Abstract
Butene-1 is one of the most important petrochemical industry products that is produced in different ways. Ethylene is an important source of Butene-1 production through the oligomerization process. In this study, to reduce the by-product of the polymer produced and to improve the catalyst yield, the dicyclopentyldimethoxysilane (DCPDS) modifier in the presence of a homogeneous titanium tetra butoxide/triethyl aluminum catalyst and a combination of dichloromethane (as a promoter) in a high-pressure Buchi reactor were used. Gas chromatography was used for liquid and gas phase analysis in the reactor. The design of experiments was performed with the Box-Behnken design technique (BBD) based on the response surface method (RSM). In this method, four effective factors of catalyst concentration, modifier, promoter, and temperature were evaluated. The results of the analysis of variance for the answers of ethylene conversion rate, selectivity, polymer production rate, and yield showed that there is a good agreement between the actual values and the values obtained from the model. Optimization using design expert software showed values of 85.6, 88.5, 2.43, and 75.78% for ethylene conversion rate, selectivity, polymer content and yield, respectively, which showed an error of less than one percent compared to the laboratory results. Comparison of the catalyst performance with and without DCPDS and DCM showed that the presence of these two compounds together with the catalyst, in addition to increasing by 4, 6, and 9% for conversion, selectivity of Butene-1 and yield, respectively, reduced the production of the undesirable polymer in the reactor from 136 mg to 2.4 mg.
Keywords: Butene-1, Homogeneous catalyst, Dimerization, ethylene conversion rate, Box-Behnken design
Terminology and abbreviations:
| Term | Chemical formula | Abb. |
|---|---|---|
| Titanium Tetrabutoxide | Ti(C4H9O)4 | TTB |
| Tetrahydrofuran | C4H8O | THF |
| Triethylaluminum | C6H15Al | TEA, AlEt3 |
| Dicyclopentyldimethoxysilane | C12H24O2Si | DCPDS |
| Dichloromethane | CH2Cl2 | DCM |
1. Introduction
In recent decades, one of the strategic areas in petrochemical processes has been the development of production processes of linear alpha olefins (LAOs) through ethylene oligomerization. Butene-1, the first member of the LAOs, is most widely used as an ethylene comonomer for the production of polyethylene resins (LLDPE and HDPE) with strength and resistance to stress and failure, which accounts for about 80% of the market [[1], [2], [3]].
The most important industrial sources of butene-1 production are crude oil refining operation, fission of four carbon (C4) hydrocarbons by steam, ethylene oligomerization processes, ethylene dimerization process, separation of C4 section output from olefin unit or butadiene unit, butene dehydrogenation, butyl alcohol dehydrogenation, pyrolysis of butyl acetate and butyl chloride [[4], [5], [6], [7]].
In the catalytic dimerization method of ethylene for the production of butene-1, the main goal is to produce long-chain polymers through the growth reaction of aluminum compounds [7,8]. In general, homogeneous and heterogeneous catalytic systems are used in this process. Heterogeneous active sites play the role of a ligand in a coordinated metal atom in a homogeneous system. Various compounds such as nickel, palladium, titanium, rhodium, zirconium, tantalum, and cobalt with (or without) alkyl of aluminum, with (or without) hydrogen atoms, and base (or baseless) are used as catalysts [[9], [10], [11], [12]].
Palladium complex, especially PdCl2, under the influence of the solvent used, easily converts ethylene to dimer butene. However, in a series of polar and non-polar solvents, no dimerization reaction occurs and the reaction proceeds gently using only oxygen-containing solvents [13].
In a study, Kusunoki et al. [14] observed a similar effect of the solvent and its selectivity to butenes. It was found that selectivity decreases in the following order: acetic acid > ethylene dichloride > benzene > chloroform > ethanol.
The dichloro-bis (benzonitrile) complex of palladium (II) in benzene solvent catalyzes dimerization exclusively to a mixture of butenes (4% butene-1, 36% cis-butene-2, and 60% transbutene-2). The reaction rate is the first order concerning ethylene [13].
Organic complexes of chromium (III) phosphine and ethyl aluminum dichloride were reported for ethylene dimerization with good conversion and selectivity. A catalytic system consisting of CrCl3(Py)3 and EtAlCl2 was used for ethylene dimerization and a mixture of butenes with a selectivity of 83% was obtained, of which 50% was butene-1 and the rest was butene-cis and trans [15].
Reaction rate between triethyl aluminum and ethylene in a range of operating conditions (temperature 80–220 °C, pressure 1–9 atm, catalyst 1–10 wt%) according to the concentration of soluble ethylene in the liquid phase and the composition of triethyl aluminum, is the first order. The reaction rate increases with increasing temperature, although it is slow at temperatures below 120 °C. Under these conditions, butene-1 is the main product in the gas phase [16].
