Abstract
HRSV is responsible for many acute lower airway infections and hospitalizations in infants, the elderly and those with weakened immune systems around the world. The strong inflammatory response that mediates viral clearance contributes to pathogenesis, and is positively correlated with disease severity. There is no specific effective therapy on hand. Antiviral synthetic stigmastanes (22S, 23S)-22,23-dihydroxystigmast-4-en-3-one (Compound 1) and 22,23-dihydroxystigmasta-1,4-dien-3-one (Compound 2) have shown to be active inhibiting unrelated virus like Herpes Simplex type 1 virus (HSV-1) and Adenovirus, without cytotoxicity. We have also shown that Compound 1 modulates the activation of cell signaling pathways and cytokine secretion in infected epithelial cells as well as in inflammatory cells activated by nonviral stimuli. In the present work, we investigated the inhibitory effect of both compounds on HRSV replication and their modulatory effect on infected epithelial and inflammatory cells. We show that compounds 1 and 2 inhibit in vitro HRSV replication and propagation and reduce cytokine secretion triggered by HRSV infection in epithelial and inflammatory cells. The compounds reduce viral loads and inflammatory infiltration in the lungs of mice infected with HRSV.
Keywords: Human respiratory syncytial virus, Synthetic stigmastanes, Antiviral, Anti-inflammatory
1. Introduction
Human respiratory syncytial virus (HRSV) is responsible for the majority of acute lower airway infections in children worldwide and a common cause of infant hospitalization in the developed world. HRSV is also responsible for lower respiratory infections and mortality in elderly and immunocompromised individuals ([1,2]).
After HRSV infection, a robust neutrophil response and IL-8 secretion begin. Although this response is needed to eliminate the virus from the site of infection, it also correlates with disease severity. CD8+ T-cell response mediates viral clearance, but it may be harmful. Cytokines and local innate immune factors contribute to pathogenesis [3]. This suggests that it is necessary not only to eliminate the virus, but also to control the inflammatory response which initiates upon infection, and which eventually triggers and is responsible of the immunopathology.
There is no specific therapy on hand. Infants with serious HRSV bronchiolitis receive oxygen supply and fluids as supportive treatment [4]. Ribavirin aerosol application was approved for severe HRSV infections in hospitalized children. However, its use is controversial since it is not easily delivered by aerosol, it may cause anemia, and no significant benefit has been demonstrated [[4], [5], [6]]. Besides, teratogenic and embryocidal effects of ribavirin have been found in almost all animal studies; this drug is contraindicated in pregnant women, and the use of contraception is required for female patients receiving Ribavirin or female partners of male receiving Ribavirin [7].
Palivizumab is a monoclonal antibody against HRSV approved for prophylactic treatment in high-risk children, its efficacy has been proven high but not complete [8] and its cost is prohibitive ([9,10]). Thus, there are no effective antivirals against HRSV until today [11].
There are currently many vaccines for HRSV being developed and tested and one vaccine has been recently approved by FDA, for HRSV prevention among the elderly (individuals of 65 years of age and older). Nevertheless, there is still no vaccine approved for HRSV in children [[12], [13], [14]].
Therefore, novel therapeutics are needed. Considering that HRSV infection triggers a potential harmful immune response to the host, a drug with antiviral and anti-inflammatory activities would be highly beneficial for the treatment of this respiratory disease.
Antiviral synthetic stigmastanes (22S, 23S)-22,23-dihydroxystigmast-4-en-3-one (Compound 1) and 22,23-dihydroxystigmasta-1,4-dien-3-one (Compound 2) have shown to be active inhibiting unrelated virus like Herpes Simplex type 1 virus (HSV-1) and Adenovirus, at non-cytotoxic concentrations ([15,16]). We have also shown that Compound 1 modulates cell signaling and cytokine secretion in infected epithelial cells and in inflammatory cells induced by non-viral stimuli ([17,18]).
With the objective of finding new potential drugs for the treatment of HRSV infections, we studied the inhibition of HRSV replication by compounds 1 and 2, and the modulatory effect of the compounds on epithelial and inflammatory cells infected with HRSV. We found that compounds 1 and 2 inhibit HRSV replication and reduce cytokine secretion triggered by HRSV infection in vitro and diminish viral loads and inflammatory infiltration in the lungs of infected mice. Hence, both compounds present themselves as promissory drugs getting together antiviral and anti-inflammatory activities for the management of immunopathologies triggered by viruses like HRSV and other respiratory viruses.
2. Materials and Methods
2.1. Cells and viruses and treatment solutions
The human HEp-2 cells (human epidermoid cancer cell line) (ATCC Number CCL-23) were grown in DMEM/F12 (Cat Number 12400-016, Gibco) supplemented with 10% inactivated fetal bovine serum (FBS) (Natocor) (DMEM/F12, 10%). THP-1 cells (human acute monocytic leukemia cell line) (ATCC Number TIB-202) and murine macrophages J774A.1 (sarcoma monocyte/macrophage cell line) (ATCC Number TIB-67), kindly provided by Dr. Osvaldo Zabal (INTA–Castelar, Buenos Aires, Argentina), were grown in RPMI 1640 (Cat Number 31800-014, Gibco) supplemented with 10% inactivated FBS (RPMI 10%). A549 cells (human lung carcinoma cell line) (ATCC Number CCL-185) and Vero cells (green monkey kidney cell line) (ATCC Number CCL-81) were grown in MEM (Cat Number 41500-067, Gibco) supplemented with 5% FBS. HRSV strains A2 and line 19 (L19) were kindly provided by Dr. Laura Talarico (INFANT–Buenos Aires, Argentina). Working stocks of HRSV were prepared as previously described [19]. Briefly, semiconfluent monolayers of HEp-2 cells were infected with HRSV strains line 19 and A2 (multiplicity of infection moi = 0.2) in serum-free MEM and incubated 3–4 days, following-up the progress of CPE daily, until CPE ≥80% of cell monolayer, but still attached to flask bottom. After supernatant was removed, 5 mL of cold 25% (w/v) sterile sucrose was added to stabilize and reduce loss of infectivity of the virus. The flask with cell surface covered with sucrose solution was frozen at −80 °C. Lysates obtained after three cycles of freezing and thawing were transferred to sterile 15 mL conical tubes and centrifuged at 500×g and 4 °C for 10 min. Cellular debris was removed, and supernatants were aliquoted and stored at −80 °C until use. Virus was titrated in Vero cells by plaque assay, in serum-free MEM with methylcellulose 1.4%.
Compounds 1 and 2, dissolved in DMSO (Cat Number 472301, Sigma), were diluted with culture medium for testing. The maximum concentration of DMSO tested was 1% v/v and it was non cytotoxic under experimental conditions.
2.2. Cytotoxicity assay
Cell viability was assayed with the tetrazolium salt MTT (3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Cat Number M2128, Sigma), as previously reported by Denizot and Lang, 1986 [20]. Cells grown in 96 microwell plates were incubated with different concentrations of the compounds, ranging from 3.1 μM to 200 μM, for 24 h at 37 °C. The absorbance of each well was measured on a microplate reader (Eurogenetics MPR-A 4i) at a test wavelength of 570 nm (reference wavelength of 630 nm). Results were plotted as percent absorbance of treated cells with respect to untreated cells. The cytotoxic concentration 50 (CC50) was defined as the concentration of the compounds which reduced cell viability by 50% relative to untreated cells incubated with medium alone.
2.3. Antiviral activity
Cells grown to monolayers in 24-well plates were infected with HRSV A2 and L19 (moi = 0.1 in A549 cells and moi = 1 in Vero cells), in serum-free MEM. After 2 h adsorption at 37 °C, the inoculum was removed and different concentrations of the compounds (0.3–40 μM) were added during 24 h, in triplicate. The cells were incubated at 37 °C until 24 h p.i. Plates were frozen and thawed and virus yields were titrated by plaque assay in Vero cells, in serum-free MEM with methylcellulose 1.4%. The effective concentration 50 (EC50) was calculated as the concentration of compounds which reduced viral yields by 50% relative to virus control. The selectivity index (SI) was then defined as CC50/EC50.
2.4. Virucidal effect
HRSV A2 and line 19 (108 and 107 PFU, respectively) were incubated in culture medium with or without 200 μM of each compound during 15, 60 and 90 min at 37 °C. After that, successive 1/10 dilutions of the samples were made up to 10−5 – 10−6 and remaining infectivity was titrated on Vero cells by plaque assay. The final compounds concentrations under these conditions were between 0.002 μM and 0.0002 μM.
2.5. Time-of-addition assays
For pre-treatment assays, A549 cells were treated with 5 μM and 25 μM of the compounds during 24 h at 37 °C before infection. After that, cells were washed with PBS, infected with HRSV A2 and L19 (moi = 0.1) in serum-free MEM, and incubated at 37 °C for 2 h. After that, inoculum was discarded, and cells were further incubated for 24 h with serum-free media.
To study the effect of the compounds during virus adsorption, cells were infected with HRSV A2 or L19 (moi = 1) and simultaneously treated with 20 μM and 40 μM of each compound. After 2 h adsorption at 4 °C, the virus-drug mixture was discarded, cells were washed, and compound free medium was added. Cells were incubated for further 24 h, at 37 °C.
To study the effect of the compounds during virus internalization, cells were infected with A2 or L19 (moi = 1) and, after 2 h of adsorption at 4 °C, the infected cells were treated with 20 μM or 40 μM of the compounds, during 15 and 60 min, for both concentrations of the compounds, at 37 °C. Non-internalized virus was inactivated with citrate buffer pH 3 and cells were washed and further incubated for 24 h at 37 °C.
