Abstract
Background
Nutritional status is one of the important factors determining the short- and long-term outcomes of surgery in cancer. This study aimed to assess the prognostic role of preoperative controlling nutritional status (CONUT) score in intrahepatic cholangiocarcinoma (iCCA) patients.
Methods
A total of 101 iCCA patients who underwent hepatectomy between 2015 and 2018 at the Srinagarind Hospital, Khon Kaen University, were included in this retrospective study. Patients were classified according to the CONUT score. Univariate and multivariate analyses were performed to determine the correlation between clinicopathological features and overall survival.
Results
Patients were categorized into normal nutrition (n = 40 or 39.5%), mild (n = 54 or 53.5%), and moderate-severe malnutrition (n = 7). Patients with high CONUT scores had significantly shorter survival (HR 2.55, 95% CI 1.04–6.25, p = 0.04). In multivariable analysis, tumor size (HR = 2.58, p < 0.01), the growth pattern of mass forming combined with periductal (HR = 4, p < 0.01), lymph node metastasis (HR = 7.20, p < 0.01) and high CONUT score (HR = 4.71, p = 0.01) were independent factors for poor survival of iCCA patients.
Conclusion
The preoperative CONUT score is a simple prognostic factor to predict the outcomes of iCCA patients undergoing hepatectomy.
Keywords: Intrahepatic cholangiocarcinoma, Controlling nutritional status score, Malnutrition, Prognosis, Preoperative evaluation, Surgery, Retrospective study
1. Introduction
Cholangiocarcinoma (CCA) is the second most common type of liver cancer arising from bile duct epithelial cells involving the intrahepatic (iCCA), perihilar (pCCA), and distal (dCCA) biliary tree [1]. Despite advances in diagnostic tools and surgical techniques, the prognosis of CCA patients remains unfavorable.
Recent evidence derived from the long-term follow-up of the BILCAP study has revealed that adjuvant capecitabine after resected biliary tract cancer provides a survival benefit [2,3]. The identification of molecular features of cholangiocarcinoma, including fibroblast growth factor receptor (FGFR)2 gene fusions and rearrangements, isocitrate dehydrogenase-1 (IDH-1) mutations, and BRAF mutations has been made possible with the next-generation sequencing technique [4,5]. However, molecular alterations such as these are not frequently exhibited in fluke-related CCA [4,6]. Recently, the US FDA has approved durvalumab (anti-PD-L1) in combination with gemcitabine and cisplatin for metastatic biliary tract cancer [7]. However, despite being statistically significant, the magnitude of the benefit is modest in unselected patients. Complete surgical resection, with or without adjuvant treatment, is currently the cornerstone of treatment for localized disease leading to long-term remission and cure [8].
The immune function, inflammatory markers, and nutritional status play pivotal roles in determining postoperative outcomes. Malnutrition negatively affects patient recovery after surgery, increases health care costs, and is associated with shorter survival in cholangiocarcinoma [9,10]. It has been observed that approximately 32% of patients diagnosed with malnutrition at the time of admission for surgery [[11], [12], [13], [14]]. Additionally, preoperative malnutrition has been significantly linked to postoperative liver failure and poor surgical outcomes [15,16].
Immuno-nutritional scores, including neutrophil-lymphocyte ratio (NLR), prognostic nutritional index (PNI), and C-reactive protein/albumin ratio (CAR) are independent factors for prognosis the survival of patients in several malignancies [[17], [18], [19], [20], [21]]. Controlling nutritional status is a simple scoring system that has been extensively studied in various solid tumors, including colon, pancreas, hepatocellular carcinoma, and CCA [[22], [23], [24], [25], [26], [27]]. It predicts both nutritional and immunological state of the individual. Moreover, the CONUT score has been deemed superior to PNI and NLR scoring systems. It is a useful tool for predicting long-term outcomes in cancer patients after curative resection [[28], [29], [30]].
Therefore, the purposes of the study are to assess the preoperative CONUT score to evaluate the prognosis of iCCA patients who have undergone hepatectomy and examine the relationship between the nutritional degree evaluated by CONUT score and the clinicopathological features of iCCA.
2. Materials and methods
2.1. Patients
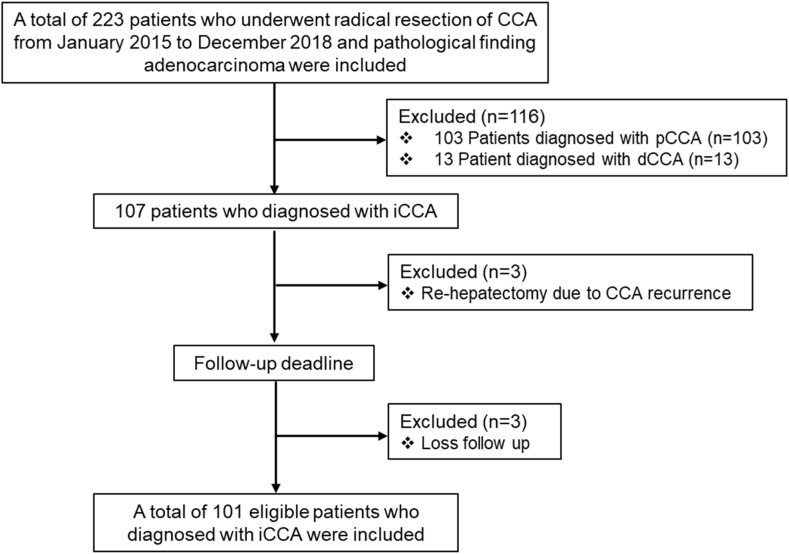
Between 2015 and 2018, a total of 223 patients underwent radical resection for CCA at Srinagarind Hospital, Khon Kaen University. Patients with perihilar or distal CCA, patients who underwent re-hepatectomy for CCA recurrence, and those who were lost to follow-up were excluded from this study. The total number of patients included in this study was 101 patients (Fig. 1). The study was approved by the Human Ethical Committee of Khon Kaen University (HE631408) per the Declaration of Helsinki.
