Abstract
Chronic pain after spine surgery (CPSS) is often characterized by intractable low back pain and/or radiating leg pain, and has been reported in 8–40% of patients that received lumbar spine surgery. We conducted a literature search of PubMed, MEDLINE/OVID with a focus on studies about the etiology and treatments of CPSS and low back pain. Our aim was to provide a narrative review that would help us better understand the pathogenesis and current treatment options for CPSS. This knowledge will aid in the development of optimal strategies for managing postoperative pain symptoms and potentially curing the underlying etiologies. Firstly, we reviewed recent advances in the mechanistic study of CPSS, illustrated both structural (e.g., fibrosis and scaring) and non-structural factors (e.g., inflammation, neuronal sensitization, glial activation, psychological factor) causing CPSS, and highlighted those having not been given sufficient attention as the etiology of CPSS. Secondly, we summarized clinical evidence and therapeutic perspectives of CPSS. We also presented new insights about the treatments and etiology of CPSS, in order to raise awareness of medical staff in the identification and management of this complex painful disease. Finally, we discussed potential new targets for clinical interventions of CPSS and future perspectives of mechanistic and translational research. CPSS patients often have a mixed etiology. By reviewing recent findings, the authors advocate that clinicians shall comprehensively evaluate each case to formulate a patient-specific and multi-modal pain treatment, and importantly, consider an early intraoperative intervention that may decrease the risk or even prevent the onset of CPSS.
Translational potential statement
CPSS remains difficult to treat. This review broadens our understanding of clinical therapies and underlying mechanisms of CPSS, and provides new insights which will aid in the development of novel mechanism-based therapies for not only managing the established pain symptoms but also preventing the development of CPSS.
Keywords: Animal models, Chronic pain after spine surgery, Failed back surgery syndrome, Low back pain, Peripheral nervous system, Sensitization
Graphical abstract
The translational potential of this article. CPSS remains difficult to treat. This review broadens our understanding of clinical therapies and underlying mechanisms of CPSS, and provides new insights which will aid in the development of novel mechanism-based therapies for not only managing the established pain symptoms but also preventing the development of CPSS.
1. Introduction
Low back pain causes substantial suffering, impairs quality of life, and is difficult to treat. It is a common presenting complaint and has an estimated lifetime prevalence of 60%–85% around the world [1,2]. In the US adult population, the prevalence of low back pain is 10%–30% [3], and causes include injury, disc herniation, aging, and other pathological conditions. Spine surgery has been used to treat degenerative and non-degenerative diseases of the spine when conservative treatment has failed, and the number of spinal surgeries has significantly increased in recent years [4]. Unfortunately, about 8%–40% of patients who undergo lumbar spine surgery develop intractable or recurrent leg pain and back pain after surgery; this is commonly known as chronic pain after spine surgery (CPSS) [5].
Symptoms of CPSS may include localized tenderness, muscle spasms, heaviness, numbness, or weakness in arms or legs, and chronic pain in the back, neck, or legs. Pain can be dull or sharp, aching, burning, or radiating. It can be ongoing, constant, or intermittent, and the pain level varies greatly among individuals and with changing in posture. Diagnosing the cause of CPSS can be challenging and is often based on the patient's symptoms, physical examination (e.g., restricted movement in the spine or neck and weakness in the arms or legs), neurological evaluation (e.g., to determine if there is potential nerve injury), sensory and pain tests to cold, heat, and mechanical stimuli, patient's medical history especially surgery report, and findings from imaging tests (e.g., X-rays, CT scans, MRIs).
Chronic pain and functional incapacities negatively affect the mental and physical well-being of patients [1]. Yet, current CPSS treatments remain inadequate, and a better understanding of the complex etiology of this disease will help to improve CPSS management [1]. The exact etiology of CPSS is not yet clear, and more than one anatomic structure (e.g., nerve roots, soft tissue, vertebrae, intervertebral discs) may serve as the potential origin of pain [6]. A better understanding of the factors that cause CPSS will help the development of new strategies, not only to manage pain but also to prevent CPSS and to improve healing and functional recovery. Residual structural factors such as bone, disc, and ligament, as well as insufficient decompression of the nerve roots and spinal cord after spine surgery (e.g., laminectomy) have been considered pathogenic factors that can lead to CPSS [[7], [8], [9]]. Yet, even patients who have received successful decompression surgery or showed no postoperative radiographic evidence of nervous tissue compression continue to experience low back pain, strongly suggesting that other, non-structural factors also contribute to CPSS [10].
Here, we review and discuss recent advances in the mechanistic study of CPSS, with an emphasis on structural factors and non-structural postoperative changes that have been overlooked in the pathogenesis of CPSS. We then summarize the clinical evidence and therapeutic perspectives of CPSS. Finally, we discuss potential new targets and future research directions, which are the first steps toward developing new therapies for CPSS.
2. Literature search
The literary search for primary and review articles in PubMed, MEDLINE/OVID, and SCOPUS was performed on October 1, 2022. Both preclinical and clinical peer-reviewed articles published in the past 5 years in indexed medical journals were included if they were related to CPSS and low back pain. We used keywords including pain, laminectomy, failed back surgery, low back pain, spine surgery, and analgesia. We limited the search articles published in English but did not apply date limits. We also examined the reference lists of the sources selected to identify additional studies not found in the original search.
3. Classification criteria for the level of evidence in clinical studies
We summarized major clinical studies on the treatment of CPSS according to the level of evidence established by the North American Spine Society. This mainly includes randomized controlled studies (RCTs) of CPSS in the past 20 years, and excludes studies with combined treatment (e.g., drug therapy plus surgery) and studies classified as weak Level Ⅴ evidence. Combination therapies may effectively alleviate CPSS pain, but determining the specific contribution and role of each treatment can be challenging. Accordingly, we focused on analyzing individual treatments to better understand their effectiveness and identify the underlying causes of the disease. Additionally, we provide a separate section to discuss the combination therapies for CPSS pain. The specific classification criteria for the level of evidence are as follows: Level Ⅰ, high-quality randomized trial or prospective study; testing of previously developed diagnostic criteria on consecutive patients; sensible costs and alternatives; values obtained from many studies with multiway sensitivity analyses; a systematic review of Level Ⅰ RCTs and studies. Level Ⅱ, lesser quality RCT; prospective comparative study; retrospective study; untreated controls from an RCT; lesser quality prospective study; development of diagnostic criteria on consecutive patients; sensible costs and alternatives; values obtained from limited studies; multiway sensitivity analyses; a systematic review of Level Ⅱ studies or Level Ⅰ studies with inconsistent results. Level Ⅲ, case–control study (therapeutic and prognostic studies); retrospective comparative study; study of nonconsecutive patients without consistently applied reference “gold” standard; analyses based on limited alternatives and costs and poor estimates; a systematic review of Level Ⅲ studies. Level Ⅳ, case series; case–control study (diagnostic studies); poor reference standard; analyses with no sensitivity analyses. Level Ⅴ, expert opinion.
4. Pathogenesis of CPSS
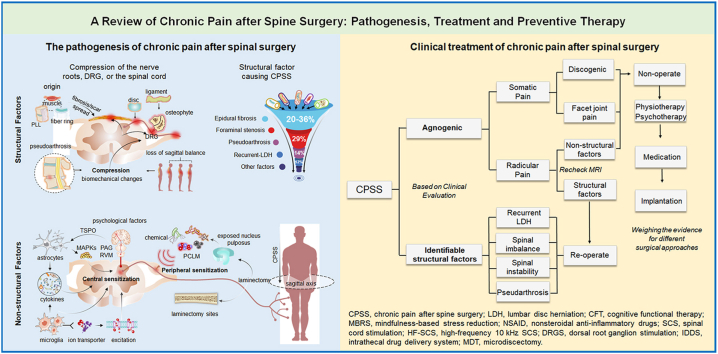
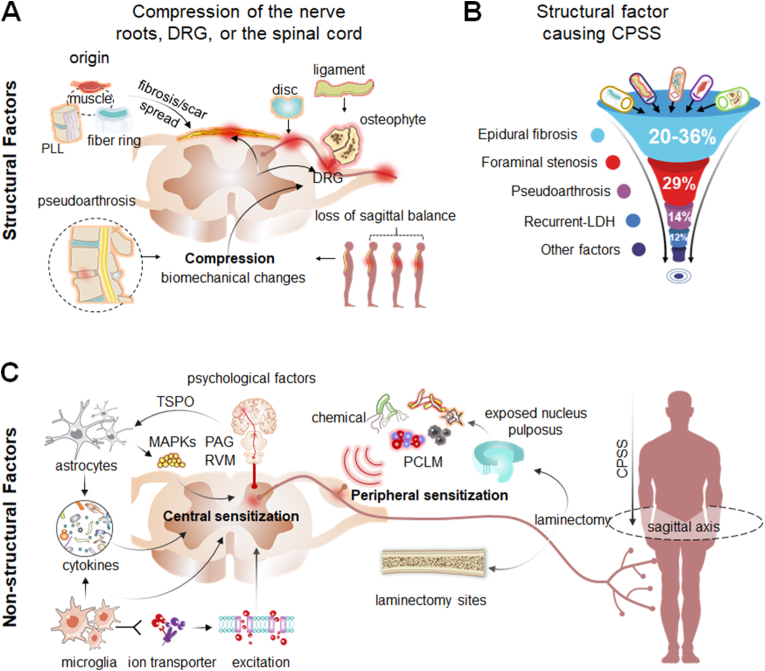
The complex pathogenesis of CPSS involves multiple factors, which are broadly classified into structural factors and non-structural factors [11]. Structural factors include bones, ligaments, intervertebral discs, fibrosis, and the postoperative epidural scar; any or all of these may cause mechanical pressure, compression, or irritation of the nerve roots, sensory ganglion, or spinal cord (Fig. 1A and B). These problems may be due to inadequate surgical decompression, foraminal stenosis, recurrent disk herniation, pseudoarthrosis, or painful disk [11,12]. The non-structural factors of the pathogenesis of CPSS include various postoperative changes in the local microenvironment (Fig. 1C), which include inflammation, maladaptive changes in the sensory nervous system (e.g., sensitization of primary sensory neurons and spinal cord dorsal horn neurons), activation of glial cells, and psychological factors [13]. To date, the specific roles of these non-structural factors in the development and course of CPSS have not been explored in depth.
Fig. 1.
Schematic drawing of the pathogenesis of chronic pain after spinal surgery (CPSS) (A) The pathogenesis of CPSS can be broadly classified into structural and non-structural factors. Schematic diagram illustrating major structural factors which refer to direct mechanical compression or irritation of the dorsal root ganglion (DRG), nerve roots, spinal cord, or dural sac (B) Major structural factors include epidural fibrosis and scar (20%–36%), foraminal stenosis (29%), pseudoarthrosis (14%), recurrent LDH (12%), iatrogenic instability, and the loss of sagittal balance (C) Schematic diagram illustrating major non-structural factors for the pathogenesis of CPSS, which refer to postoperative changes in the local environment including inflammation, increased excitability of primary sensory neurons (peripheral sensitization), spinal dorsal horn neuron hyperexcitability (central sensitization), activation of glial cells (e.g., astrocytes, microglia), the release of proinflammatory cytokines, and psychological factors. LDH, lumbar disc herniation; PLL, posterior longitudinal ligament; PCLM, postoperative changes in the local microenvironment; TSPO, translocator protein; PAG, periaqueductal gray; RVM, rostral ventromedial medulla; MAPKs, mitogen-activated protein kinase.
4.1. Structural factors
4.1.1. Structural factors that are directly related to surgical failure
Inadequate decompression in the foramen and lateral recesses during spine surgery is a common cause of CPSS, accounting for 29% of the total CPSS cases [14]. However, excessive decompression may induce low back pain and cause spinal instability. The risk is even greater if more than 50% of the superior articular process is removed, as the superior articular process is mainly involved in maintaining the stability of the spine [15]. In addition, incorrectly performed surgical procedures which account for approximately 2.1%–2.7% of total patients receiving spinal surgery, also contribute to the development of CPSS [14]. Surgical complications such as nerve damage [16], misdiagnosis, and mistreatment may also contribute to CPSS if surgery is performed on unaffected spinal segments and does not relieve nerve compression at the causative segment. Accordingly, increasing the surgery success rate is key to limiting the aforementioned structural factors directly related to surgical intervention.