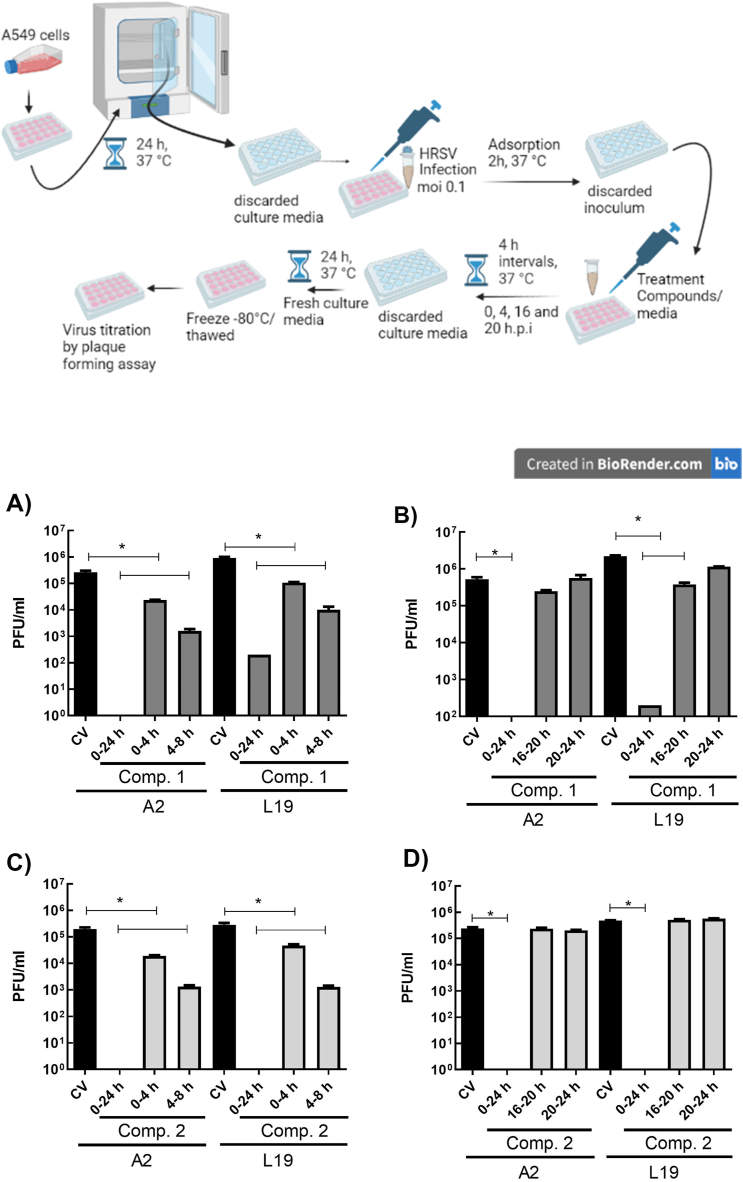
For the post-infection (p.i.) treatments (time of addition/removal assays), cells were infected with HRSV A2 and L19 (moi = 1) for 2 h at 37 °C and then treated with 40 μM of the tested compounds, during 4 h intervals, at different times p.i. (0, 4, 16 and 20 h p.i.).
Infected control not treated cells (CV) were included in all the assays. At 24 h p.i., after freezing and thawing, supernatants were titrated on Vero cells by plaque assay.
2.6. Indirect immunofluorescence
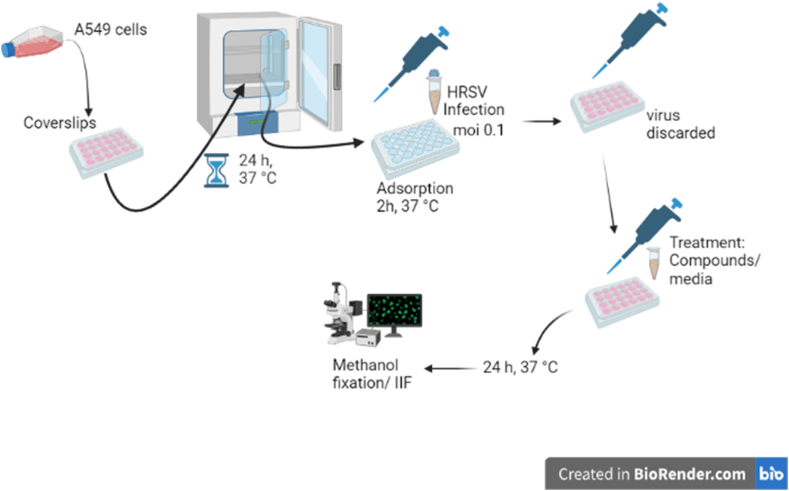
A549 cells grown in coverslips were infected or not with HRSV A2 and L19 (moi = 0.1) in serum-free MEM, with or without 10 μM of compounds 1 and 2. At different times p.i., cells were fixed in methanol for 10 min at −20 °C and processed for indirect immunofluorescence (IIF): after three washes with PBS, the coverslips were incubated for 30 min at 37 °C, inverted on a drop of diluted primary mouse monoclonal antibody anti-gF of HRSV (Cat Number R1600, US Biological Life Sciences). Then, they were subjected to three additional washes with PBS, back in the culture dishes. Afterwards, cells were incubated with diluted secondary goat anti-mouse FluoroLinkTM CyTM3 (GE Healthcare) antibody for 30 min at 37 °C. Then, coverslips were washed again with PBS, and incubated with Dapi (Cat Number D9542, Sigma) to stain cell nuclei. Coverslips were mounted, observed, and photos were taken under an Olympus IX71 with epifluorescence optics. The number of cells with gF fluorescence with respect to the total cell number stained with Dapi was determined, and the percentage of cells expressing gF was calculated, in 5 different fields, in duplicate.
2.7. Quantitative analysis of fluorescence
The images obtained with a 40X magnification were imported into the NIH ImageJ 1.34s program (designed by Wayne Rasband, NIMH, Bethesda). Immunofluorescence images were converted to an 8-bit grayscale from 0 (black) to 255 (white). The total fluorescence of the individual cells was analyzed, and we obtained a mean fluorescence density for each. To compare the distribution of fluorescence within the cell, the results were analyzed in a spreadsheet (Excel®). The total fluorescence intensity was calculated.
2.8. Cytokine determination
Mouse TNF-α and IL-6, and Human TNF-α, IL-6 and IL-8 were quantified by commercial ELISA sets (BD OptEIATM, Becton–Dickinson) according to the manufacturer's instructions.
2.9. In vivo assays in mouse model
The mouse model was approved by Comisión Institucional de Cuidado y Uso de Animales de Laboratorio (CICUAL), Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Argentina, as experimental protocol N° 43. Female Balb/C mice were purchased from Facultad de Veterinaria, Universidad de Buenos Aires, Argentina. The animals were handled according to the Animal Care Guidelines from the National Institute of Health (USA) [21]. The experiments were performed in an Animal Facility Biosafety Level 3 (ABSL-3) (UOCCB, ANLIS-Malbrán, Buenos Aires, Argentina), where animals were kept in individually ventilated cages and fed with food and water ad libitum for at least 1 week before experimental use at 6–8 week of age.
2.9.1. Toxicity evaluation
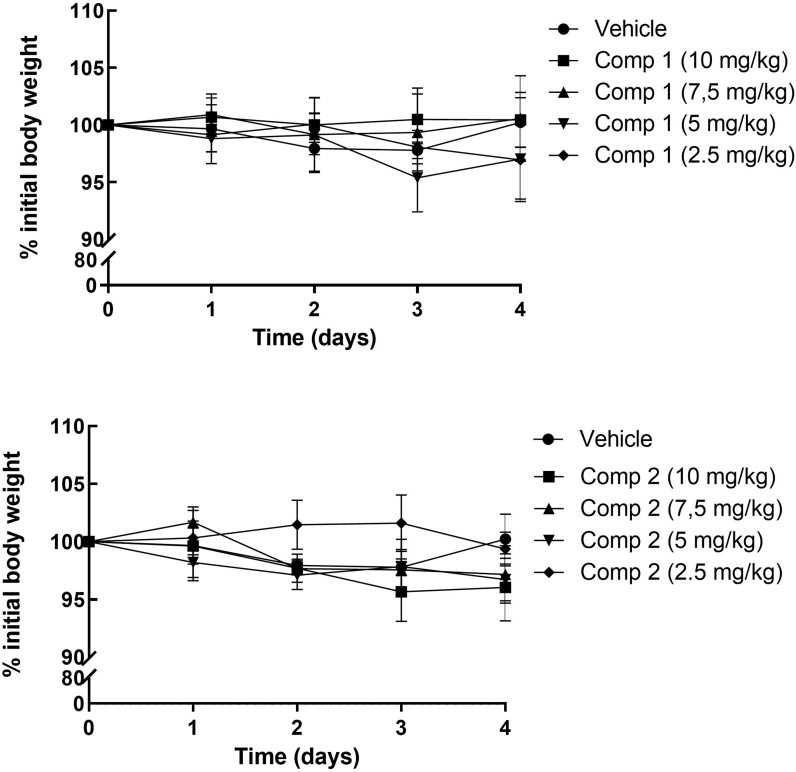
The in vivo toxicity assay was performed in 6–8-week-old female Balb/c mice, which received different doses of the compounds, from 2.5 mg/kg to 10 mg/kg, dissolved in DMSO 90%, during 4 consecutive days. Mice weights were daily registered. Signs of toxicity such as lachrymation, piloerection, stereotypies, were also evaluated.
2.9.2. Pulmonary infection
The sample size was calculated using Power and Precision software (www.Power-Analysis.com). The main objective was to compare the percentages of weight loss and viral titers between those animals infected with HRSV and those infected and treated with the antiviral.
