Abstract
The incidence and prevalence of peripheral artery disease (PAD) are increasing globally and have a marked economic burden in the United States. The American Heart Association/American College of Cardiology guidelines recommend exercise therapy as a Class 1A, but its utilization remains suboptimal. This state-of-the-art review aims to provide a comprehensive review of the most updated information available on PAD, along with its risk factors, management options, outcomes, economic burden, and the role of exercise therapy in managing PAD.
Article Highlights.
-
•
Peripheral artery disease (PAD) is a growing global epidemic and is becoming a marked economic burden in the United States.
-
•
PAD is frequently under recognized and under treated despite its high morbidity and mortality.
-
•
Increased physician awareness is necessary to improve prevention, early detection, and treatment to avoid adverse outcomes and reduce health care costs.
-
•
Exercise therapy (ET) for PAD is underutilized despite the Class 1A recommendation from the American Heart Association/American College of Cardiology guidelines.
-
•
Barriers to ET include lack of access to supervised ET programs and lack of physician awareness regarding coverage of this therapy.
Peripheral artery disease (PAD) describes the partial or complete obstruction of any arteries that supply the limbs, most likely caused by atherosclerosis.1 Historically, the term PAD was used to refer to any occlusive disease affecting any arteries other than the coronary arteries, however the contemporary use of PAD has started to specifically refer to atherosclerotic disease exclusively affecting the distal aorta and lower extremities.
Several risk factors have been associated with the development and progression of PAD. Although most patients are asymptomatic, manifestations range from exertion-induced claudication to gangrene requiring amputation.2 Medical treatment is warranted for all patients with PAD, whereas revascularization strategies are reserved for patients with more severe disease. The incidence and prevalence of PAD continue to increase globally,3 and PAD hospitalizations have a marked economic burden in the United States.4
Exercise therapy (ET) is important for the treatment and prevention of PAD and has been shown to reduce symptoms of claudication, improve functional performance, and improve quality of life (QoL) in patients with PAD.5, 6, 7 Many physiologic mechanisms of ET may be involved in the improvement of PAD. ET has been shown to improve several outcomes with minimal complications. Despite being covered by the Centers for Medicare and Medicaid Services (CMS) since 2017 and having a Class 1A recommendation by the American Heart Association or American College of Cardiology (AHA/ACC) guidelines in the management of PAD, utilization of ET in Medicare patients has been low.8, 9, 10 This state-of-the-art paper aims to be a fairly comprehensive review of the most updated information available on PAD and the role of ET in management.
Peripheral Artery Disease
Pathogenesis and Risk Factors
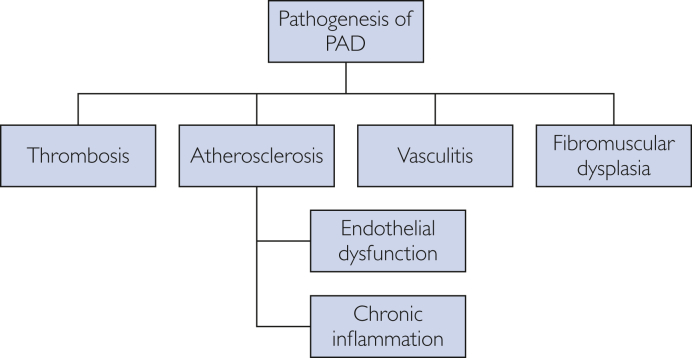
Peripheral artery disease continues to grow as a national and global health burden.11,12 The leading cause of PAD is atherosclerosis, with rare cases owing to thrombosis, vasculitis, fibromuscular dysplasia, or vessel trauma.1 Atherosclerosis leads to stenotic lesions consisting of atherosclerotic plaques with calcium deposition, thinning of the media, patchy destruction of muscle and elastic fibers, fragmentation of the internal elastic lamina, and thrombi composed of platelets and fibrin. Peripheral artery disease primarily involves the abdominal aorta and iliac arteries, the femoral and popliteal arteries, and the tibial and peroneal arteries, with preferential involvement of arterial branch points because of increased turbulence, altered shear stress, and intimal injury. Disruption of the endothelial function can cause loss of nitric oxide in skeletal muscle microvasculature, which can blunt the hyperemic responses to exercise and ischemia. This response can limit oxygen delivery under conditions of increased demand.13 Chronic ischemia harms skeletal muscle tissue, leading to reduced overall area, decreased muscle density, and increased fat content,14 and at the microscopic level, there is evidence of increased muscle apoptosis, reduced type I fibers, and reduced capillary density.15 Chronic inflammation is also believed to play a role in PAD and is associated with its progression.16 Patients develop claudication when there is an exercise-induced imbalance between oxygen supply and demand to the muscles during exertion (Figure 1).