In the case of titanium (IV) alkoxide catalysts in conjunction with aluminum trialkyl as the homogeneous selective catalyst effective in the ethylene-butene-1 dimerization reaction, triethyl aluminum should be part of the active site of the titanium homogeneous catalyst. The most important factors that affect the reaction activity and selectivity of homogeneous titanium catalytic systems for the production of butene-1 are the Al/Ti molar ratio, titanate complex concentration, nature of the modifier, amount of promoters, the pressure of ethylene, reaction temperature and reaction time [[17], [18], [19]].
Due to the nature of the process, the production of ethylene dimerization on both laboratory and industrial scales is accompanied by the production of the undesirable polymer. The rate of polymer formation will vary depending on the type of catalysts from different companies and the reactor process conditions [17,20,21]. The main problem of all catalysts is the high rate of polymer formation and the performance of adverse reactions. The formation of the polymer in the reactor and its outlets will reduce the activity of the catalyst, reduce the intensity of heat transfer, reduce the service life of the reactor and, consequently, decommission the unit and perform repairs and cleaning of all relevant equipment [22,23]. These will significantly reduce the profits of butene-1 production units and also impose high maintenance costs. In addition, decommissioning and opening of equipment will reduce the safety and risk of potential hazards for the operational unit [19,24].
Given the above, it is important to determine the conditions that improve the process selectivity and reduce the rate of polymer formation. In this research, as an applied study, the effect of dicyclopentyldimethoxysilane (DCPDS) modifier and dichloromethane (DCM) promoter on the performance of AlEt3–Ti(BuO)4 catalyst were investigated and the effect of these compounds on the conversion rate, selectivity, yield and the amount of polymer formed was investigated.
2. Materials and methods
2.1. Materials
In this study, titanium tetra butoxide, dichloromethane, triethyl aluminum and DCPDS were purchased as catalyst components from Merck. Normal heptane (as solvent) was obtained from Merck Company and ethylene gas (purity of 99.9%) was supplied from Jam Petrochemical Company.
2.2. Equipment used
A 1000 cc stainless steel Buchi reactor was used to perform the dimerization reaction of ethylene to butene-1 by a titanium catalyst in the homogeneous phase. The vessel reaction is an SS316 tank with a design pressure of 60 bar and a design temperature of 250 °C, which was equipped with temperature and pressure sensors and gas inlet and outlet paths. Also, the second wall of the reactor (thermal jacket) was made of SS304, which heats/cools the tank with the help of a fluid heat exchanger (usually an oil circulator). The reactor system was equipped with the catalyst and chemical injection system, gas flow measurement system (MFC), and pressure safety valves for emergency pressure relief (set pressure 60 bar). The flow diagram of the reactor system is shown in Fig. 1.
Fig. 1.
Schematic of the laboratory set-up of the reactor used.
All stages of the catalyst sample preparation were performed under a nitrogen gas atmosphere. For this purpose, the reactor was heated to 30–70 °C for 30 min to remove water, air and impurities. It was then cooled to ambient temperature and dried by nitrogen and then ethylene was poured into the system for 5–10 min. Then, the following steps were performed: injecting 400 ml of the solvent into the reactor and then adjusting the circulator temperature (52–55 °C), preparing the main catalyst sample and injecting it into the reactor in the amount of 0.5–1 ml, injecting hexane solution containing triethyl aluminum (TEA) under nitrogen atmosphere to the reactor (TEA concentration in hexane is 25 wt%). The molar ratio of TEA to the catalyst was also considered, and the reactor pressure was 17 bar.
The reaction started with the stirrer start and ended after 15–20 min. The discharged liquid sample was first washed with alcohol and hydrochloric acid to remove the remaining titanium and triethyl aluminum particles, and then the resulting liquid sample was distilled. The distilled product was then injected into the agilent model GC for analysis. All tests of gas exiting from the reactor have been done with a GC-450 Varian (The method for Hydrocarbons and impurities was ASTM D6159 and ASTM D4429, respectively). In each batch, the polymers formed on the reactor wall and around the stirrer blade were collected and washed with hexane or heptane. Then it was dried in a vacuum oven at 100 °C. The percentage of conversion and selectivity of the products were calculated from the mass balance of ethylene consumption, the weight of gaseous and liquid products and the volume of gas.
2.3. Experimental design
To design the experiments, effective input and output parameters of the reaction including the molar ratio of trimethylaluminium to titanium complex (Al/Ti molar ratio), the molar ratio of the new modifiers to the titanium complex (DCPDS/Ti), promoter molar ratio to titanium complex (DCM/Ti), and the reaction temperature were considered. The test results were also used to obtain the catalyst yield (%Yield), the ethylene conversion rate (%Conversion), the amount of polymer formed (PE, mg), and the selectivity (%).
Design Expert 11 software and the RSM method were used. Based on this, the Box-Behnken design method [19,24] with the information in Table 1 was used.