4.1.2. Structural factors that are not directly due to surgical failure
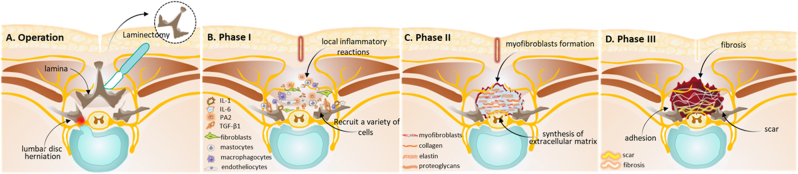
Structural factors of CPSS also involve the formation of postoperative epidural fibrosis and scarring, which are not caused by surgical failures. Local inflammatory reactions, invasion of the postoperative hematoma, aggregation of fibroblasts, and excessive deposition of extracellular matrix (ECM), cause fibrosis on the level of the fibrous periosteum and the deep paravertebral muscles after laminectomy. Fibrosis is a natural tissue reconstruction and wound-healing process to protect the relative integrity of tissues and organs after tissue damage [17]. It consists of three phases [18]. Phase I involves local inflammatory reactions that occur within the first 3 to 5 postoperative days, including the release of a large number of inflammatory factors; chemokine; and growth factors, such as interleukin (IL)-1, IL-6, phospholipase A2, and transforming growth factor-β1 (TGF-β1) at the laminectomy sites (Fig. 2A and B). These inflammatory reactions cause the aggregation of fibroblasts, mastocytes, macrophagocytes, and endotheliocytes [18,19]. The fibroblasts may arise from the paraspinal musculature, ligamentum flavum, posterior longitudinal ligament, and annulus fibrosis. Phase II is the active phase of fibroblasts, which usually lasts 2–3 weeks after surgery (Fig. 2C). In this phase, fibroblasts proliferate and differentiate into myofibroblasts which are the chief perpetrators of fibrosis and avid ECM synthesizers [20]. TGF-β1 has important implications during this process, especially in the differentiation of fibroblasts into myofibroblasts [21]. Notably, the deposition of excessive ECM and the proliferation of fibroblasts play a key role in postoperative dura fibrosis and adhesions. Phase III involves tissue reconstruction that lasts for months or longer after surgery (Fig. 2D). Fibrillar connective tissues are deposited around the defect lesion and later transform into scar tissues in this phase [19]. Excessive epidural fibrosis and scarring at the operation site can cause extradural compression and dural tethering, and it can also induce radicular pressure or stretching on the nerve roots and dorsal root ganglia (DRG) leading to mechanical radicular pain. Thus, excessive epidural fibrosis has been considered an important factor that causes recurrent radicular pain and CPSS, with a high incidence of approximately 20–36% [22].
Fig. 2.
Schematic diagram illustrating the development of epidural fibrosis after lumbar spinal laminectomy (A) Schematic diagram illustrating the laminectomy surgery for the treatment of lumbar disc herniation (B) The development of epidural fibrosis can be divided into three phases. Phase I involves local inflammatory reactions which occur in the first 3 to 5 postoperative days (C) Phase II is the active phase of fibroblasts which usually lasts 2–3 weeks after surgery. In this phase, fibroblasts proliferate and differentiate into myofibroblasts which are the chief perpetrators of fibrosis and avid extracellular matrix synthesizers (D) Phase III involves tissue reconstruction which lasts for months or longer. Epidural fibrosis and scar tissue form during this phase which may cause CPSS and functional incapacity. IL-1, interleukin-1; IL-6, interleukin-6. PA2; phospholipase A2; TGF-β1, transforming growth factor-β1.
A dural scar is generally the result of normal wound healing after tissue injury and occurs in both CPSS patients and patients without symptoms. Spinal surgery destructs a variety of tissues including muscle, ligament, fiber ring, and bone. It remains unclear which tissues are mainly responsible for the origin of dural scars. Based on Songer's three-dimensional theory [23], scar tissues around the dura mater mainly originated from sacrospinalis, the fiber ring, and the posterior longitudinal ligament. Excessive scar tissue may invade the spinal canal causing mechanical compression of nerve roots after laminectomy and spinal surgery, and it may also cause post-laminectomy epidural adhesion to the dura mater. These changes may lead to chronic back pain and functional incapacity and increase the risks of complications during revision surgery [24].
Nevertheless, a previous study suggested that dural scars may be a concomitant phenomenon of CPSS but are not necessarily related to postoperative recurrent radiating pain [25]. A recent study found that epidural scars in CPSS patients differed from those in non-CPSS patients, with the former being in a state of long-term immaturity [26]. These findings suggest that future research should focus on abnormal epidural scarring after laminectomy and examine its role in CPSS.
Adjacent segment disease refers to the constellation of symptoms associated with degeneration at a spinal level adjacent to that which received spinal fusion surgery. It may represent a longer-term sequela of an initially successful surgery. A spine that is unbalanced or balanced via a compensatory mechanism is prone to adjacent disc degeneration [11,12]. A recurrent disc herniation, causing low back pain or radiating pain in the lower extremities, may also occur in patients who have undergone lumbar surgery at the same or adjacent level. Emerging evidence suggests that recurrent disc herniation after lumbar spine surgery is often associated with unhealthy lifestyle habits, including obesity and smoking [27]. Therefore, a patient's congenital factors and lifestyle can impact the biomechanics of the spine and recurrent disc herniation which, in turn, contribute to CPSS. Aging and other pathological conditions, which could impair the normal bone repairing and remodeling processes, may also contribute to CPSS.
4.2. Non-structural factors
4.2.1. Inflammation
Chronic inflammation plays an important role in the induction of dural fibrosis, wound healing, and pain sensitization. The local inflammatory response is a complex pathological process, which not only produces pain-inducing inflammatory mediators but also accelerates the formation of dural fibrosis [28]. Previous studies have suggested that inflammation-related arachnoiditis, rather than mechanical compression, may be an important pathological mechanism of CPSS [28]. Clinical evidence has shown that numerous inflammatory mediators, including IL-6, IL-8, and prostaglandin E2 (PGE2), have been found in wound drainages in patients who have undergone spinal surgery [29]. PGE2 can elicit primary pain and is also a crucial mediator of pain sensitization [30]. Local inflammation can also spread to adjacent DRG, leading to an increased brain-derived neurotrophic factor (BDNF) expression in the DRG and neuronal sensitization, which causes hyperalgesia and radicular pain [29]. These findings are consistent with the clinical observation that radicular pain presents in patients with chemical radiculitis in the absence of compression [29]. Although the number of inflammatory factors in wound drainage fluid gradually decreases from 72 h after surgery, the acute inflammatory responses may sensitize sensory neurons or turn into chronic inflammation, which eventually develops into inflammation-related arachnoiditis [13], an important pathological mechanism in the development of CPSS.
4.2.2. Nucleus-related local environmental changes
The nucleus pulposus plays an important role in the postoperative changes in the local microenvironment. Matrix metalloproteinases, cyclooxygenase 2, nitric oxide, and PGE2 in the local microenvironment may irritate the adjacent nerve roots when the nucleus pulposus is exposed [31,32]. In a canine model, the electrical conduction velocity of the nerve root adjacent to the nucleus pulposus was significantly decreased at 7 days after the incision of the intervertebral disc to expose the nucleus pulposus without compressing the nerve root. In addition, the density and size of capillaries were significantly increased, as compared to the sham group [30]. These findings suggest that nucleus-related local microenvironmental changes, rather than mechanical compression, may alter the functional state and excitability of nerve fibers [33]. Similar changes have also occurred in patients with CPSS. Thus, local pathophysiological changes during the intraoperative extraction of the nucleus pulposus may play a role in CPSS and deserve further investigation.
4.2.3. Changes in the excitability of DRG neurons and spinal cord dorsal horn neurons
DRG neurons are primary afferent sensory neurons that play important roles in the transduction, conduction, and transmission of sensory signals. Sensitization and hyperexcitation of DRG neurons have been associated with chronic pain and hyperalgesia [34]. These changes in DRG neurons can be triggered by inflammatory mediators released at the site of tissue injury [35], which upregulate N- and T-type calcium channels in DRG neurons [34]. Recent evidence suggests that immune cells, including T-cells and macrophages, can also infiltrate into the DRG after nerve injury and increase DRG neuron excitability [36]. Spine surgery often amplifies inflammatory responses and creates a pro-inflammatory local environment, which affects adjacent DRGs and produces hyperalgesia and radicular pain [29], yet few studies have focused on DRG mechanisms in the pathogenesis of CPSS.
In addition to peripheral neuronal sensitization, central sensitization (CS) of the spinal dorsal horn neurons can also be a cause of chronic pain [37]. Glutamate/NMDA receptor-mediated dorsal horn neuronal hyperexcitability is a critical mechanism of CS [37,38], and has been suggested to be a major etiological factor in CPSS. It has been postulated that CS may be induced by a sudden increase in the concentration of extracellular glutamate, due to inadvertent stretching and compressing of the dorsal root during spine surgery [38]. A preclinical study showed that the extracellular concentrations of various amino acids, especially glutamate and aspartate, were significantly increased by 57%–744% in the dorsal spinal cord after bilateral dorsal rhizotomy in rats [38]. In addition, concentrations of monocyte chemotactic protein-1 (MCP-1) and BDNF were significantly increased in the cerebrospinal fluid of patients with CPSS and positively correlated with their pain scores [39]. The functions of these substances are closely related to increased spinal dorsal horn neuron excitability in neuropathic pain conditions. MCP-1 may also enhance NMDA receptor activity and excitatory synaptic transmission in dorsal horn neurons [40], and BDNF may enhance the spontaneous release of GABA and glycine in lamina II of the dorsal horn in response to nerve injury [41]. Based on these findings, the roles of spinal neuronal mechanisms in the pathogenesis and course of CPSS warrant further study.
4.2.4. Changes in glial cells
Injuries to the peripheral or central nervous system can trigger robust glial activation, dysfunction in neuron–glia interaction, and increased release of the pro-inflammatory cytokines, all of which contribute to the long-lasting neuronal sensitization at the periphery, spinal, and supraspinal levels [13]. In these glial cells, microglia and astrocytes, in particular, are gaining increased attention [13,40]. A rat model of chronic low back pain demonstrated a persistent activation of both microglia and astrocytes in the spinal cord and increased calcitonin gene-related peptides in the DRG [42]. Moreover, clinical evidence shows that patients with chronic lumbar pain express an increased level of brain translocator protein, which is a marker of glial activation [43]. Thus, gliosis may also contribute to chronic low back pain.
Microglial cells are known as resident macrophages in CNS and are responsible for monitoring changes in the local microenvironment [44]. Intraspinal administration of activated microglial cells produced tactile allodynia in naive rats [45]. Microglial cells respond rapidly to a wide range of stimulation that threatens physiological homeostasis, including spine surgery [13,46]. Multiple studies have provided compelling evidence that mechanical or chemical stimulation of peripheral nerves leads to a dramatic activation of microglia in the spinal dorsal horn, which shifts from a branching state to a reactive state and triggers pain hypersensitivity [46,47]. Microglia activation increases the expression of interferon regulatory factor 8 [48], which further promotes the expression of interferon regulatory factor 5 [46,48]. Then, interferon regulatory factor 5 induces P2X4R expression in microglia [49]. P2X4R + microglia can downregulate potassium-chloride transporter in dorsal horn neurons by releasing bioactive diffusible factors, which, in turn, leads to the collapse of the transmembrane anion gradient, resulting in neuron depolarization [46,49]. The increased neuronal excitability caused by microglial activation in the spinal pain circuit may be responsible for neuropathic pain [46,47], which is “pain caused by a lesion or disease of the somatosensory nervous system” according to the International Association for the Study of Pain (IASP) terminology. In addition, microglial activation is involved in spinal long-term potentiation at C-fiber synapses, which may share similar cellular mechanisms with those underlying chronic pain [50]. Behaviorally, long-term potentiation-inducting stimulation elicited prolonged pain hypersensitivity and increased CGRP. These changes were blocked by ablating spinal microglia [50], suggesting that microglia-dependent synaptic potentiation is important to chronic pain.
