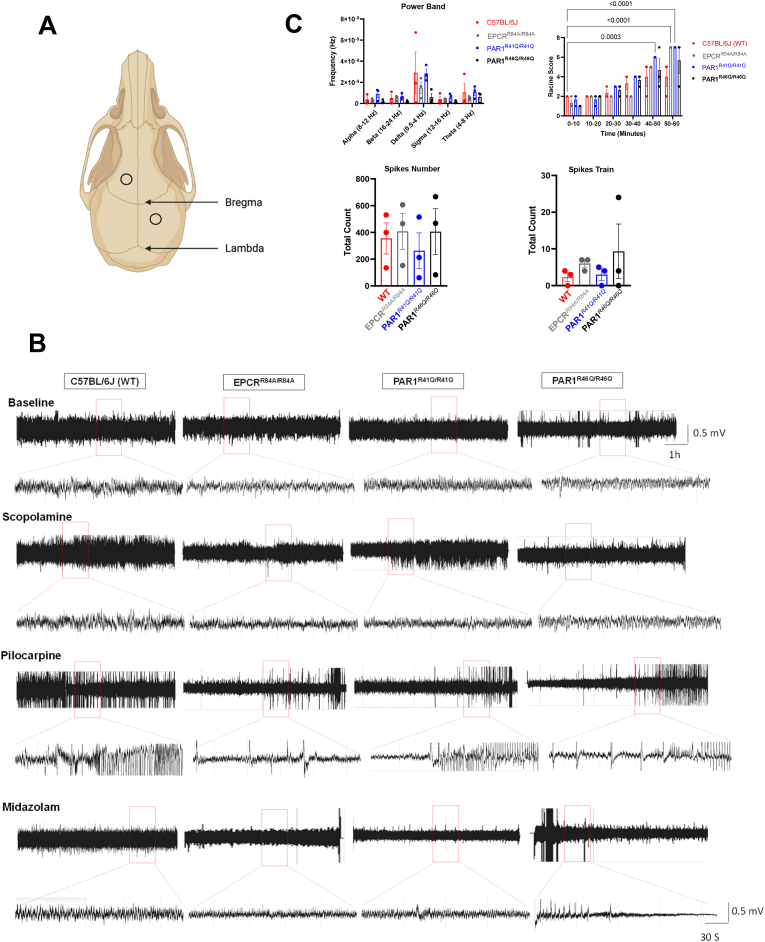
We hypothesize that the lack of APC and its binding site on the PAR1 receptor would cause an increase in seizure severity. We assessed seizure susceptibility and severity in WT C57BL/6J, EPCRR84A/R84A, PAR1R46Q/R46Q, and PAR1R41Q/R41Q (Fig. 2). During the 1 h after administration of pilocarpine (Supplementary Fig. 1A), we reported an increase in mortality in EPCRR84A/R84A, and PAR1R46Q/R46Q (Supplementary Fig. 1B). Next, we implanted electrodes (Fig. 2A) to record EEG activities (Fig. 2B). We did not record a significant change in power band or spike numbers among each group (Fig. 2C). Although there is an increase in spike trains in PAR1R46Q/R46Q, there is no statistically significant between groups. Furthermore, there was an increase in the severity of the seizure based on the Racine score (Fig. 2C).
Fig. 2.
EEG Recording and Seizure Severity. (A) Illustration of EEG electrode implantation in the mice's upper felt and lower right of the scalp. (B) Representative EEG recording of WT C57BL/6J, EPCRR84A/R84A, PAR1R41Q/R41Q, PAR1R46Q/R46Q baseline and during scopolamine, pilocarpine and midazolam injection (i.p). (C) Significant increase in the severity of the seizure compared to baseline but the difference in the power band, spikes number and spikes train between WT C57BL/6J, EPCRR84A/R84A, PAR1R41Q/R41Q, PAR1R46Q/R46Q are not significant. Data provided by DSI Harvard Bioscience NeuroScore software. Histograms bars indicate the mean ± SEM; Two-way ANOVA, post hoc analysis by Tukey's HSD for power band and Racine score, One-way ANOVA for spikes numbers and spikes train.