Table 1.
Input parameters and levels used in the BBD method.
| Independent Parameters | Lower level (−1) | Mid-level (0) | Higher level (+1) |
|---|---|---|---|
| A: Al/Ti Ratio (mol/mol) | 3 | 5 | 7 |
| B: DCPDS/Ti Ratio (mol/mol) | 3 | 5 | 7 |
| C: DCM/Ti Ratio (mol/mol) | 3 | 5 | 7 |
| D: Temperature, () | 50 | 55 | 60 |
27 experiments were performed to design the experiments by the design expert software. To test the repeatability of the results, each experiment was repeated three times with intermediate levels of input parameters.
3. Results and discussion
Table 2 reports the conditions of the 27 experiments and the response parameters. The results of the analysis of variance to determine the rate of ethylene conversion, selectivity, the polymer produced and catalyst yield are shown in Table 3.
Table 2.
Description of experiments and results for the four response parameters examined.
| Run | Al/Ti ratio (mol/mol) | DCPDS/Ti ratio (mol/mol) | DCM/Ti ratio (mol/mol) | Temperature () | Ethylene Conversion, (%) | Selectivity, (%) | PE production, (mg) | Yield, (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 55 | 80 | 85 | 2.0 | 68.00 |
| 2 | 5 | 3 | 7 | 55 | 68 | 81 | 3.5 | 55.08 |
| 3 | 7 | 3 | 5 | 55 | 84 | 77 | 1.5 | 64.68 |
| 4 | 5 | 5 | 5 | 55 | 86 | 88 | 2.3 | 75.68 |
| 5 | 5 | 7 | 7 | 55 | 82 | 84 | 3.7 | 68.88 |
| 6 | 5 | 5 | 7 | 60 | 69 | 87 | 3.5 | 60.03 |
| 7 | 3 | 7 | 5 | 55 | 83 | 81 | 2.5 | 67.23 |
| 8 | 7 | 7 | 5 | 55 | 88 | 83 | 4.6 | 73.04 |
| 9 | 7 | 5 | 7 | 55 | 76 | 84 | 3.2 | 63.84 |
| 10 | 7 | 5 | 5 | 60 | 74 | 87 | 3.5 | 64.38 |
| 11 | 5 | 5 | 5 | 55 | 84 | 88.5 | 2.1 | 74.34 |
| 12 | 7 | 5 | 5 | 50 | 75 | 80 | 2.9 | 60.00 |
| 13 | 7 | 5 | 3 | 55 | 85 | 78 | 3.3 | 66.30 |
| 14 | 5 | 7 | 3 | 55 | 77 | 82 | 4.3 | 63.14 |
| 15 | 3 | 5 | 3 | 55 | 68 | 77 | 2.1 | 52.36 |
| 16 | 5 | 3 | 5 | 60 | 66 | 84 | 4.3 | 55.44 |
| 17 | 5 | 5 | 3 | 50 | 65 | 80 | 2.5 | 52.00 |
| 18 | 5 | 3 | 3 | 55 | 73 | 75 | 2.4 | 54.75 |
| 19 | 3 | 3 | 5 | 55 | 69 | 79 | 4.0 | 54.51 |
| 20 | 5 | 3 | 5 | 50 | 65 | 81 | 4.2 | 52.65 |
| 21 | 5 | 5 | 7 | 50 | 66 | 86 | 5.2 | 56.76 |
| 22 | 5 | 7 | 5 | 50 | 71 | 83 | 5.0 | 58.93 |
| 23 | 3 | 5 | 5 | 50 | 62 | 86.5 | 2.4 | 53.63 |
| 24 | 5 | 7 | 5 | 60 | 81 | 82 | 4.4 | 66.42 |
| 25 | 5 | 5 | 5 | 55 | 86 | 88 | 2.8 | 75.68 |
| 26 | 3 | 5 | 5 | 60 | 78 | 80 | 2.6 | 62.40 |
| 27 | 5 | 5 | 3 | 60 | 72 | 79 | 4.9 | 56.88 |
Table 3.
Analysis of variance of designed test results.
