Summary
Background
No study has examined the associations between peripheral saturated long-chain fatty acids (LCFAs) and conversion from mild cognitive impairment (MCI) to Alzheimer’s disease (AD). This study aimed to examine whether circulating saturated LCFAs are associated with both risks of incident MCI from cognitively normal (CN) participants and incident AD progressed from MCI in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort.
Methods
We conducted analysis of data from older adults aged 55–90 years who were recruited at 63 sites across the USA and Canada. We examined associations between circulating saturated LCFAs (i.e., C14:0, C16:0, C18:0, C20:0) and risk for incident MCI in CN participants, and incident AD progressed from MCI.
Findings
829 participants who were enrolled in ADNI-1 had data on plasma saturated LCFAs, of which 618 AD-free participants were included in our analysis (226 with normal cognition and 392 with MCI; 60.2% were men). Cox proportional-hazards models were used to account for time-to-event/censor with a 48-month follow-up period for the primary analysis. Other than C20:0, saturated LCFAs were associated with an increased risk for AD among participants with MCI at baseline (Hazard ratios (HRs) = 1.3 to 2.2, P = 0.0005 to 0.003 in fully-adjusted models). No association of C20:0 with risk of AD among participants with MCI was observed. No associations were observed between saturated LCFAs and risk for MCI among participants with normal cognition.
Interpretation
Saturated LCFAs are associated with increased risk of progressing from MCI to AD. This finding holds the potential to facilitate precision prevention of AD among patients with MCI.
Funding
National Institutes of Health.
Keywords: Mild cognitive impairment, Alzheimer’s disease, Saturated long-chain fatty acids, The Alzheimer’s Disease Neuroimaging Initiative cohort
Research in context.
Evidence before this study
Saturated long-chain fatty acids have been linked to systemic inflammation, obesity and insulin resistance which can increase microglial activation in the brain and constitute primary risk factors for neuroinflammatory and neurodegenerative conditions, including AD. We searched PubMed and Google Scholar for studies in English published until March 1, 2023. We aimed to identify known associations between peripheral saturated long-chain fatty acids (LCFAs) and cognition and AD onset. To date, the epidemiological evidence of circulating saturated LCFAs on MCI or AD is sparse, coming mainly from cross-sectional studies. On the other hand, previous dietary studies investigating the associations between saturated fat intake and risk for MCI or AD have generated inconsistent results. Few studies have specifically examined circulating levels of saturated LCFAs and none has prospectively examined the association between circulating levels of saturated LCFAs and risk for incident AD among individuals with MCI.
Added value of this study
The current study showed that saturated LCFAs predicted the conversion from MCI to AD but not from normal cognition to MCI. One possible explanation is the variation in the relationship between systemic inflammation and the disease process at different stages of AD.
Implications of all the available evidence
This finding holds the potential to facilitate precision prevention of AD among patients with MCI. Circulating saturated LCFAs are promising markers for risk stratification among MCI patients. Future studies are warranted to examine the disease-related mechanisms and whether a modified diet and/or combined use of pharmacological agents that result in lower circulating levels of saturated LCFAs can be used to prevent or delay the onset of AD.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia, a major cause of disability and mortality among older adults in the US.1 A 5-year delay in the onset of AD, corresponding to a 50% reduction in incidence rates, would reduce the prevalence by 41% in 2050.2,3 Cerebrospinal fluid and blood biomarkers are being rapidly developed to facilitate early diagnosis of AD and identify AD-related brain changes at early stages.4 In addition to biomarkers solely for AD risk stratification, it is critical to identify modifiable risk factors that are involved in AD etiology and neuropathology.
Saturated long-chain fatty acids (LCFAs) are a group of saturated fats that include myristic acid (C14:0), palmitic acid (C16:0), stearic acid (C18:0), and arachidic acid (C20:0). In contrast to LCFAs, the other two subgroups of saturated fat, i.e., medium-chain fatty acids (MCFAs) and short-chain fatty acids (SCFAs) can rapidly cross the blood–brain barrier, directly enter the mitochondrial matrix and be directly metabolized by the brain.5 Previous evidence consistently suggest that MCFAs and SCFAs have beneficial anti-inflammatory and immunoregulatory effects on brain health and gut-brain axis.5, 6, 7, 8 However, previous dietary studies of saturated fat have not studied individual types of saturated LCFAs. Instead, LCFAs together with MCFAs and SCFAs have been examined as a whole within total saturated fat, resulting in inconsistent findings.9, 10, 11 Experimental studies found that C16:0 and C18:0 were related to β-amyloid (Aβ) accumulation and tau hyperphosphorylation through the triggering of astrocyte-mediated pro-inflammatory cascades.12,13 Elevated C16:0 and C18:0 have also been linked to altered vascular functions in the insulin resistant state, one risk factor for late-onset AD.14 However, epidemiologic evidence from human studies investigating blood concentrations of saturated LCFAs and risk for mild cognitive impairment (MCI) or AD are sparse and come mainly from cross-sectional studies. One prospective study conducted within the Ginkgo Evaluation of Memory Study with 3069 community-dwelling older adults found that plasma levels of total saturated LCFAs were not significantly associated with risk for AD, but were inversely linked to cognitive function measured by the Modified Mini-Mental State score.15
No prospective study has examined the association between circulating levels of saturated LCFAs and risk for incident AD specifically among individuals with MCI at baseline. The current study aimed to examine whether circulating saturated LCFAs are associated with both risks of incident MCI from normal cognition, and incident AD progressed from MCI among initially AD-free older adults in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort.
Methods
Study cohort
Data used in this study were obtained from the ADNI database (adni.loni.usc.edu). The ADNI was launched as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI is to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biomarkers, and clinical and neuropsychological assessment can be combined to measure the risk for and progression to MCI and early AD. The initial phase (ADNI-1) of ADNI was launched in 2003 and subsequent phases (ADNI-GO, ADNI-2, and ADNI-3) were conducted for follow-up of existing participants and additional new enrollments. The detailed design of ADNI has been described elsewhere.16 Volunteer participants between ages 55 and 90 are recruited at 63 sites across the USA and Canada. For this analysis, 829 participants who were enrolled in ADNI-1 and followed in ADNI-1, ADNI-GO, ADNI-2, or ADNI-3 between September 30th 2005 and March 4th 2019 had data on plasma saturated LCFAs (C14:0, C16:0, C18:0, C20:0). We excluded 20 duplicate records, 1 participant with a missing diagnosis, and 190 participants with preexisting AD at baseline, leaving 618 participants for analysis. Among these, 226 were cognitively normal (CN) and 392 had MCI at baseline. Consent forms were approved by each participating site’s Institutional Review Board (IRB) and all participants provided written informed consent at the time of enrollment.