3.3. Change in the level of apoptosis and inflammatory proteins
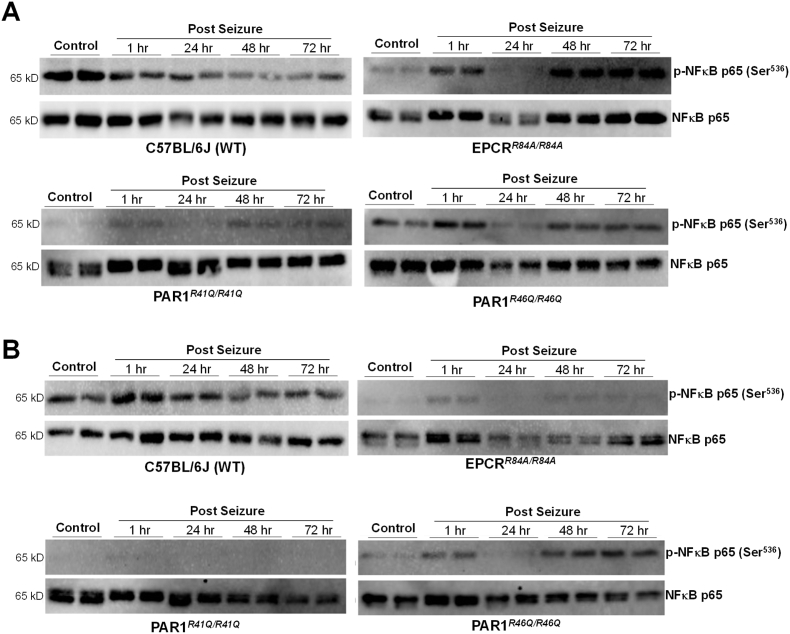
Due to the increased mortality in EPCRR84A/R84A, and PAR1R46Q/R46Q, we hypothesize that changes in levels of apoptosis proteins such as cleaved caspase 3, caspase 12, and cleaved caspase 9, and inflammatory proteins such as NF-κB and TNF-ɑ could be recorded. The protein expressions were recorded under normal conditions (1-h, 24-h, 48-h, and 72-h post-seizure) to see a gradual change in protein expression. We then conducted immunoblotting of each protein in the cortex and hippocampus regions (Fig. 3). In WT C57BL/6J, we see a decrease in p–NF–κB in both the cortex and hippocampus regions, leading to change in p–NF– κB/NF-κB expression (Fig. 3A&B). We also recorded an increase in caspase 12, cleaved caspase 9, only in the cortex region, and TNF-ɑ expression, but no change in cleaved caspase 3 (data not shown). However, we cannot conclude this statistically due to the low number of replicates. In EPCRR84A/R84A, PAR1R46Q/R46Q, and PAR1R41Q/R41Q we reported an increase in p–NF–κB at 1-h post-seizure, then a decrease at 24 h and an increase at 48- and 72-h post-seizure (Fig. 3). Interestingly, we report a low level of p–NF–κB expression in the hippocampus region in EPCRR84A/R84A, PAR1R46Q/R46Q, and no expression in PAR1R41Q/R41Q, compared to our control. We also reported that even under normal conditions, EPCRR84A/R84A, PAR1R46Q/R46Q, and PAR1R41Q/R41Q expressed a low level of p–NF–κB compared to our control group (Fig. 3A&B).
Fig. 3.
Immunoblotting of inflammatory protein. Protein expression of inflammatory proteins p–NF–κB and NF-κB in the cortex (A) and hippocampus (B) region of the brain under control or seizure conditions of WT C57BL/6J, EPCRR84A/R84A, PAR1R41Q/R41Q, PAR1R46Q/R46Q.
Again, cleaved caspase 3 was not expressed in the cortex or hippocampus regions of EPCRR84A/R84A, PAR1R46Q/R46Q, and PAR1R41Q/R41Q (data not shown). Cleaved caspase 9 was only shown in the PAR1R41Q/R41Q cortex, like in our control group, and the hippocampus (data not shown). Caspase 12 expression was low, in EPCRR84A/R84A, or not expressed in PAR1R46Q/R46Q, and PAR1R41Q/R41Q in both tested regions (data not shown). TNF-ɑ expression was low or not detected in the PAR1R41Q/R41Q cortex, hippocampus region, and PAR1R46Q/R46Q cortex region (data not shown).