The infectious dose used was as previously described for L19 strain of HRSV in the mouse model ([22,23]). Mice received 50 μl HRSV L19 (5 × 106 PFU) or 25% (w/v) sucrose by intranasal (i.n.) delivery under light general anesthesia (isoflurane) (6 per group). The mice also received different doses from 2.5 mg/kg to 10 mg/kg of compounds 1 and 2, 2.5 mg/kg of Dexamethasone (DEX) and 25 mg/kg of Ribavirin (RBV), or DMSO 90% (vehicle, control group), at 1 h p.i., intraperitoneally (i.p.). Mice further received a daily dose of compounds 1 and 2, DEX and RBV or vehicle, until day 4 p.i.. Body weights were monitored daily, and groups of mice were euthanized on day 4 or 8 p.i.. Lungs from day 4 were removed, weighed, and infectious virus was titrated. Briefly, snap frozen lungs were homogenized in glass Dounce homogenizers. Samples were centrifuged at 4 °C for 10 min at 300 g and supernatants were titrated by plaque assay in Vero cells. Lungs from day 8 were fixed by submersion in Bouin solution during 24 h and subsequently subjected to histological sectioning and staining.
2.10. Statistical analysis
CC50 and EC50 values were obtained from dose–response curves using the software GraphPad Prism 6.0. Statistical significance was assessed either using a one-way analysis of variance (ANOVA) followed by the post hoc Tukey's multiple comparison test. p-Value < 0.05 was considered significant.
3. Results
3.1. Compounds 1 and 2 inhibit HRSV replication in epithelial cells
The epithelium of the respiratory tract is both the main target and the initial defense against HRSV. HRSV replication and spread along the epithelium is dependent on the innate immune response [24]. Immortalized respiratory epithelial cell lines, like A549 cells, are commonly used as in vitro models for the study of airway epithelium (EC) response to HRSV infection. Vero cells are commonly used for the propagation and study of HRSV as well [25]. We then decided to study the effect of our compounds in these well-known epithelial cell lines infected with HRSV.
Firstly, the cytotoxic concentration 50% (CC50) for each compound was evaluated. Compounds 1 and 2 showed CC50 higher than 50 μM in A549 and Vero cells. Besides, in 549, Compound 2 showed CC50 higher than the highest concentration tested (200 μM).
Since HRSV infections caused by strain A appear to be more frequent and more transmissible than HRSV-B, we chose two laboratory A strains, A2 and L19 to perform antiviral and anti-inflammatory studies [2]. HRSV A2 strain exhibits higher replication kinetics and greater cytopathic effect in Vero and A549 cells and in respiratory epithelial cultures, than other laboratory or clinical isolates [2]. L19 strain, on the other hand, is mostly used to infect mice in which it establishes an adequate model to study lung pathophysiology and immunological responses.
Hence, both HRSV A2 and L19 strains were employed to evaluate the antiviral activity of the compounds by means of a virus yield reduction assay in A549 and Vero cells. Virus replication in infected cells treated with both compounds was inhibited in a dose-dependent manner, with the higher Selectivity Index (SI) (CC50/CE50) detected in A549 cells after 24 h p.i. The SI is a ratio which gives an idea of the range between cytotoxicity and antiviral activity of an antiviral drug/compound. The higher the SI ratio, the more effective and safer the antiviral should be. Compound 2 inhibited HRSV A2 and L19 strains with higher SI than compound 1 (Table 1).
Table 1.
Antiviral activity of compounds 1 and 2
|
COMPOUND 1 |
COMPOUND 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
|
HRSV A2 |
HRSV L19 |
HRSV A2 |
HRSV L19 |
|||||
| EC50(μM) | SI | EC5(μM) | SI | EC50(μM) | SI | EC50(μM) | SI | |
| A549 | 3.5 | 16 | 6 | 8.8 | 1.8 | >116 | 6.1 | >32.5 |
| Vero | 14.8 | 6.7 | 12 | 8.1 | 5.4 | 10.4 | 5.2 | 10.8 |
EC50: Effective Concentration 50%. EC50 were calculated by nonlinear regression. Data represent mean ± SD for 3 independent experiments, performed in duplicate.
SI: selectivity indices (ratio CC50/EC50).
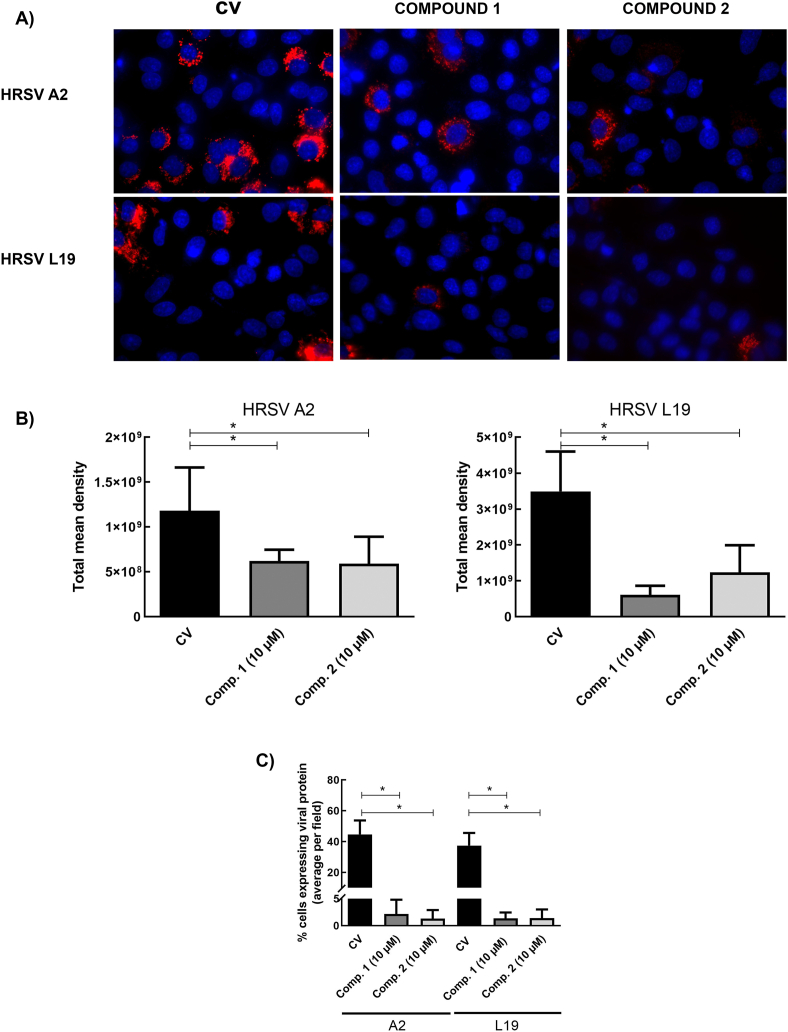
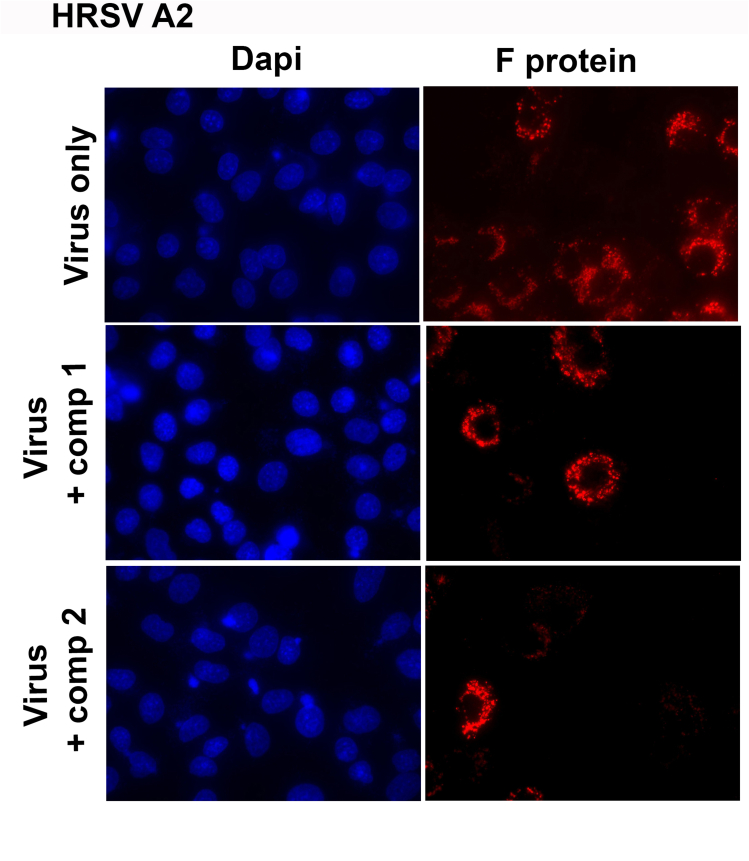
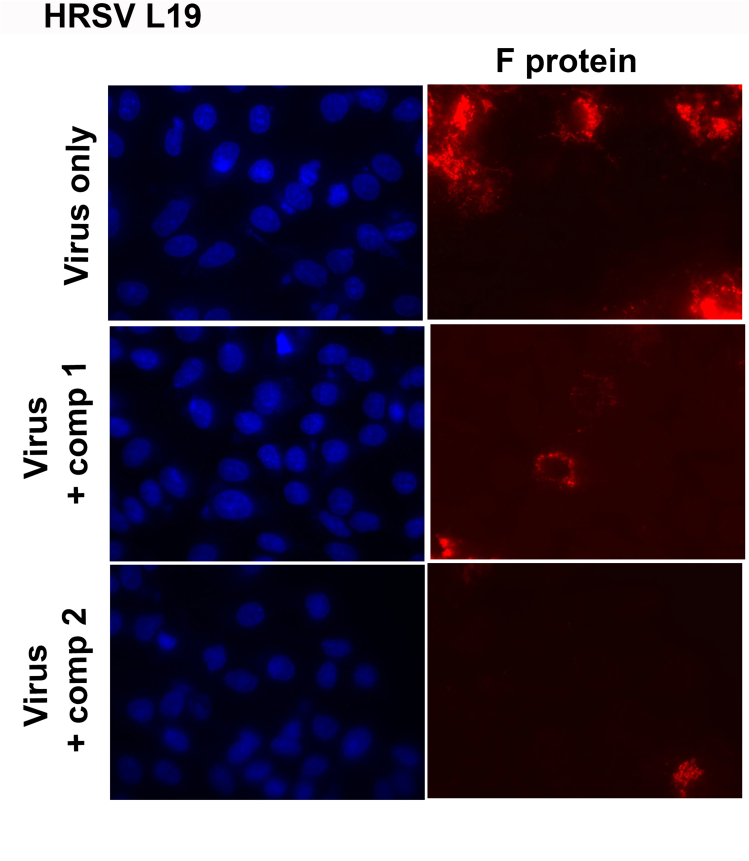
HRSV propagation was also altered. When A549 cells were infected with HRSV A2 and L19 strains and treated during 24 h with 10 μM of compounds 1 and 2, a significant decrease of fusion (F) glycoprotein expression was observed in infected cells treated with both compounds by immunofluorescence staining (Fig. 1A). This reduction was evidenced both in a lower density of fluorescence intensity per cell (Fig. 1B) and in a smaller number of fluorescent cells (Fig. 1C). Therefore, viral protein expression and virus propagation were inhibited by compounds 1 and 2. Single-color images of Fig. 1A have been included as supplementary material (Figure S1).