Fig. 1.
Enrollment and outcomes. iCCA, Intrahepatic cholangiocarcinoma.
2.2. Biomarkers and clinicopathological data
Preoperative blood samples were obtained within one month preceding hepatectomy to determine serum biomarkers including total cholesterol, albumin, white blood cell, lymphocyte, and neutrophil counts. The liver specimens were examined and formalin-fixed paraffin-embedded (FFPE) tissue blocks were sectioned at 5 μm [31] and stained with hematoxylin and eosin (H&E). Pathological diagnosis was reviewed for this study following the 2019 World Health Organization (WHO) classification criteria [32]. Under light microscopy, the following histo-morphological data were recorded - growth patterns, surgical margin, lymphovascular invasion (LVI), and lymph node metastasis (N). Intrahepatic CCA patients were classified into four groups based on the TNM staging by the 7th AJCC/UICC staging manual [33].
2.3. The definition of CONUT
The method of assessment of nutritional status according to CONUT was summarized in Table 1. Preoperative serum albumin, total Lymphocyte (T-LC), and cholesterol (T-Cho) were classified and scored accordingly. The total score of the three parameters was CONUT score and categorized into four levels of nutritional status: CONUT score, 0–1 was normal nutrition; 2–4 was mildly abnormal, 5–8 was moderately abnormal and 9–12 was severely abnormal nutrition.
Table 1.
Immuno-nutritional status assessment by controlling nutritional status (CONUT).
|
Parameters |
Undernutrition level |
|||
|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | |
| Serum Albumin (g/dL) | ≥3.50 | 3.00–3.49 | 2.5-2.99 | <2.50 |
| Score | 0 | 2 | 4 | 6 |
| Total Lymphocyte (count/mm3) | ≥1600 | 1200–1599 | 800–1199 | <800 |
| Score | 0 | 1 | 2 | 3 |
| Cholesterol (mg/dL) | ≥180 | 140–179 | 100–139 | <100 |
| Score | 0 | 1 | 2 | 3 |
| Screening Total Score | 0–1 | 2–4 | 5–8 | 9–12 |
2.4. Growth pattern proportion
Growth pattern identification in iCCA was followed by Sa-ngiamwibool P et al. [34]. Briefly, the liver resection specimens were serially sectioned, photographed, and tumor growth patterns were recorded at the time of the gross examination. The growth patterns were classified as a single pattern (intraductal (ID), periductal (PI), or mass-forming (MF) or a combination of patterns (ID + PI, ID + MF, PI + MF, and ID + PI + MF). The growth pattern was also confirmed by microscopic examination by pathologists.
2.5. Statistical analysis
The Statistical Package for the Social Sciences (SPSS) software v.25 (SPSS, Inc., Chicago, IL, USA). was performed to analyze the data. Descriptive characteristic data for continuous variables were presented as the mean ± standard deviation when the variables showed a normal distribution, while the median (interquartile range) was shown when the variables did not present a normal distribution. The correlation analysis of the CONUT score and clinicopathological features was applied by Chi-square test (or Fisher's exact tests, as appropriate). Three groups of continuous variables with a normal distribution were compared by a one-way ANOVA test, while those with a non-normal distribution were compared by the Kruskal-Wallis test. The survival curves were calculated by the Kaplan-Meier method, and comparisons among groups were evaluated by the log-rank test. Multivariate analysis was performed using the Cox regression model. A p-value of less than 0.05 was regarded as statistically significant.
3. Results
3.1. Clinicopathological characteristics
A total of 101 iCCA patients were included. The median age of the patients was 63 years (ranging from 42 to 83 years), and a majority of them (72.3%) were male. The preoperative BMI mean ± SD was 23.1 ± 3.4 kg/m2. The major growth pattern observed was mass-forming CCA, which accounted for 54.6% of the cases. The median tumor size was 60 mm (range, 10–130 mm). The surgical margin was assessed microscopically to be involved by tumor (R1) in 38.6%, and lymphovascular invasion (LVI) was observed in 50% of the patients. Lymph node metastasis was found in 37.4% of the patients, with most of them being in stage III (55.4%) (Table 2).
Table 2.