Astrocytes are thought principally to provide structural and nutritional support to neurons and comprise approximately 20%–40% of all glial cells in CNS [51]. Many lines of evidence support the role of astrocytes in neuropathic pain [52,53]. Preclinical studies show astrocyte hypertrophy as indicated by increased GFAP expression, which is associated with pain hypersensitivity after nerve injury [53]. Although research on human astrocytes has been limited by the availability of tissue, autopsy studies show astrocyte activation in the spinal cord dorsal horn in patients with longstanding chronic pain [52]. In addition, by using integrated positron emission tomography-magnetic resonance imaging and emerging radioligand [11C]-PBR28 [54], clinical research shows increased levels of brain translocator protein in patients with chronic lumbar pain. This evidence suggests that astrocyte hyperplasia is also associated with chronic pain in humans. Mechanistically, astrocytic intracellular signaling and the release of neuromodulators from astrocytes have attracted increasing attention for the part they play in neuropathic pain. These neuromodulators include those affecting kinase and protease pathways, transporters, and gap–junction proteins, which play important roles in neuron–astrocyte and microglia–astrocyte interactions [51]. In addition, pro-inflammatory cytokines and chemokines can also be produced and released by astrocytes, affecting the induction and maintenance of CS and chronic pain.
4.2.5. Psychological factors
Common psychological and psychosocial factors, including stress, depression, anxiety, catastrophizing, and a lack of support, have been suggested to predispose patients to chronic pain conditions. Clinical evidence shows that patients with psychological distress are more likely to experience poorer outcomes after spinal surgery [55]. Thus, psychological factors may produce similar detrimental effects on CPSS. These psychological factors may affect pain by attenuating descending pain inhibitory systems and/or activating descending pain facilitatory systems in CNS [56]. For example, the stimulation or activation of neurons in the periaqueductal gray can induce descending pain inhibition through projections to the rostral ventromedial medulla in the brain stem, which then project to the dorsal horn of the spinal cord [56]. The rostral ventromedial medulla contains serotonergic neurons that can exert a bidirectional regulation on spinal pain transmission and pain perception [57]. In patients with depression, the pain-inhibitory effect is reduced in part due to the depletion of serotonin in this pathway. Therefore, poor psychosocial well-being may be a strong predictor or risk factor of CPSS [58,59].
5. Current treatment of CPSS
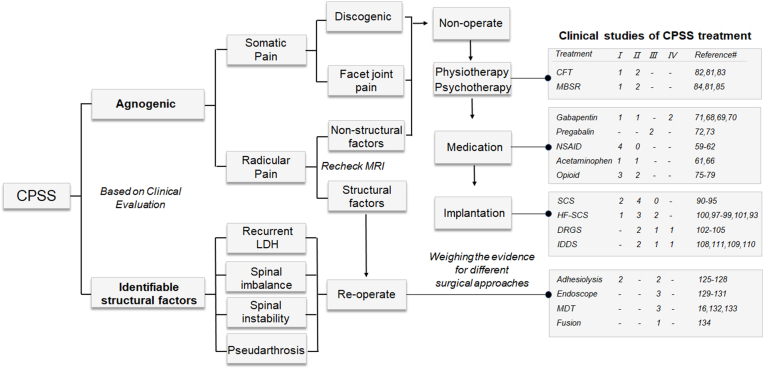
Recent clinical studies of CPSS treatment were categorized according to the proposed algorithm and the levels of evidence (Fig. 3). Current CPSS therapies can be broadly divided into three categories: conservative, surgical, and neuromodulation management.
Fig. 3.
Clinical studies on CPSS treatment. Recent clinical studies of CPSS were categorized according to the proposed algorithm and the levels of evidence. These studies were mainly randomized controlled clinical trials over the past 20 years, excluding those using the combined treatment and studies classified as Level V evidence. CPSS, chronic pain after spine surgery; LDH, lumbar disc herniation; CFT, cognitive functional therapy; MBRS, mindfulness-based stress reduction; NSAID, nonsteroidal anti-inflammatory drugs; SCS, spinal cord stimulation; HF-SCS, high-frequency 10 kHz SCS; DRGS, dorsal root ganglion stimulation; IDDS, intrathecal drug delivery system; MDT, microdiscectomy [[60], [61], [62], [63], [64], [65], [66], [67], [68], [69]].
5.1. Conservative treatment
Conservative treatments include pharmacologic therapy, physical therapy, and psychotherapy [6]. Due to their good safety profiles, conservative treatments should always be attempted first in CPSS patients who do not require urgent surgical intervention [6].
5.1.1. Pharmacologic therapy
Non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs are often used as the first-line drug to attenuate low back pain. A high-quality study suggested that NSAIDs were superior to placebo for patients with chronic low back pain (Level Ⅰ) [70]. Another study showed that NSAIDs reduced pain intensity scores by an average of 6.97 points on a 0 to 100 visual analog scale (Level Ⅰ) [71]. However, a systematic review found that the analgesic effect of NSAIDs was not superior to other conservative treatments such as muscle relaxants, physical therapy, or bed rest (Level Ⅰ) [72]. For back pain with radiating leg pain, a previous study found no difference between NSAIDs and placebo treatment (Level Ⅰ) [73]. Due to limited and conflicting evidence, it is difficult to ascertain the effectiveness of NSAIDs in relieving complex pain symptoms in CPSS patients. NSAIDs should not be prescribed to patients with a history of gastric reflux or heart problems. Although some side effects including gastrointestinal ulcerations and kidney dysfunction were reported in the study of aspirin for thromboprophylaxis [74], significant adverse events of using NSAIDs for low back pain treatment were rarely reported.
Acetaminophen/paracetamol. Acetaminophen/paracetamol was often used to treat mild to moderate pain and fever [75] Some reviews recommend listing acetaminophen as one of the medications for CPSS [6,76]. Previous studies showed that acetaminophen at doses of 3–4 g/day relieved chronic low back pain, but its analgesic effect was not significantly different from NSAIDs (Level Ⅰ) [72]. However, for acute low back pain, it was shown that pain relief from acetaminophen treatment (3 g/day) was not superior to the non-treatment group (Level Ⅱ) [77]. Adverse events (e.g., gastrointestinal bleeding) with acetaminophen were rare.
Anticonvulsants. Anticonvulsants such as gabapentin and pregabalin are increasingly being used in the treatment of neuropathic pain which can be caused by damage or disease of the somatosensory nervous system [78]. Gabapentin and pregabalin were also tested in clinical trials for CPSS treatment [[79], [80], [81], [82]]. Two low-quality case reports showed that perioperative pregabalin treatment produced an analgesic effect and improved the functionality and sleep quality in CPSS patients (Level Ⅲ) [83,84]. Nevertheless, these two studies only evaluated the clinical efficacy in the early postoperative period (2–6 months post-surgery), but the medium to longer-term follow-up result was unclear. For gabapentin at a maximum daily dose of 1800 mg, it was found to be significantly more efficient than naproxen for alleviating CPSS (Level Ⅳ) [79]. Another two lower-quality case reports also reached a similar conclusion (Level Ⅱ, Ⅳ) [80,81]. However, one high-quality study found no significant difference between gabapentin (1800 mg/day) and placebo using multiple outcome measures of pain including the Numerical Pain Intensity Scale, the McGill Pain Questionnaire, and the Patient Global Impression of Change (Level Ⅰ) [82]. In summary, while some low-quality studies suggested that anticonvulsants may be effective, these studies were mostly based on small sample sizes and case reports, and hence these findings may not be generalizable. Overall, there is scant high-quality evidence on the efficacy of using anticonvulsants for CPSS treatment. Accordingly, large-scale, multicenter, randomized clinical trials are recommended to rigorously investigate the effectiveness of anticonvulsants in the future.
Opioids. Opioid prescription for low back pain has increased and is now the most commonly prescribed drug class [85]. A systematic review of 11 trials showed that opioids reduced pain intensity by an average of 30% in neuropathic and musculoskeletal pain conditions (Level Ⅰ) [86]. Multiple systematic reviews also agreed that opioids are superior to placebo to provide acute analgesia in chronic low back pain patients (Level Ⅰ-Ⅱ) [[87], [88], [89], [90]]. In some chronic pain patients, opioids are often the only analgesic that can help to relieve pain. However, the aforementioned systematic review reported that approximately 80% of patients experienced at least one major side effect, with constipation (41%), nausea (32%), and somnolence (29%) being the most common ones [24]. In addition, opioids are highly addictive and susceptible to abuse, and more than half of regular opioid users still suffer significant low back pain and radicular pain [85]. Because the adverse events associated with opioid use are alarmingly high, opioid use should be carefully weighed against the patient's pain severity, expectations, medical history, and risk of adverse events. Opioid trials rarely lasted over four months and had a high dropout rate (>20%), mainly because of these adverse effects and tolerance [85]. Accordingly, the effectiveness and safety of long-term opioid use remains unclear. In light of the significant limitations associated with opioid analgesics, it is urgent to develop non-opioid drugs and novel strategies to manage pain and opioid use in CPSS patients. These strategies may include using a combination of analgesics, neuromodulation, physical and psychological care, rehabilitation, etc. as those described in other sections.
Summary and clinical significance. For the pharmacological management of CPSS, it is important to understand the characteristics and etiology of pain symptoms, such as the presence of neuropathic, irradiation, or nociceptive component. For example, although NSAIDs are used as the first-line drugs to relieve chronic low back pain, they exert only limited therapeutic effects on radicular pain. Opioids may have short-term analgesic efficacy for chronic back pain, but their long-term efficacy and safety in CPSS patients remain questionable. Muscle relaxants, acetaminophen, antidepressants, and anticonvulsants are also used in CPSS patients [88]. Yet, evidence for their clinical effectiveness has been weak and sometimes contradictory. Overall, choosing which pharmacotherapy should be considered as part of a larger multidisciplinary program for managing CPSS. In this regard, an important future task is to create best practice guidelines and establish gold standards for clinicians to choose analgesic treatment for CPSS.
5.1.2. Physical/psychosocial therapy
Cognitive functional therapy (CFT). CFT represents an integrated approach for individualizing pain treatment by changing the perception of pain, quitting a bad lifestyle habit, and reducing negative psychological factors [91]. Clinical evidence showed that CFT resulted in a greater improvement in the quality of life in patients with chronic low back pain and functional limitations, as compared to usual care (Level Ⅱ) [92]. Yet, other studies found that CFT only reduced disability, but not pain, during short-term follow-ups (Level Ⅰ) [93], and CFT failed to alleviate pain even with extended follow-up to 3 years (Level Ⅱ) [94]. Therefore, the clinical implications of CFT in CPSS remain elusive due to conflicting data.
Mindfulness-based stress reduction (MBSR). MBSR is often used to treat pain by sequentially shifting attention to different parts of the body, through meditation, hatha yoga and body scans, and sustained mindfulness practices [95]. MBSR increased pain acceptance with a mean of 7.0-point (on a 108-point scale) in patients with CPSS at 12-week follow-up (Level Ⅱ) [96]. Another study reached the same conclusion but showed that MBSR was similar to CFT in improving back pain (Level Ⅱ) [92]. Importantly, long-term follow-up results showed no significant difference between MBSR and usual care (Level Ⅰ) [95]. Therefore, more high-quality long-term RCTs are needed to conclude the efficacy of MBSR in the management of CPSS.
Summary and clinical significance. Physical/psychosocial therapies are also commonly recommended for CPSS patients [1]. Both CFT and MBSR are comprehensive treatments that involve physical therapy and psychotherapy. Physical rehabilitation may help to maintain and improve physical function, but there is a lack of high-quality evidence to support its long-term efficacy [6]. For psychosocial therapy, several low-quality studies have demonstrated that lowering stress levels can reduce the risk of postoperative pain [[97], [98], [99]]. CFT and MBSR are also widely recommended for patients with chronic low back pain. Yet, the efficacy of physical/psychosocial therapy for CPSS still lacks high-level evidence support, and its utility for CPSS with severe pain may be limited. Therefore, some authors recommend that physical/psychosocial therapies should be considered as a supplemental treatment for CPSS [6,76].
5.2. Neuromodulation
Neuromodulation via a surgically implanted spinal cord, DRG, or peripheral nerve stimulation device is gaining increased popularity owing to its substantial superiority over pharmacological and surgical management.