| Source | Ethylene Conversion |
Selectivity |
PE production |
Yield |
||||
|---|---|---|---|---|---|---|---|---|
| F-value | p-value | F-value | p-value | F-value | p-value | F-value | p-value | |
| Model | 36.30 | <0.0001 | 13.73 | <0.0001 | 8.86 | 0.0003 | 52.08 | <0.0001 |
| A-Al/Ti | 51.25 | <0.0001 | 0.0116 | 0.9160 | 4.71 | 0.0508 | 50.06 | <0.0001 |
| B-DCPDS/Ti | 75.57 | <0.0001 | 15.06 | 0.0022 | 11.88 | 0.0048 | 157.63 | <0.0001 |
| C-DCM/Ti | 0.0291 | 0.8675 | 60.22 | <0.0001 | 1.47 | 0.2485 | 31.74 | 0.0001 |
| D-Temp | 37.66 | <0.0001 | 0.2904 | 0.5998 | 0.9168 | 0.3572 | 42.91 | <0.0001 |
| AB | 8.72 | 0.0121 | 2.23 | 0.1611 | 25.87 | 0.0003 | 2.45 | 0.1432 |
| AC | 38.44 | <0.0001 | 0.5576 | 0.4696 | 0.0000 | 1.0000 | 42.28 | <0.0001 |
| AD | 25.19 | 0.0003 | 25.41 | 0.0003 | 0.1956 | 0.6662 | 2.49 | 0.1407 |
| BC | 5.58 | 0.0359 | 2.23 | 0.1611 | 2.40 | 0.1476 | 3.78 | 0.0758 |
| BD | 7.06 | 0.0209 | 2.23 | 0.1611 | 1.76 | 0.2093 | 2.85 | 0.1171 |
| CD | 1.39 | 0.2605 | 0.5576 | 0.4696 | 20.55 | 0.0007 | 0.3346 | 0.5737 |
| A2 | 5.43 | 0.0381 | 39.08 | <0.0001 | 2.41 | 0.1463 | 50.73 | <0.0001 |
| B2 | 27.33 | 0.0002 | 55.31 | <0.0001 | 20.93 | 0.0006 | 107.39 | <0.0001 |
| C2 | 80.59 | <0.0001 | 37.74 | <0.0001 | 6.09 | 0.0296 | 191.35 | <0.0001 |
| D2 | 223.31 | <0.0001 | 5.62 | 0.0353 | 25.22 | 0.0003 | 312.24 | <0.0001 |
| Lack of Fit | 2.38 | 0.3318 | 25.63 | 0.0381 | 1.69 | 0.4287 | 3.68 | 0.2323 |
| R2 | 0.9769 | 0.9412 | 0.9118 | 0.9838 | ||||
| Adjusted R2 | 0.9500 | 0.8727 | 0.8090 | 0.9649 | ||||
| Predicted R2 | 0.8734 | 0.7631 | 0.7249 | 0.9097 | ||||
3.1. Investigation of ethylene conversion rate
According to the variance analysis for the conversion rate, the parameters A, B, D, AB, AC, AD, BC, BD, A2, B2, C2, and D2 are significant. Based on the results, of analysis and design, the obtained model for the conversion rate is as Eq. (1):
| (1) |
The predicted R2 of 0.8734 in Table 3 is in reasonable agreement with the adjusted R2 of 0.9500; i.e. the difference is less than 0.2.
Predicted R-squared is a measure of how well the model predicts a response value. It is computed as Eq. (2):
| (2) |
Where PRESS stands for the Predicted Residual Sum of squares. R-squared adjusted for the number of parameters in the model relative to the number of points in the design. A measure of the amount of variation about the mean explained by the model. The Adjusted R-squared is obtained Eq. (3):
| (3) |
The adjusted R-squared and predicted R-squared should be within approximately 0.20 of each other to be in “reasonable agreement”. If they do not exist, there may be a problem with the data or the model.
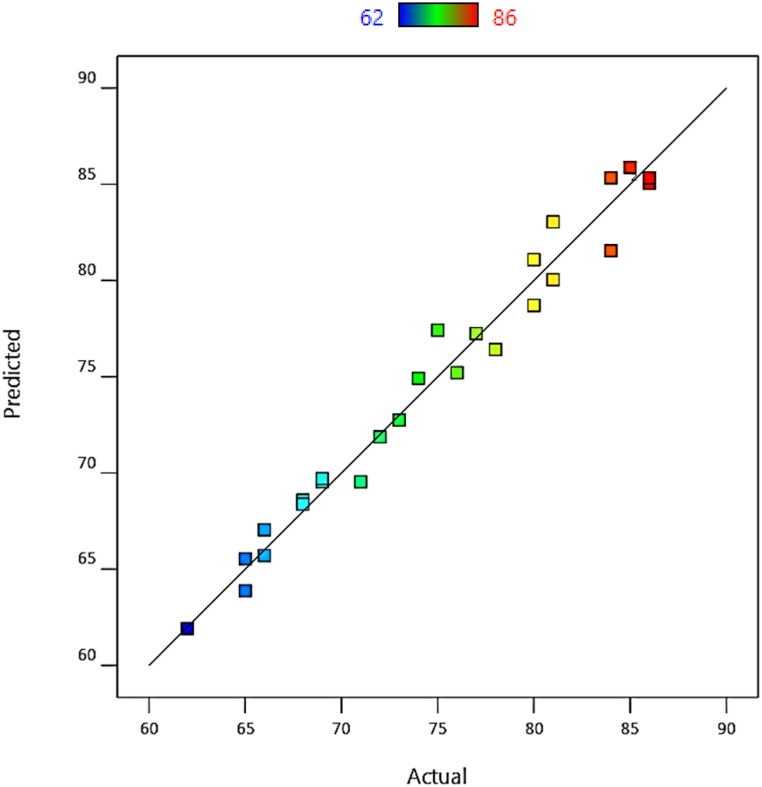
The value of R2 for the conversion rate is 0.9769, which indicates the acceptable accuracy of the model. Fig. 2 shows the predicted values of the ethylene conversion rate in terms of the actual values obtained from the experiments. The closer the points are to the line y = x, the higher the model accuracy. Therefore, the model obtained in Eq. (1) can predict the conversion rate of ethylene with high accuracy.