Baseline characteristics of intrahepatic cholangiocarcinoma patients.
|
Features |
Number of cases (n) | percentage (%) |
|---|---|---|
| Age (range, 42–83 years) | ||
| <63 years | 47 | 46.5 |
| ≥63 years | 54 | 53.5 |
| Sex (n, %) | ||
| Male | 73 | 72.3 |
| Female | 28 | 27.7 |
| BMI (kg/m2) | ||
| <23.1 | 49 | 48.5 |
| ≥23.1 | 52 | 51.5 |
| Tumor size (mm) | ||
| <60 | 46 | 45.5 |
| ≥60 | 55 | 54.5 |
| Growth pattern | ||
| Intraductal (ID) | 16 | 15.8 |
| Periductal (PI) | 7 | 6.9 |
| Mass-forming (MF) | 55 | 54.6 |
| ID + MF | 7 | 6.9 |
| MF + PI | 16 | 15.8 |
| Surgical margin (R) | ||
| R0 | 62 | 61.4 |
| R1 | 39 | 38.6 |
| Lymphovascular invasion (LVI)a | ||
| Negative | 49 | 50 |
| Positive | 49 | 50 |
| Lymph metastasis (N)a | ||
| Negative | 62 | 62.6 |
| Positive | 37 | 37.4 |
| TNM Stage by 7th AJCC/UICC | ||
| I | 22 | 21.9 |
| II | 16 | 15.8 |
| III | 56 | 55.4 |
| IV | 7 | 6.9 |
| CONUT score | ||
| Normal (0–1) | 40 | 39.6 |
| Mild (2–4) | 54 | 53.5 |
| Moderate-severe (5–12) | 7 | 6.9 |
The data was not recorded in some cases.
The Correlation of preoperative CONUT score with clinicopathological features in intrahepatic cholangiocarcinoma.
According to the CONUT score, patients were classified into three groups: (1) 40 (39.6%) patients had a CONUT score of 0–1 which represented the normal nutritional status; (2) 54 (53.5%) patients had a CONUT score of 2–4, the mild malnutritional status, and 7 (6.9%) patients were the moderate-severe malnutritional status (CONUT score 5–12) (Table 2).
The correlation analysis of the CONUT score and clinicopathological features using chi-square tests (χ2-Test) was shown in Table 3. There was no statically significant correlation between preoperative CONUT score with age, sex, BMI, tumor size, surgical margin, LVI, lymph node metastasis, and TNM staging. Interestingly, the CONUT score, especially 5–12 represented mod-severe malnutrition and was significantly correlated with growth pattern (MF and PI + MF, p = 0.004). Almost 70% of iCCA patients with mod-severe malnutritional status related to MF and PI + MF which are the poor prognostic growth pattern types. Notably, 71.4% of patients with CONUT scores 5–12 had BMI <23.1 kg/m2, although this correlation had no statical significance.
Table 3.
The relationship between clinicopathological features with nutritional status by CONUT score.
|
Features |
CONUT score (immuno-nutritional status) |
p-value | ||
|---|---|---|---|---|
| 0-1 (Normal) n = 40 (%) | 2-4 (Mild) n = 54 (%) | 5-12 (Mod-Severe) n = 7 (%) | ||
| Age (range, 42–83 years) | ||||
| <63 years | 24 (60) | 21 (38.9) | 2 (28.6) | |
| ≥63 years | 16 (40) | 33 (61.1) | 5 (71.4) | 0.078 |
| Gender | ||||
| Male | 29 (72.5) | 28 (70.4) | 6 (85.7) | |
| Female | 11 (27.5) | 16 (29.6) | 1 (14.3) | 0.830a |
| BMI (kg/m2) | ||||
| <23.1 | 16 (40) | 28 (51.9) | 5 (71.4) | |
| ≥23.1 | 24 (60) | 26 (48.1) | 2 (28.6) | 0.238 |
| Tumor size (mm) | ||||
| <60 | 21 (52.5) | 23 (42.6) | 2 (28.6) | |
| ≥60 | 19 (47.5) | 31 (57.4) | 5 (71.4) | 0.410 |
| Growth pattern | ||||
| Intraductal (ID) | 8 (20) | 6 (11.1) | 2 (28.6) | |
| Periductal (PI) | 5 (12.5) | 2 (3.7) | 0 (0) | |
| Mass-forming (MF) | 19 (47.5) | 34 (63.0) | 2 (28.6) | 0.028a |
| ID + MF | 7 (17.5) | 9 (16.7) | 0 (0) | |
| MF + PI | 1 (2.5) | 3 (5.6) | 3 (42.8) | |
| Surgical margin (R) | ||||
| R0 | 26 (65) | 31 (57.4) | 5 (71.4) | |
| R1 | 14 (35) | 23 (42.6) | 2 (28.6) | 0.644 |
| Lymphovascular invasion (LVI) | ||||
| Negative | 23 (59) | 23 (44.2) | 3 (42.9) | |
| Positive | 16 (41) | 29 (55.8) | 4 (57.1) | 0.358a |
| Lymph metastasis (N) | ||||
| Negative | 24 (60) | 35 (66) | 3 (50) | |
| Positive | 16 (40) | 18 (34) | 3 (50) | 0.673a |
| TNM Stage by 7th AJCC/UICC | ||||
| I | 9 (22.5) | 11 (20.4) | 2 (28.6) | |
| II | 6 (15) | 10 (18.5) | 0 (0) | |
| III | 22 (55) | 31 (57.4) | 3 (42.8) | 0.380a |
| IV | 3 (7.5) | 2 (3.7) | 2 (28.6) | |
Fisher's exact test.