Fig. 1.
Inhibitory activity of compounds 1 and 2 on HRSV F protein expression. Diagram of the experimental setup. A) A549 cell monolayers grown in coverslips in 24-well plates (for IIF) were infected with HRSV A2 and L19 strains (moi = 0.1) and incubated for 2 h at 37 °C. The inoculum was discarded, and cells were further incubated with fresh medium or treated with 10 μM of the compounds for further 24 h. Cells were processed for an IIF to detect HRSV F protein (red), as described in Materials and Methods. Cells nuclei were stained with Dapi (blue).
Quantification of the IIF. (B) Mean fluorescence intensity of F was quantified as described in Materials and Methods. (C) The number of fluorescent cells expressing F was determined in ten different fields of the control and each treatment condition, in duplicate, and it was expressed as percentage of cells expressing F protein with respect to the total cells in the field. * Significantly different (p < 0.05), One-way ANOVA with post-hoc Tukey test.
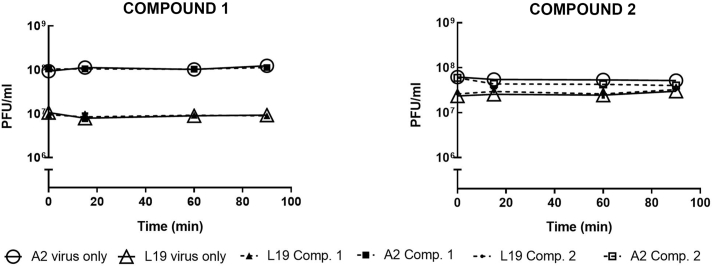
To rule out the possibility that both compounds would be exerting a direct inhibitory effect on HRSV infectivity, we performed a virucidal assay. Viral suspensions were incubated with 200 μM of the compounds during 15, 60 and 90 min at 37 °C and the residual infectivity was titrated by plaque assay in Vero cells. The compounds exhibited no virucidal activity at any of the time points evaluated (Fig. 2).
Fig. 2.
Effect of compounds 1 and 2 on HRSV infectivity. Aliquots of 108 PFU/mL and 107 PFU/mL of HRSV A2 and L19 strains, respectively, were incubated for 15, 60 and 90 min at 37 °C in the presence or absence of 200 μM of compounds 1 and 2. Remaining infectivity was determined by plaque assay in Vero cells, as described in Materials and Methods. Data represent mean ± SD for 3 independent experiments, performed in duplicate.
3.2. Inhibitory activity dependence on the lapse of addition of the compounds
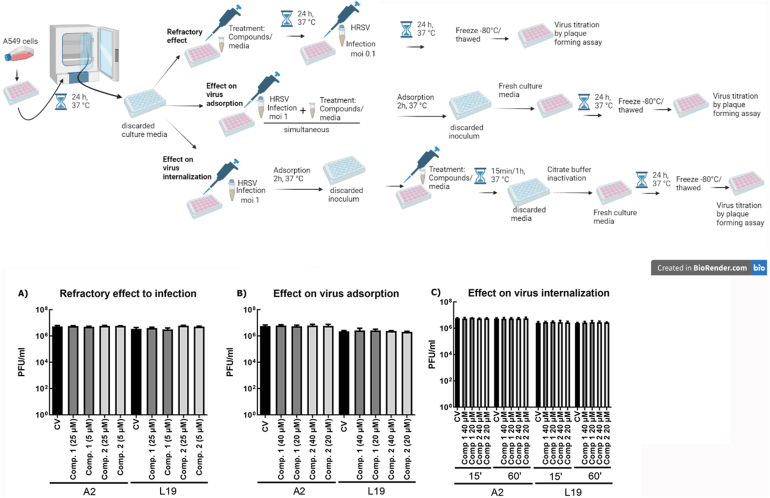
We chose A549 cells to continue with the characterization of the inhibitory activity of compounds 1 and 2 since this cell line is derived from pulmonary tissue. We first performed time-of-addition experiments. A549 cells were treated with the compounds before infection (pre-treatment) or at virus entry (adsorption/internalization), and virus yields were determined at 24 h p.i. Neither compound 1 nor 2 inhibited HRSV replication when added before infection (Fig. 3A) and/or during virus entry into the cells (Fig. 3B and C).
Fig. 3.
Effect of compounds 1 and 2 on HRSV entry into the cells. Diagram of the experimental setup. A). Refractory effect to infection. A549 cell monolayers were treated with compounds 1 and 2 during 24 h before infection. Then, the compounds were removed, and cells were washed 3 times and they were infected with HRSV. After 24 h virus yields were collected and titrated by plaque assay in Vero cells. B). Effect of the compounds on virus adsorption to the cells. A549 cell monolayers were infected with HRSV (moi = 1) together with 20 μM and 40 μM of the compounds, and incubated for 2 h at 4 °C. The inoculum was discarded, and cells were washed, supplemented with fresh culture media, and further incubated for 24 h at 37 °C. Virus yields were collected and titrated by plaque assay in Vero cells. C). Effect of the compounds on virus internalization. A549 cell monolayers were infected with HRSV (moi = 1) and incubated for 2 h at 4 °C. The inoculum was discarded, and cells were washed and treated with 20 μM and 40 μM of the compounds for 15 min and 1 h at 37 °C. Afterwards, cells were washed, and non-internalized virus was inactivated with citrate buffer (pH 3) for 1 min. Cells were further incubated with fresh culture media for 24 h at 37 °C. Virus yields were collected and titrated by plaque assay in Vero cells. Data represent mean ± SD for 3 independent experiments, performed in duplicate.
One-way ANOVA with post-hoc Tukey test. No significant differences were found.
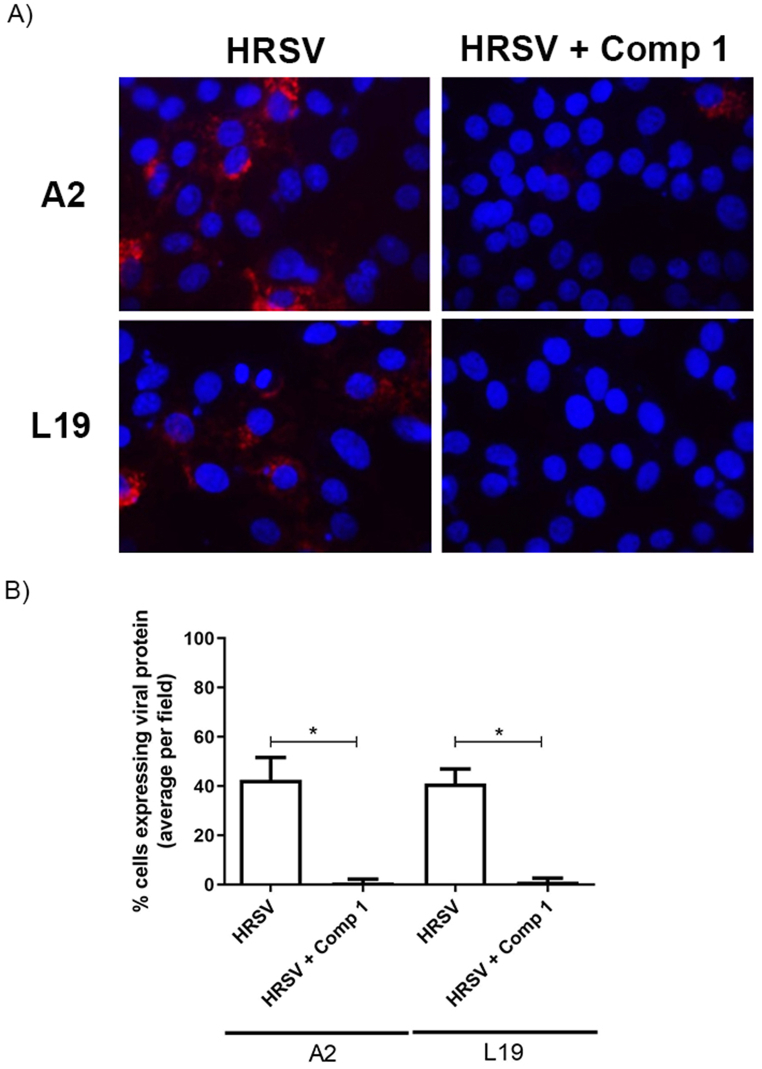
To determine whether the compounds would be exerting their antiviral effect at an early or late step of virus replication cycle, we performed a time-of-addition and removal assay. Viral replication was significantly inhibited in the presence of both compounds during a 24 h period (Fig. 4A–D), with viral yields of or less than 100 PFU/mL. Compounds 1 and 2 displayed their antiviral effect along the first 8 h p.i. when added during 4 h intervals starting immediately after infection and from 4 h p.i. onwards, being the higher inhibition between 4 and 8 h p.i. (Fig. 4 A and C). Furthermore, when infected cells were treated with either of the compounds during the first 8 h p.i., viral protein F expression was considerably reduced at 24 h p.i. (Fig. 5). No inhibitory effect was observed when the compounds were added at the 16–24 h p.i. interval (Fig. 4 B and D).