Fig. 2.
Predicted conversion rate of ethylene to actual values, T = 50–60 °C, Al/Ti = 3–7, DCPDS/Ti = 3–7, DCM/Ti = 3–7.
Fig. 3 shows the effect of input parameters on the ethylene conversion rate. According to these graphs, at DCPDS/Ti = 3–5 ratios, ethylene conversion increases with increasing Al/Ti ratio. But in ratios higher than DCPDS/Ti = 5, the amount of ethylene conversion will decrease with the increase of the Al/Ti ratio. In a constant ratio of DCM/Ti = 5 and a constant temperature of 55 °C, as well as a constant ratio of Al/Ti, the amount of ethylene conversion increases continuously with an increase in the DCPDS/Ti ratio. It should be noted that this increase was seen for low ratios of Al/Ti = 3–5, but the trend is different for ratios higher than Al/Ti = 5.
Fig. 3.
Ethylene conversion rate changes according to a) temperature and DCM/Ti ratio, b) DCPDS/Ti ratio and Al/Ti ratio, and c) all parameters.
By increasing the DCPDS/Ti ratio up to 5, the conversion rate increases, but at values higher than Al/Ti = 5 and by increasing the DCPDS/Ti ratio, the ethylene conversion decreases. At a fixed DCM/Ti = 5 ratio and a fixed temperature of 55 °C, Al/Ti = 5 and DCPDS/Ti = 5 ratios, the maximum ethylene conversion is obtained.
As a Lewis acid, triethylaluminum plays the role of releasing the free coordination sites in the titanate complex, recovering the electron density around the central titanium metal, and producing one or more Ti–C bonds by exchanging alkyl groups with the alkoxide groups of the titanate complex. In dimerization reactions without promoters, the dimer decomposition constant to triethylaluminum monomer is very slight and only dimers participate in the reaction. Chloro geminal groups in DCM prefer to react with triethylaluminum, which is usually known as a dimer with two ethyl bridge bonds and two Al Lewis acid sites with a specific arrangement, and monomers of triethyl aluminum are produced. It is expected that the presence of triethylaluminum monomers is effective for the formation of active titanium species, as a result of which the improvement of catalytic activity is observed with the performance of triethylaluminum monomers [25].
The selectivity of butene-1 depends on the number of chlorine groups in the promoting compound. One of the reasons for the formation of heavy compounds in the ethylene dimerization reaction can be considered due to be the participation and re-reaction of butene-1 in the catalytic reaction cycle, as a result, the decrease in the selectivity of butene-1 with the increase in the number of chlorine groups (increasing the DCM/Ti ratio) should be attributed to the effective interaction of the chlorine compound with the active species in the reaction. Therefore, based on the rational confirmed reaction schemes and the chemical species involved in the catalytic cycle, it can be shown that the rapid and easy decomposition of dimeric triethylaluminum into monomer type, which is supposed to affect the formation of an increasing number of active titanium species, as one of the roles suggested the chemical importance of chlorine compounds in the ethylene dimerization catalyst system.
