Summary
Background
Complex patterns of cross-reactivity exist between flaviviruses, yet there is no precise understanding of how sequential exposures due to flavivirus infections or vaccinations impact subsequent antibody responses.
Methods
We investigated whether B cell priming from Japanese encephalitis virus (JEV) or yellow fever virus (YFV) vaccination impacted binding and functional antibody responses to flaviviruses following vaccination with a Zika virus (ZIKV) purified inactivated virus (ZPIV) vaccine. Binding antibody responses and Fc gamma receptor engagement against 23 flavivirus antigens were characterized along with neutralization titres and Fc effector responses in 75 participants at six time points.
Findings
We found no evidence that priming with JEV or YFV vaccines improved the magnitude of ZPIV induced antibody responses to ZIKV. Binding antibodies and Fc gamma receptor engagement to ZIKV antigens did not differ significantly across groups, while antibody-dependent cellular phagocytosis (ADCP) and neutralizing responses were higher in the naïve group than in the JEV and YFV primed groups following the second ZPIV immunization (p ≤ 0.02). After a third dose of ZPIV, ADCP responses remained higher in the naïve group than in the primed groups. However, priming affected the quality of the response following ZPIV vaccination, as primed individuals recognized a broader array of flavivirus antigens than individuals in the naïve group.
Interpretation
While a priming vaccination to either JEV or YFV did not boost ZIKV-specific responses upon ZIKV vaccination, the qualitatively different responses elicited in the primed groups highlight the complexity in the cross-reactive antibody responses to flaviviruses.
Funding
This work was supported by a cooperative agreement between The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of the Army [W81XWH-18-2-0040]. The work was also funded in part by the National Institute of Allergy and Infectious Diseases (NIAID) R01AI155983 to SJK and KM.
Keywords: Japanese encephalitis virus (JEV), Yellow fever virus (YFV), Zika virus (ZIKV), Vaccination, Antibody responses, Cross-reactivity
Research in context.
Evidence before this study
There are conflicting reports on the impact of prior flavivirus immunity on the antibody response elicited by vaccination against flaviviruses.
Added value of this study
Our results showed that priming with either a JEV or a YFV vaccine did not boost antibody responses to a subsequent ZIKV vaccine. As such, neutralizing responses were actually lower in the JEV and YFV primed groups than in the naïve group. Nonetheless, antibody responses were qualitatively different in the primed groups than in the naïve group. Primed individuals targeted a diverse array of flavivirus antigens and immune profiles post-ZIKV vaccination showed that participants segregated based on their priming vaccination.
Implications of all the available evidence
Our results emphasized that priming with one flavivirus vaccine does not necessarily promote humoral immunity to a subsequent vaccine against another flavivirus and warrant further studies to better define features that can limit interference between different flavivirus vaccines.
Introduction
Zika virus (ZIKV) is a single-stranded ribonucleic acid (RNA) virus. This flavivirus was discovered in 1947 in a rhesus macaque in Uganda.1 ZIKV is endemic in Africa and Asia and the past fifteen years saw the expansion of its distribution with outbreaks in Yap islands2 and French Polynesia3 before its detection in Brazil in 20154 and its subsequent rapid spread throughout the Americas.
ZIKV is primarily transmitted by the bite of infected mosquitoes (Aedes genus) but transmission via sexual contact, blood transfusion, during pregnancy and peri-partum can also occur.5, 6, 7 While most ZIKV infections cause mild and self-limited illness, the infection can cause Guillain–Barré syndrome in adults and, if occurring during pregnancy, different foetal abnormalities, including microcephaly.8
The rapid spread of ZIKV in the Americas prompted the development of multiple candidate vaccines. There is currently no licensed vaccine against ZIKV infection. Clinical trials of different candidate vaccines showed safety and immunogenicity in a limited number of participants but their efficacy in humans has not yet been tested.9, 10, 11, 12, 13 Among them, three phase 1, randomized, placebo-controlled, double-blind trials tested a Zika purified inactivated virus (ZPIV) vaccine and showed that the vaccine was safe and well-tolerated.10 The three studies evaluated either the vaccine dose (5 μg, 2.5 μg, 10 μg), the vaccination schedule or the impact of prior flavivirus vaccination. Stephenson and colleagues enrolled 36 participants to receive a single dose or two doses given with an interval of either two or four weeks.11 Neutralizing responses were not induced following a single dose; neutralization titres peaked two weeks after the second dose in the two-dose groups (geometric mean of 1153.9 and 517.7 for the 4- vs 2-week interval) and had declined to baseline at week 28. In the third study, the vaccine was administered (two or three doses) to participants who received a Japanese encephalitis virus (JEV) vaccine or a yellow fever virus (YFV) vaccine prior to the ZPIV immunization series; both the JEV vaccine and ZPIV are inactivated vaccines while the YFV vaccine is live-attenuated.14 This vaccine regimen also demonstrated a favourable safety, tolerability and immunogenicity profile. Our study was designed to characterize the antibody-mediated immunity in participants with or without prior flavivirus vaccination and to evaluate whether a priming vaccination to a different flavivirus (either JEV or YFV) would modify the antibody response to ZPIV vaccination. In the study by Stephenson and colleagues,11 one participant who showed remarkably high ZIKV neutralization titres following vaccination was found to have had high pre-existing neutralization titres to dengue virus serotypes 1–4 (DENV-1–4) and West Nile virus (WNV) prior to the first ZPIV vaccination.15 Strong responses to ZIKV following priming to another flavivirus may be expected as ZIKV infection can activate new B cell specificities as well as cross-reactive memory B cell responses in participants previously infected with DENV.16, 17, 18 Additionally, some epidemiologic studies suggested that prior DENV infections afforded some protection against ZIKV.19 Nonetheless, different studies suggested that ZIKV vaccination in individuals with prior flavivirus exposure could induce cross-reactive binding antibodies towards distinct flaviviruses, yet, these antibodies may only be poorly neutralizing.19, 20, 21
To evaluate how prior flavivirus vaccination shaped the immune response to the ZPIV vaccine, we characterized the antibody-mediated immune profile elicited by the vaccines and investigated the impact of potentially cross-reactive responses (due to prior vaccination) on ZPIV-induced immunity using a systems serology approach.22, 23, 24 We found no evidence that priming with JEV or YFV vaccines improved the magnitude of the antibody response elicited by ZPIV, yet antibody-mediated responses showed qualitative differences across groups with more flaviviruses being recognized in the primed groups.
Methods
Ethics statement
All participants were enrolled in the clinical trial NCT02963909 registered at clinicaltrial.gov; NIAID/DMID was the regulatory sponsor for the trial. The phase 1 clinical trial was approved by WRAIR as RV 478/WRAIR #2350 and by the NIH as DMID 16–0062 since DMID/NIAID NIH was the Regulatory Sponsor of this study. Clinical trial approval had to first pass the WRAIR Scientific Review Committee and then the pre-IRB process of the Human Subjects Protection Branch and then the WRAIR Institutional Review Board (IRB). The IRB provided ethics approval and all participants provided written informed consent. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25.
Samples and antigens
To measure the antibody binding responses, we obtained 371 plasma samples from 75 participants enrolled in a phase 1, placebo-controlled, double-blind trial at the Walter Reed Army Institute of Research (WRAIR). Samples were collected with different schedules based on the priming group and the number of ZPIV doses that the participants received (Fig. S1). Binding responses were measured on day 1 (1st ZPIV), 57 (28 days after 2nd ZPIV), 252 (28 days after 3rd ZPIV), and 392 (168 days after 3rd ZPIV) for participants who received 3 doses of ZPIV or placebo and day 1, 57, 196, 364 for participants who received 2 doses of ZPIV or placebo. For participants in the two primed groups, additional samples were collected on the day of priming vaccine (110 and 82 days prior ZPIV for JEV-primed and YFV-primed respectively) and 28 days after the completion of priming vaccine (54 days prior ZPIV) (Fig. S1). Negative control samples were a pooled human plasma (Innovative Research), a ZIKV negative plasma (SeraCare) and a non-human primate plasma. Positive control samples were a pooled ZIKV positive human plasma (BEI), a JEV human serum (JEV H08/335 from VDB) and a pooled YFV positive non-human primate serum (BEI).