Fig. 4.
Inhibitory activity of compounds 1 and 2 in time of addition/removal assays. Diagram of the experimental setup. A549 cell monolayers were infected with HRSV (moi = 0.1) and incubated for 2 h at 37 °C. The inoculum was discarded, and cells were incubated with fresh medium or treated with 40 μM of the compounds, in 4 h intervals, at different times p.i. (0, 4, 16 and 20 h p.i.). Cells were washed after each Interval and further incubated with fresh medium at 37 °C. At 24 h p.i. virus yields were collected and titrated by plaque assay in Vero cells. CV: control virus. Data represent mean ± SD for 3 independent experiments, performed in duplicate. * Significantly different (p < 0.05), One-way ANOVA with post-hoc Tukey test.
Fig. 5.
A) HRSV F protein expression after early treatments with Compound 1. A549 cell monolayers were infected with HRSV A2 and L19 (moi = 0.1) and incubated in the absence and presence of 40 μM of Compound 1, during 8 h, immediately after infection. The compound was removed, cells were washed, and they were further incubated for 24 h. Cells were processed for an IIF to detect HRSV F protein, as described in Materials and Methods. Cells nuclei were stained with Dapi. CV: control virus. Quantification of the IIF. B) The number of fluorescent cells expressing F was determined in ten different fields of the control and each treatment condition, and it was expressed as percentage of cells expressing F protein with respect to the total cells in the field. * Significantly different (p < 0.05), One-way ANOVA with post-hoc Tukey test.
3.3. In vitro immunomodulatory activity of the compounds
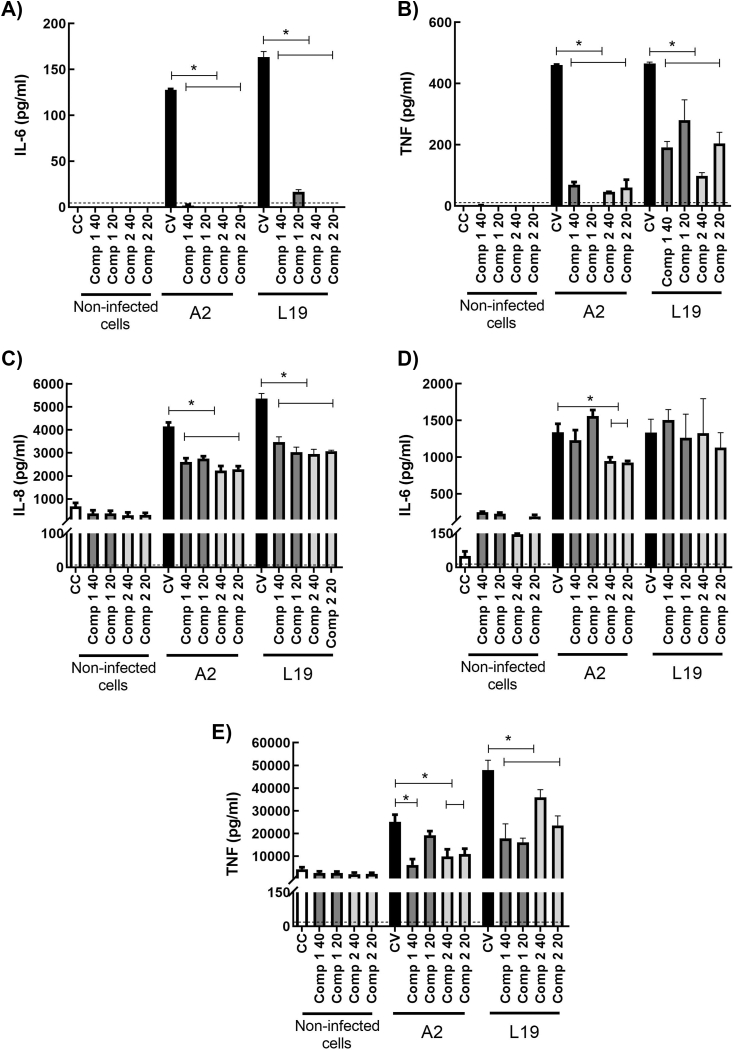
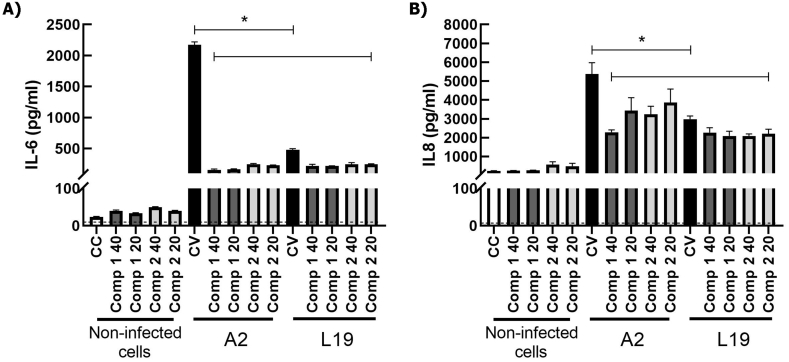
It has been extensively described that HRSV induces the expression of proinflammatory cytokines like IL-6 and TNF-α, and chemokines like IL-8, which participate in inflammation and the related immunopathology ([1,3,26]). Therefore, we studied the effect of the compounds on the secretion of these immune mediators in infected epithelial and immune cells. As we have previously reported, HRSV does not multiply in murine macrophages J774A.1 [27]. Moreover, virus yields are low in human macrophages THP-1 (unpublished data). However, HRSV induces cytokine secretion in both cell lines. A2 and L19 strains induced a significant increase of IL-6 (Fig. 6 A), TNF-α (Fig. 6 B) and IL-8 (Fig. 6C) in THP-1. In all cases, both compounds significantly diminished these cytokines secretion. Mice do not express IL-8, therefore we only studied IL-6 and TNF- α secretion in murine macrophages J774A.1. When J774A.1 were infected with HRSV A2 and L19 strains, a significant increase in IL-6 (Fig. 6 D) and TNF-α (Fig. 6 E) secretion was detected too. Compounds 1 and 2 significantly reduced TNF-α secretion in treated-infected cells (Fig. 6 E), but only compound 2 could also reduce IL-6 secretion in A2 infected macrophages (Fig. 6 D). In the case of epithelial cells, we observed no TNF- α secretion in A549 after HRSV-infection. HRSV A2 and L19 elicited IL-6 and IL-8 secretion (Fig. 7) and both compounds were able to significantly reduce both cytokines in HRSV A2 and L19 infected cells (p < 0.05) (Fig. 7). Therefore, compounds 1 and 2 modulate cytokine secretion in epithelial and inflammatory cells infected with HRSV. In most cases, compounds 1 and 2 were able to reduce cytokine secretion in HRSV infected cells. Only IL-6 secretion was unaltered in J774A.1 infected with L19 strain and treated with the compounds.
Fig. 6.
Effect of compounds 1 and 2 on cytokine production in HRSV infected THP-1 (A) and J774A.1 (B) cells. J774A.1 and THP-1 cells were infected with HRSV A2 and L19 strains (moi = 1). After virus adsorption, cells were incubated at 37 °C in the presence or absence of the compounds for 24 h. Supernatants were processed for cytokine quantification. *Significantly different, (p-value<0.05), One-way ANOVA. Data represent mean ± SD for 3 independent experiments, performed in triplicate. CC: control non-infected cells, CV: HRSV infected cells.
Fig. 7.
Effect of compounds 1 and 2 on cytokine production in epithelial cells infected with HRSV. A549 were infected with HRSV A2 and L19 (moi = 1). After virus adsorption, cells were incubated at 37 °C in the presence or absence of the compounds for 24 h. Supernatants were harvested, and IL-6 and IL-8 were determined by ELISA. * Significantly different from HRSV infected cells (CV), (p-value<0.05), ANOVA. Data represent mean ± SD for 3 independent experiments, performed in triplicate.
3.4. In vivo antiviral and anti-inflammatory activity of compounds 1 and 2
Given the antiviral and immunomodulatory activity of the compounds in epithelial and inflammatory infected cells in vitro, we investigated the ability of both steroid analogs to reduce infective virus load and lung inflammation in a murine model of HRSV infection.
A TH2-type antiviral response prevails upon infection of mice with strain L19 in the same way as in HRSV-infected patients. This makes them a suitable model to study lung pathophysiology and immunological responses [2].