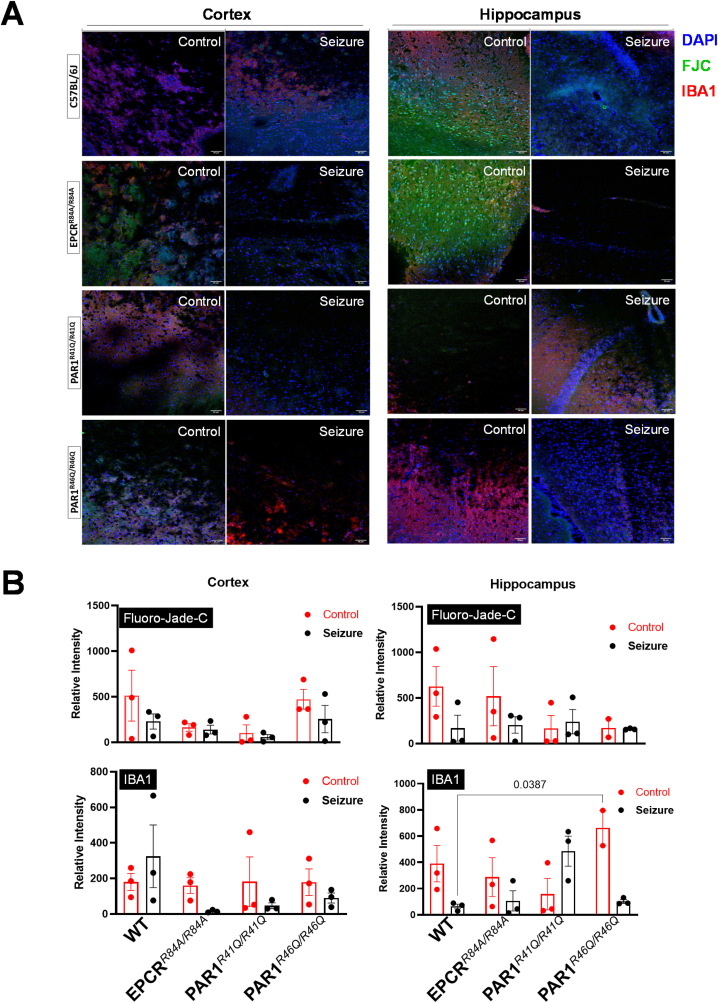
3.4. Presence of inflammatory markers and degenerative neurons
To further investigate inflammatory and apoptosis mechanisms in our seizure-induced model, we stained for IBA1, the marker for activated microglia, and the FJC marker for degenerative neurons (Fig. 4). We look at the hippocampus and cortex regions of the brain. We hypothesize that the greater change in cell population would be more noticeable between normal conditions and 72-h post-seizure. We did not report any statistical change in staining intensity among each group in the hippocampus and cortex (Fig. 4B). However, we reported an increase in IBA1 in the WT C57BL/6J cortex region and in the PAR1R41Q/R41Q hippocampus region, although it was not statistically significant (Fig. 4B).
Fig. 4.