The antigen panel included 23 viral antigens corresponding to the envelope (E) and non-structural 1 (NS1) proteins from different flaviviruses (Zika (ZIKV), Dengue 1–4 (DENV), yellow fever (YFV), West Nile (WNV), Japanese encephalitis (JEV) and Tick-Borne encephalitis (TBEV) viruses). The envelope protein (E1) from the alphavirus CHIKV was also included as a control antigen that would not cross-react with flaviviruses; CHIKV is also mosquito-borne and co-circulates with ZIKV (Table S1). JEV, YFV and ZIKV E antigens matched the sequence used for the vaccine inserts; some proteins were produced in-house as previously described.23
Bead-based multiplex assay
Antibody responses were profiled using an assay adapted from previous studies.23,24 Per million beads, 10 μg of antigen was coupled; 1200 conjugated beads of each antigen per well were used and samples were ran in triplicate at 2 dilutions, 1:200 and 1:800. Binding to seven antibody isotypes and subclasses (IgA, total IgG, IgG1-4 and IgM) and four Fc gamma receptors (FcγR) was measured. Biotinylated FcγR2A, FcγR2B, FcγR3A (Sino Biological), and FcγR3B (in house) were tagged with a 1:4 M ratio of Streptavidin-R-Phycoetherin (Agilent). Tagged FcγR were stored at 4 °C and used within 24 h of conjugation. FcγR binding was detected by using 20 μL of Streptavidin-R-Phycoethrerin–bound FcγR (3 μg/mL). A minimum of 100 beads per antigen and per well were acquired on a FlexMap-3D (Luminex Corporation) using the xPONENT® software (Luminex Corporation) to measure the median fluorescence intensity (MFI) from the beads. Seven plates per detection were assayed with three negative and three positive plasma controls per plate. The mean MFI was calculated from the triplicates for each sample. Fold change from the baseline responses was used in the analysis and the positive threshold was set at three times the response higher than the baseline response (as a commonly used and previously described threshold for positivity23).
Neutralization assay
A high-throughput ZIKV MN assay was used for measuring ZIKV-, YFV- and JEV-specific neutralizing antibodies, as described in previous studies.10,25,26 Seropositivity was defined as a MN50 titre ≥1:10, measuring the 50% reduction of infection in Vero cells. The strains used for the microneutralization testing corresponded to the vaccine inserts: ZIKV (Strain PRVABC59); YFV (Strain 17-D); JEV (Strain SA-14-14-2).
ADCP
ADCP was measured as previously described.27 Briefly, biotinylated Zika E protein was incubated with yellow-green streptavidin-fluorescent beads (Molecular Probes) for 2 h (h) at 37 °C. 10 μl of a 100-fold dilution of beads–protein was incubated 2 h at 37 °C with 100 μl of diluted plasma (100-fold) before addition of THP-1 cells (20,000 cells per well; Millipore Sigma, Burlington, MA, USA). After a 19 h incubation at 37 °C, the cells were fixed with 2% formaldehyde solution (Tousimis, Rockville MD USA) and fluorescence was evaluated on a LSRII (BD Bioscience). The phagocytic score was calculated by multiplying the percentage of bead-positive cells by the geometric mean fluorescence intensity (MFI) of the bead-positive cells and dividing by 104.
RF-ADCC and trogocytosis
CEM.NKR ZIKV NS1 expressing cells were generated by transfection with linearized plasmid (pcDNA3.1) encoding codon-optimized the ZIKV NS1 protein corresponding to a sequence sampled in Thailand in 2014 (GenBank id: KU681081). Stable transfectants were single-cell sorted and selected to obtain a high-level NS1 surface expressing clone (CEM.NKR.NS1). RF-ADCC was measured using a previously described assay.28 Briefly, CEM. NKR.NS1 cells were stained with PKH26 (Sigma–Aldrich, St-Louis, MO, USA). Cells were then washed twice with R10 and incubated with 100-fold diluted plasma samples for 1 h at room temperature (RT). Effector cells (PBMCs) were next added in R10 at an effector to target (E:T) cell ratio of 50:1 and then incubated for 5 h at 37 °C. After the incubation, cells were washed, stained with Live/dead aqua fixable cell stain (Life Technologies, Eugene, OR, USA) and CD14 APC-Cy7 (BD Bioscience, clone MφP9) for 15 min at RT, washed again, and fixed with 4% formaldehyde (Tousimis, Rockville, MD) for 15 min at RT. Trogocytosis was evaluated by measuring the MFI of the PKH26 on the live CD14+ cells. Cytotoxicity was evaluated by measuring the percentage of Live/dead aqua positive cells within the PKH26 bright target cell population.
Statistical analysis
Data analysis and visualization were performed in R version 4.1.2 (2021-11-01) with the packages moach, ggplot2, ComplexHeatmap, ggpubr, and randomForest.
Univariate analysis
Wilcoxon rank sum tests were performed to compare responses in placebo and vaccine groups as well as the pairwise differences among the three priming groups at each time point. Wilcoxon signed rank tests were performed to compare responses between different time points. Kruskal–Wallis tests were performed to compare responses across the three priming groups. Spearman's rho was used to estimate correlations between assays. For multiple testing, the Benjamini–Hochberg procedure was used to adjust p values. Linear mixed-effects models using restricted maximum likelihood with a random intercept and slope were utilized to assess the priming effect on an individual binding feature longitudinally. Log transformed signal to noise ratio at enrollment baseline, four weeks after second and third ZPIV immunization were used and the interaction between time and priming groups was included. p values < 0.05 were considered statistically significant.
Classification of the study groups
Random forest classification models were applied to select binding features that differentiated the priming groups at four weeks after the second and third dose of immunization using the randomForest function with the number of trees set to 1000. Importance score of a feature was calculated based on mean decrease in accuracy. Partial least squares discriminant analysis was performed on the binding features with importance score above 50% and the functional data to evaluate and visualize the predictive ability for classifying the priming groups. To assess the binding responses at the study enrolment, principal component (PC) analysis was applied using prcomp function on scaled signal to noise ratio data with no group separation and low variance explained from the first two PCs due to limited responses which reflected the enrolment criteria of no neutralization of selected flaviviruses.
Role of funders
The funders had no role in study design, data collection, data analyses, interpretation, or writing of report.
Results
Study design
We investigated the antibody-mediated immune response elicited by an experimental purified inactivated Zika vaccine (ZPIV, strain PRVABC59) in 75 individuals randomized in three groups of 25 participants with five placebo participants per group (Fig. S1, Table S2).14 Participants received ZPIV on day 1 and 29, with an optional third dose at day 224. Our study included samples from the participants who received at least 2 doses of ZPIV (n = 73).
The first group of individuals, or naïve group, received only ZPIV while participants in groups 2 and 3 were immunized against other flaviviruses prior to ZPIV vaccination. Participants in group 2 received the recommended two doses of a purified formalin inactivated JEV vaccine (IXIARO®, Valneva, strain SA-14-14-2) 110 and 82 days prior to ZPIV vaccination. Participants in group 3 received the recommended single dose live attenuated YFV vaccine (YF-VAX®, Sanofi Pasteur, strain 17-D) 82 days prior to ZPIV vaccination.