Assay of metabolomics
The metabolomics database for plasma biospecimens was obtained from the Alzheimer Disease Metabolomics Consortium (ADMC) funded by the National Institute on Aging. Details of sample preparation, data generation, quality control (QC), data filtering and normalization have been described elsewhere.17 In brief, the NIH-West Coast Metabolomics Center used an ultra-high-performance liquid chromatography quadruple/time-of-flight mass spectrometry (UHPLC-QTOF MS) instrument to measure the lipid metabolite profile of blood specimens of subjects from ADNI at baseline. Metabolomics lab staff were blinded to diagnostic data. After unblinding and data release, metabolite profiles went through QC checks and data preprocessing including batch-effect adjustment, missing value imputation, and log-transformation. All serum LCFAs data are unitless normalized intensities.
Outcome ascertainment
The ADNI diagnostic criteria for determining CN, MCI and AD were previously reported.18 In brief, ADNI investigators used conventional Petersen/Winblad criteria to categorize participants into CN, MCI or AD. Endpoints were measured using categorical response variables: any incident MCI among participants with normal cognition at baseline, or any incident AD among participants with MCI at baseline. There were no incident dementias resulting from causes other than AD during the follow-up after enrollment, and only AD-related dementia was included in the outcome assessment.
Measurement of covariates
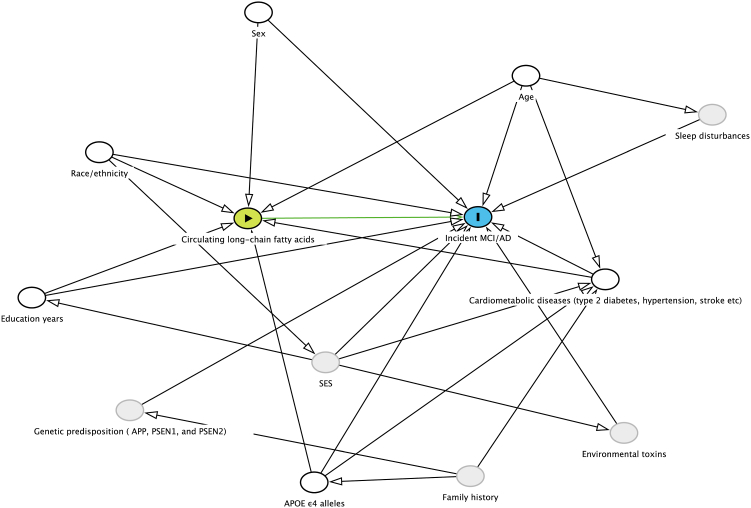
Covariates were determined based on a causal directed acyclic graph (DAG, Fig. 1). Demographic characteristics were obtained from the ADNI data repository. During baseline interviews, self-identified demographic characteristics including age (date of birth), sex (male/female, self-reported by study participants), race (American Indian or Alaskan Native/Asian/Native Hawaiian or Other Pacific Islander/Black or African American/White/More than one race, self-reported by study participants), ethnicity (Hispanic or Latino/Not Hispanic or Latino), and years of education (years) were collected in the case report form. A self-reported physician-diagnosed history of medical conditions including type 2 diabetes, hypertension, stroke, cardiovascular disease, and endocrine-metabolic diseases were also collected in the case report form (yes/no). If yes, additional questions captured information about whether the condition was current and the age at diagnosis as well as details of medication use. APOE ε4 genotyping was performed at the time of participant enrollment. The two SNPs (rs429358, rs7412) that define the epsilon 2, 3, and 4 alleles were genotyped and the copy numbers of APOE ε4 alleles for each individual were calculated based on genotyping data.
Fig. 1.
Directed acyclic graph. A directed acyclic graph represents associations between circulating long-chain fatty acids, covariates and incident MCI/AD. Green circle represents exposure and blue circle represents outcome. Hollow black circles represent adjusted confounders and grey circles represent unobserved (ie, latent) variables which may not associate with exposure. Green lines represent causal paths, and black lines represent biasing paths. The minimally sufficient adjustment set represents covariates such that the adjustment for this set of variables will minimize confounding bias when estimating the association between the exposure and the outcome. The minimally sufficient adjustment set was determined using the DAGitty software.19 The final minimally sufficient adjustment set comprised age, sex, race, ethnicity, years of education, APOE ε4 alleles and cardiometabolic diseases (type 2 diabetes, hypertension, stroke).