In addition, we directly compared the serum biomarkers in different CONUT scores. The results showed that CONUT score 5–12 had significantly lower cholesterol, albumin, and lymphocyte levels (p < 0.001) than CONUT score 0–1 and 2–4. However, there was no markedly significant difference in these parameters between CONUT score 0–1 (normal) and 2–4 (mild). The neutrophil level, which represents high inflammation, in CONUT score 5–12 was significantly higher than CONUT score 0–1 and 2–4 (p = 0.005) (Table 4).
Table 4.
The comparison of serum immuno-nutritional status between the CONUT score.
|
Features |
CONUT score |
p-value | ||
|---|---|---|---|---|
| 0–1 Normal (n = 78) |
2–4 Mild (n = 20) |
5–12 Mod-severe (n = 7) |
||
| Total cholesterol | 215.8 | 187.3 | 142 | <0.001 |
| Total albumin | 4.3 | 4.2 | 3.1 | <0.001 |
| Total WBC | 8570 | 8349 | 10154 | 0.22 |
| Total lymphocytes | 2372 | 1817 | 1038 | <0.001 |
| Total neutrophils | 4946 | 4871 | 6550 | 0.005 |
3.2. CONUT scores and clinicopathological impact on survival of iCCA
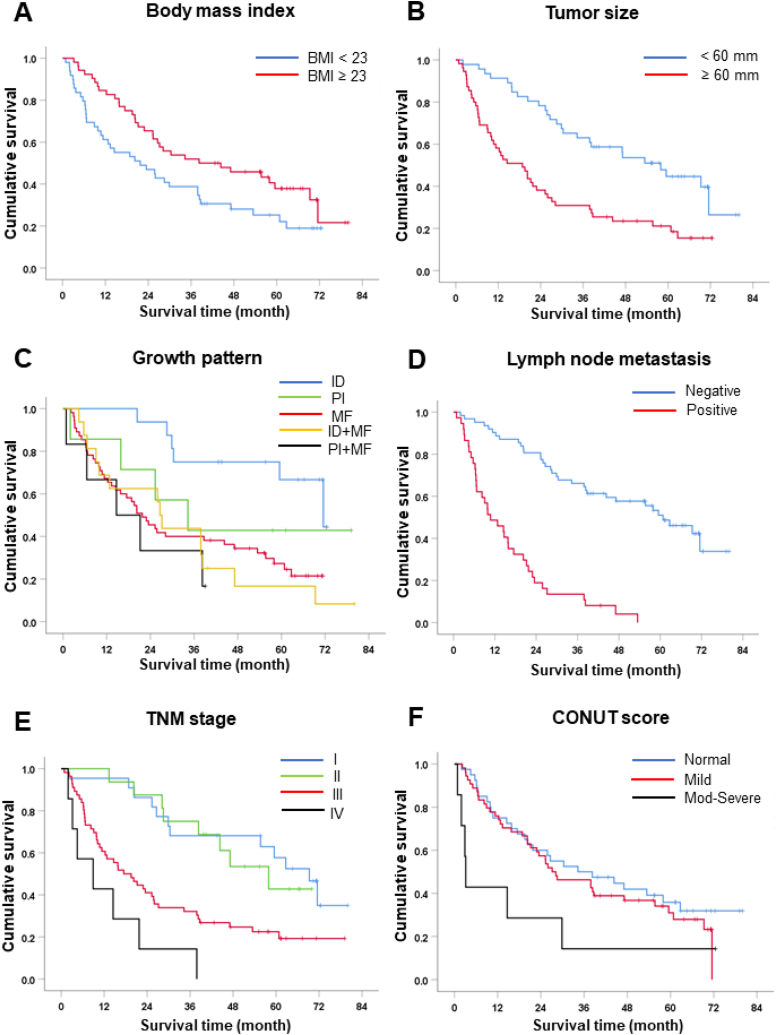
By using univariate analysis for overall survival (OS), we identified factors that were significantly associated with OS. Patients with lower preoperative BMI (<23.1 kg/m2) had shorter survival than those with higher BMI (OS 22 vs 38 months, hazard ratio (HR) = 0.61, p = 0.036, Fig. 2A). Larger tumor size (≥60 mm) was also a poor prognostic factor with OS of 19 vs 58 months compared to those with smaller size (HR = 2.44, p < 0.001, Fig. 2B).
Fig. 2.
Kaplan–Meier survival curves for iCCA patients treated with surgery. (A) Body mass index (BMI) (B) Tumor size, median size 60 mm (mm) as cut-off value. (C) Growth pattern. (D) Lymph node metastasis. (E) TNM stage by 7th AJCC/UICC staging system. (F) CONUT score where: normal, CONUT score 0–1; mild, CONUT score 2–4 and moderate-severe, CONUT score 5–12.