Spinal cord stimulation (SCS). SCS is comprised of an implantable pulse generator which is connected to an electrode that is placed into the epidural space over the spinal cord dorsal columns [100]. Conventional low-frequency SCS was shown to be more effective than reoperation for CPSS treatment, and the number of patients that required increasing opiate analgesics after reoperation surgery was significantly more than those who underwent SCS, with a mean follow-up period of 3 years (Level Ⅰ) [101]. Another Level Ⅰ study suggested that CPSS patients who received conventional SCS treatment reported superior pain relief and functional recovery at the 2-year follow-up, as compared to those treated with conventional medical management [102]. Evidence also suggests that SCS exerted significant long-term pain reduction in CPSS patients, with a mean follow-up of 8.3–10.6 years (Level Ⅱ) [[103], [104], [105]]. Nevertheless, a study found that permanent SCS implantation may not provide consistent long-term pain relief (Level Ⅱ) [106]. Although increasing evidence supports the effectiveness of conventional SCS in the treatment of CPSS, findings of its long-term outcome remain conflicting [100,106,107]. Reported adverse events were limited, with lead migration (7%), implantable pulse generator pocket pain (4%), and muscle spasm or cramp (2%) being the most common complications (Level Ⅱ) [106].
High-frequency SCS (HF-SCS) delivers electrical stimulation pulses of 1–10 kHz to the spinal cord and induces pain inhibition without paraesthesia [100]. An increasing amount of evidence supports the effectiveness of the new SCS paradigm in the treatment of chronic back and leg pain (Level Ⅱ-Ⅲ) [104,108,109]. Yet, studies that compared the efficacy of HF-SCS with conventional SCS have yielded conflicting results [110]. Previous studies showed its long-term superiority to the conventional SCS in treating chronic back and leg pain (Level Ⅰ) [111], and an average pain reduction of 49% following HF-SCS in patients who failed conventional SCS therapy (Level Ⅱ) [112]. However, another study found no significant difference between HF-SCS and conventional SCS in a one-year follow-up (Level Ⅱ) [110].
Dorsal root ganglion (DRG) stimulation. DRG stimulation involves surgically placing an electrode near the DRG to block nociceptive signals in peripheral sensory units, thereby alleviating pain [100]. It was found that pain was reduced from a score of 8.64 at baseline to 2.40 after 12 months of DRG stimulation in CPSS patients, a 72% average reduction (Level Ⅱ) [113]. Another study also showed that DRG stimulation was effective in attenuating both leg pain and back pain in CPSS patients at 1-year follow-up (Level Ⅱ) [114]. Thus, an increasing amount of evidence supports the utility of DRG stimulation in the treatment of chronic back and leg pain (Level Ⅲ, Ⅳ) [115,116]. Nevertheless, more high-quality clinical evidence of DRG stimulation in CPSS treatment, especially for long-term efficacy (>1 year), remains warranted.
Summary and clinical significance. Overall, evidence that demonstrated the effectiveness of SCS utilization in CPSS and its superiority over conservative management and repeated surgery is strong [[100], [101], [102],112,113]. Low-to-moderate clinical evidence also showed that DRG stimulation was effective against low back pain, as it relieved pain and improved the quality of life of CPSS patients (Level Ⅱ) [113]. CPSS patients often rely on long-term treatment for pain control, but the efficacy of neuromodulation therapies may decrease over time [13,117]. In addition, SCS also leads to high upfront costs for the device and surgical implantation. Accordingly, SCS may be recommended to CPSS patients when conservative therapies have not provided meaningful benefits, after weighing the expected outcomes, risks, and benefits of SCS.
5.3. Surgical intervention
CPSS in many patients may have complex etiologies that include both structural and non-structural factors. Surgical intervention may be an option for patients with clearly identified structural factors but is often associated with low success rates. Reoperation is often recommended in patients if structural factors are identified on postoperative imaging of the previous spinal operation [54]. For example, CPSS due to recurrent disc herniation and postoperative sagittal imbalance usually requires surgical intervention [1]. Several surgical modalities have been recommended such as posterior lumbar interbody fusion, microendoscopic discectomy, adhesiolysis, and percutaneous endoscopic lumbar discectomy [1,14]. Although reoperation may be a good option if imaging data indicate that nerve roots are compressed or irritated due to structural factors [54], it may not help to alleviate CPSS caused by non-structural factors. Notably, the success rate may decrease as the number of surgeries increases [31]. For example, compared to a success rate of over 50% after the first spine surgery, the success rate decreased to 30%, 15%, and 5% following the second, third, and fourth surgical interventions, respectively [31]. This may be due in part to increased tissue injury, inflammation, and other non-structural factors that can cause low back pain.
5.4. Other treatment options for CPSS
Some low-to-moderate evidence has shown that the intrathecal drug delivery system (IDDS) was effective against low back pain, which delivers low-dose drugs directly to the spinal cord target (Level Ⅱ, Ⅲ, Ⅳ) [[118], [119], [120]]. Some authors recommended the application of IDD for CPSS when SCS and DRG stimulation treatment cannot provide satisfying pain relief (Level Ⅱ) [121]. In addition, a recent study showed that non-invasive painless signaling therapy (NPST) relieved the pain of CPSS patients [122]. NPST is an electrodermal therapy that converts pain information into synthetic non-pain information [122]. Electroencephalography data suggest that pain reduction by NPST in CPSS patients was associated with increased activity in the right anterior cingulate gyrus. In addition, pulsed radiofrequency treatment (PRF) also showed pain-inhibitory effects in CPSS patients [123]. PRF can inhibit the transmission of nerve impulses without the damaging heating effect [123]. These new technologies are non-invasive or minimally invasive and devoid of tolerance, which is a common disadvantage of pharmacological treatments, and hence are worthy of further in-depth study.
6. Preventative treatment of CPSS and clinical significance
CPSS is difficult to treat once established. Excessive spinal epidural fibrosis after laminectomy represents a common cause of CPSS. In a subset of CPSS patients, the fibrosis after spine surgery spreads from the operative region and presses on the nerve root, DRG, or the dura mater, resulting in functional incapacity (e.g., motor deficits) and low back pain [18,23]. Once fibrosis is formed, revision surgery, which is associated with an increased risk of complications, is often required to reduce extensive epidural scar adhesions and decompress the tethered nerve roots [8]. Unlike other chronic low back pain conditions, CPSS is a postoperative syndrome and may be preventable through postoperative interventions [18,124], such as the local and immediate application of drugs or biomaterials which aim to improve the local microenvironment to reduce inflammation, adhesion, and fibrosis, and to attenuate neuronal sensitization.
6.1. Preemptive drug treatment
Several pharmaceutical agents, including mitomycin C [125], rosuvastatin [126], dexamethasone [127], hydroxycamptothecine [19], colchicine, steroid hormones, doxycycline, NSAIDs [128], and rapamycin have been tested to reduce inflammation, adhesion, fibrosis, and neuronal sensitization with topical or systemic administration [126]. Local inflammation, deposition of ECM, fibroblast proliferation, and differentiation play key roles in fibrosis and scar formation. TGF-β1 is a pluripotent growth factor and plays a vital role in the development of fibrosis, especially in the differentiation of fibroblasts into myofibroblasts [21]. A TGF-β1 inhibitor, decorin, was shown to exert a preemptive effect on epidural fibrosis and epidural adhesions after laminectomy in rats [20]. This effect of decorin depends on inhibitions of TGF-β1-Induced ECM synthesis, proliferation, transdifferentiation, and extracellular matrix production in primary fibroblasts through the inhibition of the Smad2/3 signaling pathway. In a mouse model of laminectomy, metformin, which was supplied via drinking water after the surgery, also reduced the hyperproliferation of epidural scars [129]. This drug effect involves inhibition of both TGF-β1/Smad3 and the high mobility group box 1 (HMGB1)/Toll-like receptor 4 (TLR4) signaling pathways, which reduced fibronectin and collagen deposition. In addition, bevacizumab was shown to reduce spinal epidural fibrosis and adhesion in rats after laminectomy, by decreasing the new blood vessel formation by inhibiting vascular endothelial growth factor (VEGF) signaling [130]. Most recently, long non-coding RNAs (LncRNAs) that inhibit COX2 expression (LncRNA-COX2) were shown to be decreased in epidural tissues after laminectomy and in activated fibrotic fibroblasts. Importantly, overexpression of LncRNA-COX2 attenuated laminectomy-induced epidural fibrosis in mice, by decreasing the expression of EGR1 and inhibiting fibroblasts differentiation, proliferation, and migration [24]. Thus, postoperative treatment with LncRNA-COX2 may prevent aberrant epidural fibrosis. Although these drugs and treatments have demonstrated the ability to alleviate postoperative adhesion and reduce fibrosis in animal models, their therapeutic effects and safety still require validation through clinical studies.
6.2. Preemptive treatment with biomaterials and synthetic materials
Synthetic materials, including polymethyl methacrylate, polylactic acid, and silastic silicone, were shown to exert anti-fibrotic effects. Biomaterials, such as Silastic-Dacron gelatin sponge, animal collagen membranes, Adcon-L, autologous lipid graft, and omentum graft, are also gaining attention with interdisciplinary backgrounds. They can act as physical barriers to limit fibroblast infiltration into epidural space and the adhesion of the dura mater to the surgical wound [18]. One clinical study showed that the placement of a polytetrafluoroethylene membrane over the laminectomy wound prevented the invasion of fibrous tissue into the vertebral canal and the formation of seromas. Importantly, patients who received the treatment developed less postoperative radicular pain. E8002, which has a three-layered structure, also exerted an anti-adhesive effect in a rat laminectomy model [124]. Certain materials can also act as carriers to control local drug release [18]. For example, electrospun polycaprolactone (PCL) fibrous membranes loaded with celecoxib (CEL) induced a slow release of CEL for 2 weeks and attenuated fibrosis and inflammation associated with CPSS in rats [28].
6.3. Preemptive treatment with biomaterials from human tissues
Human amniotic membrane and umbilical cord particulate were also shown to reduce epidural scar amount and adhesion after laminectomy. Clinical studies demonstrated that these human birth tissue products, which exert natural anti-inflammatory and regenerative actions [1,2], may have multiple beneficial effects to treat various diseases, such as ocular surface disorders (e.g., burns, infections, corneal scars, post-surgical trauma), painful bullous keratopathy, complex diabetic foot ulcers, burn injury, discogenic pain, neuropathic corneal pain, and facet joint syndrome [4,13,14,17,22]. In a canine model, scar amount and adhesion tenacity were significantly lower in dogs treated with a cross-linked amniotic membrane covering the dura after lumbar laminectomy, compared to the control groups [131]. Additionally, amniotic membrane and umbilical cord particulate induced pain relief in patients with discogenic low back pain, and reduced back pain caused by facet joint syndrome following intra-articular injection. Human birth tissue products may exert multiple beneficial effects for post-laminectomy epidural adhesion, including a physical barrier to reduce fibroblast infiltration and consequent epidural fibrosis, the limitation of inflammation and disc degeneration, reduction of pain, and promotion of regeneration. Similarly, a dual-layer, chorion-free amnion patch (DLAM; ViaShield®, Globus Medical Inc., Audubon, PA, USA), which was developed from human amniotic membrane, effectively reduced fibroblast infiltration and tissue tenacity after lumbar laminectomy in sheep [132]. Recently, a new bacterial cellulose (bc) anti-adhesion membrane, composed of exosomes from human umbilical cord mesenchymal stem cells, also demonstrated the prevention effect on epidural fibrosis post-laminectomy in a rabbit model, without notable cytotoxicity [133]. Because these bioceuticals are prepared from immune-privileged tissue, they rarely caused immunologic rejection and show better capability and biocompatibility in the body, as compared to fat grafts [11]. Accordingly, these bioceuticals may be suitable for preventing postoperative epidural fibrosis and adhesion and minimizing postoperative complications and risk of CPSS. More well-designed studies using human birth tissue products for CPSS prevention, wound healing, and regeneration remain warranted.