Statistical analysis
Our analysis included 392 participants with MCI at baseline and 226 CN participants at baseline. Cox proportional-hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) with incident MCI and AD as dependent variables, respectively. Test of proportional-hazards assumption was conducted by creating and including time dependent covariates, and no violation was observed. We tested nonlinearity for continuous predictors (age, education years) and the linearity assumptions for Cox regression were satisfied. In the primary analyses, time-to-event/censoring assessments were conducted over 48-month intervals of follow-up. In secondary analyses, time-to-event/censoring evaluations were performed over follow-up intervals of 24 and 120 months, respectively. For each participant, the number of months of follow-up was calculated from the date of enrollment (i.e., the date of the first clinical visit at baseline) until the date of conversion to MCI or AD, the date of loss to follow-up or stopping providing information on outcomes (i.e., the last clinical visit before the end of follow-up), or the end of follow-up period (24 or 48 or 120 months), whichever comes first. For cognitively normal participants, date of conversion to MCI was calculated as the midpoint between their last visit without MCI and their first visit with MCI.20 For MCI patients, date of conversion to AD was calculated as the midpoint between their last visit without AD and their first visit with AD.20
Log2-transformed circulating levels of saturated LCFAs served as independent variables. Associations between log2-transformed circulating levels of saturated LCFAs and risk of incident MCI among CN participants, and risk of incident AD among MCI participants were examined in 3 models. In Model 1, we present the crude ORs (95% CIs) without adjustment. In Model 2, we adjusted for age, sex, race, ethnicity, years of education, and APOE ε4 alleles. In multivariable-adjusted model 3, we additionally adjusted for type 2 diabetes, hypertension, stroke, cardiovascular disease, and metabolic diseases. Because we were interested in differential associations by sex, age, presence of cardiometabolic diseases and APOE ε4 alleles, we conducted stratified analyses for these variables. The significance of multiplicative interactions was evaluated by adding corresponding interaction terms in the models. Relative excess risk due to interaction (RERI) and 95% CIs were calculated and reported for additive interactions.21 Restricted cubic spline analyses with 3 knots (10th, 50th, and 90th percentiles) were used to examine possible nonlinear relationships between circulating levels of saturated LCFAs and risk of incident MCI and AD within the values between the 5th and 95th percentile to minimize the influence of potential outliers. Statistical significance of nonlinearity was tested using Wald Chi-Square by inclusion of saturated LCFA as a quadratic term in the model together with LCFA as a continuous variable, and P values of < 0.05 were regarded as statistically significant nonlinear relationship between the exposure and the outcome. Statistical significance of linearity was tested by comparing the linear model to the model including only the covariates, both using likelihood ratio tests. We also conducted sensitivity analyses after excluding the MCI/AD cases that occurred within the first year of follow-up. All analyses were conducted using Statistical Analysis Software, version 9.4 (SAS Institute Inc., Cary, North Carolina) and restricted cubic splines were plotted using R 4.2.0 (https://cran.r-project.org/). All hypothesis testing was 2-sided with P < 0.05 indicating a statistically significant finding.
Role of the funder/sponsor
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Results
During the 24-month follow-up (a total of 12,067 person-month of observation), 133 (33.9%) of MCI participants developed incident AD while 6 (2.6%) of CN participants converted from CN to MCI. During the 48-month follow-up (a total of 19,273 person-month of observation), 183 (46.7%) of MCI participants developed incident AD while 23 (10.2%) of CN participants converted from CN to MCI. During the 120-month follow-up (a total of 28,382 person-month of observation), 210 (53.6%) of MCI participants developed incident AD while 59 (26.1%) of CN participants converted from CN to MCI. Seventeen out of 226 (7.5%) baseline participants with CN who developed MCI and later converted to incident AD during the follow-up interval were not included in the analysis for the risk of MCI to AD. Additionally, three out of 226 (1.3%) CN participants who directly converted from CN to incident AD without observing an MCI phase were excluded from the analyses for both the risk of CN to MCI and the risk of MCI to AD (Supplemental Fig. S1).
Baseline characteristics of CN and MCI participants were presented separately (Table 1). Age, race, ethnicity, years of education, plasma levels of saturated LCFAs (C14:0, C16:0, C18:0, C20:0), and comorbidity of cardiometabolic diseases did not differ significantly between CN and MCI participants, whereas participants with MCI were more likely to be male (118 [52.2%] vs 254 [64.8%]) and APOE ε4 carriers (60 [26.5%] vs 209 [53.3%]).
Table 1.
Baseline characteristics of ADNI participants.
| Variables | Cognitively Normal |
Mild Cognitive Impairment |
P-values |
|---|---|---|---|
| n = 226 | n = 392 | ||
| Age, years | 76.0 (5.0) | 75.0 (7.5) | 0.34 |
| Sex, n, % | |||
| Male | 118 (52.2) | 254 (64.8) | 0.0021 |
| Female | 108 (47.8) | 138 (35.2) | |
| Race, n, % | 0.13 | ||
| White | 207 (91.6) | 367 (93.6) | |
| Black | 16 (7.1) | 15 (3.8) | |
| Other | 3 (1.3) | 10 (2.5) | |
| Ethnicity, n, % | 0.16 | ||
| Hispanic or Latino | 2 (0.9) | 13 (3.3) | |
| Not Hispanic or Latino | 223 (98.7) | 377 (96.2) | |
| Unknown | 1 (0.4) | 2 (0.5) | |
| Education, years | 16.1 (2.9) | 15.6 (3.0) | 0.08 |
| APOE ε4 alleles, n, % | <0.0001 | ||
| 0 | 166 (73.4) | 183 (46.7) | |
| 1 | 55 (24.3) | 163 (41.6) | |
| 2 | 5 (2.2) | 46 (11.7) | |
| Circulating LCFAs levels | |||
| Myristic acid (C14:0) | 1346 (533) | 1527 (2080) | 0.23 |
| Palmitic acid (C16:0) | 22,752 (6235) | 22,952 (6160) | 0.66 |
| Stearic acid (C18:0) | 137,370 (28,928) | 141,037 (32,298) | 0.14 |
| Arachidic acid (C20:0) | 1232 (405) | 1261 (403) | 0.55 |
| Comorbidity of cardiometabolic diseases | |||
| Any one or more cardiometabolic diseases, n, % | 185 (81.9) | 333 (84.9) | 0.36 |
| Type 2 diabetes, n, % | 29 (12.8) | 48 (12.2) | 0.83 |
| Hypertension, n, % | 104 (46.0) | 135 (34.4) | 0.0044 |
| Stroke, n, % | 12 (5.3) | 21 (5.4) | 0.98 |
| Any cardiovascular diseases, n, % | 153 (67.7) | 283 (72.2) | 0.24 |
| Any endocrine-metabolic diseases, n, % | 89 (39.4) | 145 (37.0) | 0.55 |
Data are presented as mean (SD) for continuous variables and % for categorical variables. All serum LCFAs data are unitless normalized intensities. P values are calculated using Chi-square test for categorical variables and Wilcoxon rank sum tests for continuous variables.
ADNI, the Alzheimer’s Disease Neuroimaging Initiative.