We characterized antibody binding responses in 371 samples collected longitudinally using a previously developed multiplex bead-based immunoassay designed to assess Envelope (E) and non-structural protein 1 (NS1) responses against human flaviviruses (ZIKV (x8), DENV(x8), JEV (x2), YFV (x2), TBEV, WNV (x2)) and one alphavirus (Chikungunya virus CHIKV as a negative control).23,24 Profiling isotypes (IgG, IgA, IgM), subclasses (IgG1-4) and binding to four Fc gamma receptors (FcγR2A, FcγR2B, FcγR3A, FcγR3B) resulted in 587,664 data points (Fig. S2). We analysed antibody binding responses together with neutralizing and Fc effector responses via a systems serology approach (Fig. S1).
Vaccination with JEV or YFV vaccines elicited virus specific responses
Only participants without prior flavivirus exposure who demonstrated a lack of neutralization toward all four dengue virus serotypes (DENV-1-4), ZIKV, YFV, JEV and West Nile (WNV) virus were enrolled in the study. In addition to the lack of neutralizing responses to these flaviviruses, we verified that there was no separation of the study groups based on binding antibody responses. A PC analysis showed that the first two PCs described little variance, further indicating that there was no prior flavivirus immunity history that segregated participants at study entry (Fig. S3).
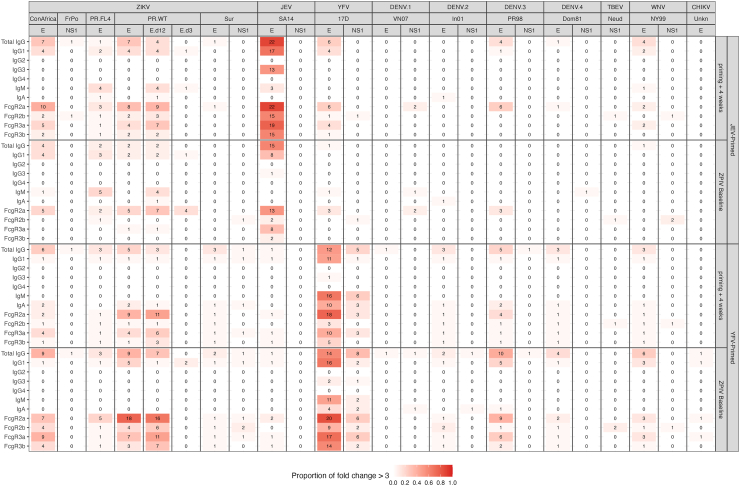
Four weeks after two doses of the JEV vaccine, individuals showed strong JEV E IgG responses with 22 of 25 individuals showing responses at least 3-fold above the baseline (median fold over baseline = 17.78, range = 1–54.76); these responses had declined significantly (Wilcoxon signed rank test p < 0.0001) eight weeks later when they received the first ZPIV immunization (4.69 [1–25.68]), yet levels at both time points were significantly higher than at enrolment (Fig. 1A). Responses against other antigens were detected at week four post second JEV vaccination but declined to background level after twelve weeks (fold change below 3). One individual had detectable IgG responses across ZIKV E antigens (week 4: 4.21–26.15, week 12: 1–4.07), knowing that this outlier individual had IgM responses towards DENV1-3, JEV, YFV, ZIKA WT E and DENV1-2 NS1 at enrolment. Other participants had responses closer to the background level. IgG1 showed similar patterns. IgG2 and IgG4 showed no significant responses (Fig. 2).
Fig. 1.
IgG responses against 24 antigens following JEV (A) and YFV (B) vaccination. Binding antibody responses are shown as fold over baseline at the enrolment visit (JEV1/YFV vaccination), four weeks after the second dose of JEV or the single dose of YFV vaccination (JEV2/YFV+4 W) and eight weeks later at the first ZPIV vaccination (ZPIV Baseline). The outlier participant in each priming group is shown with a coloured line.
Fig. 2.
Proportion of positive samples after the priming vaccinations. Number of positive samples using a fold over baseline >3 across the 24 antigens and 11 detection reagents tested. Positivity rates are shown for the JEV- and YFV-primed groups four weeks after vaccination and at the baseline for ZPIV.
YFV vaccination also elicited YFV-specific IgG responses, with responses to YFV E increasing between week 4 and week 12 after YFV vaccination which corresponded to the ZPIV baseline visit (median = 2.95 vs 5.33, p = 0.01) (Fig. 1B). At week 12, 14 of 24 individuals had responses at least 3-fold above the baseline. YFV vaccination also elicited NS1 responses in one-third of the participants (fold over baseline >3) by 12 weeks after YFV vaccination (Fig. 2). Some individuals showed responses towards other flavivirus antigens above the background threshold: DENV-3 E, ZIKV_ConAfrica E, ZIKV_PR.WT E and E domains 1 and 2 were recognized most frequently with, respectively, 10, 9, 9, 7 participants showing over 3-fold changes in their responses (Fig. 2). In particular, one outlier individual had a high response towards all E antigens except DENV-1 (and also to the alphavirus CHIKV), reactivity was high at 4 weeks post YFV vaccination and increased further at the baseline time point for ZPIV vaccination; of note, this individual was not an outlier based on responses at enrolment (except for a borderline IgM response against ZIKV_FrPo_NS1 (MFI: 882.40 and S/N: 3.15) (Fig. 1B). Similar profiles with one outlier were seen for FcγR binding responses. IgM responses were also detected against YFV E antigen at 4 weeks post YFV vaccination with a median fold over baseline of 6.94 (less than 1.62-fold for all other antigens). There was only a low signal for IgA responses (median of 2.31-fold change for YFV E and below 1.13-fold change for all other antigens) (Fig. 2). Overall, the JEV and YFV vaccinations elicited virus-specific responses with a subset of individuals showing cross-reactive binding responses, principally IgG and FcγR2A responses towards ZIKV E antigens (Figs. 1 and 2).
Binding antibodies to ZIKV were not boosted in the JEV or YFV primed groups compared to the naïve group following ZPIV vaccination
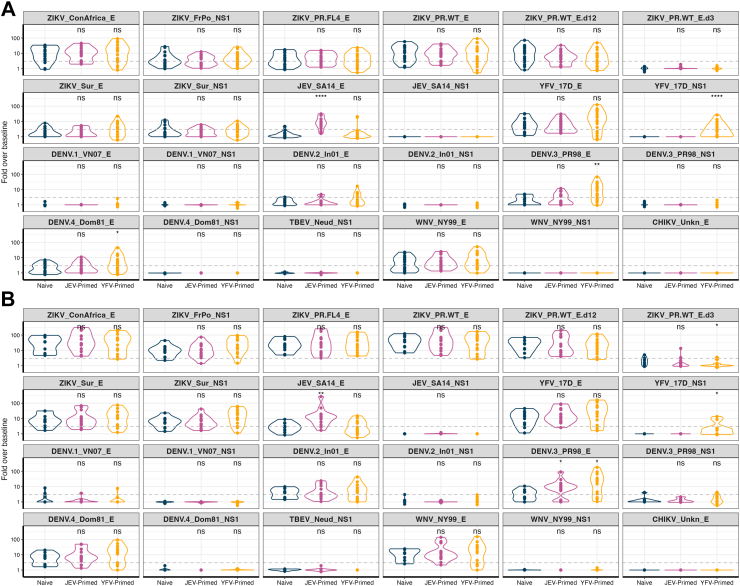
Peak antibody responses were observed four weeks after the third ZPIV vaccination at day 252 (Fig. S4, Fig. 3). In the naïve group, vaccinees’ responses were significantly higher than those from placebo recipients against ZIKV E and NS1 proteins with a median of 37.69-fold change for E towards the cognate antigen (PR_WT_E) (range 6.89–122.71) and a median increase between 5.52 and 9.14 for NS1 for IgG (Fig. S4). Responses against other flavivirus E antigens were also significantly higher in ZPIV recipients than in the placebo recipients although at levels lower than for ZIKV E with median fold increases ranging between 3.2-fold for DENV3 and 12.78-fold for WNV. There was no significant difference for NS1 antigens. Similar profiles were observed in both the JEV and YFV primed groups when compared to placebo recipients in each group: there was a significant increase in ZIKV responses against E (with a median of 34.62 (JEV group) and 45.05 (YFV group) fold change against PR_WT_E) and NS1 (median fold change ranged between 4.4 and 8.78 across antigens for the JEV group and between 11.1 and 15.64 for the YFV group), paralleled with responses at lower levels for E antigens for other flaviviruses and absent NS1 responses for other flaviviruses (Fig. S5). ZIKV responses were likely primarily focused on E domains 1 and 2, as no significant difference in responses to domain 3 were detected (max fold increase = 5.13, with 8 out of 10 participants below the 3-fold increase in the naïve group). Across all groups, responses were detected against E domain 3 in 5 individuals (2 in the naïve group, 2 in the JEV-primed group and 1 in the YFV-primed group) who had responses above 3-fold over baseline (Fig. S6).