7. Future research
7.1. Establishing a suitable, clinically relevant CPSS model
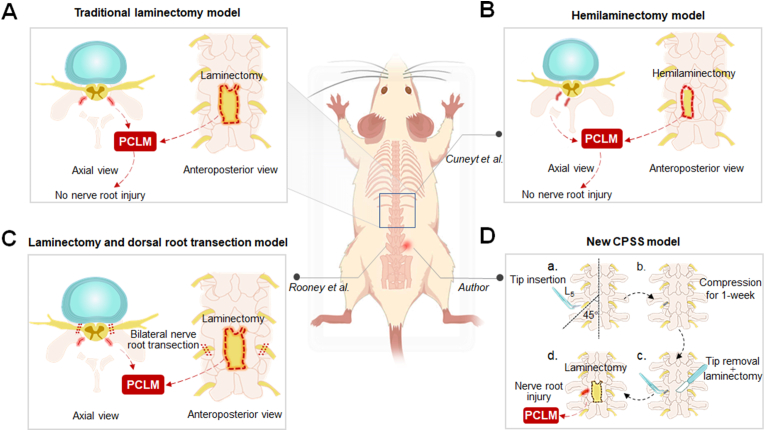
The lack of clinically relevant models is an important rate-limiting step in the mechanism and treatment studies of CPSS. The most common CPSS model involves a laminectomy (Fig. 4A) or hemilaminectomy (Fig. 4B) in the thoracic or lumbar spine vertebra (e.g., T10, L1, L4-6) of naïve animals [20], without irritating the nerve root and spinal cord. However, unlike in healthy animals, the nerve roots that are oppressed by epidural fibrosis after laminectomy in patients often have certain pre-existing pathological changes before spinal surgery. To address the limitation of the laminectomy and hemilaminectomy models, Rooney et al. [38] established a new CPSS model by both performing the laminectomy and cutting the bilateral L4 and L5 dorsal roots (Fig. 4C). However, an acute or severe dorsal root rupture rarely occurred in clinical practice; rather, chronic compression and irritation of the nerve roots or dorsal spinal cord are common pre-existing conditions before surgical revision. Currently, none of the current CPSS models closely mimics this clinical situation. Therefore, establishing a suitable, clinically relevant CPSS model to mimic this situation is important for preclinical mechanistic research.
Fig. 4.
Schematic illustration of animal models of CPSS (A) The conventional CPSS model involves a full laminectomy in the thoracic or lumbar spine vertebra without irritating or damaging the nerve roots (B) Cuneyt et al. modified the traditional CPSS model by performing only a hemilaminectomy in the L4 spine vertebra (C) Rooney et al. established a new CPSS model by performing both laminectomy and cutting of bilateral L4 and L5 dorsal roots (D) We propose a new model to simulate the clinical CPSS. (a) A plastic tip will be first inserted into the left foramina at L5 spine vertebra level in rats to induce a chronic compression of the nerve root and DRG, which simulates lumbar disc herniation causing low back pain (b–c) One week later, the plastic tip will be removed and a laminectomy will then be performed at the L5 vertebra to relieve the compression. (d) This new model mimics CPSS which may develop after decompression surgery performed in patients with lumbar disc herniation. PCLM, postoperative changes in the local microenvironment.
To better simulate CPSS in the clinical setting, laminectomy may be performed in animals that have preexisting spine conditions, such as low back pain, nerve root compression, or disc herniation. This may be more suitable for exploring the etiology of CPSS and its treatment (Fig. 4D). Future studies using new CPSS models are needed to ascertain important non-structural factors in CPSS pathogenesis, which may provide novel targets for clinical interventions. Close collaboration between basic scientists and front-line clinicians is required to achieve this goal.
7.2. Establishing clinical-relevant behavioral pain testing in CPSS models
In addition to the lack of a suitable CPSS model, the behavioral measurement of CPSS-like symptoms in animal models represents another challenge in the translation of preclinical findings to the development of novel clinical treatment. CPSS in animal models is usually inferred by measuring paw withdrawal responses to external test stimulation. In particular, mechanical stimulus-evoked behavioral responses have been the most commonly measured outcome. However, withdrawal responses represent a spinal reflex to test stimulation and may not accurately indicate CPSS in subjects, which often experience ongoing pain, background pain, spontaneous pain, and movement-induced pain. However, these important clinical symptoms have been difficult to measure in animal studies [134]. Previous studies of neuropathic pain conditions suggested that behavioral tests of reflex responses alone do not correlate well with the effectiveness of drug treatment in subjects [134]. Accordingly, comprehensive assays using non-reflex outcome measures, such as cerebral-dependent conditioned place preference, place escape or avoidance paradigm, operate behavior test, voluntary wheel running activity, and gait analysis are warranted for evaluating the effectiveness and studying the mechanisms of new therapies for alleviating ongoing pain and movement-related manifestations of CPSS in order to facilitate future translational studies.
7.3. The research on the pathogenesis of CPSS
CPSS is characterized by a cluster of symptoms, including intractable or recurrent low back pain or leg pain, and it is a major cause of morbidity in patients after spinal surgery. To date, CPSS remains difficult to treat and represents a therapy-refractory clinical condition. Understanding the etiology of CPSS plays a critical role in determining the course of the treatment. However, the exact cause of CPSS is often difficult to ascertain in patients and may involve multiple factors, including the formation of epidural fibrosis, chronic arachnoiditis, nucleus-related local environmental changes, sensitization of DRG neurons and spinal cord dorsal horn neurons, changes in glial cell activation, psychological factors, and other structural factors.
7.4. New treatment strategies of CPSS
Although the intra-operative placement of drugs, biomaterials, and adipose-derived mesenchymal stem cells on the surface of the dura mater has been tested to improve the postoperative microenvironment and reduce fibrosis, the long-term efficacy and the metabolism of allogeneic biomaterials in humans remain to be determined. Therefore, more clinical studies are required to determine the efficacy and safety of these preventive treatment strategies and to develop new strategies to promote repair processes under a regenerative condition other than scar formation. In addition to the repair of soft tissue, how these treatments affect new bone formation from the margins of laminectomy defects also warrants attention.
In addition to the aforementioned individual treatments and re-operation [[60], [61], [62], [63], [64], [65], [66], [67], [68], [69]], a promising new strategy for treating CPSS is the use of combination therapies. This approach may tailor the patient-specific treatment and reduce the limitations and potential side effects of individual treatments. There are several combined approaches for CPSS management, including the combined use of opioids with regional anesthetics such as spinal or epidural analgesia [135], infusions of ketamine and lidocaine, NSAIDs [136], and other non-opioid analgesics such as gabapentin [137] and acetaminophen [138], cognitive and physical therapies. These approaches can optimize pain relief, reduce opioid usage, and minimize opioid-related adverse effects [[135], [136], [137], [138]]. The clinical efficacy of some combined treatments for CPSS has been demonstrated. A meta-analysis of ten studies showed that the combined use of opioids and NSAIDs following spinal surgery decreased both the total amount of narcotics consumed and pain levels, compared to the use of opioid medications alone [136]. Additionally, a retrospective review of 139 patients undergoing spine surgery found that those who received multimodal analgesia had lower rates of inpatient narcotics consumption than those treated with patient-controlled analgesia [135]. Despite the recommendation for multimodal analgesia as a better strategy for postoperative analgesia, there is a lack of consensus on appropriate protocols or algorithms for multimodal analgesic treatment [139]. Accordingly, identifying and utilizing effective strategies should also be a focus for future research.
8. Conclusion and clinical significance
This review aimed to broaden our understanding of the mechanisms underlying CPSS, which will aid in the appropriate application and development of optimal strategies for not only managing pain symptoms but also potentially curing the underlying etiology for long-term functional recovery. Because CPSS patients often have a mixed etiology, the authors advocate that clinicians should examine all available evidence and comprehensively evaluate each case in order to formulate a patient-specific and multi-modal pain treatment. In addition, physicians should consider an early, intra-operative intervention that may decrease the risk or even prevent the onset of CPSS.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authorship
Indicate the specific contributions made by each author (list the authors’ initials followed by their surnames, e.g., Y.L. Cheung). The name of each author must appear at least once in each of the three categories below.
Category 1
Conception and design of study: Yun Guan, Lei Zang; acquisition of data: Qichao Wu; analysis and/or interpretation of data.
Category 2
Drafting the manuscript: Qichao Wu, Xiang Cui, Leo C Guan, Chi Zhang, Jing Liu, Neil C Ford
Revising the manuscript critically for important intellectual content: Shaoqiu He, Xueming Chen, Xu Cao.
Category 3
Approval of the version of the manuscript to be published (the names of all authors must be listed):
Qichao Wu, Xiang Cui, Leo C Guan, Chi Zhang, Jing Liu, Neil C Ford, Shaoqiu He, Xueming Chen, Xu Cao, Lei Zang, Yun Guan.
Declaration of competing interest
A conflict of interest occurs when an individual's objectivity is potentially compromised by a desire for financial gain, prominence, professional advancement or a successful outcome. The Editors of the Journal of Orthopaedic Translation strive to ensure that what is published in the Journal is as balanced, objective and evidence-based as possible. Since it can be difficult to distinguish between an actual conflict of interest and a perceived conflict of interest, the Journal requires authors to disclose all and any potential conflicts of interest.
Acknowledgements
All persons who have made substantial contributions to the work reported in the manuscript (e.g., technical help, writing and editing assistance, general support), but who do not meet the criteria for authorship, are named in the Acknowledgements and have given us their written permission to be named. If we have not included an Acknowledgements, then that indicates that we have not received substantial contributions from non-authors.
This work was conducted at the Johns Hopkins University School of Medicine. The authors thank Dr. Courtney McQueen (Ph.D., Senior Science Writer, Research Development Team, Office of the Vice Provost for Research, Johns Hopkins University) for editing the manuscript. Q.W’s effort was supported by a training grant from the China Scholarship Council. Funders had no role in study design, data collection, data interpretation, or in the decision to submit the work for publication.
Contributor Information
Lei Zang, Email: zanglei@ccmu.edu.cn.
Yun Guan, Email: yguan1@jhmi.edu.