Associations between circulating levels of saturated LCFAs and risk for incident MCI among CN participants are presented in Table 2. No associations were observed between log2-transformed circulating levels of saturated LCFAs (C14:0, C16:0, C18:0, C20:0) and risk for incident MCI among CN participants in any of the three models with 24-, 48- or 120-month follow-up (Table 2, Supplemental Table S1).
Table 2.
Multivariable-adjusted HR (95%CIs) for per 2-fold increase in circulating saturated LCFAs and risk of incident MCI among individuals with normal cognition at baseline with 48-month follow-up interval (n = 223).
| LCFAsa | Cases/Person-month | HR (95%CI) | P value |
|---|---|---|---|
| Myristic acid (C14:0) | 23/8778 | ||
| Model 1 | 1.0 (0.7–1.4) | 0.8393 | |
| Model 2 | 1.0 (0.7–1.4) | 0.9321 | |
| Model 3 | 1.0 (0.7–1.5) | 0.9529 | |
| Palmitic acid (C16:0) | 23/8778 | ||
| Model 1 | 1.1 (0.4–3.1) | 0.9042 | |
| Model 2 | 1.0 (0.3–3.2) | 0.9361 | |
| Model 3 | 1.2 (0.4–3.8) | 0.7054 | |
| Stearic acid (C18:0) | 23/8778 | ||
| Model 1 | 1.3 (0.3–5.1) | 0.6639 | |
| Model 2 | 1.3 (0.3–4.9) | 0.702 | |
| Model 3 | 1.5 (0.4–5.7) | 0.5291 | |
| Arachidic acid (C20:0) | 23/8778 | ||
| Model 1 | 1.3 (0.6–2.9) | 0.5188 | |
| Model 2 | 1.3 (0.6–3.1) | 0.5111 | |
| Model 3 | 1.2 (0.5–2.9) | 0.6232 |
All CN individuals at baseline excluded three participants with diagnoses converting directly from CN to AD without observing MCI phase.
Model 1 presented crude values; model 2 was adjusted for age, sex, race, ethnicity, education years, and APOE ε4 alleles; model 3 was model 2 additionally adjusted for type 2 diabetes, hypertension, stroke, cardiovascular disease, and metabolic diseases.
LCFAs, long-chain saturated fatty acids; MCI, mild cognitive impairment.
Circulating saturated LCFAs levels were log2 transformed.
Associations between circulating levels of saturated LCFAs and risk for incident AD among MCI participants with 48-month follow-up are presented in Table 3. For the primary analyses with 48-month follow-up, per 2-fold increases in circulating C14:0, C16:0 and C18:0 levels were associated with a 1.3-, 2.1- and 2.2-fold increased risk of incident AD after adjusting for all covariates with HRs (95% CIs) of 1.3 (1.1–1.6) (P = 0.0029), 2.1 (1.4–3.1) (P = 0.0005) and 2.2 (1.3–3.5) (P = 0.0013), respectively. In contrast, circulating levels of C20:0 was not significantly linked to risk for AD with 48-month follow-up.
Table 3.
Multivariable-adjusted HR (95%CIs) for per 2-fold increase in circulating saturated LCFAs and risk of incident AD among individuals with MCI at baseline with 48-month follow-up interval (n = 392).
| LCFAsa | Cases/Person-month | HR (95%CI) | P value |
|---|---|---|---|
| Myristic acid (C14:0) | 183/10,495 | ||
| Model 1 | 1.4 (1.1–1.7) | 0.0015 | |
| Model 2 | 1.3 (1.1–1.6) | 0.0037 | |
| Model 3 | 1.3 (1.1–1.6) | 0.0029 | |
| Palmitic acid (C16:0) | 183/10,495 | ||
| Model 1 | 2.2 (1.5–3.3) | 0.0001 | |
| Model 2 | 2.0 (1.3–3.1) | 0.0008 | |
| Model 3 | 2.1 (1.4–3.1) | 0.0005 | |
| Stearic acid (C18:0) | 183/10,495 | ||
| Model 1 | 2.4 (1.5–3.9) | 0.0003 | |
| Model 2 | 2.1 (1.3–3.4) | 0.0017 | |
| Model 3 | 2.2 (1.3–3.5) | 0.0013 | |
| Arachidic acid (C20:0) | 183/10,495 | ||
| Model 1 | 1.2 (0.9–1.6) | 0.2506 | |
| Model 2 | 1.2 (0.8–1.6) | 0.3289 | |
| Model 3 | 1.2 (0.9–1.6) | 0.2653 |
Model 1 presented crude values; model 2 was adjusted for age, sex, race, ethnicity, education years, and APOE ε4 alleles; model 3 was model 2 additionally adjusted for type 2 diabetes, hypertension, stroke, cardiovascular disease, and metabolic diseases.
LCFAs, long-chain saturated fatty acids; MCI, mild cognitive impairment; AD, Alzheimer’s disease.
Circulating saturated LCFAs levels were log2 transformed.
For secondary analyses with 24-month follow-up, per 2-fold increases in circulating C14:0 level were associated with a 1.3-fold increased risk of incident AD after adjusting for all covariates with an HR (95% CI) of 1.3 (1.0–1.6) (P = 0.0134). The associations between per 2-fold increases in circulating levels of C16:0 and C18:0 with risk of incident AD were of borderline significance with HRs (95% CIs) of 1.6 (1.0–2.6) (P = 0.0622) and 1.6 (0.9–2.7) (P = 0.0926), respectively. In contrast, circulating C20:0 was not significantly linked to risk for AD with 24-month follow-up (Supplemental Table S2).