Fig. 3.
IgG binding responses against 24 antigens four weeks after the second (A) and third (B) ZPIV vaccination. Antibody responses shown as fold over baseline at enrolment visit are compared between the naïve group and the two primed groups. The significance of Wilcoxon rank sum test is shown in each panel. ns: p > 0.05; ∗: p ≤ 0.05; ∗∗: p ≤ 0.01; ∗∗∗: p ≤ 0.001; ∗∗∗∗: p ≤ 0.0001.
When comparing IgG responses against ZIKV antigens in the vaccinees from the three groups, we did not observe significant differences four weeks after the second or third ZPIV immunization. To account for the potential time interaction with the priming groups, linear mixed-effects model was used to evaluate the total IgG responses to the antigen corresponding to the vaccine insert (ZIKV PR_WT_E). As expected, given the ZPIV immunization, the change from baseline to peak immunity was significant. However, the priming groups and the interaction with time were not significant (p > 0.085). In addition to the ZIKV E binding responses, there were ZIKV NS1 responses induced by ZPIV vaccination. These responses are likely due to the presence of some NS1 in the vaccine preparation; an optimized purification process has allowed a more efficient removal of NS1 in the second generation ZPIV-SP.29 As expected, the two primed groups showed significantly higher responses against their cognate JEV or YFV antigens while also showing higher responses against DENV-3 E (median of 9.15- and 21.6-fold over baseline in JEV- and YFV-primed groups, respectively, vs 3.2 in the naïve group, p values < 0.05) (Fig. 3). Overall, there were no significant differences across groups and no evidence that either JEV- or YFV-vaccine priming boosted ZIKV antibody responses following ZPIV vaccination.
Broader patterns of flavivirus binding antibody responses in the primed groups than in the naïve group
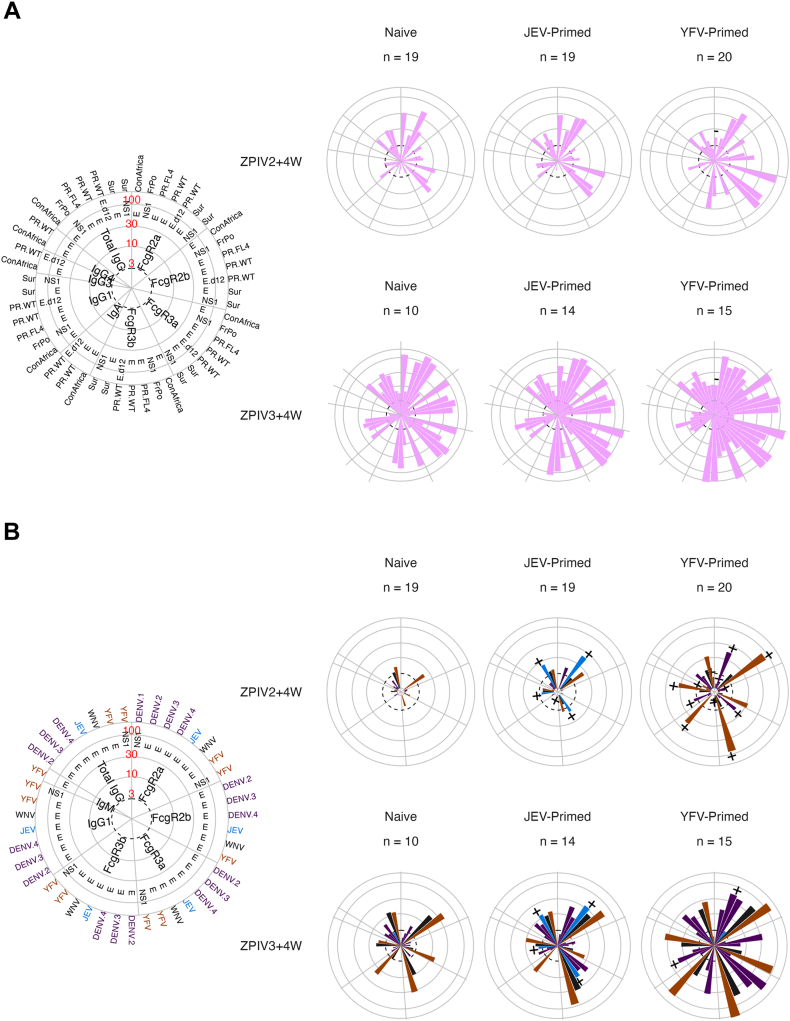
Examination of ZIKV-specific responses across the 11 isotypes, subclasses and Fcγ receptors revealed similar antibody binding profiles across groups (Fig. 4A) with few exceptions. For example, IgG1 responses towards ZIKV_ConAfrica E were significantly higher in the naïve group (median of 18.43-fold over baseline) than in the YFV-primed group (7.43-fold, p value = 0.04); likewise, FcγR2A responses against ZIKV_ConAfrica E were significantly higher in the naïve group (128.77-fold) than in the YFV-primed group (median of 10.55-fold over baseline, p value < 0.0001) four weeks after third ZPIV. Similarly, when positivity rates were considered, binding antibodies to ZIKV revealed no significant difference between groups across isotypes, subclasses and Fcγ receptors (Fig. S6).
Fig. 4.
Antibody binding responses for all antigens and detections four weeks after the second and third ZPIV vaccinations. Median fold over baseline against ZIKV antigens (A) and against DENV, YFV, WNV and JEV antigens (B) measured four weeks after the second (day 57) and third (day 252) ZPIV vaccinations. The polar plot on the left indicates the location of each antigen and detection with circles representing median folds over baseline ranging between 3 and 100. Viruses are colour coded. Features with near zero variance were excluded. The ± signs on top of the bars indicate significantly higher/lower responses in the priming groups than in the naïve group with Wilcoxon rank sum test p value < 0.05 adjusted using the Benjamini–Hochberg procedure.
In contrast to ZIKV-specific responses, binding antibodies to other flaviviruses were higher in the JEV- and YFV-primed groups than in the naïve group following ZPIV vaccinations, with statistically significant differences observed for JEV, YFV and DENV antigens (Fig. 4B). We evaluated how the ZPIV vaccination boosted JEV and YFV responses in the primed groups by looking at the fold increase from the first ZPIV dose (Fig. S7). Median fold increases at the different time points (four weeks after the second and third doses and at the last time point) were lower for JEV responses (maximum fold increase = 3.17) than for YFV responses (maximum fold increase = 82.93). When comparing the small increases in JEV responses across vaccine groups, they tended to increase slightly more in the JEV-primed group than in the naïve or YFV-primed group. When comparing increases in YFV responses across vaccine groups, responses tended to increase more in the naïve or JEV-primed group than in the YFV-primed group, possibly reflecting the higher initial level of YFV responses following the priming vaccination.