Growth pattern type is another significant prognostic factor. Results showed that ID (OS = 72 mo) had a significantly better survival time than MF (OS 22 mo, HR = 3.68, p = 0.003), ID + MF (OS 27 mo, HR = 5.63, p = 0.005) and PI + MF (OS 15 mo, HR = 4.09, p = 0.004, Fig. 2C). Patients with nodal metastasis had remarkably shorter OS compared to those with negative node, (OS = 11 vs 61 mo, HR = 6.14, p < 0.001, Fig. 2D). Moreover, patients with stage I (OS 69 mo) had markedly better survival than patients with stage III (OS 20 months, HR = 2.92, p = 0.001) and IV (OS 9 mo, HR = 7.15, p < 0.001, Fig. 2E).
Patients who had a CONUT score between 5 and 12 which represented moderate-severe malnutrition had significantly shorter survival compared to those with normal status or CONUT score 0–1 (OS = 3 vs 34 mo, HR = 2.55, p = 0.040). (Table 5 and Fig. 2F).
Table 5.
Univariate and multivariate analysis for overall survival.
|
Features |
Median survival (months) | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | p-value | HR | 95% CI | p-value | ||
| Age (years) | |||||||
| <63 | 34 | 1 | – | ||||
| ≥63 | 26 | 1.24 | (0.8–1.98) | 0.370 | – | – | – |
| Gender | |||||||
| Male | 29 | 1 | |||||
| Female | 25 | 1.21 | (0.73–2.02) | 0.470 | – | – | – |
| BMI (kg/m2) | |||||||
| <23 | 22 | 1 | 1 | ||||
| ≥23 | 38 | 0.61 | (0.38–0.97) | 0.036 | 0.83 | (0.48–1.43) | 0.497 |
| Tumor size (mm) | |||||||
| <60 | 58 | 1 | 1 | ||||
| ≥60 | 19 | 2.44 | (1.50–3.97) | <0.001 | 2.58 | (1.47–4.52) | 0.001 |
| Growth pattern | |||||||
| Intraductal (ID) | 72 | 1 | 1 | ||||
| Periductal (PI) | 34 | 2.15 | (0.60–7.61) | 0.238 | – | – | – |
| Mass-forming (MF) | 22 | 3.68 | (1.54–8.76) | 0.003 | 4.43 | (1.45–13.49) | 0.009 |
| ID + MF | 27 | 5.63 | (1.69–18.78) | 0.005 | 3.57 | (0.86–14.80) | 0.079 |
| PI + MF | 15 | 4.09 | (1.56–10.72) | 0.004 | 4.38 | (1.30–14.79) | 0.017 |
| Lymph node metastasis (N) | |||||||
| N0 | 61 | 1 | 1 | ||||
| N1 | 11 | 6.14 | (3.60–10.48) | <0.001 | 7.20 | (3.30–15.69) | <0.001 |
| Surgical Margin | |||||||
| Negative | 38 | 1 | – | ||||
| Positive | 18 | 1.59 | (0.99–2.55) | 0.055 | – | – | – |
| LVI | |||||||
| Negative | 38 | 1 | – | ||||
| Positive | 20 | 1.61 | (0.99–2.61) | 0.054 | – | – | – |
| TNM Stage | |||||||
| I | 69 | 1 | 1 | ||||
| II | 58 | 1.07 | 0.43-2.66 | 0.877 | – | – | – |
| III | 20 | 2.92 | 1.52-5.64 | 0.001 | 0.73 | (0.29–1.85) | 0.507 |
| IV | 9 | 7.15 | 2.72-18.84 | <0.001 | 0.75 | (0.20–2.78) | 0.663 |
| CONUT | |||||||
| Normal | 34 | 1 | 1 | ||||
| Mild | 27 | 1.21 | 0.74-1.99 | 0.456 | – | – | – |
| Mod-Severe | 3 | 2.55 | 1.04–6.25 | 0.040 | 4.71 | (1.44–15.45) | 0.011 |
Other clinicopathological features including age, gender, surgical margin, and LVI showed no statistically significant difference in the survival times when compared to the references (p = 0.370, 0.470, 0.055, and 0.054, respectively) (Table 5).
Subsequently, all the statically significant factors in univariate analysis were further selected to perform for multivariate analysis by Cox Regression test which included BMI, tumor size, growth pattern, lymph node metastasis, TNM stage, and preoperative CONUT score. Interestingly, we found that there were four independent factors for shorter survival including larger tumor size (HR = 2.58, p = 0.001), growth pattern (MF and PI + MF, HR = 4.43, p = 0.009 and HR = 4.38, p = 0.017, respectively), lymph node metastasis (HR = 7.20, p < 0.001) and preoperative CONUT score (5–12, moderate-severe status, HR = 4.71, p = 0.011). Therefore, our cohort showed that the preoperative CONUT score was a strong and independent prognostic factor for the survival of iCCA patients who underwent hepatectomy. In addition, we also confirmed the finding that the tumor size, growth pattern, and lymph node metastasis are independent risk factors for survival in iCCA (Table 5).