3.2. Selectivity
Another important parameter in the study of reactions is selectivity. A catalyst with a higher selectivity can produce a higher desired amount of product than other by-products. Analysis of variance based on the results obtained for selectivity according to Table 2 shows that the model is significant and the terms B, C, AD, A2, B2, C2, and D2 are significant in the model. Also, the value of R2 equal to 0.9412 indicates the acceptable accuracy of the obtained model. The resulting model equation for the selectivity of the catalyst used is as follows:
| (4) |
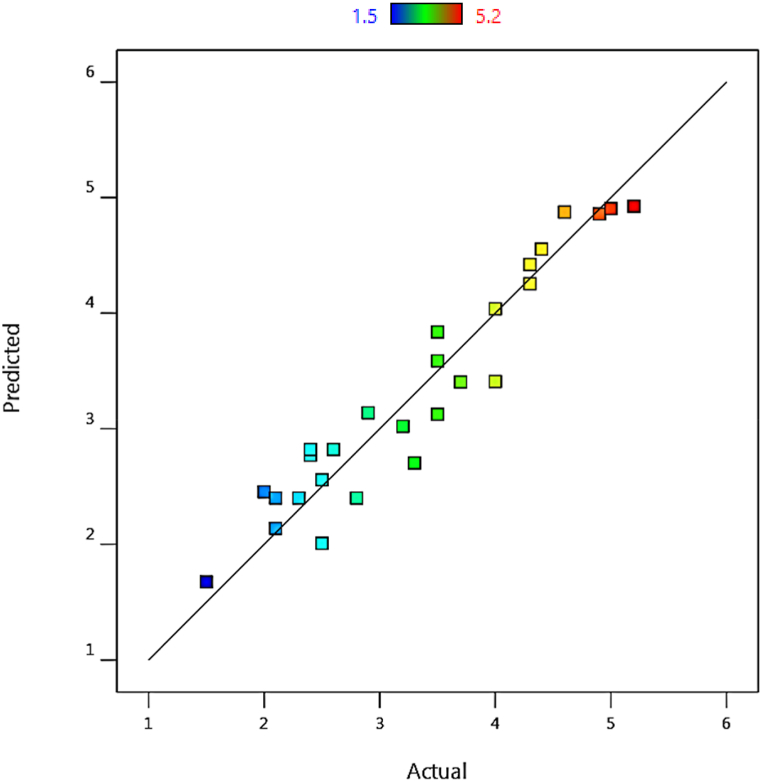
A comparison of the predictable selectivity values with Eq. (4) compared to the actual values obtained from the design of the experiments (as shown in Table 2) is shown in Fig. 4. It is observed that the points are reasonably close to the line y = x and therefore confirm the model's accuracy. Fig. 5 shows the selectivity changes according to the studied parameters. According to these graphs, the selectivity for all parameters is first ascending and then descending. Therefore, despite the higher conversion rate of Al/Ti and DCPDS/Ti parameters, there is a decrease in selectivity. But for DCM/Ti and temperature variables, the highest selectivity is still observed in moderate values.
Fig. 4.
Predicted selectivity values compared to actual values, T = 50–60 °C, Al/Ti = 3–7, DCPDS/Ti = 3–7, DCM/Ti = 3-7.
Fig. 5.
Selectivity changes according to a) temperature and DCM/Ti ratio, b) DCPDS/Ti ratio and Al/Ti ratio, and c) all parameters.
3.3. The amount of polymer formed
One of the problems in dimerization processes is the expansion of the reaction chain and production of polyethylene polymer inside the reactor. This phenomenon, on the one hand, causes the consumption of raw material to produce an undesirable product, and on the other hand, sticking the polymer to the mixer and the container wall, may cause clogged ducts and prevent the mixing of the reactor contents. Therefore, the reduction of polymer content was investigated as an output parameter. Analysis of variance of the amount of polymer produced according to Table 2 indicates the significance of the model B, AB, CD, B2, C2, and D2 are the significant terms of this model. A value of R2 equal to 0.9118 indicates good model accuracy. Also, the predicted R2 of 0.7249 is in reasonable agreement with the Adjusted R2 of 0.8090. The model equation obtained for the produced polymer is as Eq. (5):
| (5) |
The predicted polymer values in terms of the actual values obtained from the experiments are shown in Fig. 6, which have a relatively acceptable agreement. Also, Fig. 7 shows the effect of the studied parameters on the amount of polymer produced. According to this diagram, the produced polymer first decreases and then increases according to the parameters of DCPDS/Ti, DCM/Ti ratio, and temperature. Therefore, in the middle values of these parameters, the least amount of polymer will be formed, which is desirable. For the Al/Ti factor, first the amount of polymer produced increases and then decreases, so at the higher limit of this factor, the higher the conversion rate, the less selectivity and the amount of polymer produced will be less. At the low Al/Ti limit, the amount of polymer produced is lower than at other levels but is not recommended due to the low conversion rate under these conditions.
Fig. 6.
Predicted production polymer values in terms of actual values, T = 50–60 °C, Al/Ti = 3–7, DCPDS/Ti = 3–7, DCM/Ti = 3-7.
Fig. 7.
Changes in the amount of polymer produced according to a) temperature and DCM/Ti ratio, b) DCPDS/Ti ratio and Al/Ti ratio, and c) all parameters.
3.4. Catalyst efficiency (yield)
The last factor to be considered is the catalyst yield, calculated from Eq. (6):
| (6) |
This parameter is obtained by multiplying the conversion rate and selectivity and thus can be considered a major factor. Analysis of variance for catalyst yield has a p-value less than 0.0001 and is therefore significant. Terms A, B, C, D, AC, A2, B2, C2, and D2 will also be significant in the model. A value of R2 equal to 0.9838 indicates the high accuracy of the model. Also, the predicted R2 of 0.9097 is in reasonable agreement with the adjusted R2 of 0.9649. The equation obtained for catalyst yield is as Eq. (7):
| (7) |
Fig. 8 shows the yield values predicted based on the above equation versus the actual values in Table 3. In this diagram, the data are close enough to the line y = x, so the model has high accuracy in predicting the yield. Also, Fig. 9 shows the yield changes in terms of other parameters studied. According to this graph, the Yield value for all parameters first increases and then decreases. Therefore, the highest returns will occur around the median values of each of the factors.