Immunofluorescence of degenerative neuron and microglia activity. (A) Representative staining of DAPI (blue), FJC (green), and IBA1 (red) in the cortex and hippocampus under control or 72-h post-seizure of WT C57BL/6J, EPCRR84A/R84A, PAR1R41Q/R41Q, PAR1R46Q/R46Q. Scale bars are 50 μm. (B) Relative intensity of FJC and IBA1 comparing control, and 72-h post-seizure of WT C57BL/6J, EPCRR84A/R84A, PAR1R41Q/R41Q, PAR1R46Q/R46Q. Histogram bars indicate the mean ± SEM; Two-way ANOVA, post hoc analysis by Tukey's HSD. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Epilepsy is the most common serious neurological disorder, affecting millions [26]. Although it is one of the oldest neurological disorders, its mechanisms are not fully understood [27]. Plus, pharmaco-resistant has been reported in a few patients [28]. It is necessary to have a better understanding of epilepsy pathophysiology and find alternative treatment targets. APC and its recombinant variant have proven to have beneficial effects in the preclinical rodent model of Alzheimer's disease, stroke, sepsis, brain trauma, and multiple sclerosis [[17], [18], [19], [20], [21]]. We were interested in understanding the role of APC in seizure-induced models, with a focus on inflammation and apoptosis mechanisms. We first hypothesize that APC is associated with impaired anxiety-like behavior, a phenotype reported in epileptic patients and mouse models [24,29]. We did not report any anxiety-like behavior in our groups of mice since their time spent on the perimeter was not significantly different. However, we reported a significant difference in locomotion in EPCRR84A/R84A and PAR1R46Q/R46Q compared to WT C57BL/6J, suggesting they could have motor impairments. For instance, neurodevelopmental disorders such as impaired motor skills could lead to the development of seizures [30]. The fact that EPCRR84A/R84A and PAR1R46Q/R46Q exhibited locomotion-impaired behavior suggests APC and its binding receptor PAR1 could play a role in motor nerves or neurons. However, further investigation into this parameter is required. To investigate, seizure susceptibility and severity, we recorded EEG activity. Although we reported many EPCRR84A/R84A and PAR1R46Q/R46Q mice that did not survive pilocarpine injection, their brain activity recording did not significantly differ. This suggests animal mortality might not be associated with brain activity; further investigation is required. Next, we investigate inflammatory and apoptosis mechanisms and their association with APC in a seizure-induced model. Interestingly, we saw a change in NF-κB among each group. This shows APC activity acts on NF-κB during seizure induction in the brain. APC seems to also alter cleaved caspase 12, and cleaved caspase 9, TNF-ɑ, as shown in our illustration; however, larger sample sizes and further investigation are required.
Plus, the number of activated microglia, signs of neuronal inflammation, and degenerative neurons undergoing apoptosis or necrosis were not significant among each group. This suggests that at the cellular level, there were not many changes 72-h post-seizure. Changes in cell activity could be more noticeable after 72 h. Further investigation and an increased sample size for these parameters are required. This study presents major limitations to take into consideration. The sample sizes in the EEG, immunoblotting, and immunofluorescence are low, which could affect their significance. Plus, the drug used to induce seizures acts on the muscarinic receptors present in all major organs. It is administered intraperitoneally, which means it can act on organs other than the brain. Future work will be to conduct local injections and increase the sample size.
Inflammation and apoptosis are parameters reported in epilepsy [31]. In addition, APC acts on this parameter in different neurological disorders, including Alzheimer's disease, stroke, and brain trauma [[17], [18], [19], [20], [21]]. However, this activity has not been significantly reported in our seizure-induced model. Based on our findings, the next logical step will be to confirm our findings and determine if other organs, such as the heart, could be associated with the phenotype we reported. If proven beneficial, this could provide a better understanding of the role of APC in seizures and could be a new treatment target for epilepsy.
Author contributions
Linda Ines Zoungrana and Ji Li designed and conducted the study; Meredith Krause-Hauch, Hao Wang, Zehui Li, Adewale James, Lily Slotabec, Steven Didik, Mohammad Kasim Fatmi and Ji Li performed data collection and analysis; Linda Ines Zoungrana and Ji Li interpreted the data. Linda Ines Zoungrana and Ji Li drafted the manuscript. All authors contributed feedback to the manuscript.