References
- 1.Alizadeh R., Sharifzadeh S.R. Pathogenesis, etiology and treatment of failed back surgery syndrome. Neurochirurgie. 2021 doi: 10.1016/j.neuchi.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Hoy D., March L., Brooks P., Blyth F., Woolf A., Bain C., et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):968–974. doi: 10.1136/annrheumdis-2013-204428. [DOI] [PubMed] [Google Scholar]
- 3.Brox J.I., Reikerås O., Ø Nygaard, Sørensen R., Indahl A., Holm I., et al. Lumbar instrumented fusion compared with cognitive intervention and exercises in patients with chronic back pain after previous surgery for disc herniation: a prospective randomized controlled study. Pain. 2006;122(1–2):145–155. doi: 10.1016/j.pain.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 4.Grotle M., Småstuen M.C., Fjeld O., Grøvle L., Helgeland J., Storheim K., et al. Lumbar spine surgery across 15 years: trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open. 2019;9(8) doi: 10.1136/bmjopen-2018-028743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guyer R.D., Patterson M., Ohnmeiss D.D. Failed back surgery syndrome: diagnostic evaluation. J Am Acad Orthop Surg. 2006;14(9):534–543. doi: 10.5435/00124635-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Amirdelfan K., Webster L., Poree L., Sukul V., McRoberts P. Treatment options for failed back surgery syndrome patients with refractory chronic pain: an evidence based approach. Spine. 2017;42(14):S41–S52. doi: 10.1097/BRS.0000000000002217. [DOI] [PubMed] [Google Scholar]
- 7.Jang J.S., Lee S.H., Min J.H., Kim S.K., Han K.M., Maeng D.H. Surgical treatment of failed back surgery syndrome due to sagittal imbalance. Spine (Phila Pa 1976. 2007;32(26):3081–3087. doi: 10.1097/BRS.0b013e31815cde71. [eng] [DOI] [PubMed] [Google Scholar]
- 8.Kim S.S., Michelsen C.B. Revision surgery for failed back surgery syndrome. Spine (Phila Pa 1976. 1992;17(8):957–960. doi: 10.1097/00007632-199208000-00015. [eng] [DOI] [PubMed] [Google Scholar]
- 9.Avellanal M., Diaz-Reganon G., Orts A., Gonzalez-Montero L., Riquelme I. Transforaminal epiduroscopy in patients with failed back surgery syndrome. Pain Physician. 2019;22(1):89–95. [eng] [PubMed] [Google Scholar]
- 10.Daniell J.R., Osti O.L. Failed back surgery syndrome: a review article. Asian Spine Journal. 2018;12(2):372–379. doi: 10.4184/asj.2018.12.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y.-C., Lee C.-Y., Chen S.-J. Narcotic addiction in failed back surgery syndrome. Cell Transplant. 2019;28(3):239–247. doi: 10.1177/0963689718796072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schofferman J., Reynolds J., Herzog R., Covington E., Dreyfuss P., O'Neill C. Failed back surgery: etiology and diagnostic evaluation. Spine J. 2003;3(5):400–403. doi: 10.1016/s1529-9430(03)00122-0. [DOI] [PubMed] [Google Scholar]
- 13.Echeverria-Villalobos M., Mitchell J., Fiorda-Diaz J., Weaver T. Effects of dorsal column spinal cord stimulation on neuroinflammation: revisiting molecular mechanisms and clinical outcomes on chronic lumbar/leg pain and failed back surgery syndrome. J Pain Res. 2021;14:2337–2345. doi: 10.2147/JPR.S309872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sebaaly A., Lahoud M.-J., Rizkallah M., Kreichati G., Kharrat K. Etiology, evaluation, and treatment of failed back surgery syndrome. Asian Spine Journal. 2018;12(3):574–585. doi: 10.4184/asj.2018.12.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natarajan R.N., Andersson G.B., Patwardhan A.G., Andriacchi T.P. Study on effect of graded facetectomy on change in lumbar motion segment torsional flexibility using three-dimensional continuum contact representation for facet joints. J Biomech Eng. 1999;121(2):215–221. doi: 10.1115/1.2835106. [DOI] [PubMed] [Google Scholar]
- 16.Bokov A., Isrelov A., Skorodumov A., Aleynik A., Simonov A., Mlyavykh S. An analysis of reasons for failed back surgery syndrome and partial results after different types of surgical lumbar nerve root decompression. Pain Physician. 2011;14(6):545–557. [eng] [PubMed] [Google Scholar]
- 17.Henderson N.C., Rieder F., Wynn T.A. Fibrosis: from mechanisms to medicines. Nature. 2020;587(7835):555–566. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H., Sun W., Fu D., Shen Y., Chen Y-y, Wang L-l. Update on biomaterials for prevention of epidural adhesion after lumbar laminectomy. Journal of Orthopaedic Translation. 2018;13:41–49. doi: 10.1016/j.jot.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y., Wang L., Sun S., Liu B., Wu N., Cao X. The effect of 10-hydroxycamptothecine in preventing fibroblast proliferation and epidural scar adhesion after laminectomy in rats. Eur J Pharmacol. 2008;593(1–3):44–48. doi: 10.1016/j.ejphar.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Ding Q., Wei Q., Sheng G., Wang S., Jing S., Ma T., et al. The preventive effect of decorin on epidural fibrosis and epidural adhesions after laminectomy. Front Pharmacol. 2021:12. doi: 10.3389/fphar.2021.774316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohan R.R., Gupta R., Mehan M.K., Cowden J.W., Sinha S. Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp Eye Res. 2010;91(2):238–245. doi: 10.1016/j.exer.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samy A.M., Hardy R.W., Jr. Epidural fibrosis and the failed back surgery syndrome: history and physical findings. Neurol Res. 1999;21(Suppl 1):S5–S8. doi: 10.1080/01616412.1999.11758603. [DOI] [PubMed] [Google Scholar]
- 23.Songer M.N., Ghosh L., Spencer D.L. Effects of sodium hyaluronate on peridural fibrosis after lumbar laminotomy and discectomy. Spine (Phila Pa 1976. 1990;15(6):550–554. doi: 10.1097/00007632-199006000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Yang L., Zheng S., Ge D., Xia M., Li H., Tang J. LncRNA-COX2 inhibits fibroblast activation and epidural fibrosis by targeting EGR1. Int J Biol Sci. 2022;18(4):1347–1362. doi: 10.7150/ijbs.67974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Einhaus S.L., Robertson J.T., Dohan F.C., Jr., Wujek J.R., Ahmad S. Reduction of peridural fibrosis after lumbar laminotomy and discectomy in dogs by a resorbable gel (ADCON-L) Spine. 1997;22(13):1440–1446. doi: 10.1097/00007632-199707010-00003. discussion 46-7. [DOI] [PubMed] [Google Scholar]
- 26.Ross J.S., Robertson J.T., Frederickson R.C., Petrie J.L., Obuchowski N., Modic M.T., et al. Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation. ADCON-L European Study Group. Neurosurgery. 1996;38(4):855–861. ; discussion 61-3. [PubMed] [Google Scholar]
- 27.Siccoli A., Staartjes V.E., Klukowska A.M., Muizelaar J.P., Schröder M.L. Overweight and smoking promote recurrent lumbar disk herniation after discectomy. Eur Spine J. 2022;31(3):604–613. doi: 10.1007/s00586-022-07116-y. [DOI] [PubMed] [Google Scholar]
- 28.Wang W., Wang Y., Lou T., Ding M., Li J., Xiong H., et al. Celecoxib-loaded electrospun fibrous antiadhesion membranes reduce COX-2/PGE2 induced inflammation and epidural fibrosis in a rat failed back surgery syndrome model. Neural Plast. 2021;2021:1–8. doi: 10.1155/2021/6684176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amaya F., Samad T.A., Barrett L., Broom D.C., Woolf C.J. Periganglionic inflammation elicits a distally radiating pain hypersensitivity by promoting COX-2 induction in the dorsal root ganglion. Pain. 2009;142(1):59–67. doi: 10.1016/j.pain.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayama S., Konno S., Olmarker K., Yabuki S., Kikuchi S. Incision of the anulus fibrosus induces nerve root morphologic, vascular, and functional changes. An experimental study. Spine (Phila Pa 1976. 1996;21(22):2539–2543. doi: 10.1097/00007632-199611150-00002. [DOI] [PubMed] [Google Scholar]
- 31.Das U.N. Bioactive lipids in intervertebral disc degeneration and its therapeutic implications. Biosci Rep. 2019;39(10) doi: 10.1042/BSR20192117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C., Liang G., Deng Z., Tan J., Zheng Q., Lyu F.J. The upregulation of COX2 in human degenerated nucleus pulposus: the association of inflammation with intervertebral disc degeneration. Mediat Inflamm. 2021;2021 doi: 10.1155/2021/2933199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogerson A., Aidlen J., Jenis L.G. Persistent radiculopathy after surgical treatment for lumbar disc herniation: causes and treatment options. Int Orthop. 2019;43(4):969–973. doi: 10.1007/s00264-018-4246-7. [DOI] [PubMed] [Google Scholar]
- 34.Liem L., van Dongen E., Huygen F.J., Staats P., Kramer J. The dorsal root ganglion as a therapeutic target for chronic pain. Reg Anesth Pain Med. 2016;41(4):511–519. doi: 10.1097/AAP.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 35.Berta T., Qadri Y., Tan P.-H., Ji R.-R. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin Ther Targets. 2017;21(7):695–703. doi: 10.1080/14728222.2017.1328057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berger A.A., Liu Y., Possoit H., Rogers A.C., Moore W., Gress K., et al. Dorsal root ganglion (DRG) and chronic pain. Anesthesiol Pain Med. 2021;11(2) doi: 10.5812/aapm.113020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z.Y., Song Z.W., Guo S.W., He J.S., Wang S.Y., Zhu J.G., et al. CXCL12/CXCR4 signaling contributes to neuropathic pain via central sensitization mechanisms in a rat spinal nerve ligation model. CNS Neurosci Ther. 2019;25(9):922–936. doi: 10.1111/cns.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rooney B.A., Crown E.D., Hulsebosch C.E., McAdoo D.J. Preemptive analgesia with lidocaine prevents failed back surgery syndrome. Exp Neurol. 2007;204(2):589–596. doi: 10.1016/j.expneurol.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy K.F., Connor T.J., McCrory C. Cerebrospinal fluid levels of vascular endothelial growth factor correlate with reported pain and are reduced by spinal cord stimulation in patients with failed back surgery syndrome. Neuromodulation: Technology at the Neural Interface. 2013;16(6):519–522. doi: 10.1111/j.1525-1403.2012.00527.x. [DOI] [PubMed] [Google Scholar]
- 40.Latremoliere A., Latini A., Andrews N., Cronin S.J., Fujita M., Gorska K., et al. Reduction of neuropathic and inflammatory pain through inhibition of the tetrahydrobiopterin pathway. Neuron. 2015;86(6):1393–1406. doi: 10.1016/j.neuron.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Y.J., Zhang L., Samad O.A., Suter M.R., Yasuhiko K., Xu Z.Z., et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29(13):4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyagi M., Ishikawa T., Orita S., Eguchi Y., Kamoda H., Arai G., et al. Disk injury in rats produces persistent increases in pain-related neuropeptides in dorsal root ganglia and spinal cord glia but only transient increases in inflammatory mediators. Spine. 2011;36(26):2260–2266. doi: 10.1097/BRS.0b013e31820e68c7. [DOI] [PubMed] [Google Scholar]
- 43.Loggia M.L., Chonde D.B., Akeju O., Arabasz G., Catana C., Edwards R.R., et al. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138(Pt 3):604–615. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 45.Tsuda M., Shigemoto-Mogami Y., Koizumi S., Mizokoshi A., Kohsaka S., Salter M.W., et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 46.Tsuda M. Microglia in the spinal cord and neuropathic pain. J Diabetes Investig. 2016;7(1):17–26. doi: 10.1111/jdi.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu N., Peng J., Murugan M., Wang X., Eyo U.B., Sun D., et al. Spinal microgliosis due to resident microglial proliferation is required for pain hypersensitivity after peripheral nerve injury. Cell Rep. 2016;16(3):605–614. doi: 10.1016/j.celrep.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masuda T., Tsuda M., Yoshinaga R., Tozaki-Saitoh H., Ozato K., Tamura T., et al. IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep. 2012;1(4):334–340. doi: 10.1016/j.celrep.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuda M., Masuda T., Tozaki-Saitoh H., Inoue K. Microglial regulation of neuropathic pain. J Pharmacol Sci. 2013;121(2):89–94. doi: 10.1254/jphs.12r14cp. [DOI] [PubMed] [Google Scholar]