Fig. 8.
Graph of predicted yield values in terms of actual values, T = 50–60 °C, Al/Ti = 3–7, DCPDS/Ti = 3–7, DCM/Ti = 3-7.
Fig. 9.
Changes in the yield produced according to a) temperature and DCM/Ti ratio, b) DCPDS/Ti ratio and Al/Ti ratio, and c) all parameters.
3.5. Determining the optimal parameters
After examining the quadratic models to predict the quadratic responses using a design expert software optimization tool, the optimal values of each factor to obtain the highest yield, conversion and selectivity values and the lowest amount of polymer produced were obtained in Table 4. Due to the small number of catalysts, modifiers and promoters, it is a little difficult to accurately control the concentration of materials in the optimal range. It can be concluded that the median values of each parameter were close to the optimal values obtained from the software.
Table 4.
Optimal values of factors under different conditions.
| Parameters |
Conventional Catalyst |
Model Results |
Experimental Results with Modifier and Promotor |
|||
|---|---|---|---|---|---|---|
| Input Factors | ||||||
| Al/Ti ratio | 5 | 4.67 | 5 | |||
| DCPDS/Ti ratio | 0 | 5.3 | 5 | |||
| DCM/Ti ratio | 0 | 5.3 | 5 | |||
| Temperature (oC) | 55 | 55.5 | 55 | |||
| Output Parameters | Run 4 | Run 11 | Run 25 | Average | ||
| Ethylene Conversion, % | 81.2 | 85.58 | 86 | 84 | 86 | 85.3 |
| Selectivity, % | 82.3 | 88.5 | 88 | 88.5 | 88 | 88.2 |
| PE production, mg | 136 | 2.43 | 2.3 | 2.1 | 2.8 | 2.4 |
| Yield, % | 66.1 | 75.78 | 75.68 | 74.34 | 75.68 | 75.2 |
The results of comparing the optimal catalyst proposed by the software with the results of the actual catalyst close to the optimal conditions (according to Table 4) show the high predictive power of the model. With this method, the optimal values of the studied factors were determined. Also, in Table 4, the results of experiments with the single catalyst, without the presence of the modifier and promoter, which were performed at 55 °C, are reported. According to these results, without the use of a modifier and promotor, the maximum ethylene conversion was about 81.2%, while in the new complex and with the presence of a modifier and promotor, the ethylene conversion increased to about 85.3%. With the presence of modifier and promotor, the selectivity of Butene-1 has increased from 82.3% to about 88.2%. The yield of the catalyst without modifier and promotor is about 66.1%. After using modifier and promotor, this value has increased to about 75.2%. The amount of polymer in the reaction in conventional systems without modifier and promotor has been obtained up to about 136 mg, which has been reduced to about 2.4 mg with the presence of modifier and promotor.
4. Conclusion
In this study, the process of producing butene-1 from ethylene in the presence of a homogeneous catalyst in a laboratory high-pressure Buchi reactor was investigated. For this purpose, ethylene was introduced at a temperature of 50–60 °C and a pressure of 17 bar in a reactor containing a liquid catalyst and a heptane solvent. DCPDS modifier and dichloromethane promoter were used to improve the catalyst performance. A Box-Behnken design method based on response surface methodology was used for experiments. Four factors affecting the process with three adjustable levels were studied. Process responses such as ethylene conversion rate, selectivity, polymer production and yield were all obtained from the results of gas and liquid sample analysis at the beginning and end of the reaction time. The optimal results obtained from the design of the experiments showed a very good agreement with the laboratory data. It can be concluded that by using a DCPDS modifier and dichloromethane promoter while achieving the desired catalyst yield, the amount of polymer formed, which is an undesirable product of the process, is greatly reduced.