The JEV- and YFV-primed groups showed a more diverse pattern of binding responses than the naïve group, with more robust responses towards JEV and YFV antigens as well as against DENV1-4 and WNV antigens whether isotype or FcγR binding was considered (Fig. 4B). This pattern of multi-reactivity across flaviviruses expanded between day 57 (4 weeks after the second dose) and day 252 (4 weeks after the third dose) with both primed groups showing stronger multifaceted profiles than the naïve group. To assess whether there was an increase in binding antibody responses towards flavivirus that were not included in the vaccine, i.e. DENV1-4 and WNV, we measured the fold change in binding antibody responses in the JEV and YFV groups when compared to the naïve group using as a reference either the baseline at enrolment or the baseline prior to the first ZPIV vaccination. We found an increase in binding responses, particularly for DENV3 specific responses (Fig. S8). The increase was statistically significant only in the YFV primed group when comparing to the levels at enrolment, suggesting that these responses were in part induced by YFV vaccination.
The priming vaccination determined the immune profile of the participants following ZPIV vaccination
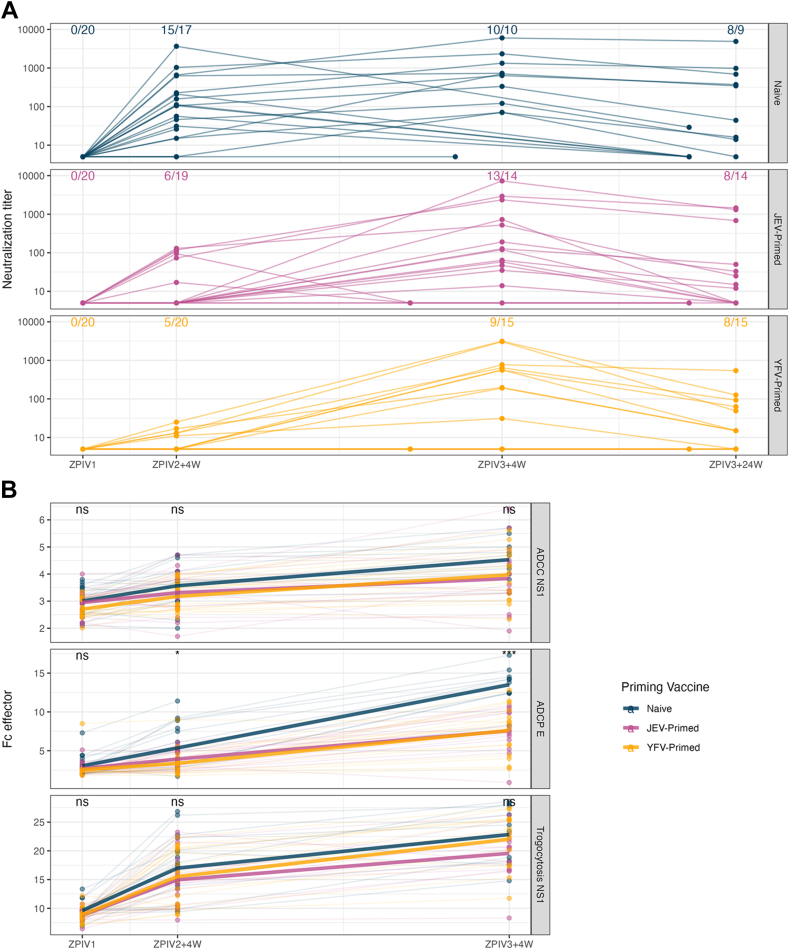
In addition to binding antibody responses, neutralization and Fc effector responses against ZIKV were measured longitudinally (Fig. 5, Fig. S9). The Fc effector functions included antibody dependent cellular phagocytosis (ADCP) measured against ZIKV E as well as antibody dependent cellular toxicity (ADCC) and trogocytosis measured against NS1. Four weeks after the second ZPIV immunization, neutralization responses were significantly higher in the naïve group than in the primed groups (p value = 0.0008 (JEV) and <0.0001 (YFV)), while ADCP responses were significantly higher in the naïve group only when compared to the YFV-primed group (p value = 0.161 (JEV) and = 0.012 (YFV)) (Fig. S9). Following the third ZPIV immunization, the difference was no longer significant for the neutralization (p value = 0.172 (JEV) and = 0.088 (YFV)). However, ADCP responses remained significantly higher in the naïve group than in the primed groups (p value = 0.0002 (JEV) and = 0.0004 (YFV)) (Fig. S9). Unlike neutralization and ADCP which were measured against the ZIKV E antigen, ADCC and trogocytosis were measured against ZIKV NS1. There was no significant difference across groups for ADCC and trogocytosis (p > 0.15), as could be expected given the rare NS1 binding responses measured in participants. Neutralization and ADCP responses were correlated with each other (Spearman's rho: 0.65 and 0.64 four weeks after second and third ZPIV, p < 0.001) (Fig. S10). All three groups showed strong correlations between binding to ZIKV E and neutralizing responses (Spearman's rho >0.66 for IgG against the cognate antigen) with a slightly higher value in the naïve group (Fig. S11). For Fc effector responses, we observed different patterns in the three groups. Strong correlations between ADCP and FcγRs binding to ZIKV E were seen in the naïve and YFV-primed groups. The JEV-primed group showed overall limited correlations between binding antibodies and all three Fc effector responses. In the YFV-primed group, ADCC and trogocytosis associated with binding responses against both ZIKV E and NS1. Across all binding and functional measurements, there was no difference based on sex between the three groups at each time point.
Fig. 5.
Neutralizing antibody responses (A) and Fc effector function responses (B) against ZIKV in vaccine recipients. The number of seropositive participants (neutralization titre >10) and total number within each group are reported in panel A. Neutralization and ADCP were measured against ZIKV E, while ADCC and trogocytosis were measured against ZIKV NS1. Significant difference of Fc effector responses among the three vaccine regimens were reported using Kruskal–Wallis test with asterisks: ∗: p ≤ 0.05; ∗∗: p ≤ 0.01; ∗∗∗: p ≤ 0.001; ∗∗∗∗: p ≤ 0.0001.
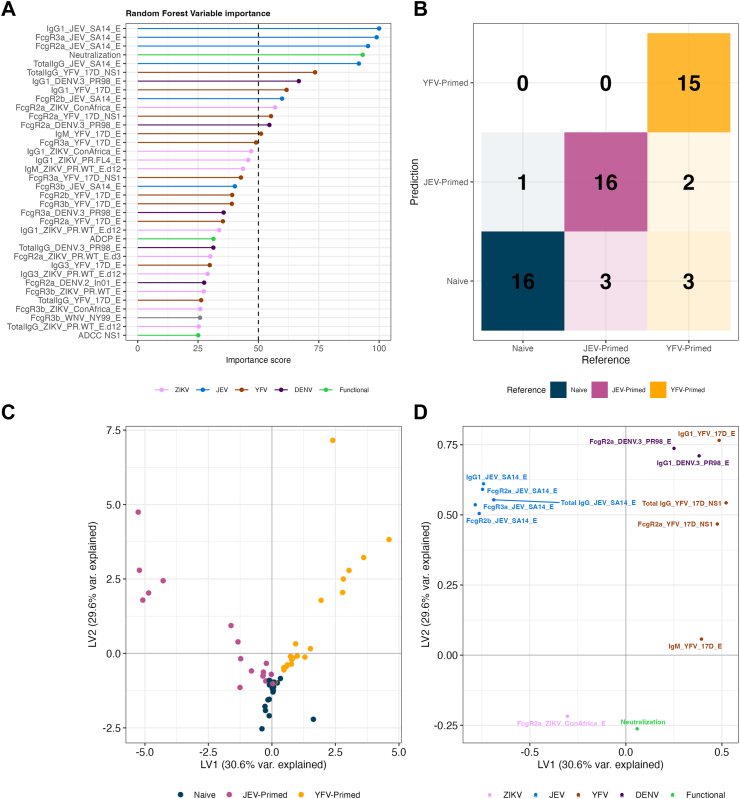
We used random forest models to distinguish the three groups based on binding and functional responses four weeks after the second (Day 57) (Fig. 6) and third ZPIV immunization (Day 252) (Fig. S12). Consistent with the univariate analysis, the features that were selected with the highest importance at Day 57 were binding responses against JEV, YFV, DENV3 and the neutralization titre against ZIKV; the only ZIKV-specific binding feature with an importance score over 50 was FcγR2A towards ConAfrica E (Fig. 6A). At Day 252, ADCP was the top feature selected by the model followed by binding responses against ZIKV, DENV3, JEV, and YFV (Fig. S12A). The model was able to classify the participants to the priming groups with an accuracy of 83.93% using data from four weeks after the second ZPIV immunization (Fig. 6B) and a reduced accuracy of 74.36% after the third ZPIV immunization (Fig. S12B), indicating that the impact of the priming-induced immunogenicity had waned over time. Features with importance score over 50 were selected and used in partial least squares discriminant analysis (PLSDA) to evaluate and visualize the separation of the three groups (Fig. 6C and D, Fig. S12C and D). At Day 57, the first dimension (LV1) captured the difference between the JEV- and YFV-primed groups which was driven by the binding responses towards antigens corresponding to the priming vaccines. The second dimension (LV2) illustrated that the elevated FcγR2A ConAfrica E responses and neutralization titre as well as the absence of DENV3 contributed to the separation of the naïve group from the two primed groups. At Day 252, the clusters corresponding to each group were less prominent (Fig. S12C and D).