- 50.Zhou L.-J., Peng J., Xu Y.-N., Zeng W.-J., Zhang J., Wei X., et al. Microglia are indispensable for synaptic plasticity in the spinal dorsal horn and chronic pain. Cell Rep. 2019;27(13):3844. doi: 10.1016/j.celrep.2019.05.087. 59.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji R.-R., Donnelly C.R., Nedergaard M. Astrocytes in chronic pain and itch. Nat Rev Neurosci. 2019;20(11):667–685. doi: 10.1038/s41583-019-0218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Del V.L., Schwartzman R.J., Alexander G. Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain Behav Immun. 2009;23(1):85–91. doi: 10.1016/j.bbi.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Garrison C.J., Dougherty P.M., Kajander K.C., Carlton S.M. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991;565(1):1–7. doi: 10.1016/0006-8993(91)91729-k. [DOI] [PubMed] [Google Scholar]
- 54.Kogias E., Franco J.P., Klingler J.H., Hubbe U. Minimally invasive redo discectomy for recurrent lumbar disc herniations. J Clin Neurosci. 2015;22(9):1382–1386. doi: 10.1016/j.jocn.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 55.Gum J.L., Glassman S.D., Carreon L.Y. Is type of compensation a predictor of outcome after lumbar fusion? Spine (Phila Pa 1976. 2013;38(5):443–448. doi: 10.1097/BRS.0b013e318278ebe8. [DOI] [PubMed] [Google Scholar]
- 56.Michaelides A., Zis P. Depression, anxiety and acute pain: links and management challenges. Postgrad Med. 2019;131(7):438–444. doi: 10.1080/00325481.2019.1663705. [DOI] [PubMed] [Google Scholar]
- 57.Fields H.L. Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res. 2000;122:245–253. doi: 10.1016/s0079-6123(08)62143-3. [DOI] [PubMed] [Google Scholar]
- 58.Kahere M., Ginindza T. The prevalence and psychosocial risk factors of chronic low back pain in KwaZulu-Natal. Afr J Prim Health Care Fam Med. 2022;14(1):e1–e8. doi: 10.4102/phcfm.v14i1.3134. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burgel B.J., Elshatarat R.A. Psychosocial work factors and low back pain in taxi drivers. Am J Ind Med. 2017;60(8):734–746. doi: 10.1002/ajim.22732. [eng] [DOI] [PubMed] [Google Scholar]
- 60.Manchikanti L., Singh V., Cash K.A., Pampati V. Assessment of effectiveness of percutaneous adhesiolysis and caudal epidural injections in managing post lumbar surgery syndrome: 2-year follow-up of a randomized, controlled trial. J Pain Res. 2012;5:597–608. doi: 10.2147/JPR.S38999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerdesmeyer L., Noe C., Prehn-Kristensen A., Harrasser N., Muderis M.A., Weuster M., et al. Long-term efficacy of percutaneous epidural neurolysis of adhesions in chronic lumbar radicular pain: 10 Year follow-up of a randomized controlled trial. Pain Physician. 2021;24(5):359–367. [PubMed] [Google Scholar]
- 62.Chun-jing H., Hao-xiong N., Jia-xiang N. The application of percutaneous lysis of epidural adhesions in patients with failed back surgery syndrome. Acta Cir Bras. 2012;27(4):357–362. doi: 10.1590/s0102-86502012000400013. [DOI] [PubMed] [Google Scholar]
- 63.Lee J.H., Lee S.H. Clinical effectiveness of percutaneous adhesiolysis versus transforaminal epidural steroid injection in patients with postlumbar surgery syndrome. Reg Anesth Pain Med. 2014;39(3):214–218. doi: 10.1097/AAP.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 64.Ahn Y., Keum H.J., Son S. Percutaneous endoscopic lumbar foraminotomy for foraminal stenosis with postlaminectomy syndrome in geriatric patients. World Neurosurg. 2019;130:e1070–e1076. doi: 10.1016/j.wneu.2019.07.087. [DOI] [PubMed] [Google Scholar]
- 65.Telfeian A.E., Oyelese A., Fridley J., Camara-Quintana J.Q., Niu T., Gokaslan Z.L. Transforaminal endoscopic surgical treatment for postlaminectomy lumbar radiculopathy: case series. World Neurosurgery. 2021;150:e577–e584. doi: 10.1016/j.wneu.2021.03.058. [DOI] [PubMed] [Google Scholar]
- 66.Yeung A., Gore S. Endoscopic foraminal decompression for failed back surgery syndrome under local anesthesia. Internet J Spine Surg. 2014;8 doi: 10.14444/1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ozer A.F., Oktenoglu T., Sasani M., Bozkus H., Canbulat N., Karaarslan E., et al. Preserving the ligamentum flavum in lumbar discectomy: a new technique that prevents scar tissue formation in the first 6 months postsurgery. Neurosurgery. 2006;59(1) doi: 10.1227/01.NEU.0000220078.90175.E6. Suppl 1):ONS126-O133;discussion ONS26-33. [DOI] [PubMed] [Google Scholar]
- 68.Shamim M.S., Parekh M.A., Bari M.E., Enam S.A., Khursheed F. Microdiscectomy for lumbosacral disc herniation and frequency of failed disc surgery. World Neurosurg. 2010;74(6):611–616. doi: 10.1016/j.wneu.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 69.AlShazli A.B.A.D., Amer A.Y., Sultan A.M., Barakat A.S., Koptan W., ElMiligui Y., et al. Minimally invasive transforaminal lumbar interbody fusion for the surgical management of post-discectomy syndrome. Asian Spine Journal. 2020;14(2):148–156. doi: 10.31616/asj.2019.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schnitzer T.J., Ferraro A., Hunsche E., Kong S.X. A comprehensive review of clinical trials on the efficacy and safety of drugs for the treatment of low back pain. J Pain Symptom Manag. 2004;28(1):72–95. doi: 10.1016/j.jpainsymman.2003.10.015. [eng] [DOI] [PubMed] [Google Scholar]
- 71.Enthoven W.T., Roelofs P.D., Deyo R.A., van Tulder M.W., Koes B.W. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2(2) doi: 10.1002/14651858.CD012087. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Tulder M.W., Scholten R.J., Koes B.W., Deyo R.A. Non-steroidal anti-inflammatory drugs for low back pain. Cochrane Database Syst Rev. 2000;(2) doi: 10.1002/14651858.CD000396. [eng] [DOI] [PubMed] [Google Scholar]
- 73.Vroomen P.C., de Krom M.C., Slofstra P.D., Knottnerus J.A. Conservative treatment of sciatica: a systematic review. J Spinal Disord. 2000;13(6):463–469. doi: 10.1097/00002517-200012000-00001. [eng] [DOI] [PubMed] [Google Scholar]
- 74.Derry S., Loke Y.K. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. Br Med J. 2000;321(7270):1183–1187. doi: 10.1136/bmj.321.7270.1183. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warwick C. Paracetamol and fever management. J R Soc Promot Health. 2008;128(6):320–323. doi: 10.1177/1466424008092794. [eng] [DOI] [PubMed] [Google Scholar]
- 76.Orhurhu V.J., Chu R., Gill J. StatPearls. Treasure island (FL) StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023. Failed back surgery syndrome. [Google Scholar]
- 77.Milgrom C., Finestone A., Lev B., Wiener M., Floman Y. Overexertional lumbar and thoracic back pain among recruits: a prospective study of risk factors and treatment regimens. J Spinal Disord. 1993;6(3):187–193. doi: 10.1097/00002517-199306030-00001. [eng] [DOI] [PubMed] [Google Scholar]
- 78.Kaur J., Ghosh S., Sahani A.K., Sinha J.K. Mental imagery training for treatment of central neuropathic pain: a narrative review. Acta Neurol Belg. 2019;119(2):175–186. doi: 10.1007/s13760-019-01139-x. [eng] [DOI] [PubMed] [Google Scholar]
- 79.Khosravi M.B., Azemati S., Sahmeddini M.A. Gabapentin versus naproxen in the management of failed back surgery syndrome; a randomized controlled trial. Acta Anaesthesiol Belg. 2014;65(1):31–37. [PubMed] [Google Scholar]
- 80.Wu Y.T., Lai M.H., Lu S.C., Chang S.T. Beneficial response to gabapentin portraying with interval change of brain SPECT imaging in a case with failed back surgery syndrome. J Clin Pharm Therapeut. 2011;36(4):525–528. doi: 10.1111/j.1365-2710.2010.01200.x. [DOI] [PubMed] [Google Scholar]
- 81.Braverman D.L., Slipman C.W., Lenrow D.A. Using gabapentin to treat failed back surgery syndrome caused by epidural fibrosis: a report of 2 cases. Arch Phys Med Rehabil. 2001;82(5):691–693. doi: 10.1053/apmr.2001.21867. [DOI] [PubMed] [Google Scholar]
- 82.Gewandter J.S., Frazer M.E., Cai X., Chiodo V.F., Rast S.A., Dugan M., et al. Extended-release gabapentin for failed back surgery syndrome: results from a randomized double-blind cross-over study. Pain. 2019;160(5):1029–1036. doi: 10.1097/j.pain.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 83.Gianesello L., Pavoni V., Barboni E., Galeotti I., Nella A. Perioperative pregabalin for postoperative pain control and quality of life after major spinal surgery. J Neurosurg Anesthesiol. 2012;24(2):121–126. doi: 10.1097/ANA.0b013e31823a885b. [DOI] [PubMed] [Google Scholar]
- 84.Canos A., Cort L., Fernández Y., Rovira V., Pallarés J., Barberá M., et al. Preventive analgesia with pregabalin in neuropathic pain from “failed back surgery syndrome”: assessment of sleep quality and disability. Pain Med. 2016;17(2):344–352. doi: 10.1111/pme.12895. [DOI] [PubMed] [Google Scholar]
- 85.Deyo R.A., Von Korff M., Duhrkoop D. Opioids for low back pain. Br Med J. 2015;350:g6380. doi: 10.1136/bmj.g6380. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalso E., Edwards J.E., Moore A.R., McQuay H.J. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112(3):372–380. doi: 10.1016/j.pain.2004.09.019. [eng] [DOI] [PubMed] [Google Scholar]
- 87.Chaparro L.E., Furlan A.D., Deshpande A., Mailis-Gagnon A., Atlas S., Turk D.C. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane Review. Spine (Phila Pa 1976. 2014;39(7):556–563. doi: 10.1097/BRS.0000000000000249. [eng] [DOI] [PubMed] [Google Scholar]
- 88.Chou R., Huffman L.H. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):505–514. doi: 10.7326/0003-4819-147-7-200710020-00008. [DOI] [PubMed] [Google Scholar]
- 89.Martell B.A., O'Connor P.G., Kerns R.D., Becker W.C., Morales K.H., Kosten T.R., et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [eng] [DOI] [PubMed] [Google Scholar]
- 90.Kuijpers T., van Middelkoop M., Rubinstein S.M., Ostelo R., Verhagen A., Koes B.W., et al. A systematic review on the effectiveness of pharmacological interventions for chronic non-specific low-back pain. Eur Spine J. 2011;20(1):40–50. doi: 10.1007/s00586-010-1541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Avila L., Neves M.L., Abreu A.R., Fiuza C.R., Fukusawa L., Meziat-Filho N., et al. Cognitive functional therapy (CFT) compared with core training exercise (CTE) in patients with failed back surgery syndrome (FBSS): a study protocol for a randomized controlled trial. J Bodyw Mov Ther. 2021;26:428–434. doi: 10.1016/j.jbmt.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 92.Cherkin D.C., Sherman K.J., Balderson B.H., Cook A.J., Anderson M.L., Hawkes R.J., et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315(12):1240–1249. doi: 10.1001/jama.2016.2323. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O'Keeffe M., O'Sullivan P., Purtill H., Bargary N., O'Sullivan K. Cognitive functional therapy compared with a group-based exercise and education intervention for chronic low back pain: a multicentre randomised controlled trial (RCT) Br J Sports Med. 2020;54(13):782–789. doi: 10.1136/bjsports-2019-100780. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vibe Fersum K., Smith A., Kvåle A., Skouen J.S., O'Sullivan P. Cognitive functional therapy in patients with non-specific chronic low back pain-a randomized controlled trial 3-year follow-up. Eur J Pain. 2019;23(8):1416–1424. doi: 10.1002/ejp.1399. [eng] [DOI] [PubMed] [Google Scholar]
- 95.Anheyer D., Haller H., Barth J., Lauche R., Dobos G., Cramer H. Mindfulness-based stress reduction for treating low back pain: a systematic review and meta-analysis. Ann Intern Med. 2017;166(11):799–807. doi: 10.7326/M16-1997. [eng] [DOI] [PubMed] [Google Scholar]
- 96.Esmer G., Blum J., Rulf J., Pier J. Mindfulness-based stress reduction for failed back surgery syndrome: a randomized controlled trial. J Am Osteopath Assoc. 2010;110(11):646–652. [PubMed] [Google Scholar]