Funding information
This work was supported by National Institute of Health grants P20GM104357, P30GM149404, R01HL158515, Department of Veterans Affairs Merit Award I01BX005625 and I01CX002406. The views of the authors do not necessarily represent the opinions of the VA or United States Government.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2023.101550.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Center for Disease Control and Prevention . 2023. Epilepsy.https://www.cdc.gov/epilepsy/index.html [Google Scholar]
- 2.World Health Organization . 2023. Epilepsy.https://www.who.int/news-room/fact-sheets/detail/epilepsy [Google Scholar]
- 3.Yuen A.W.C., Keezer M.R., Sander J.W. Epilepsy is a neurological and a systemic disorder. Epilepsy Behav. 2018;78:57–61. doi: 10.1016/j.yebeh.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Kumar P., Lim A., Hazirah S.N., Chua C.J.H., Ngoh A., Poh S.L., Yeo T.H., Lim J., Ling S., Sutamam N.B., Petretto E., Low D.C.Y., Zeng L., Tan E.-K., Arkachaisri T., Yeo J.G., Ginhoux F., Chan D., Albani S. Single-cell transcriptomics and surface epitope detection in human brain epileptic lesions identifies pro-inflammatory signaling. Nat. Neurosci. 2022;25:956–966. doi: 10.1038/s41593-022-01095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akyuz E., Polat A.K., Eroglu E., Kullu I., Angelopoulou E., Paudel Y.N. Revisiting the role of neurotransmitters in epilepsy: an updated review. Life Sci. 2021;265 doi: 10.1016/j.lfs.2020.118826. [DOI] [PubMed] [Google Scholar]
- 6.Lerche H. Drug-resistant epilepsy — time to target mechanisms. Nat. Rev. Neurol. 2020;16:595–596. doi: 10.1038/s41582-020-00419-y. [DOI] [PubMed] [Google Scholar]
- 7.Raut D., Bhatt L.K. Evolving targets for anti-epileptic drug discovery. Eur. J. Pharmacol. 2020;887 doi: 10.1016/j.ejphar.2020.173582. [DOI] [PubMed] [Google Scholar]
- 8.Meldrum B.S., Rogawski M.A. Molecular targets for antiepileptic drug development. Neurotherapeutics. 2007;4:18–61. doi: 10.1016/j.nurt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kambli L., Bhatt L.K., Oza M., Prabhavalkar K. Novel therapeutic targets for epilepsy intervention. Seizure. 2017;51:27–34. doi: 10.1016/j.seizure.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Wang L., Liu Y.-H., Huang Y.-G., Chen L.-W. Time-course of neuronal death in the mouse pilocarpine model of chronic epilepsy using Fluoro-Jade C staining. Brain Res. 2008;1241:157–167. doi: 10.1016/j.brainres.2008.07.097. [DOI] [PubMed] [Google Scholar]
- 11.Griffin J.H., Zlokovic B.V., Mosnier L.O. Activated protein C, protease activated receptor 1, and neuroprotection. Blood. 2018;132:159–169. doi: 10.1182/blood-2018-02-769026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin J.H., Fernández J.A., Liu D., Cheng T., Guo H., Zlokovic B.V. Activated protein C and ischemic stroke. Crit. Care Med. 2004;32 doi: 10.1097/01.ccm.0000126127.87484.2b. [DOI] [PubMed] [Google Scholar]
- 13.Griffin J.H., Mosher D.F., Zimmerman T.S., Kleiss A.J. Protein C, an antithrombotic protein, is reduced in hospitalized patients with intravascular coagulation. Blood. 1982;60:261–264. doi: 10.1182/blood.V60.1.261.261. [DOI] [PubMed] [Google Scholar]
- 14.Riewald M., Petrovan R.J., Donner A., Mueller B.M., Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 15.Griffin J.H., Zlokovic B.V., Mosnier L.O. Activated protein C: biased for translation. Blood. 2015;125:2898–2907. doi: 10.1182/blood-2015-02-355974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepler L., Yu P., Dwivedi D.J., Trigatti B.L., Liaw P.C. Characterization of mice harboring a variant of EPCR with impaired ability to bind protein C: novel role of EPCR in hematopoiesis. Blood. 2015;126:673–682. doi: 10.1182/blood-2014-02-558940. [DOI] [PubMed] [Google Scholar]