We first determined the safe range of doses of both compounds intraperitoneally (i.p.) administered. An in vivo toxicity assay was performed in Balb/c mice which received different doses of the compounds from 2.5 mg/kg to 10 mg/kg, dissolved in DMSO 90%, during 4 consecutive days. Daily registration of mice weights showed no significant differences between treated and untreated animals (Figure S2 - Supplementary material). No other signs of toxicity were observed in mice which received any of the doses of the compounds with respect to control animals.
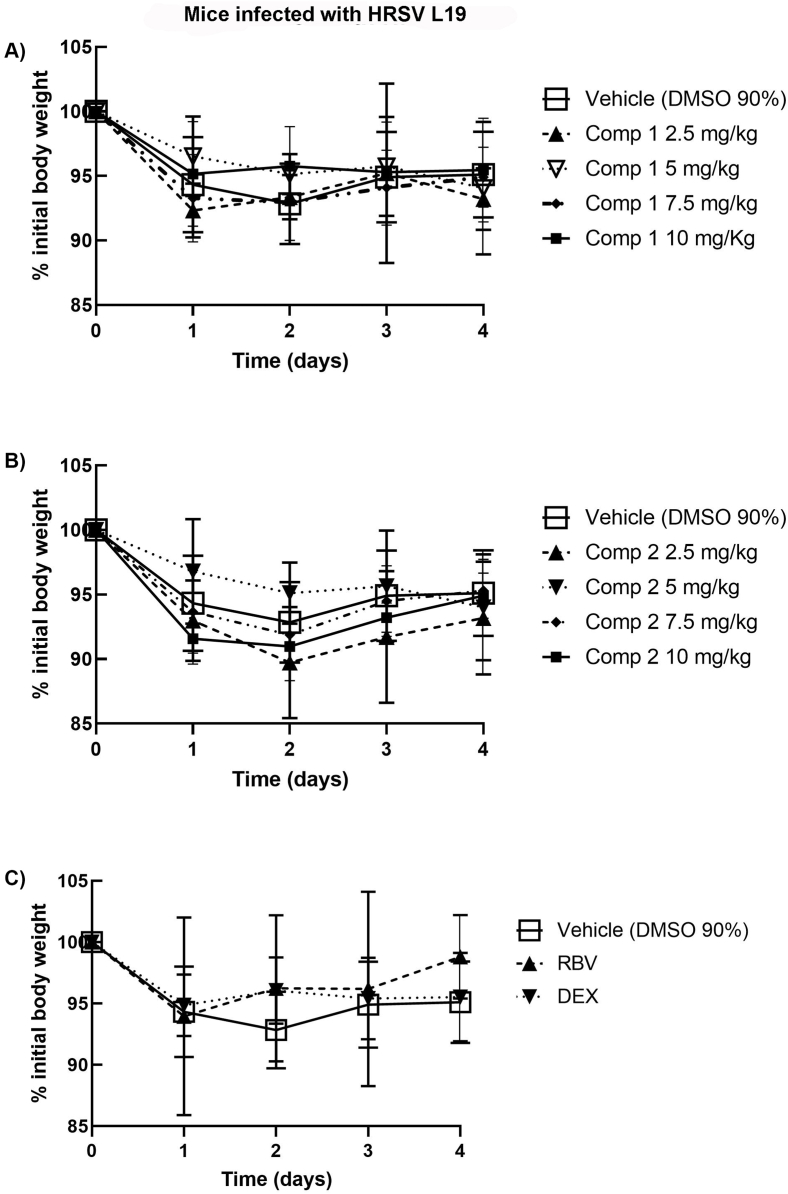
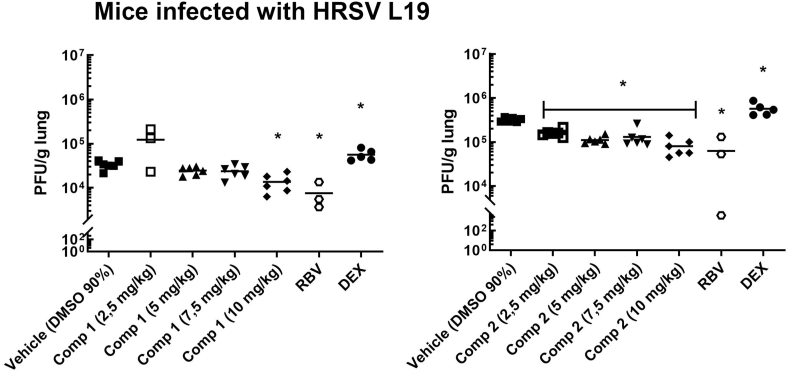
Then, we evaluated the activity of the compounds as antivirals and as anti-inflammatory drugs in vivo. Doses of 2.5, 5, 7.5 and 10 mg/kg of compounds 1 and 2 were administered i.p. immediately after mice intranasal inoculation with HRSV L19, and subsequently treated with daily administration of the compounds for 3 days. 25 mg/kg Ribavirin (RBV) and 2.5 mg/kg Dexamethasone (DEX) were used as controls of antiviral and anti-inflammatory activity, respectively. Mice injected i.p. with DMSO 90% were used as infection controls. Loss of weight was measured every day, virus infectivity was determined in the lungs at day 4 p.i., and pulmonary histopathology was studied at day 8 p.i. Infected mice treated either with compounds 1 (Fig. 8A), 2 (Fig. 8B), RBV or DEX (Fig. 8C) showed an early weight reduction between days 1 and 2 p.i., comparable to the weight reduction observed for HRSV-infected control mice [28]. Nevertheless, HRSV L19 titers in the lung were significantly lower in animals treated with 10 mg/kg of compounds 1 and 2 as well as in animals treated with RBV, with respect to untreated animals, at day 4 p.i.. Furthermore, compound 2 was also effective in reducing viral load at all the doses tested (Fig. 9). Treatment with DEX led to a significant increase of HRSV titers in the lungs of infected animals with respect to infected mice which receive vehicle (DMSO 90%) (Fig. 9). As we previously reported, the anti-inflammatory steroid was not able to eliminate the virus from the site of infection [15] and, in this case, we observed an increase in HRSV replication. Despite having a steroid structure, compounds 1 and 2 exert their effects through a mechanism of action different from that of glucocorticoids like DEX, since they do not bind to glucocorticoid receptors [29].
Fig. 8.
Female Balb/c mice were infected with HRSV line 19 (5 × 106 PFU) by intranasal instillation, concomitant with different doses of compounds 1 and 2 in DMSO 90%, Dexamethasone (DEX) and Ribavirin (RBV) by i.p. injection on day 0. On days 1–4, all mice received further inoculations of compounds 1 and 2, DEX and RBV i.p. Body weights were monitored and registered daily, until 4 day p.i. and the percentage of initial body weight was calculated and graphed for every condition. Data show mean ± SD from a minimum of n = 3 mice/condition.
Fig. 9.
Female Balb/c mice were infected with HRSV line 19 (5 × 106 PFU) by intranasal instillation, concomitant with different doses of compounds 1 and 2 in DMSO 90%, Dexamethasone (DEX) and Ribavirin (RBV) by i.p. injection on day 0. On days 1–4, all mice received further inoculations of compounds 1 and 2, DEX and RBV i.p. The animals were killed on day 4 and the lungs were used for titration of infectious virus. Data show mean ± SD from n = 6 mice/condition, for compounds 1 and 2, and 3 mice/condition for RBV and DEX.. *Significantly different from HRSV infected mice (p-value<0.05), ANOVA. Saline solution: infected animals, treated with saline solution by i.p. injection. DMSO 90%: infected animals, treated with DMSO 90% by i.p. inyection.
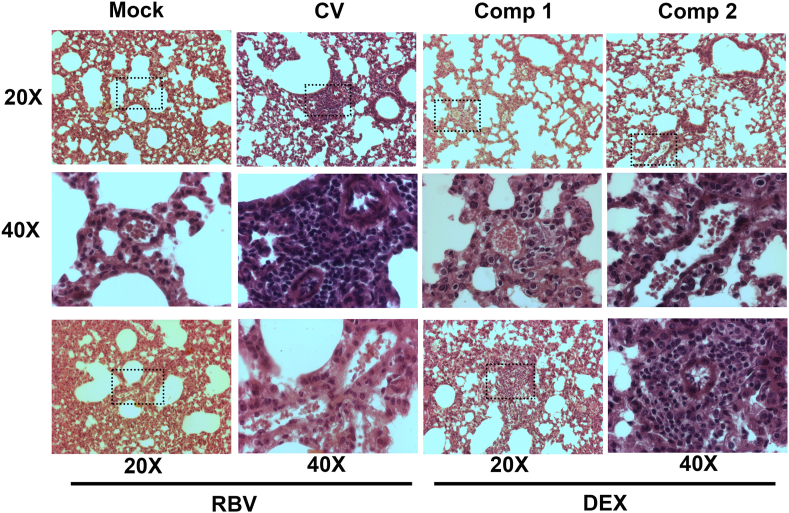
The histopathology of the lungs in infected control mice showed moderate to severe inflammatory infiltrates in the alveolar walls and spaces. The lungs from infected mice treated with compounds 1 and 2 showed a histologic pattern more like that of the uninfected controls. Thus, compounds 1 and 2 reduced lung inflammation and inflammatory cell infiltration. On the contrary, lung sections from mice treated with RBV and DEX were similar to that of infected untreated controls (Fig. 10). Although RBV administration achieved a reduction in viral loads in the lungs of infected mice, the treatment with the antiviral alone was not enough to reduce the inflammatory infiltrates since the drug has no anti-inflammatory activity.
Fig. 10.