- 97.Schoell K., Wang C., D'Oro A., Heindel P., Lee L., Wang J.C., et al. Depression increases the rates of neurological complications and failed back surgery syndrome in patients undergoing lumbar spine surgery. Clin Spine Surg. 2019;32(2):E78–e85. doi: 10.1097/BSD.0000000000000730. [eng] [DOI] [PubMed] [Google Scholar]
- 98.Suzuki K., Ebina Y., Shindo T., Takano T., Awata S., Matsuoka H. Repeated electroconvulsive therapy courses improved chronic regional pain with depression caused by failed back syndrome. Med Sci Mon Int Med J Exp Clin Res. 2009;15(4):Cs77–C79. [eng] [PubMed] [Google Scholar]
- 99.Stanton E., Fresquez Z., Muehlbauer E.J., Wang J.C., Buser Z. Onset of mental disorders in patients who developed failed back surgery syndrome. Eur Spine J. 2022;31(10):2612–2618. doi: 10.1007/s00586-022-07334-4. [eng] [DOI] [PubMed] [Google Scholar]
- 100.Knotkova H., Hamani C., Sivanesan E., Le Beuffe M.F.E., Moon J.Y., Cohen S.P., et al. Neuromodulation for chronic pain. Lancet. 2021;397(10289):2111–2124. doi: 10.1016/S0140-6736(21)00794-7. [eng] [DOI] [PubMed] [Google Scholar]
- 101.North R.B., Kidd D.H., Farrokhi F., Piantadosi S.A. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98–106. doi: 10.1227/01.neu.0000144839.65524.e0. ; discussion 06-7. [DOI] [PubMed] [Google Scholar]
- 102.Eldabe S., Kumar K., Buchser E., Taylor R.S. An analysis of the components of pain, function, and health-related quality of life in patients with failed back surgery syndrome treated with spinal cord stimulation or conventional medical management. Neuromodulation. 2010;13(3):201–209. doi: 10.1111/j.1525-1403.2009.00271.x. [eng] [DOI] [PubMed] [Google Scholar]
- 103.Nissen M., Ikäheimo T.M., Huttunen J., Leinonen V., von Und Zu Fraunberg M. Long-term outcome of spinal cord stimulation in failed back surgery syndrome: 20 Years of experience with 224 consecutive patients. Neurosurgery. 2019;84(5):1011–1018. doi: 10.1093/neuros/nyy194. [eng] [DOI] [PubMed] [Google Scholar]
- 104.Do T.T., Smet I., Jerjir A., Vandamme K., Devos M., Van Buyten J.P. Real-world analysis: long-term effect of spinal cord stimulation with different waveforms for patients with failed back surgery syndrome. Pain Pract. 2021;21(2):215–225. doi: 10.1111/papr.12952. [eng] [DOI] [PubMed] [Google Scholar]
- 105.Puylaert M., Nijs L., Buyse K., Vissers K., Vanelderen P., Nagels M., et al. Long-term outcome in patients with spinal cord stimulation for failed back surgery syndrome: a 20-year audit of a single center. Neuromodulation. 2022 doi: 10.1016/j.neurom.2022.03.006. [eng] [DOI] [PubMed] [Google Scholar]
- 106.Mekhail N., Levy R.M., Deer T.R., Kapural L., Li S., Amirdelfan K., et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020;19(2):123–134. doi: 10.1016/S1474-4422(19)30414-4. [eng] [DOI] [PubMed] [Google Scholar]
- 107.Rigoard P., Basu S., Desai M., Taylor R., Annemans L., Tan Y., et al. Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: a multicenter randomized controlled trial. Pain. 2019;160(6):1410–1420. doi: 10.1097/j.pain.0000000000001510. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vajramani G.V. High frequency (HF10) spinal cord stimulation for chronic neuropathic pain. Neurol India. 2020;68(Supplement):S337. doi: 10.4103/0028-3886.302470. s39. [eng] [DOI] [PubMed] [Google Scholar]
- 109.Kapural L., Yu C., Doust M.W., Gliner B.E., Vallejo R., Sitzman B.T., et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851–860. doi: 10.1097/ALN.0000000000000774. [eng] [DOI] [PubMed] [Google Scholar]
- 110.De Andres J., Monsalve-Dolz V., Fabregat-Cid G., Villanueva-Perez V., Harutyunyan A., Asensio-Samper J.M., et al. Prospective, randomized blind effect-on-outcome study of conventional vs high-frequency spinal cord stimulation in patients with pain and disability due to failed back surgery syndrome. Pain Med. 2017;18(12):2401–2421. doi: 10.1093/pm/pnx241. [DOI] [PubMed] [Google Scholar]
- 111.Kapural L., Yu C., Doust M.W., Gliner B.E., Vallejo R., Sitzman B.T., et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery. 2016;79(5):667–677. doi: 10.1227/NEU.0000000000001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Russo M., Verrills P., Mitchell B., Salmon J., Barnard A., Santarelli D. High frequency spinal cord stimulation at 10 kHz for the treatment of chronic pain: 6-month Australian clinical experience. Pain Physician. 2016;19(4):267–280. [PubMed] [Google Scholar]
- 113.Kallewaard J.W., Nijhuis H., Huygen F., Wille F., Zuidema X., van de Minkelis J., et al. Prospective cohort analysis of DRG stimulation for failed back surgery syndrome pain following lumbar discectomy. Pain Pract. 2019;19(2):204–210. doi: 10.1111/papr.12734. [DOI] [PubMed] [Google Scholar]
- 114.Liem L., Russo M., Huygen F.J., Van Buyten J.P., Smet I., Verrills P., et al. One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation. 2015;18(1):41–48. doi: 10.1111/ner.12228. ; discussion 48-9. [eng] [DOI] [PubMed] [Google Scholar]
- 115.Chapman K.B., Groenen P.S., Patel K.V., Vissers K.C., van Helmond N. T12 dorsal root ganglion stimulation to treat chronic low back pain: a case series. Neuromodulation. 2020;23(2):203–212. doi: 10.1111/ner.13047. [DOI] [PubMed] [Google Scholar]
- 116.Morgalla M.H., Fortunato M., Lepski G., Chander B.S. Dorsal root ganglion stimulation (DRGS) for the treatment of chronic neuropathic pain: a single-center study with long-term prospective results in 62 cases. Pain Physician. 2018;21(4):E377–E387. [PubMed] [Google Scholar]
- 117.Fontaine D. Spinal cord stimulation for neuropathic pain. Rev Neurol (Paris) 2021;177(7):838–842. doi: 10.1016/j.neurol.2021.07.014. [DOI] [PubMed] [Google Scholar]
- 118.Hamza M., Doleys D., Wells M., Weisbein J., Hoff J., Martin M., et al. Prospective study of 3-year follow-up of low-dose intrathecal opioids in the management of chronic nonmalignant pain. Pain Med. 2012;13(10):1304–1313. doi: 10.1111/j.1526-4637.2012.01451.x. [DOI] [PubMed] [Google Scholar]
- 119.Jain S., Malinowski M., Chopra P., Varshney V., Deer T.R. Intrathecal drug delivery for pain management: recent advances and future developments. Expet Opin Drug Deliv. 2019;16(8):815–822. doi: 10.1080/17425247.2019.1642870. [eng] [DOI] [PubMed] [Google Scholar]
- 120.Deer T., Chapple I., Classen A., Javery K., Stoker V., Tonder L., et al. Intrathecal drug delivery for treatment of chronic low back pain: report from the National Outcomes Registry for Low Back Pain. Pain Med. 2004;5(1):6–13. doi: 10.1111/j.1526-4637.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- 121.Caraway D., Walker V., Becker L., Hinnenthal J. Successful discontinuation of systemic opioids after implantation of an intrathecal drug delivery system. Neuromodulation. 2015;18(6):508–515. doi: 10.1111/ner.12318. ; discussion 15-6. [DOI] [PubMed] [Google Scholar]
- 122.Lee C.H., Kim H.S., Kim Y.-S., Jung S., Yoon C.H., Kwon O.-Y. Cerebral current-source distribution associated with pain improvement by non-invasive painless signaling therapy in patients with failed back surgery syndrome. The Korean Journal of Pain. 2021;34(4):437–446. doi: 10.3344/kjp.2021.34.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baronio M.M., Baglivo M.M., Natalini G.G., Notaro P.P., Dautaj A.A., Paolacci S.S., et al. Genetic and physiological autonomic nervous system factors involved in failed back surgery syndrome: a review of the literature and report of nine cases treated with pulsed radiofrequency. Acta Bio-Med Ateneo Parmense. 2020;91(Suppl 13) doi: 10.23750/abm.v91i13-S.10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kikuchi K., Setoyama K., Terashi T., Sumizono M., Tancharoen S., Otsuka S., et al. Application of a novel anti-adhesive membrane, E8002, in a rat laminectomy model. Int J Mol Sci. 2018;19(5) doi: 10.3390/ijms19051513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun Y., Wang L.X., Wang L., Sun S.X., Cao X.J., Wang P., et al. A comparison of the effectiveness of mitomycin C and 5-fluorouracil in the prevention of peridural adhesion after laminectomy. J Neurosurg Spine. 2007;7(4):423–428. doi: 10.3171/SPI-07/10/423. [DOI] [PubMed] [Google Scholar]
- 126.Gürer B., Kahveci R., Gökçe E.C., Ozevren H., Turkoglu E., Gökçe A. Evaluation of topical application and systemic administration of rosuvastatin in preventing epidural fibrosis in rats. Spine J. 2015;15(3):522–529. doi: 10.1016/j.spinee.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 127.Tian F., Dou C., Qi S., Zhao L., Chen B., Yan H., et al. Preventive effect of dexamethasone gelatin sponge on the lumbosacral epidural adhesion. Int J Clin Exp Med. 2015;8(4):5478–5484. [PMC free article] [PubMed] [Google Scholar]
- 128.Sae-Jung S., Jirarattanaphochai K. Prevention of peridural fibrosis using a cyclooxygenase-2 inhibitor (nonsteroidal anti-inflammatory drug) soaked in absorbable gelatin sponge: an experimental comparative animal model. Spine (Phila Pa 1976. 2013;38(16):E985–E991. doi: 10.1097/BRS.0b013e318297c795. [DOI] [PubMed] [Google Scholar]
- 129.Song Z., Wu T., Sun J., Wang H., Hua F., Nicolas Y.S.M., et al. Metformin attenuates post-epidural fibrosis by inhibiting the TGF-β1/Smad3 and HMGB1/TLR4 signaling pathways. J Cell Mol Med. 2021;25(7):3272–3283. doi: 10.1111/jcmm.16398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Karatay M., Erdem Y., Koktekir E., Erkoc Y.S., Caydere M., Bayar M.A. The effect of bevacizumab on spinal epidural fibrosis in a postlaminectomy rat model. Turk Neurosurg. 2012;22(6):753–757. doi: 10.5137/1019-5149.JTN.6155-12.1. [DOI] [PubMed] [Google Scholar]
- 131.Tao H., Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J. 2009;18(8):1202–1212. doi: 10.1007/s00586-009-1013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cunningham B.W., Seiber B., Riggleman J.R., Van Horn M.R., Bhat A. An investigational study of a dual-layer, chorion-free amnion patch as a protective barrier following lumbar laminectomy in a sheep model. J Tissue Eng Regen Med. 2019;13(9):1664–1671. doi: 10.1002/term.2920. [DOI] [PubMed] [Google Scholar]
- 133.Wang B., Li P., Shangguan L., Ma J., Mao K., Zhang Q., et al. A novel bacterial cellulose membrane immobilized with human umbilical cord mesenchymal stem cells-derived exosome prevents epidural fibrosis. Int J Nanomed. 2018;13:5257–5273. doi: 10.2147/IJN.S167880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.King T., Porreca F. Preclinical assessment of pain: improving models in discovery research. Curr Top Behav Neurosci. 2014;20:101–120. doi: 10.1007/7854_2014_330. [DOI] [PubMed] [Google Scholar]
- 135.Singh K., Bohl D.D., Ahn J., Massel D.H., Mayo B.C., Narain A.S., et al. Multimodal analgesia versus intravenous patient-controlled analgesia for minimally invasive transforaminal lumbar interbody fusion procedures. Spine (Phila Pa 1976. 2017;42(15):1145–1150. doi: 10.1097/BRS.0000000000001992. [DOI] [PubMed] [Google Scholar]
- 136.Jirarattanaphochai K., Jung S. Nonsteroidal antiinflammatory drugs for postoperative pain management after lumbar spine surgery: a meta-analysis of randomized controlled trials. J Neurosurg Spine. 2008;9(1):22–31. doi: 10.3171/SPI/2008/9/7/022. [DOI] [PubMed] [Google Scholar]
- 137.Pandey C.K., Navkar D.V., Giri P.J., Raza M., Behari S., Singh R.B., et al. Evaluation of the optimal preemptive dose of gabapentin for postoperative pain relief after lumbar diskectomy: a randomized, double-blind, placebo-controlled study. J Neurosurg Anesthesiol. 2005;17(2):65–68. doi: 10.1097/01.ana.0000151407.62650.51. [DOI] [PubMed] [Google Scholar]
- 138.Rivkin A., Rivkin M.A. Perioperative nonopioid agents for pain control in spinal surgery. Am J Health Syst Pharm. 2014;71(21):1845–1857. doi: 10.2146/ajhp130688. [DOI] [PubMed] [Google Scholar]
- 139.Devin C.J., McGirt M.J. Best evidence in multimodal pain management in spine surgery and means of assessing postoperative pain and functional outcomes. J Clin Neurosci. 2015;22(6):930–938. doi: 10.1016/j.jocn.2015.01.003. [DOI] [PubMed] [Google Scholar]