Fig. 6.
Immune profiles four weeks after the second ZPIV vaccination (day 57). Random Forest models selected features that are reported with their importance score (A) and used to classify participants in each vaccine group B). PLSDA models are shown with PLSDA scores (C) and loadings (D).
Post-ZPIV vaccination, participants were separated according to their priming status, yet, there were important individual differences within groups. We sought to identify if certain features characterized the inter-individual variability. For example, six of the 15 participants in the YFV-primed group never had detectable ZPIV neutralization titres (Fig. S13A). We investigated the responses after the priming vaccination and found that there was a trend towards higher IgA and IgM responses against YFV in those who did not develop neutralization to ZPIV (Fig. S13B) although we cannot rule out the possibility that this was driven by a small number of outliers. Similarly, six of the 19 participants in the JEV-primed group were early responders who had detectable neutralization titre 4 weeks after second ZPIV (Fig. S14). After the priming vaccination, these individuals were distinguished by higher binding responses to JEV E antigens than the late responders albeit without any significant difference suggesting that early JEV E binding responses associated with early ZIKV neutralization titres in these primed individuals.
Discussion
We investigated the influence of priming vaccinations to either JEV or YFV on the antibody responses induced subsequently by another flavivirus vaccine, the Zika purified inactivated virus (ZPIV) vaccine. This vaccine was previously demonstrated to be safe and well tolerated.10 We characterized the antibody binding responses, Fc gamma receptor engagement, Fc effector functions and neutralization responses at six time points spanning the priming and ZPIV immunization series until day 392 after ZPIV vaccination. Strikingly, there was no evidence that priming vaccinations boosted subsequent responses to ZPIV vaccination. On the contrary, participants in the naïve group had significantly higher or similar neutralizing and ADCP responses to ZIKV when compared to participants in the JEV- and YFV-primed groups. While ZIKV-specific binding antibodies were not different in magnitude across groups, binding antibodies to other flavivirus antigens were higher and more diverse in the JEV or YFV primed groups than in the naïve group; these responses did not only focus on JEV or YFV antigens (corresponding to the priming immunization) but also targeted DENV or WNV antigens, for which no vaccination was received. Our goal was to investigate potential differences across primed and naïve groups; yet, since individuals received multiple immunizations at different time points, our analyses evaluate the vaccine-induced immune responses at key immunity time points. We employed a comprehensive systems serology analysis that integrated all antibody biophysical profiling and functional responses. This revealed that, four weeks after the second dose, participants were distinguished by their priming vaccination resulting in three clusters separating each group. This clustering, albeit with less demarcation, remained at day 252, four weeks after the third vaccination.
Our results indicated that the priming with JEV or YFV vaccination did not confer an advantage in the elicitation of ZIKV responses of high magnitude–a somewhat unexpected finding given that, in theory, a priming vaccination series would be thought to boost responses. A landmark study in 1983 showed that a live attenuated DENV vaccine in individuals previously vaccinated against YFV yielded higher and more durable antibody titres than in non-immune participants.30 Similarly, different studies showed that pre-existing immunity due to vaccination or prior flavivirus exposure enhanced cross-reactive responses in secondary ZIKV infections.16,31, 32, 33, 34, 35 However, some recent studies have shown equivocal results regarding the boosting yielded through consecutive flavivirus immunizations. Bradt and colleagues showed that prior vaccination against YFV had a negative impact on the neutralizing antibody response elicited following TBEV vaccination.36 Glass and colleagues reported that JEV vaccination prior to a tetravalent DENV vaccine (Dengvaxia) did not increase neutralizing antibody titres.37 Larocca and colleagues showed that rhesus macaques and mice immunized with a live attenuated DENV vaccine developed neutralizing antibodies to multiple DENV serotypes but not against ZIKV and found no evidence that prior DENV vaccination improved outcomes following ZIKV vaccination.38
Several factors could explain the lack of evidence of boosting of ZIKV binding and functional responses by prior immunizations against JEV or YFV. First, the similar neutralizing responses across the three groups might be due to the preferential targeting of epitopes conserved across flaviviruses but with limited neutralization potency such as the fusion loop in E Domain 2 (which are also associated with enhancement). While neutralizing antibodies can target all three domains of E, many of the most potent antibodies target E Domain 3 and are virus-specific.39 In our study, binding antibody responses principally targeted E Domains 1 and 2 although 5 individuals (2 from JEV-primed group, 1 in YFV-primed group) showed responses above 3-fold over baseline towards ZIKV E Domain 3. However, Domain 3 responses did not show particular importance in the clustering analyses. It has previously been reported that binding antibody levels may not align with neutralization titres: several studies found binding antibodies that cross-reacted with DENV and ZIKV but only poorly neutralized both viruses.16,40,41 It is also likely that conformational epitopes of E were better preserved in live attenuated vaccines (YFV) than in inactivated ones (JEV, ZPIV). Second, binding antibody characteristics could be preferentially associated with non-neutralizing rather than neutralizing antibody features. However, we found no evidence that this vaccine regimen favoured boosting of non-neutralizing Fc effector functions at the detriment of neutralizing antibody responses, as assays characterizing Fc effector, specifically ADCP, and neutralization responses showed similar patterns with superior or similar responses in the naïve group after the second ZPIV vaccination and no evidence of boosting in the JEV- and YFV-primed groups. In addition, neutralizing and ADCP responses were strongly correlated in all study groups. Hence, we found no evidence suggesting that prior flavivirus vaccination directed antibody responses towards non-neutralizing Fc effector specificities. It is possible that the diversification of the binding antibody response observed in the primed groups resulted in epitope spread but that these epitopes were not necessarily functional against ZIKV.