Lung histology. Light micrographic images of pulmonary histology of H&E-stained lungs collected at day 8 p.i., shown at magnification × 20 and x40, representative of n = 3/condition. CV: control virus.
HRSV-infected mice treated with compounds 1 and 2 exhibited reduced viral loads as well as reduced inflammatory infiltrates in the lungs, which confirmed that this dual bioactivity (antiviral and anti-inflammatory) would be responsible for the improvement of the signs of pathology.
4. Discussion and Conclusions
Although much knowledge exists about HRSV virology, there are still no effective approaches available to prevent and treat HRSV infection [30]. The COVID-19 pandemic has exposed the crucial necessity of broad-spectrum drugs against respiratory viruses [31]. A broad-spectrum antiviral against different virus types could help in the clinical approach of different acute respiratory virus infections [32]. Many broad-spectrum drugs which exert antiviral activity against different respiratory virus like HRSV were tested during the last 2 years for antiviral activity against SARS-CoV2 [[31], [32], [33], [34]]. We have already described the inhibitory effect of compounds 1 and 2 against two unrelated viruses: an enveloped virus such as HSV-1, and a naked one, Adenovirus (ADV), which has cellular tropism in human respiratory tract, among other tissues. The described inhibitory activity of compounds 1 and 2 provides evidence of a broad spectrum of antiviral activity [[15], [16], [17], [18]]. In this work, we show these compounds are also effective against the respiratory virus HRSV in vitro and in vivo conditions. The inhibitory activity of the compounds on virus replication is displayed when they are added after infection, with the highest inhibition observed during the first 8 h p.i. (Fig. 4 A and C). In a similar manner, Compound 1 has shown to inhibit HSV-1 and Adenovirus when it was added up to 8 h after infection. In the case of Adenovirus, an inhibitory effect of Compound 1 was evident even when the compound was added at 15 h or 24 h post infection [16].
We observed that compounds 1 and 2 inhibited the expression of F protein when administered during 24 h to the infected cells, as well as at a shorter incubation time of 8 h immediately after infection (Fig. 1, Fig. 5). HRSV binds to cellular receptors through the fusion (F) glycoprotein, which induces pH-independent fusion between the viral envelope and the cellular plasma membrane. Infected cells fuse their membranes with the membranes of adjacent cells forming syncitia (giant multinucleate cells), also through the F glycoprotein. This results in viral propagation through the cells ([35,36]). The inhibition of F protein expression observed, especially after 8 h treatment with compounds 1 and 2, correlates with the major inhibition in viral yields obtained when infected cells received an early treatment with the compounds after infection (Fig. 4 A and C). It is possible that the compounds are inhibiting HRSV F protein synthesis, among other viral proteins, with the consequent inhibition of virus particles assembly and propagation through the cells. This would be, at least, one of the compounds' action mechanisms since they showed no direct inactivating effect on the virus particles (Fig. 2), they did not induce a refractory state against infection in the cells (Fig. 3A) and they did not inhibit virus entry into the cells (Fig. 3 B and C), as previously observed with HSV-1 and ADV infections [[14], [15], [16], [17]]. Preugschas et al. [37] describe that ERK activation is necessary for F protein trafficking to the plasma membrane. We have previously reported that our compounds inhibit ERK signalling pathway [29]. We can hypothesize that the compounds are restraining HRSV F protein and viral propagation through ERK inhibition. Future studies on the effects of the compounds on ERK activation by HRSV infection should let us corroborate this possible mechanism of action.
The epithelial cells of the respiratory tract are the primary target cells for HRSV infection. The epithelium is not only a physical barrier but has the potential to synthesize a variety of cytokines, like IL-8 and IL-6 [38]. A549 cells secrete these cytokines under HRSV infection [39]. On the other hand, monocytes are the first inflammatory cells activated after HRSV infection [1]. Alveolar macrophages from bronchoalveolar fluid from HRSV-infected patients secret proinflammatory cytokines, exerting a local immune-regulatory role ([1,3]). Our results show that both compounds are beneficial not only as inhibitors of virus replication and propagation but also as modulators of the immune response which develops after HRSV infection, and which is closely related with the lung pathology (Fig. 6, Fig. 7).
The lungs of mice infected with HRSV L19 strain and treated with compounds 1 and 2 showed a reduction in viral load, being the latter more effective (Fig. 9). As happens with other common respiratory viruses, HRSV viral load correlates with the severity of disease which, in turn, dictates the degree of inflammation [30]. This was evident in our work since we found that infected mice treated with the compounds showed reduced inflammatory infiltration in the lungs (Fig. 10). However, the reduction of lung inflammation would not only be ascribed to the compound's antiviral activity. Unlike Ribavirin that reduced viral titers in the infected lungs but had no effect in avoiding inflammation, Compounds 1 and 2 exert antiviral activity and reduce HRSV induced lung damage as well (Fig. 10). Compounds 1 and 2 would be then also exerting an anti-inflammatory activity which, together with the antiviral activity, reduce HRSV induced lung pathology. Both activities would be necessary to ameliorate the signs of disease in the infected mice since DEX treatment alone did not reduce lung inflammation and induced an increase in viral titers.
It is well known that damage to the pulmonary epithelium caused by HRSV is due to a disproportionate inflammatory response at the airways which ends in a cytokine storm that might result in organ failure and fatal respiratory distress [40]. This kind of response is similar to that caused by SARS-CoV-2 [41].
Our work shows that compounds 1 and 2 reduce viral yields and proinflammatory cytokines secretion induced by HRSV infection in vitro and these results are strengthened by the demonstrated effectiveness of both compounds to reduce HRSV immunopathology in vivo. Further toxicity studies with higher doses of the compounds would extend the security range of the doses administered in vivo. Hence, compounds 1 and 2, with both, antiviral and anti-inflammatory activities, represent an advantage over other drugs for the management of immunopathologies triggered by viruses like HRSV. And they would be promissory candidates to be tested against SARS-CoV-2 and other respiratory viruses.
Author contributions statement
Carlos Alberto Bueno; Franco Maximiliano Salinas: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Luciana Vazquez: Performed the experiments.
Laura Edith Alché; Flavia Mariana Michelini: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Funding
This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT2013-2281, PICT2018-0733, PICT2018-01588), CONICET (PIP 1007), Universidad de Buenos Aires (UBACyT 20,020,100,100,522) and Instituto Massone S.A., Argentina.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to thank Instituto Massone S.A. for kindly providing compounds 1 and 2, and to Guillermo Assad Ferek for his technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e20148.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
figs2.
figs3.
References
- 1.Nuriev R., Johansson C. vol. 8. F1000 Research Ltd; 2019. Chemokine regulation of inflammation during respiratory syncytial virus infection [version 1; peer review: 3 approved. (F1000Research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandya M.C., Callahan S.M., Savchenko K.G., Stobart C.C. vol. 8. MDPI AG; 2019. A contemporary view of respiratory syncytial virus (RSV) biology and strain-specific differences. (Pathogens). Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell C.D., Unger S.A., Walton M., Schwarze J. vol. 30. American Society for Microbiology; 2017. The human immune response to respiratory syncytial virus infection; pp. 481–502. (Clinical Microbiology Reviews). Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fearns R., Deval J. vol. 134. Elsevier B.V; 2016. New antiviral approaches for respiratory syncytial virus and other mononegaviruses: inhibiting the RNA polymerase; pp. 63–76. (Antiviral Research). [DOI] [PubMed] [Google Scholar]
- 5.Tejada S., Martinez-Reviejo R., Karakoc H.N., Peña-López Y., Manuel O., Rello J. vol. 39. 2022. Ribavirin for treatment of subjects with respiratory syncytial virus-related infection: a systematic review and meta-analysis; pp. 4037–4051. (Advances in Therapy). 9. [DOI] [PubMed] [Google Scholar]