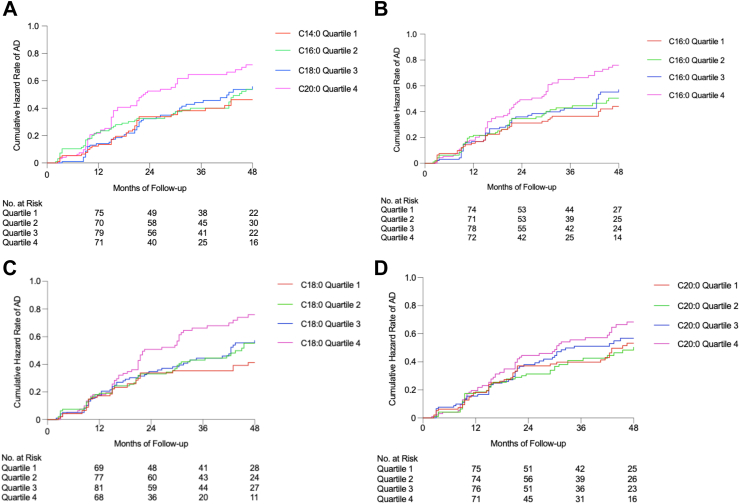
For 120-month follow-up, per 2-fold increases in circulating C14:0, C16:0 and C18:0 levels were associated with a 1.2-, 2.0- and 2.2-fold increased risk of incident AD after adjusting for all covariates with HRs (95% CIs) of 1.2 (1.0–1.4) (P = 0.0153), 2.0 (1.3–2.9) (P = 0.0005) and 2.2 (1.4–3.4) (P = 0.0007), respectively. The association between circulating C20:0 and risk of AD with 120-month follow-up was of borderline significance with HRs (95% CIs) of 1.3 (1.0–1.8) (P = 0.0811) (Supplemental Table S2). Kaplan–Meier estimates of cumulative hazard rates of MCI or AD by quartiles of log2-transformed LCFAs were presented in Fig. 2 and Supplemental Figs. S2–S4.
Fig. 2.
Kaplan–Meier estimates of cumulative hazard rates of AD by quartiles of LCFAs among MCI participants (n = 392). Quartiles of LCFAs levels were used for plotting Kaplan–Meier estimates of cumulative hazard ratios of AD.
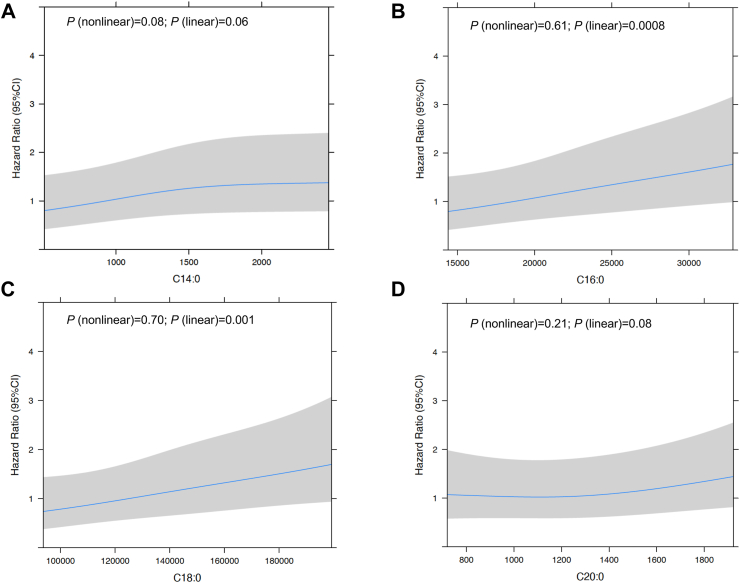
Restricted cubic spline analyses suggested no evidence of nonlinear associations between circulating LCFAs (C14:0, C16:0, C18:0, C20:0) and risk of AD while test for linearity showed positive linear associations between C16:0 and C18:0 and risk for AD among participants with MCI at baseline (Fig. 3 A,B,C,D).
Fig. 3.
Multivariable-adjusted Hazard Ratios and 95% CIs of Incident AD Associated with Circulating Saturated LCFAs among participants with MCI at Baseline (n = 392). Restricted cubic spline analyses used 3 knots (10th, 50th, 90th percentile) and all models were adjusted for age, sex, race, ethnicity, education years, APOE ε4 alleles, type 2 diabetes, hypertension, stroke, cardiovascular disease, and metabolic diseases. MCI, mild cognitive impairment; AD, Alzheimer’s disease; LCFAs: long-chain fatty acids.
We conducted sensitivity analyses by excluding the 66 participants who converted to AD within the first year of follow-up after enrollment. The results from sensitivity analyses are consistent with the main results (Supplemental Table S3).
In stratified analyses, the associations between plasma levels of C14:0, C16:0 and C18:0 and risk of incident AD were not significantly modified by preexisting cardiometabolic diseases, sex, age and APOE ε4 genotype (Supplemental Tables S4–S7). A significant positive additive interaction between C14:0 and age were observed with RERI (95%CI) of 0.05 (0.02, 0.07), suggesting that age and C14:0 are acting synergistically to increase the risk of AD among MCI participants. None of the tests for multiplicative interactions were statistically significant (Supplemental Table S4).
Discussion
This study examined the relationships between circulating levels of saturated LCFAs and risk for incident MCI and AD among 618 AD-free older adults in the ADNI dataset. We found that higher circulating levels of saturated LCFAs (i.e., C14:0, C16:0, C18:0), not including C20:0, were associated with a 1.3- to 2.2-fold increased risk for incident AD among participants with MCI at baseline. However, no associations were observed between circulating levels of saturated LCFAs (i.e., C14:0, C16:0, C18:0, C20:0) and risk for incident MCI among participants who were cognitively normal at baseline.
Lipids constitute a great proportion of dry mass of human brain and lipids dysregulation plays an integral role in AD pathology.22 Yet, the epidemiologic evidence of saturated LCFAs on MCI or AD is sparse, and come mainly from cross-sectional studies.23 No previous study has prospectively examined the association between circulating levels of saturated LCFAs and risk of incident AD specifically among individuals with MCI at baseline. On the other hand, previous dietary studies investigating the associations between saturated fat intake and risk of MCI or AD have generated inconsistent results. For example, a prospective cohort meta-analysis found that dietary intakes of saturated fatty acids were not associated with incident MCI,11 while another meta-analysis concluded that higher dietary saturated fat intake was associated with an increased risk for dementia including AD.9,24 However, the Rotterdam Study conducted among 5395 cognitively normal older adults with a mean follow-up interval of 6 years found the rate ratios (95% CI) per standard deviation increase in intakes of saturated fatty acids were 0.9 (0.8–1.0) for total dementia and 0.8 (0.7–1.0) for AD.25 A possible explanation for the inconsistency is that these previous studies of dietary intakes did not separately evaluate individual saturated LCFAs. Furthermore, none of previous studies has specifically examined conversions from normal to MCI and from MCI to AD.