There are additional hypotheses that we could not evaluate in this study. The timing of the prior flavivirus experience may dictate the strength of the response to a subsequent flavivirus immunization (or infection) as a longer time span between immunizations could yield more mature antibody responses. A prior study showed that intervals between prime and boost of three or six months were more immunogenic than a one month interval for a DENV vaccine42; the benefits of longer intervals between prime and boost have also been reported for COVID-19 or HIV-1 vaccines. It is possible that different intervals between the JEV or YFV priming vaccinations and the ZPIV vaccination could have modified the ZIKV-induced immunity. Distinct combinations of flavivirus antigens, more or less distantly related, could interact to elicit superior or inferior antibody-mediated immunity upon a secondary vaccination series. Here, JEV is closer to ZIKV than YFV is but our study was not powered to distinguish differences according to the priming group. Another hypothesis is that the lack of ZIKV-boosting in the primed groups could be due to the formation of immune complexes between pre-existing antibodies and the ZPIV antigen which could lead to competition between naïve and memory B cells, potentially hampering the induction of ZPIV-specific immune responses. This could also happen through epitope masking by cross-reactive JEV- or YFV-vaccination induced antibodies. Several reports highlighted that previous ZIKV and DENV antibody responses may pose a risk of exacerbated disease upon secondary exposure to a heterologous virus through antibody dependent enhancement (ADE).32,43, 44, 45 In this instance, antibodies would fail to neutralize but may opsonize the secondary virus and enhance its capture by FcγR expressing cells, leading to enhanced viral replication and activation of cross-reactive memory T cells and possibly a cytokine storm.46 While our data did not show evidence that FcγR engagement with ZPIV-induced antibodies negatively impacted neutralization, it can be hypothesized that these vaccine elicited antibodies could mediate ADE. This hypothesis would need to be tested experimentally to better define whether and how vaccine induced antibodies can mediate both protection and enhancement. A prior study evaluated T cell responses in this cohort and identified CD4 T cell responses but no CD8 T cell responses following ZPIV immunization.47 Participants in the JEV-primed group tended to have more durable CD4 T cell responses than those in the naïve or YFV-primed groups. Unlike the inactivated JEV and ZPIV vaccines that targeted E, the YFV vaccine is a live-attenuated vaccine that induced responses, preferentially CD8 T cells, to structural and non-structural proteins (particularly towards the immunogenic NS1). The distinct T cell immunodominance patterns seen after the JEV and YFV vaccinations may explain the more limited cross-reactive T cell responses observed in the YFV group compared to the JEV group following ZPIV; however, it is unclear whether it affected neutralizing responses in these participants.
While we demonstrated that JEV- or YFV-priming yielded a broader array of flavivirus-specific antibody binding specificities, understanding the link between these multi-faceted binding responses and the lack of boosting in the primed groups requires additional investigations. Our knowledge of the potential interactions between flavivirus vaccinations is limited and, at the individual level, often mired in an unknown flavivirus exposure history. More studies are needed to evaluate the impact of consecutive flavivirus vaccinations and to characterize interactions between specific flaviviruses that may yield different patterns and possibly synergistic ones. An interesting aspect of our analyses is the strong variability of individual responses observed in each group suggesting that some individual specificities may have masked broader patterns that were not measurable due to the small number of participants per group in our study. One limitation of our study was the small group sizes (n = 25 participants per group and n ≤ 15 vaccinees per group by the third dose of ZPIV) inherent to a phase 1 clinical trial, and we recognize that this small sample size poses a constraint on our choice of analysis method. The limitation of power has been acknowledged for systems serology analyses.48 Although we can lose power and thus statistical rigor with this framework, a systems serology analysis offers an unbiased and comprehensive approach to survey an array of biophysical features and functions of the humoral response with high resolution. There is a trade off between statistical power and the likelihood of identifying novel responses that can advance vaccine development. To advance an antibody-omic understanding while ensuring a robust analysis, we employed rigorous machine learning methods such as feature selection and correlation filtering methods.
In summary, the priming immunization had a lasting effect on the immune profile of the participants, as binding, Fc effector and neutralizing features segregated participants in three clusters even after the third ZPIV vaccination. Moreover, the fact that there was no boosting of ZIKV responses in the JEV- or YFV-primed groups can suggest that the JEV- or YFV-priming interfered negatively with the development of ZIKV responses following the subsequent ZIPV vaccination, possibly due to masking, competition or dilution of ZPIV antibody targets. However, whether the constrained ZIKV responses in the primed groups has clinical relevance is unknown. Importantly, individuals in the primed groups had a more diverse pattern of reactivity across flaviviruses and it can be hypothesized that a more balanced immunity towards flaviviruses could have benefits in a clinical setting. Our results demonstrated that antibody responses to one flavivirus can modulate the development of antibody responses to another flavivirus and warrant future studies to elucidate which factors can potentiate or harm the development of protective immune responses in serial combinations of flavivirus vaccinations. The increasing prevalence of flaviviruses should renew interest in deciphering the mechanisms behind how prior immunity influences recall and de novo responses between related flaviviruses that present highly conserved (e.g., the fusion loop) as well as virus-specific epitopes.
Contributors
Conceptualization: YL, MR.
Data curation: YL, MM, SWR, TM.
Had access to and verified all the original data: YL, MM, SWR, TM, MR.
Software: YL, TM.
Formal Analysis: YL.
Visualization: YL.
Investigation: YL, MM, SWR, BB, TM, DJC, JRC, RDLB, DPP, MAE.
Supervision: MR.
Produced antigens as described in 23: VD, LMR, RDLB, SJK.
Conducted the RV478 clinical trial: NLM, MAK, KM.
Writing—original draft: YL, MR.
Writing—review & editing: YL, MM, SWR, BB, TM, JRC, VD, SJK, RDLB, NLM, DPP, MAK, KM, MR. All authors read and approved the final version of the manuscript.
Data sharing statement
All data are available in the main text or the supplementary materials.
Declaration of interests
Kayvon Modjarrad is an employee of Pfizer. Rafael De La Barrera is one of the inventors for the WO2017210215A1 Patent: Zika virus vaccine and methods of production. All other authors declare that they have no competing interests. The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army, the Department of Defense, or the Department of Health and Human Services.
Acknowledgements
We thank the trial participants and clinical team, we also thank those involved in the development of the vaccine candidate and design of the phase 1 trial: Stephen Thomas, Richard Jarman, Kenneth Eckels, Leyi Lin. We also thank Julie Ake, Nathalie Collins, Paul Edlefsen, Leilani Francisco, Morgan Geniviva, Shida Shangguan, Glenna Schluck and Sandhya Vasan.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104815.