4. Discussion
In this study, we found that preoperative controlling nutritional status (CONUT) score is associated with shorter survival time for intrahepatic cholangiocarcinoma patients who underwent surgical resection.
Curative-intent surgery has been the mainstay treatment for localized cholangiocarcinoma [8]. However, the operation could also lead to several adverse events including delayed wound healing, post-operative infection, and postoperative liver failure. Malnutrition is associated with poor survival of patients after surgical treatment, causes the speed up of cancer progression, and leads to reduced quality of life [16,[35], [36], [37], [38]]. In this study, we found that nearly 60% of the patients had malnutrition evaluated by CONUT score which is comparable to earlier studies on cholangiocarcinoma and hepatocellular carcinoma [25,26]. With the new evidence of improved survival outcomes with adjuvant capecitabine after surgery [3], it is thus very crucial for speedy recovery after liver resection so the patients can be healthy enough to receive chemotherapy.
The CONUT score is an easy and simple screening tool to determine nutritional status. Currently, there are several preoperative immuno-nutritional scoring systems such as neutrophil-lymphocyte ratio (NLR), prognostic nutritional index (PNI), CRP-albumin ratio (CAR), and CONUT to assign and evaluate the patients’ outcomes after curative surgery. However, multiple studies revealed that the CONUT scoring system outperformed NLR, PNI, and CAR in terms of predictive performance [[28], [29], [30]]. In hilar CCA or pCCA, three immuno-nutritional score systems including CONUT, NLR, and PNI were compared by Wang A et al. [30]. They demonstrated that in pCCA, the preoperative CONUT score can be used as a prognostic indicator to predict both overall survival and recurrence-free survival.
In this study, we found that the CONUT score was associated with tumor growth pattern which has been suggested as a prognostic factor for iCCA. A high CONUT score was correlated with MF and PI + MF patterns. Moreover, we found that a high CONUT score was an independent prognostic factor for survival outcomes in iCCA patients after surgical treatment. These findings support the findings from other stuies including patients who underwent hepatic surgery for cholangiocarcinoma and hepatocellular carcinoma [25,26]. Thus, this study confirms the importance of the evaluation of a patient's nutritional status and nutritional intervention before surgery.
To improve the outcome of cholangiocarcinoma surgery, the nutritional screening tool designated especially for cholangiocarcinoma patients is warranted. A preoperative nutritional support model, either high-protein oral supplementation or parenteral nutrition for patients with preexisting malnutrition or a high CONUT score is necessary and needed validation as studies in other gastrointestinal malignancies and hepatocellular carcinoma [39,40].
There are several limitations to this study. First, it is a retrospective study, and the number of patients is relatively small, especially patients with CONUT 5–12, and there was no external validation. Thus, the results should be interpreted with caution. Second, we did not compare the performance to predict the outcome between other immune-nutritional scores such as prognostic nutritional index (PNI) or neutrophil-lymphocyte ratio (NLR). Third, most of the patients did not receive adjuvant treatment since it was not a standard treatment at the time.
5. Conclusion
This finding indicated that the preoperative CONUT score is a simple tool to evaluate the nutritional status of iCCA patients undergoing curative resection by hepatectomy, and a high CONUT score is strongly associated with a poor prognosis.
Funding
None.
Author contribution statement
Attapol Titapun; Aumkhae Sookprasert Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yanin Sripanuskul: Conceived and designed the experiments; Performed the experiments.
Piyakarn Watcharenwong: Performed the experiments.
Watcharin Loilome; Tharatip Srisuk: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Prin Twinprai: Performed the experiments; Wrote the paper.
Piya Prajumwongs: Analyzed and interpreted the data; Wrote the paper.