Findings from the current study indicate that circulating levels of saturated LCFAs predict the risk of incident AD among individuals with MCI. In previous studies conducted in other cohorts, the participants at baseline included both cognitively normal and MCI participants. Thus, the strength of the associations between levels of saturated LCFAs and incident AD could be dependent on the proportion of MCI patients in the cohorts at baseline. Also in previous studies, saturated LCFAs were classified into total saturated fatty acids together with SCFAs and MCFAs. However, in our recent report, circulating levels of MCFA C8:0 were associated with a reduced risk of incident MCI but not related to risk of converting from incident MCI to AD.26
The observed positive associations in this analysis with C14:0, C16:0 and C18:0, not including C20:0, are supported by studies that examined the lipid profiles of serum, cerebrospinal fluid (CSF) and brain tissues. Kotlega et al. examined the relationship between serum free fatty acids and post-stroke (seven days and six months after stroke) cognitive function among 72 ischemic stroke patients. They found that C14:0 was inversely associated with cognitive performance, whereas C20:0 had a beneficial influence on cognitive outcome and played a positive role in promoting neuroplasticity.27 Analysis of brain tissue from postmortem specimens found that C14:0, C16:0, and C18:0 were substantially higher in AD patients.28 Another human study assaying brain-derived, membrane-rich nanoparticles in CSF from 139 older adults also reported significantly higher levels of C14:0, C16:0, and C18:0 in AD patients compared with CN and MCI participants, suggesting that elevated saturated LCFAs (C14:0, C16:0, and C18:0) are involved in AD neuropathology.29
The main finding of this study is the absence of an association with conversion to MCI among cognitively normal participants and presence of an association with conversion to AD among those with MCI at baseline. This difference may be explained by a differential relationship between systemic inflammation and the disease process at different stages of AD. Pascoal et al. have demonstrated a strong correlation between Braak stage and microglial activation.30 Earlier stages of the disease, like MCI, are associated with less microglial activation; while more advanced stages of the disease are associated with heightened activation and increase spread of tau. Studies have shown that systemic inflammation can increase microglial activation in the brain.31 Saturated LCFAs have been linked to the development of obesity, insulin resistance and type 2 diabetes, all of which are primary risk factors for neuroinflammatory and neurodegenerative conditions, including AD.32 Increases in microglial activation present in later vs earlier stages of the disease could facilitate the spread of pathological tau, leading to cognitive deficits that cause conversion from MCI to dementia. While MCI is considered a transitional stage from normal cognitive aging to dementia, our findings showed longitudinal associations between circulating saturated LCFAs and AD risk among MCI individuals.
In the current study, the association strength of saturated LCFAs and risk of conversion from MCI to AD intensified as the carbon chain length increased from 14 to 18, while the linear association disappeared with 20 carbons or more (C22:0 data in Supplemental Table S8), suggesting differential associations as transition from saturated LCFAs to very long chain saturated fatty acids (VLSFAs) with a chain-length of ≥22 carbons. Saturated LCFAs including palmitic and stearic acids (C16:0 and C18:0) were related to β-amyloid (Aβ) accumulation and tau hyperphosphorylation, mainly through triggering the astrocytes-mediated pro-inflammatory cascades.12,13 Also, C14:0, C16:0, and C18:0 are endogenously converted into monounsaturated fatty acids myristoleic acid (C14:1), palmitoleic acid (C16:1n7) and oleic acid (C18:1n9cis), respectively. This conversion is catalyzed by the Delta-9 desaturase, the key enzyme that has been associated with insulin resistance.33 Elevated C16:0 and C18:0 and impaired desaturase activity have been linked to insulin resistance,34 whereas C20:0 along with VLSFAs such as behenic acid (C22:0) and lignoceric acid (C24:0) have been shown to have a protective effect against insulin resistance, diabetes, and heart failure.35,36 Furthermore, a cohort study conducted among the Atherosclerosis Risk in Communities (ARIC) participants (n = 3229) found that higher plasma total VLSFAs and each individual VLSFA were associated with less 20-year cognitive decline in the Word Fluency Test.37 This may explain our findings that no associations were observed for C20:0 and C22:0 in relation to AD risk among participants with MCI (Fig. 3D and Supplemental Table S8). Furthermore, in vitro studies in cultured aortic smooth muscle cells showed that C16:0 and C18:0 stimulated de novo diacylglycerol (DAG) synthesis and subsequently activated protein kinase C (PKC) and mitogen-activated protein kinase (MAP), resulting in altered vascular functions in the insulin resistant state.14 Of note, as the carbon-chain number increases, the effects of C16:0 and C18:0 increased by five- and eight-fold, respectively, in stimulating de novo DAG synthesis whereas this effect was not observed for C20:0, which is again consistent with our findings.
This study has a few strengths. First, ADNI is a rich dataset incorporating clinical, imaging, genetic, and biochemical biomarkers for the early detection and tracking of AD progression. Based on this unique resource, we examined the association between circulating levels of saturated LCFAs and risk for incident MCI and AD over 24-month, 48-month, and 120-month follow-up intervals, respectively. Second, prevalent AD patients at baseline were excluded prior to study entry and multiple clinic visits per person were performed to confirm and track the diagnoses during the follow-up, which minimized the possibility of outcome misclassification.