Appendix A. Supplementary data
References
- 1.Dick G.W., Kitchen S.F., Haddow A.J. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 2.Duffy M.R., Chen T.H., Hancock W.T., et al. Zika virus outbreak on yap island, federated states of Micronesia. N Engl J Med. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 3.Cao-Lormeau V.M., Roche C., Teissier A., et al. Zika virus, French polynesia, South pacific, 2013. Emerg Infect Dis. 2014;20(6):1085–1086. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campos G.S., Bandeira A.C., Sardi S.I. Zika virus outbreak, bahia, Brazil. Emerg Infect Dis. 2015;21(10):1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen L.R., Jamieson D.J., Powers A.M., Honein M.A. Zika virus. N Engl J Med. 2016;374(16):1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 6.D'Ortenzio E., Matheron S., Yazdanpanah Y., et al. Evidence of sexual transmission of zika virus. N Engl J Med. 2016;374(22):2195–2198. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
- 7.Driggers R.W., Ho C.Y., Korhonen E.M., et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med. 2016;374(22):2142–2151. doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- 8.Cauchemez S., Besnard M., Bompard P., et al. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet. 2016;387(10033):2125–2132. doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaudinski M.R., Houser K.V., Morabito K.M., et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet. 2018;391(10120):552–562. doi: 10.1016/S0140-6736(17)33105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modjarrad K., Lin L., George S.L., et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet. 2018;391(10120):563–571. doi: 10.1016/S0140-6736(17)33106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephenson K.E., Tan C.S., Walsh S.R., et al. Safety and immunogenicity of a Zika purified inactivated virus vaccine given via standard, accelerated, or shortened schedules: a single-centre, double-blind, sequential-group, randomised, placebo-controlled, phase 1 trial. Lancet Infect Dis. 2020;20(9):1061–1070. doi: 10.1016/S1473-3099(20)30085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han H.H., Diaz C., Acosta C.J., Liu M., Borkowski A. Safety and immunogenicity of a purified inactivated Zika virus vaccine candidate in healthy adults: an observer-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21(9):1282–1292. doi: 10.1016/S1473-3099(20)30733-7. [DOI] [PubMed] [Google Scholar]
- 13.Tebas P., Roberts C.C., Muthumani K., et al. Safety and immunogenicity of an anti-zika virus DNA vaccine. N Engl J Med. 2021;385(12):e35. doi: 10.1056/NEJMoa1708120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koren M.A., Lin L., Eckels K.H., et al. Safety and immunogenicity of a purified inactivated Zika virus vaccine candidate in adults primed with a Japanese encephalitis virus or yellow fever virus vaccine in the USA: a phase 1, randomised, double-blind, placebo-controlled clinical trial. Lancet Infect Dis. 2023;S1473-3099(23):00192. doi: 10.1016/S1473-3099(23)00192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dussupt V., Sankhala R.S., Gromowski G.D., et al. Potent Zika and dengue cross-neutralizing antibodies induced by Zika vaccination in a dengue-experienced donor. Nat Med. 2020;26(2):228–235. doi: 10.1038/s41591-019-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers T.F., Goodwin E.C., Briney B., et al. Zika virus activates de novo and cross-reactive memory B cell responses in dengue-experienced donors. Sci Immunol. 2017;2(14) doi: 10.1126/sciimmunol.aan6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Barraquer I., Costa F., Nascimento E.J.M., et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science. 2019;363(6427):607–610. doi: 10.1126/science.aav6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon A., Gresh L., Ojeda S., et al. Prior dengue virus infection and risk of Zika: a pediatric cohort in Nicaragua. PLoS Med. 2019;16(1) doi: 10.1371/journal.pmed.1002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitehead S.S., Pierson T.C. Effects of dengue immunity on Zika virus infection. Nature. 2019;567(7749):467–468. doi: 10.1038/d41586-019-00868-6. [DOI] [PubMed] [Google Scholar]
- 20.Burgomaster K.E., Foreman B.M., Aleshnick M.A., et al. Limited flavivirus cross-reactive antibody responses elicited by a zika virus deoxyribonucleic acid vaccine candidate in humans. J Infect Dis. 2021;224(9):1550–1555. doi: 10.1093/infdis/jiab185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malafa S., Medits I., Aberle J.H., et al. Impact of flavivirus vaccine-induced immunity on primary Zika virus antibody response in humans. PLoS Negl Trop Dis. 2020;14(2) doi: 10.1371/journal.pntd.0008034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mdluli T., Jian N., Slike B., et al. RV144 HIV-1 vaccination impacts post-infection antibody responses. PLoS Pathog. 2020;16(12) doi: 10.1371/journal.ppat.1009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merbah M., Wollen-Roberts S., Shubin Z., et al. A high-throughput multiplex assay to characterize flavivirus-specific immunoglobulins. J Immunol Methods. 2020;487 doi: 10.1016/j.jim.2020.112874. [DOI] [PubMed] [Google Scholar]
- 24.Li Y., Merbah M., Wollen-Roberts S., et al. Coronavirus antibody responses before COVID-19 pandemic, Africa and Thailand. Emerg Infect Dis. 2022;28(11):2214–2225. doi: 10.3201/eid2811.221041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbink P., Larocca R.A., De La Barrera R.A., et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353(6304):1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larocca R.A., Abbink P., Peron J.P., et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536(7617):474–478. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ackerman M.E., Moldt B., Wyatt R.T., et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366(1-2):8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alrubayyi A., Schuetz A., Lal K.G., et al. A flow cytometry based assay that simultaneously measures cytotoxicity and monocyte mediated antibody dependent effector activity. J Immunol Methods. 2018;462:74–82. doi: 10.1016/j.jim.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Lecouturier V., Bernard M.C., Berry C., et al. Immunogenicity and protection conferred by an optimized purified inactivated Zika vaccine in mice. Vaccine. 2019;37(20):2679–2686. doi: 10.1016/j.vaccine.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Scott R.M., Eckels K.H., Bancroft W.H., et al. Dengue 2 vaccine: dose response in volunteers in relation to yellow fever immune status. J Infect Dis. 1983;148(6):1055–1060. doi: 10.1093/infdis/148.6.1055. [DOI] [PubMed] [Google Scholar]
- 31.Lanciotti R.S., Kosoy O.L., Laven J.J., et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stettler K., Beltramello M., Espinosa D.A., et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353(6301):823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 33.Priyamvada L., Suthar M.S., Ahmed R., Wrammert J. Humoral immune responses against zika virus infection and the importance of preexisting flavivirus immunity. J Infect Dis. 2017;216(suppl_10):S906–S911. doi: 10.1093/infdis/jix513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai L., Rouphael N., Xu Y., et al. Innate, T-, and B-cell responses in acute human zika patients. Clin Infect Dis. 2018;66(1):1–10. doi: 10.1093/cid/cix732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Souza N., Felix A.C., de Paula A.V., Levi J.E., Pannuti C.S., Romano C.M. Evaluation of serological cross-reactivity between yellow fever and other flaviviruses. Int J Infect Dis. 2019;81:4–5. doi: 10.1016/j.ijid.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 36.Bradt V., Malafa S., von Braun A., et al. Pre-existing yellow fever immunity impairs and modulates the antibody response to tick-borne encephalitis vaccination. NPJ Vaccines. 2019;4:38. doi: 10.1038/s41541-019-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glass A., Polhemus M., Wang D., et al. The effects of Japanese encephalitis vaccine and accelerated dosing scheduling on the immunogenicity of the chimeric yellow fever derived tetravalent dengue vaccine: a phase II, randomized, open-label, single-center trial in adults aged 18 to 45 Years in the United States. J Infect Dis. 2020;221(7):1057–1069. doi: 10.1093/infdis/jiz592. [DOI] [PubMed] [Google Scholar]
- 38.Larocca R.A., Abbink P., Ventura J.D., et al. Impact of prior Dengue immunity on Zika vaccine protection in rhesus macaques and mice. PLoS Pathog. 2021;17(6) doi: 10.1371/journal.ppat.1009673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierson T.C., Fremont D.H., Kuhn R.J., Diamond M.S. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe. 2008;4(3):229–238. doi: 10.1016/j.chom.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhaumik S.K., Priyamvada L., Kauffman R.C., et al. Pre-existing dengue immunity drives a DENV-biased plasmablast response in ZIKV-infected patient. Viruses. 2018;11(1):19. doi: 10.3390/v11010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katzelnick L.C., Zambrana J.V., Elizondo D., et al. Dengue and Zika virus infections in children elicit cross-reactive protective and enhancing antibodies that persist long term. Sci Transl Med. 2021;13(614) doi: 10.1126/scitranslmed.abg9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin L., Lyke K.E., Koren M., et al. Safety and immunogenicity of an AS03(B)-Adjuvanted inactivated tetravalent dengue virus vaccine administered on varying schedules to healthy U.S. Adults: a phase 1/2 randomized study. Am J Trop Med Hyg. 2020;103(1):132–141. doi: 10.4269/ajtmh.19-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dejnirattisai W., Supasa P., Wongwiwat W., et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016;17(9):1102–1108. doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bardina S.V., Bunduc P., Tripathi S., et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science. 2017;356(6334):175–180. doi: 10.1126/science.aal4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katzelnick L.C., Narvaez C., Arguello S., et al. Zika virus infection enhances future risk of severe dengue disease. Science. 2020;369(6507):1123–1128. doi: 10.1126/science.abb6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halstead S.B. Dengue. Lancet. 2007;370(9599):1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 47.Lima N.S., Moon D., Darko S., et al. Pre-existing immunity to Japanese encephalitis virus alters CD4 T cell responses to zika virus inactivated vaccine. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.640190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackerman M.E., Barouch D.H., Alter G. Systems serology for evaluation of HIV vaccine trials. Immunol Rev. 2017;275(1):262–270. doi: 10.1111/imr.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.