Jarin Chindaprasirt: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
The authors do not have permission to share data.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Banales J.M., Marin J.J.G., Lamarca A., Rodrigues P.M., Khan S.A., Roberts L.R., et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020;17(9):557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bridgewater J., Fletcher P., Palmer D.H., Malik H.Z., Prasad R., Mirza D., et al. Long-term outcomes and exploratory analyses of the randomized phase III BILCAP study. J. Clin. Oncol. 2022;40(18):2048–2057. doi: 10.1200/JCO.21.02568. [DOI] [PubMed] [Google Scholar]
- 3.Primrose J.N., Fox R.P., Palmer D.H., Malik H.Z., Prasad R., Mirza D., et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 4.Jusakul A., Cutcutache I., Yong C.H., Lim J.Q., Huang M.N., Padmanabhan N., et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017;7(10):1116–1135. doi: 10.1158/2159-8290.CD-17-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth G.S., Neuzillet C., Sarabi M., Edeline J., Malka D., Lievre A. Cholangiocarcinoma: what are the options in all comers and how has the advent of molecular profiling opened the way to personalised medicine. Eur. J. Cancer. 2023;179:1–14. doi: 10.1016/j.ejca.2022.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Kongpetch S., Jusakul A., Lim J.Q., Ng C.C.Y., Chan J.Y., Rajasegaran V., et al. Lack of targetable FGFR2 fusions in endemic fluke-associated cholangiocarcinoma. JCO Glob Oncol. 2020;6:628–638. doi: 10.1200/GO.20.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh D.Y., Lee K.H., Lee D.W., Yoon J., Kim T.Y., Bang J.H., et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol. 2022;7(6):522–532. doi: 10.1016/S2468-1253(22)00043-7. [DOI] [PubMed] [Google Scholar]
- 8.Vogel A., Bridgewater J., Edeline J., Kelley R.K., Klumpen H.J., Malka D., et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023;34(2):127–140. doi: 10.1016/j.annonc.2022.10.506. [DOI] [PubMed] [Google Scholar]
- 9.Lee D.U., Wang E., Fan G.H., Hastie D.J., Addonizio E.A., Chou H., et al. Malnutrition as a risk factor of adverse postoperative outcomes in patients undergoing hepatic resection: analysis of US hospitals. Br. J. Nutr. 2021:1–9. doi: 10.1017/S0007114521003809. [DOI] [PubMed] [Google Scholar]
- 10.Limpawattana P., Theerakulpisut D., Wirasorn K., Sookprasert A., Khuntikeo N., Chindaprasirt J. The impact of skeletal muscle mass on survival outcome in biliary tract cancer patients. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0204985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardito F., Coppola A., Rinninella E., Razionale F., Pulcini G., Carano D., et al. Preoperative assessment of skeletal muscle mass and muscle quality using computed tomography: incidence of sarcopenia in patients with intrahepatic cholangiocarcinoma selected for liver resection. J. Clin. Med. 2022;11(6) doi: 10.3390/jcm11061530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ardito F., Lai Q., Rinninella E., Mimmo A., Vellone M., Panettieri E., et al. The impact of personalized nutritional support on postoperative outcome within the enhanced recovery after surgery (ERAS) program for liver resections: results from the NutriCatt protocol. Updates Surg. 2020;72(3):681–691. doi: 10.1007/s13304-020-00787-6. [DOI] [PubMed] [Google Scholar]
- 13.Sungurtekin H., Sungurtekin U., Balci C., Zencir M., Erdem E. The influence of nutritional status on complications after major intraabdominal surgery. J. Am. Coll. Nutr. 2004;23(3):227–232. doi: 10.1080/07315724.2004.10719365. [DOI] [PubMed] [Google Scholar]
- 14.Tangvik R.J., Tell G.S., Eisman J.A., Guttormsen A.B., Henriksen A., Nilsen R.M., et al. The nutritional strategy: four questions predict morbidity, mortality and health care costs. Clin Nutr. 2014;33(4):634–641. doi: 10.1016/j.clnu.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Cornide-Petronio M.E., Alvarez-Mercado A.I., Jimenez-Castro M.B., Peralta C. Current knowledge about the effect of nutritional status, supplemented nutrition diet, and gut microbiota on hepatic ischemia-reperfusion and regeneration in liver surgery. Nutrients. 2020;12(2) doi: 10.3390/nu12020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walcott-Sapp S., Billingsley K.G. Preoperative optimization for major hepatic resection. Langenbeck's Arch. Surg. 2018;403(1):23–35. doi: 10.1007/s00423-017-1638-x. [DOI] [PubMed] [Google Scholar]
- 17.He H., Guo W., Song P., Liu L., Zhang G., Wang Y., et al. Preoperative systemic immune-inflammation index and prognostic nutritional index predict prognosis of patients with pulmonary neuroendocrine tumors after surgical resection. Ann. Transl. Med. 2020;8(10):630. doi: 10.21037/atm-19-4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudou K., Nakashima Y., Haruta Y., Nambara S., Tsuda Y., Kusumoto E., et al. Comparison of inflammation-based prognostic scores associated with the prognostic impact of adenocarcinoma of esophagogastric junction and upper gastric cancer. Ann. Surg Oncol. 2021;28(4):2059–2067. doi: 10.1245/s10434-020-08821-y. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z., Shi H., Chen L. Prognostic role of pre-treatment C-reactive protein/albumin ratio in esophageal cancer: a meta-analysis. BMC Cancer. 2019;19(1):1161. doi: 10.1186/s12885-019-6373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Liu Y., Mi X., Shao M., Liu L. The prognostic value of prognostic nutritional index (PNI) and neutrophil to lymphocyte ratio (NLR) for advanced non-small cell lung cancer treated with platinum-based chemotherapeutics. Ann. Palliat. Med. 2020;9(3):967–978. doi: 10.21037/apm.2020.04.31. [DOI] [PubMed] [Google Scholar]