There are also some limitations for the current study. First, the sample size is limited in stratified analyses by comorbidity of cardiometabolic diseases, sex and APOE genotype. Thus, the non-significant interactions by APOE genotype, sex, and comorbidity of cardiometabolic diseases may be due to Type II error. Further, the current study only had 20 incident AD developed from normal cognition which may not have sufficient statistical power to study whether levels of saturated LCFAs in CN participants are associated with risk of incident AD. Although we separately examined the associations between circulating levels of saturated LCFAs and risks for incident MCI among CN participants and incident AD among MCI participants, differences between these two groups by age and sex could have led to the differential findings regarding the associations of saturated LCFAs. Given that the CN and MCI groups are not entirely comparable, prudence is advised when comparing results from these two analyses. While the current study is a prospective cohort study with clear temporal sequence, we acknowledge the risk of endowing a HR with a causal interpretation and cannot fully rule out the possibility of built-in selection bias. Further, a single measure of circulating LCFAs may not be able to reflect the variations in a long-term follow-up period. Despite our efforts to adjust for multiple covariates and conduct sensitivity analysis, it is important to acknowledge that we cannot eliminate the potential influence of residual confounding. In addition, there is a lack of racial and ethnic diversity in the current study. The risk and burden of AD are greater among African Americans and Hispanics compared to non-Hispanic Whites, yet these racial/ethnic minority groups are underrepresented in most AD observational/clinical studies including ADNI. ADNI recruited predominantly Caucasians and >90% have a four-year college education, which may impact the generalizability of conclusions from the current study.
Conclusion
In summary, higher circulating levels of saturated LCFAs (i.e., C14:0, C16:0, C18:0), but not C20:0, were associated with an increased risk for incident AD among participants with MCI at baseline. We did not find an increased risk for MCI related to saturated LCFAs among those with normal cognition. If confirmed, these findings may help to stave off the conversion from MCI to AD. Further studies are necessary to confirm whether peripheral saturated LCFAs can be used as biomarkers to screen patients with MCI for incident AD risk. Future studies are also required to understand the mechanisms and investigate whether the changes in plasma saturated LCFAs are due to endogenous sources, diet or both and whether saturated LCFAs serve as potential targets for intervention.
Contributors
QD and LF designed this study jointly with ARB, SW and XZ. LF and XZ coordinated the study. QD supervised the study. LF downloaded the data and LF and QD have accessed and verified the data reported in the manuscript. LF, ARB, SW, KN, XZ, WW, XH, JAM, MJS and QD analyzed the data and interpreted the results. LF, QD and ARB wrote the original draft. All authors revised the manuscript. All authors read and approved the final version of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
The results published here are obtained from the AD Knowledge Portal with an approved project application via https://adknowledgeportal.org.
Declaration of interests
KN received payment or honoraria for lectures and/or presentations from AIS symposium, outside the submitted work. SW received book royalties from American Psychiatric Publishing, Inc. and participated in DSMB for NIA funded study, not related to the submitted work. All other authors declare that they have no competing interests.
Acknowledgments
Funding: The data available in the AD Knowledge Portal would not be possible without the participation of research volunteers and the contribution of data by collaborating researchers. Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). Metabolomics data is provided by the Alzheimer’s Disease Metabolomics Consortium (ADMC) and funded wholly or in part by the following grants and supplements thereto: NIA R01AG046171, RF1AG051550, RF1AG057452, R01AG059093, RF1AG058942, U01AG061359, U19AG063744 and FNIH: #DAOU16AMPA awarded to Dr. Kaddurah-Daouk at Duke University in partnership with a large number of academic institutions. As such, the investigators within the ADMC, not listed specifically in this publication’s author’s list, provided data along with its pre-processing and prepared it for analysis, but did not participate in analysis or writing of this manuscript. A complete listing of ADMC investigators can be found at: https://sites.duke.edu/adnimetab/team/. QD and MJS are supported by R01 DK110166 from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). KN is supported by R01 LM012535, U01 AG072177, and U19 AG0748790 from National Institutes of Health (NIH). LF is supported by the Vanderbilt Ingram Cancer Center Endowment Fund.
Collaborators
ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Graphical abstract was created with BioRender.com.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104818.
Appendix ASupplementary data
References
- 1.Liang C.-S., Li D.-J., Yang F.-C., et al. Mortality rates in Alzheimer’s disease and non-Alzheimer’s dementias: a systematic review and meta-analysis. Lancet Healthy Longev. 2021;2:e479–e488. doi: 10.1016/S2666-7568(21)00140-9. [DOI] [PubMed] [Google Scholar]
- 2.Zissimopoulos J., Crimmins E., St Clair P. The value of delaying alzheimer’s disease onset. Forum Health Econ Policy. 2015;18:25–39. doi: 10.1515/fhep-2014-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brookmeyer R., Gray S., Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zetterberg H. Biofluid-based biomarkers for Alzheimer’s disease-related pathologies: an update and synthesis of the literature. Alzheimers Dement. 2022;18:1687–1693. doi: 10.1002/alz.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augustin K., Khabbush A., Williams S., et al. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018;17:84–93. doi: 10.1016/S1474-4422(17)30408-8. [DOI] [PubMed] [Google Scholar]
- 6.Grammatikopoulou M.G., Goulis D.G., Gkiouras K., et al. To Keto or not to Keto? A systematic review of randomized controlled trials assessing the effects of ketogenic therapy on alzheimer disease. Adv Nutr. 2020;11:1583–1602. doi: 10.1093/advances/nmaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avgerinos K.I., Egan J.M., Mattson M.P., Kapogiannis D. Medium chain triglycerides induce mild ketosis and may improve cognition in Alzheimer’s disease. A systematic review and meta-analysis of human studies. Ageing Res Rev. 2020;58 doi: 10.1016/j.arr.2019.101001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva Y.P., Bernardi A., Frozza R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan Y., Tang J., Guo X., Li K., Li D. Dietary fat intake and risk of alzheimer’s disease and dementia: a meta-analysis of cohort studies. Curr Alzheimer Res. 2018;15:869–876. doi: 10.2174/1567205015666180427142350. [DOI] [PubMed] [Google Scholar]