- 6.Turner T.L., Kopp B.T., Paul G., Landgrave L.C., Hayes D., Thompson R. vol. 6. Dove Medical Press; 2014. Respiratory syncytial virus: current and emerging treatment options; pp. 217–225. (ClinicoEconomics and Outcomes Research). Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H., Zhao H., Lin H., Shen P., Liu C., Zhan S. Utilization of intravenous ribavirin among reproductive age adults in 2010–2017: a population-based study in the yinzhou district, ningbo city of China. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.678785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viguria N., Navascués A., Juanbeltz R., Echeverría A., Ezpeleta C., Castilla J. Effectiveness of palivizumab in preventing respiratory syncytial virus infection in high-risk children. Hum. Vaccines Immunother. 2021;17(6):1867–1872. doi: 10.1080/21645515.2020.1843336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domachowske J.B., Anderson E.J., Goldstein M. The future of respiratory syncytial virus disease prevention and treatment. Infect. Dis. Ther. 2021;10:47–60. doi: 10.1007/s40121-020-00383-6. Adis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang R., Zhang Y., Zheng W., Shang W., Wu Y., Li N., Xiong J., Jiang H., Shen J., Xiao G., Xie Y., Zhang L. vol. 7. Springer Nature; 2022. Oral remdesivir derivative VV116 is a potent inhibitor of respiratory syncytial virus with efficacy in mouse model. (Signal Transduction and Targeted Therapy). Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sake S.M., Kosch C., Blockus S., Haid S., Gunesch A.P., Zhang X., Friesland M., Trummer S.B., Grethe C., Kühnel A., Rückert J., Duprex W.P., Huang J., Rameix-Welti M.A., Empting M., Fischer N., Hirsch A.K.H., Schulz T.F., Pietschmann T. Respiratory syncytial virus two-step infection screen reveals inhibitors of early and late Life cycle stages. Antimicrob. Agents Chemother. 2022;66(12) doi: 10.1128/aac.01032-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blunck B.N., Rezende W., Piedra P.A. Profile of respiratory syncytial virus prefusogenic fusion protein nanoparticle vaccine. Expet Rev. Vaccine. 2021;20(4):351–364. doi: 10.1080/14760584.2021.1903877. [DOI] [PubMed] [Google Scholar]
- 13.Mejias A., Rodríguez-Fernández R., Oliva S., Peeples M.E., Ramilo O. vol. 125. American College of Allergy, Asthma and Immunology; 2020. The journey to a respiratory syncytial virus vaccine; pp. 36–46. (Annals of Allergy, Asthma and Immunology). Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmoele-Thoma B., Zareba A.M., Jiang Q., Maddur M.S., Danaf R., Mann A., Eze K., Fok-Seang J., Kabir G., Catchpole A., Scott D.A., Gurtman A.C., Jansen K.U., Gruber W.C., Dormitzer P.R., Swanson K.A. Vaccine efficacy in adults in a respiratory syncytial virus challenge study. N. Engl. J. Med. 2022;386(25):2377–2386. doi: 10.1056/nejmoa2116154. [DOI] [PubMed] [Google Scholar]
- 15.Michelini F.M., Ramírez J.A., Berra A., Galagovsky L.R., Alché L.E. Anti-herpetic and anti-inflammatory activities of two new synthetic 22,23-dihydroxylated stigmastane derivatives. J. Steroid Biochem. Mol. Biol. 2008;111(1–2):111–116. doi: 10.1016/j.jsbmb.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Michelini F.M., Bueno C.A., Areco Y.B., Alché L.E. A synthetic stigmastane displays antiadenoviral activity and reduces the inflammatory response to viral infection. Antivir. Res. 2020;183 doi: 10.1016/j.antiviral.2020.104879. [DOI] [PubMed] [Google Scholar]
- 17.Michelini F.M., Zorrilla P., Robello C., Alché L.E. Immunomodulatory activity of an anti-HSV-1 synthetic stigmastane analog. Bioorg. Med. Chem. 2013;21(2):560–568. doi: 10.1016/j.bmc.2012.10.054. [DOI] [PubMed] [Google Scholar]
- 18.Michelini F.M., Lombardi M.G., Bueno C.A., Berra A., Sales M.E., Alché L.E. Synthetic stigmasterol derivatives inhibit capillary tube formation, herpetic corneal neovascularization and tumor induced angiogenesis: antiangiogenic stigmasterol derivatives. Steroids. 2016;115:160–168. doi: 10.1016/j.steroids.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Caidi H., Harcourt J.L., Haynes L.M. vol. 1442. Humana Press Inc; 2016. RSV growth and quantification by microtitration and qRT- PCR assays; pp. 13–32. (Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- 20.Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Journal oflmmunologicalMethods. 1986;89 doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 21.National Research Council (Us) eighth ed. National Academies Press (US); Washington (DC): 2011. Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals.https://www.ncbi.nlm.nih.gov/books/NBK54050/ Available from: [DOI] [Google Scholar]
- 22.Shi H., Ren K., L B., Zhang W., Zhao Y., Tan R.X., Li E. Baicalin from Scutellaria baicalensis blocks respiratory syncytial virus (RSV) infection and reduces inflammatory cell infiltration and lung injury in mice. Sci. Rep. 2016;21(6) doi: 10.1038/srep35851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudd P.A., Chen W., Mahalingam S. Mouse and cotton rat models of human respiratory syncytial virus. Methods Mol. Biol. 2016;1442:209–217. doi: 10.1007/978-1-4939-3687-8_15. [DOI] [PubMed] [Google Scholar]
- 24.Hillyer, P., Shepard, R., Uehling, M., Krenz, M., Sheikh, F., Thayer, K. R., Huang, L., Yan, L., Panda, D., Luongo, C., Buchholz, U. J., Collins, P. L., Donnelly, R. P., & Rabin, R. L. (n.d.). Differential Responses by Human Respiratory Epithelial Cell Lines to Respiratory Syncytial Virus Reflect Distinct Patterns of Infection Control. 10.1128/JVI. [DOI] [PMC free article] [PubMed]
- 25.Jorquera P.A., Anderson L., Tripp R.A. vol. 1442. Humana Press Inc; 2016. Human respiratory syncytial virus: an introduction; pp. 1–12. (Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- 26.Becker Y. Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy - a review. Virus Gene. 2006;33(Issue 2):235–252. doi: 10.1007/s11262-006-0064-x. [DOI] [PubMed] [Google Scholar]
- 27.Salinas F.M., Vázquez L., Gentilini M.V., O′Donohoe A., Regueira E., Nabaes Jodar M.S., Viegas M., Michelini F.M., Hermida G., Alché L.E., Bueno C.A. Aesculus hippocastanum L. seed extract shows virucidal and antiviral activities against respiratory syncytial virus (RSV) and reduces lung inflammation in vivo. Antivir. Res. 2019;164:1–11. doi: 10.1016/j.antiviral.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Stokes K.L., Chi M.H., Sakamoto K., Newcomb D.C., Currier M.G., Huckabee M.M., Lee S., Goleniewska K., Pretto C., Williams J.v., Hotard A., Sherrill T.P., Peebles R.S., Moore M.L. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J. Virol. 2011;85(12):5782–5793. doi: 10.1128/jvi.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michelini F., Bueno C., Molinari A., Galigniana M., Galagovsky L., Alché L., Ramírez J. Synthetic stigmastanes with dual antiherpetic and immunomodulating activities inhibit ERK and Akt signaling pathways without binding to glucocorticoid receptors. Biochimica et Biophysica Acta - General Subjects. 2016;1860(1):129–139. doi: 10.1016/j.bbagen.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths C., Drews S.J., Marchant D.J. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin. Microbiol. Rev. 2017;30(1):277–319. doi: 10.1128/CMR.00010-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sourimant J., Lieber C.M., Aggarwal M., Cox R.M., Wolf J.D., Yoon J.-J., Toots M., Ye C., Sticher Z., Kolykhalov A.A., Martinez-Sobrido L., Bluemling G.R., Natchus M.G., Painter G.R., Plemper R.K. 4′-Fluorouridine is an oral antiviral that blocks respiratory syncytial virus and SARS-CoV-2 replication. Science. 2022;375(6567):161–167. doi: 10.1126/science.abj5508. https://www.science.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Beltagi S., Preda C.A., Goulding L.v., James J., Pu J., Skinner P., Jiang Z., Wang B.L., Yang J., Banyard A.C., Mellits K.H., Gershkovich P., Hayes C.J., Nguyen-Van-tam J., Brown I.H., Liu J., Chang K.C. Thapsigargin is a broad-spectrum inhibitor of major human respiratory viruses: coronavirus, respiratory syncytial virus and influenza a virus. Viruses. 2021;13(2) doi: 10.3390/v13020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray J., Bergeron H.C., Jones L.P., Chapman T., Reener Z.B., Trippbio D.E.M., Sancilio F.D., Tripp R.A. Probenecid Inhibits Respiratory Syncytial Virus (RSV) Replication. 2022 doi: 10.21203/rs.3.rs-1280404/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., Leist S.R., Schafer A., Dinnon K.H., Stevens L.J., Chappell J.D., Lu X., Hughes T.M., George A.S., Hill C.S., Montgomery S.A., Brown A.J., Bluemling G.R., Natchus M.G., Baric R.S. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020;12(541) doi: 10.1126/SCITRANSLMED.ABB5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckardt-Michel J., Lorek M., Baxmann D., Grunwald T., Keil G.M., Zimmer G. The fusion protein of respiratory syncytial virus triggers p53-dependent apoptosis. J. Virol. 2008;82(7):3236–3249. doi: 10.1128/jvi.01887-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leemans A., Boeren M., van der Gucht W., Martinet W., Caljon G., Maes L., Cos P., Delputte P. Characterization of the role of N-glycosylation sites in the respiratory syncytial virus fusion protein in virus replication, syncytium formation and antigenicity. Virus Res. 2019;266:58–68. doi: 10.1016/j.virusres.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Preugschas H., Hrincius E., Mewis C., Tran G., Ludwig S., Ehrhardt C. Late activation of the Raf/MEK/ERK pathway is required for translocation of the respiratory syncytial virus F protein to the plasma membrane and efficient viral replication. Cell Microbiol. 2019;21(1) doi: 10.1111/cmi.12955. [DOI] [PubMed] [Google Scholar]
- 38.Smith K.G., Kamdar A.A., Stark J.M. Lung defenses. Kendig’s Disorders of the Respiratory Tract in Children. 2019 doi: 10.1016/b978-0-323-44887-1.00008-0. 120–133.e2. [DOI] [Google Scholar]
- 39.Arnold R., Humbert B., Werchau H., Gallatit H., Konig W. Interleukin-8, interleukin-6, and soluble tumour necrosis factor receptor type I release from a human pulmonary epithelial cell line (A549) exposed to respiratory syncytial virus. Immunology. 1994;82(1):126–133. [PMC free article] [PubMed] [Google Scholar]
- 40.Bohmwald K., Gálvez N.M.S., Canedo-Marroquín G., Pizarro-Ortega M.S., Andrade-Parra C., Gómez-Santander F., Kalergis A.M. vol. 10. Frontiers Media S.A; 2019. Contribution of cytokines to tissue damage during human respiratory syncytial virus infection. (Frontiers in Immunology). MAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hafezi B., Chan L., Knapp J.P., Karimi N., Alizadeh K., Mehrani Y., Bridle B.W., Karimi K. vol. 10. MDPI; 2021. Cytokine storm syndrome in sars‐cov‐2 infections: a functional role of mast cells. (Cells). Issue 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.