- 21.Yu J., Liu H., Zeng X., Zhao Y., Jiang D., Lu H., et al. Prognostic and clinicopathological significance of C-reactive protein/albumin ratio (CAR) in patients with gastric cancer: a meta-analysis. PLoS One. 2021;16(4) doi: 10.1371/journal.pone.0250295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Cui P., Pang Q., Wang Y., Qian Z., Hu X., Wang W., et al. Nutritional prognostic scores in patients with hilar cholangiocarcinoma treated by percutaneous transhepatic biliary stenting combined with 125I seed intracavitary irradiation: a retrospective observational study. Medicine (Baltim.) 2018;97(22) doi: 10.1097/MD.0000000000011000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ignacio de Ulibarri J., Gonzalez-Madrono A., de Villar N.G., Gonzalez P., Gonzalez B., Mancha A., et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005;20(1):38–45. [PubMed] [Google Scholar]
- 24.Iseki Y., Shibutani M., Maeda K., Nagahara H., Ohtani H., Sugano K., et al. Impact of the preoperative controlling nutritional status (CONUT) score on the survival after curative surgery for colorectal cancer. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyata T., Yamashita Y.I., Higashi T., Taki K., Izumi D., Kosumi K., et al. The prognostic impact of controlling nutritional status (CONUT) in intrahepatic cholangiocarcinoma following curative hepatectomy: a retrospective single institution study. World J. Surg. 2018;42(4):1085–1091. doi: 10.1007/s00268-017-4214-1. [DOI] [PubMed] [Google Scholar]
- 26.Takagi K., Yagi T., Umeda Y., Shinoura S., Yoshida R., Nobuoka D., et al. Preoperative controlling nutritional status (CONUT) score for assessment of prognosis following hepatectomy for hepatocellular carcinoma. World J. Surg. 2017;41(9):2353–2360. doi: 10.1007/s00268-017-3985-8. [DOI] [PubMed] [Google Scholar]
- 27.Wang A., Sun B., Wang M., Shi H., Huang Z., He T., et al. Predictive value of CONUT score combined with serum CA199 levels in postoperative survival of patients with pancreatic ductal adenocarcinoma: a retrospective study. PeerJ. 2020;8 doi: 10.7717/peerj.8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W., Li M., Wang T., Ma G., Deng Y., Pu D., et al. Controlling Nutritional Status (CONUT) score is a prognostic factor in patients with resected breast cancer. Sci. Rep. 2020;10(1):6633. doi: 10.1038/s41598-020-63610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao Y.S., Hao S.J., Zou C.F., Xie Z.B., Fu D.L. Controlling Nutritional Status score is superior to Prognostic Nutritional Index score in predicting survival and complications in pancreatic ductal adenocarcinoma: a Chinese propensity score matching study. Br. J. Nutr. 2020;124(11):1190–1197. doi: 10.1017/S0007114520002299. [DOI] [PubMed] [Google Scholar]
- 30.Wang A., He Z., Cong P., Qu Y., Hu T., Cai Y., et al. Controlling nutritional status (CONUT) score as a new indicator of prognosis in patients with hilar cholangiocarcinoma is superior to NLR and PNI: a single-center retrospective study. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.593452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel P.G., Selvarajah S., Boursalie S., How N.E., Ejdelman J., Guerard K.P., et al. Preparation of formalin-fixed paraffin-embedded tissue cores for both RNA and DNA extraction. J. Vis. Exp. 2016;114 doi: 10.3791/54299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagtegaal I.D., Odze R.D., Klimstra D., Paradis V., Rugge M., Schirmacher P., et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edge S.B., Compton C.C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 34.Sa-Ngiamwibool P., Aphivatanasiri C., Sangkhamanon S., Intarawichian P., Kunprom W., Thanee M., et al. Modification of the AJCC/UICC 8th edition staging system for intrahepatic cholangiocarcinoma: proposal for an alternative staging system from cholangiocarcinoma-prevalent Northeast Thailand. HPB (Oxford) 2022;24(11):1944–1956. doi: 10.1016/j.hpb.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Borre M., Dam G.A., Knudsen A.W., Gronbaek H. Nutritional status and nutritional risk in patients with neuroendocrine tumors. Scand. J. Gastroenterol. 2018;53(3):284–292. doi: 10.1080/00365521.2018.1430848. [DOI] [PubMed] [Google Scholar]
- 36.Li D., Yuan X., Liu J., Li C., Li W. Prognostic value of prognostic nutritional index in lung cancer: a meta-analysis. J. Thorac. Dis. 2018;10(9):5298–5307. doi: 10.21037/jtd.2018.08.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwegler I., von Holzen A., Gutzwiller J.P., Schlumpf R., Muhlebach S., Stanga Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br. J. Surg. 2010;97(1):92–97. doi: 10.1002/bjs.6805. [DOI] [PubMed] [Google Scholar]
- 38.Zheng H.L., Lu J., Li P., Xie J.W., Wang J.B., Lin J.X., et al. Effects of preoperative malnutrition on short- and long-term outcomes of patients with gastric cancer: can we do better? Ann. Surg Oncol. 2017;24(11):3376–3385. doi: 10.1245/s10434-017-5998-9. [DOI] [PubMed] [Google Scholar]
- 39.Bozzetti F., Gavazzi C., Miceli R., Rossi N., Mariani L., Cozzaglio L., et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. JPEN J Parenter Enteral Nutr. 2000;24(1):7–14. doi: 10.1177/014860710002400107. [DOI] [PubMed] [Google Scholar]
- 40.Fan S.T., Lo C.M., Lai E.C., Chu K.M., Liu C.L., Wong J. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N. Engl. J. Med. 1994;331(23):1547–1552. doi: 10.1056/NEJM199412083312303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.