- 17.Lazic D., Sagare A.P., Nikolakopoulou A.M., Griffin J.H., Vassar R., Zlokovic B.V. 3K3A-activated protein C blocks amyloidogenic BACE1 pathway and improves functional outcome in mice. J. Exp. Med. 2019;216:279–293. doi: 10.1084/jem.20181035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha R.K., Wang Y., Zhao Z., Xu X., Burnier L., Gupta N., Fernández J.A., Martin G., Kupriyanov S., Mosnier L.O., Zlokovic B.V., Griffin J.H. PAR1 biased signaling is required for activated protein C in vivo benefits in sepsis and stroke. Blood. 2018;131:1163–1171. doi: 10.1182/blood-2017-10-810895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker C.T., Marky A.H., Petraglia A.L., Ali T., Chow N., Zlokovic B.V. Activated protein C analog with reduced anticoagulant activity improves functional recovery and reduces bleeding risk following controlled cortical impact. Brain Res. 2010;1347:125–131. doi: 10.1016/j.brainres.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han M.H., Hwang S.-I., Roy D.B., Lundgren D.H., Price J.V., Ousman S.S., Fernald G.H., Gerlitz B., Robinson W.H., Baranzini S.E., Grinnell B.W., Raine C.S., Sobel R.A., Han D.K., Steinman L. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- 21.Petraglia A.L., Marky A.H., Walker C., Thiyagarajan M., Zlokovic B.V. Activated protein C is neuroprotective and mediates new blood vessel formation and neurogenesis after controlled cortical impact. Neurosurgery. 2010;66 doi: 10.1227/01.NEU.0000363148.49779.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Erum J., Van Dam D., De Deyn P.P. PTZ-induced seizures in mice require a revised Racine scale. Epilepsy Behav. 2019;95:51–55. doi: 10.1016/j.yebeh.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 23.Ikenari T., Kurata H., Satoh T., Hata Y., Mori T. Evaluation of fluoro-jade C staining: specificity and application to damaged immature neuronal cells in the normal and injured mouse brain. Neuroscience. 2020;425:146–156. doi: 10.1016/j.neuroscience.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Jeste S.S., Tuchman R. Autism spectrum disorder and epilepsy:two sides of the same coin? J. Child Neurol. 2015;30:1963–1971. doi: 10.1177/0883073815601501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith G., Ahmed N., Arbuckle E., Lugo J.N. Early-life status epilepticus induces long-term deficits in anxiety and spatial learning in mice. Int. J. Epilepsy. 2017;4:36–45. doi: 10.1016/j.ijep.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacoby A., Snape D., Baker G.A. Epilepsy and social identity: the stigma of a chronic neurological disorder. Lancet Neurol. 2005;4:171–178. doi: 10.1016/S1474-4422(05)01014-8. [DOI] [PubMed] [Google Scholar]
- 27.Patel P., Moshé S.L. The evolution of the concepts of seizures and epilepsy: what's in a name? Epilepsia Open. 2020;5:22–35. doi: 10.1002/epi4.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fattorusso A., Matricardi S., Mencaroni E., Dell'Isola G.B., Di Cara G., Striano P., Verrotti A. The pharmacoresistant epilepsy: an overview on existant and new emerging therapies. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.674483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Alvarez N., Jimenez-Mateos E.M., Dunleavy M., Waddington J.L., Boylan G.B., Henshall D.C. Effects of hypoxia-induced neonatal seizures on acute hippocampal injury and later-life seizure susceptibility and anxiety-related behavior in mice. Neurobiol. Dis. 2015;83:100–114. doi: 10.1016/j.nbd.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Fallah M.S., Eubanks J.H. Seizures in mouse models of rare neurodevelopmental disorders. Neuroscience. 2020;445:50–68. doi: 10.1016/j.neuroscience.2020.01.041. [DOI] [PubMed] [Google Scholar]
- 31.Kegler A., Caprara A.L.F., Pascotini E.T., Arend J., Gabbi P., Duarte M.M.M.F., Furian A.F., Oliveira M.S., Royes L.F.F., Fighera M.R. Apoptotic markers are increased in epilepsy patients: a relation with manganese superoxide dismutase Ala16Val polymorphism and seizure type through IL-1β and IL-6 pathways. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/6250429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.