Author contribution statement
Sajjad Bahrami Reyhan: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Seyed Mahdi Alavi: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Davood Soudbar: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Al-Jarallah A., et al. Ethylene dimerization and oligomerization to butene-1 and linear α-olefins: a review of catalytic systems and processes. Catal. Today. 1992;14(1):1–121. [Google Scholar]
- 2.Al-Sa'doun A.W. Dimerization of ethylene to butene-1 catalyzed by Ti (OR') 4-AlR3. Appl. Catal. Gen. 1993;105(1):1–40. [Google Scholar]
- 3.Platzer N. Branched polyethylene. LDPE and LLDPE. Ind. Eng. Chem. Prod. Res. Dev. 1983;22(1):158–160. [Google Scholar]
- 4.Behr A., Keim W. The nickel-complex catalyzed synthesis of α-olefins. Arabian J. Sci. Eng. 1985;10(4):377–390. [Google Scholar]
- 5.Job R. Google Patents; 2002. Magnesium-zirconium Alkoxide Complexes and Polymerization Catalysts Made Therefrom. [Google Scholar]
- 6.Jolly P.W. Elsevier; 2012. The Organic Chemistry of Nickel: Organonickel Complexes. [Google Scholar]
- 7.Alenezi H., et al. Recent developments on ethylene dimerization with focus on alphabutol optimization. Int. J. Innovative Technol. Explor. Eng. 2019;8:3969–3975. [Google Scholar]
- 8.Xu Z., et al. Ethylene dimerization and oligomerization to 1-butene and higher olefins with chromium-promoted cobalt on carbon catalyst. ACS Catal. 2018;8(3):2488–2497. [Google Scholar]
- 9.Pillai S.M., Ravindranathan M., Sivaram S. Dimerization of ethylene and propylene catalyzed by transition-metal complexes. Chem. Rev. 1986;86(2):353–399. [Google Scholar]
- 10.Abel E.W., et al. 1995. Comprehensive Organometallic Chemistry II: a Review of the Literature 1982-1994. [Google Scholar]
- 11.Lashchinskaya Z.N., et al. Selective dimerization of ethene to 2-butene on Zn2+-modified ZSM-5 zeolite. J. Phys. Chem. C. 2022;126(15):6570–6577. [Google Scholar]
- 12.Mancuso J.L., et al. Singlet-to-Triplet spin transitions facilitate selective 1-butene formation during ethylene dimerization in Ni (II)-MFU-4 l. J. Phys. Chem. C. 2021;125(40):22036–22043. [Google Scholar]
- 13.Kitamura T., et al. Dimerization and isotopic mixing of ethylene by a palladium complex catalyst. Bull. Chem. Soc. Jpn. 1972;45(5):1457–1460. [Google Scholar]
- 14.Kusunoki Y., et al. The dimerization of ethylene using palladous chloride as the catalyst. Bull. Chem. Soc. Jpn. 1966;39(9):2021–2023. [Google Scholar]
- 15.Zuech E. Polymerizations with homogeneous chromium catalysts. J. Polym. Sci. 1 Polym. Chem. 1972;10(12):3665–3672. [Google Scholar]
- 16.Albright L.F., Smith C.S. Reactions of ethylene with triethyl aluminum: effect of operating variables and kinetics of reaction. AIChE J. 1968;14(2):325–330. [Google Scholar]
- 17.Bigdeli P., Abdouss M., Abedi S. Ti alkoxide-based catalyst system in selective ethylene dimerization: high performance through modifying by alkylsilanes. Chem. Eng. Commun. 2018;205(1):102–109. [Google Scholar]
- 18.Pillai S.M., et al. Dimerization of ethylene to 1-butene catalyzed by the titanium alkoxide-trialkylaluminum system. Ind. Eng. Chem. Res. 1988;27(11):1971–1977. [Google Scholar]
- 19.Mahdaviani S., Parvari M., Soudbar D. 2012. Production of 1-butene via Selective Ethylene Dimerization by Addition of Bromoethane as a New Promoter to Titanium-Based Catalyst in the Presence of Tetrahydropyran Modifier and Triethylaluminum Co-catalyst. [Google Scholar]
- 20.Alzamly A., et al. Linear α-olefin oligomerization and polymerization catalyzed by metal-organic frameworks. Coord. Chem. Rev. 2022;462 [Google Scholar]
- 21.Liu J., et al. Beyond the active site: tuning the activity and selectivity of a metal–organic framework-supported Ni catalyst for ethylene dimerization. J. Am. Chem. Soc. 2018;140(36):11174–11178. doi: 10.1021/jacs.8b06006. [DOI] [PubMed] [Google Scholar]
- 22.Deshmukh S.S., et al. Pd‐Iminocarboxylate complexes and their behavior in ethylene polymerization. Chem.--Asian J. 2020;15(3):398–405. doi: 10.1002/asia.201901501. [DOI] [PubMed] [Google Scholar]
- 23.Phung T.K., et al. (Bio) Propylene production processes: a critical review. J. Environ. Chem. Eng. 2021;9(4) [Google Scholar]
- 24.Bigdeli P., Abdouss M., Abedi S. Alkoxysilane modification of a T i‐based catalyst system for ethylene dimerization: a step forward in enhancing productivity and selectivity. J. Appl. Polym. Sci. 2017;134(12) [Google Scholar]
- 25.Nataj S.M.M., Alavi S.M., Mazloom G. Modeling and optimization of methane dry reforming over Ni–Cu/Al2O3 catalyst using Box–Behnken design. J. Energy Chem. 2018;27(5):1475–1488. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.