- 10.Cao G.-Y., Li M., Han L., et al. Dietary fat intake and cognitive function among older populations: a systematic review and meta-analysis. J Prev Alzheimers Dis. 2019;6:204–211. doi: 10.14283/jpad.2019.9. [DOI] [PubMed] [Google Scholar]
- 11.Zhu R.-Z., Chen M.-Q., Zhang Z.-W., Wu T.-Y., Zhao W.-H. Dietary fatty acids and risk for Alzheimer’s disease, dementia, and mild cognitive impairment: a prospective cohort meta-analysis. Nutrition. 2021;90 doi: 10.1016/j.nut.2021.111355. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S., Knight A.G., Gupta S., Keller J.N., Bruce-Keller A.J. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J Neurochem. 2012;120:1060–1071. doi: 10.1111/j.1471-4159.2012.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patil S., Chan C. Palmitic and stearic fatty acids induce Alzheimer-like hyperphosphorylation of tau in primary rat cortical neurons. Neurosci Lett. 2005;384:288–293. doi: 10.1016/j.neulet.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Yu H.Y., Inoguchi T., Kakimoto M., et al. Saturated non-esterified fatty acids stimulate de novo diacylglycerol synthesis and protein kinase c activity in cultured aortic smooth muscle cells. Diabetologia. 2001;44:614–620. doi: 10.1007/s001250051668. [DOI] [PubMed] [Google Scholar]
- 15.Koch M., Furtado J.D., DeKosky S.T., et al. Case-cohort study of plasma phospholipid fatty acid profiles, cognitive function, and risk of dementia: a secondary analysis in the Ginkgo Evaluation of Memory Study. Am J Clin Nutr. 2021;114:154–162. doi: 10.1093/ajcn/nqab087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiner M.W., Veitch D.P., Aisen P.S., et al. Recent publications from the Alzheimer’s Disease Neuroimaging Initiative: reviewing progress toward improved AD clinical trials. Alzheimers Dement. 2017;13:e1–e85. doi: 10.1016/j.jalz.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barupal D.K., Fan S., Wancewicz B., et al. Generation and quality control of lipidomics data for the alzheimer’s disease neuroimaging initiative cohort. Sci Data. 2018;5 doi: 10.1038/sdata.2018.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bondi M.W., Edmonds E.C., Jak A.J., et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. 2014;42:275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Textor J., van der Zander B., Gilthorpe M.S., Liskiewicz M., Ellison G.T. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45:1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 20.Gomar J.J., Bobes-Bascaran M.T., Conejero-Goldberg C., Davies P., Goldberg T.E., Alzheimer’s Disease Neuroimaging Initiative Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s Disease Neuroimaging Initiative. Arch Gen Psychiatr. 2011;68:961–969. doi: 10.1001/archgenpsychiatry.2011.96. [DOI] [PubMed] [Google Scholar]
- 21.Li R., Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17:227–236. doi: 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Chew H., Solomon V.A., Fonteh A.N. Involvement of lipids in alzheimer’s disease pathology and potential therapies. Front Physiol. 2020;11:598. doi: 10.3389/fphys.2020.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosseini M., Poljak A., Braidy N., Crawford J., Sachdev P. Blood fatty acids in Alzheimer’s disease and mild cognitive impairment: a meta-analysis and systematic review. Ageing Res Rev. 2020;60 doi: 10.1016/j.arr.2020.101043. [DOI] [PubMed] [Google Scholar]
- 24.Barnard N.D., Bunner A.E., Agarwal U. Saturated and trans fats and dementia: a systematic review. Neurobiol Aging. 2014;35(Suppl 2):S65–S73. doi: 10.1016/j.neurobiolaging.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Engelhart M.J., Geerlings M.I., Ruitenberg A., et al. Diet and risk of dementia: does fat matter?: The Rotterdam Study. Neurology. 2002;59:1915–1921. doi: 10.1212/01.wnl.0000038345.77753.46. [DOI] [PubMed] [Google Scholar]
- 26.Fan L., Zhu X., Borenstein A.R., et al. Association of circulating caprylic acid with risk of mild cognitive impairment and Alzheimer’s disease in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort. J Prev Alzheimers Dis. 2023;10:513–522. doi: 10.14283/jpad.2023.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotlęga D., Peda B., Palma J., et al. Free fatty acids are associated with the cognitive functions in stroke survivors. Int J Environ Res Public Health. 2021;18:6500. doi: 10.3390/ijerph18126500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasaruddin M.L., Hölscher C., Kehoe P., Graham S.F., Green B.D. Wide-ranging alterations in the brain fatty acid complement of subjects with late Alzheimer’s disease as detected by GC-MS. Am J Transl Res. 2016;8:154–165. [PMC free article] [PubMed] [Google Scholar]
- 29.Fonteh A.N., Cipolla M., Chiang J., Arakaki X., Harrington M.G. Human cerebrospinal fluid fatty acid levels differ between supernatant fluid and brain-derived nanoparticle fractions, and are altered in Alzheimer’s disease. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascoal T.A., Benedet A.L., Ashton N.J., et al. Microglial activation and tau propagate jointly across Braak stages. Nat Med. 2021;27:1592–1599. doi: 10.1038/s41591-021-01456-w. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y., Koyama Y., Shimada S. Inflammation from peripheral organs to the brain: how does systemic inflammation cause neuroinflammation? Front Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.903455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howe A.-M., Burke S., O’Reilly M.E., McGillicuddy F.C., Costello D.A. Palmitic acid and oleic acid differently modulate TLR2-mediated inflammatory responses in microglia and macrophages. Mol Neurobiol. 2022;59:2348–2362. doi: 10.1007/s12035-022-02756-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurotani K., Sato M., Ejima Y., et al. High levels of stearic acid, palmitoleic acid, and dihomo-γ-linolenic acid and low levels of linoleic acid in serum cholesterol ester are associated with high insulin resistance. Nutr Res. 2012;32:669–675.e3. doi: 10.1016/j.nutres.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Mayneris-Perxachs J., Guerendiain M., Castellote A.I., et al. Plasma fatty acid composition, estimated desaturase activities, and their relation with the metabolic syndrome in a population at high risk of cardiovascular disease. Clin Nutr. 2014;33:90–97. doi: 10.1016/j.clnu.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Lemaitre R.N., Fretts A.M., Sitlani C.M., et al. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2015;101:1047–1054. doi: 10.3945/ajcn.114.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemaitre R.N., McKnight B., Sotoodehnia N., et al. Circulating very long-chain saturated fatty acids and heart failure: the cardiovascular health study. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li D., Misialek J.R., Jing M., et al. Plasma phospholipid very-long-chain SFAs in midlife and 20-year cognitive change in the Atherosclerosis Risk in Communities (ARIC): a cohort study. Am J Clin Nutr. 2020;111:1252–1258. doi: 10.1093/ajcn/nqaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.