Figure 1.
Pathogenesis of PAD. PAD, peripheral artery disease.
Risk factors for PAD include smoking, diabetes mellitus (DM), chronic kidney disease, age, hypertension, and dyslipidemia (Figures 2 and 3).17, 18, 19 Although genetics may play a role in the development of PAD, lifestyle risk factors appear to outweigh genetic factors in the development of vascular disease.20
Figure 2.
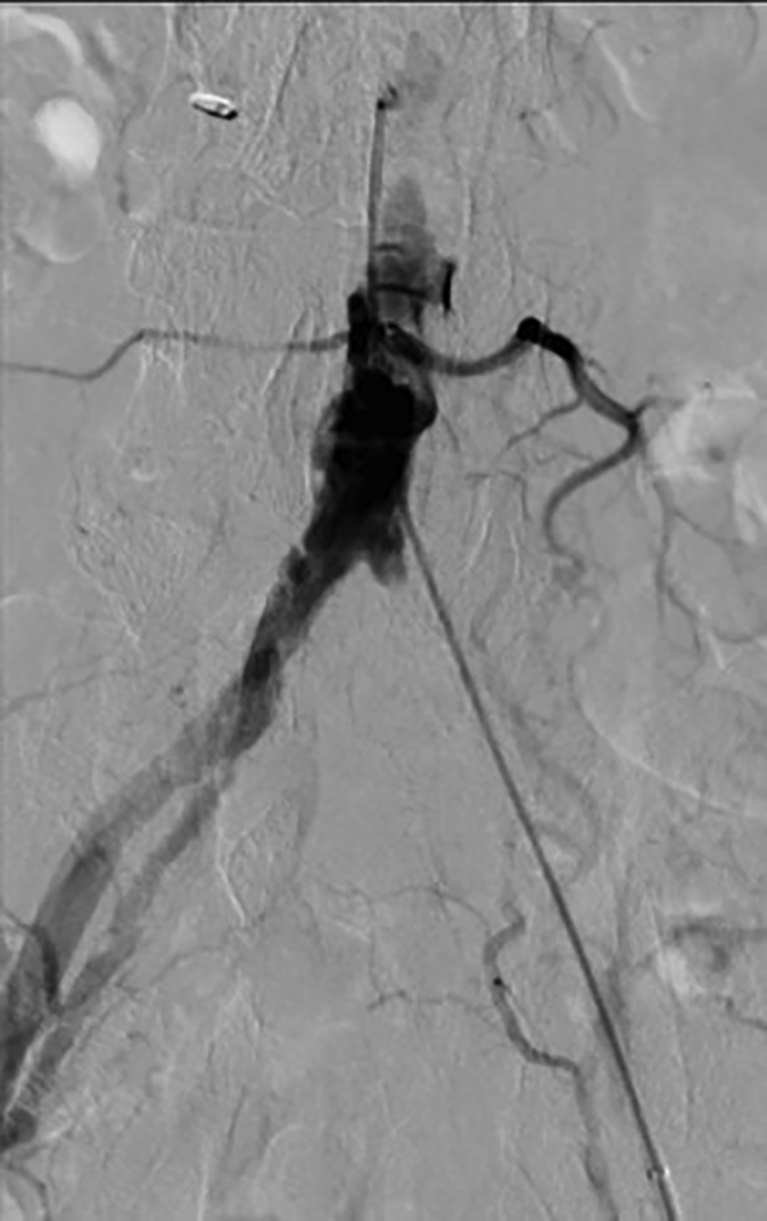
58-year-old male hypertensive smoker with occluded left common iliac artery, with distal reconstitution by collaterals. Proximal PAD is common among smokers. PAD, peripheral artery disease.
Figure 3.
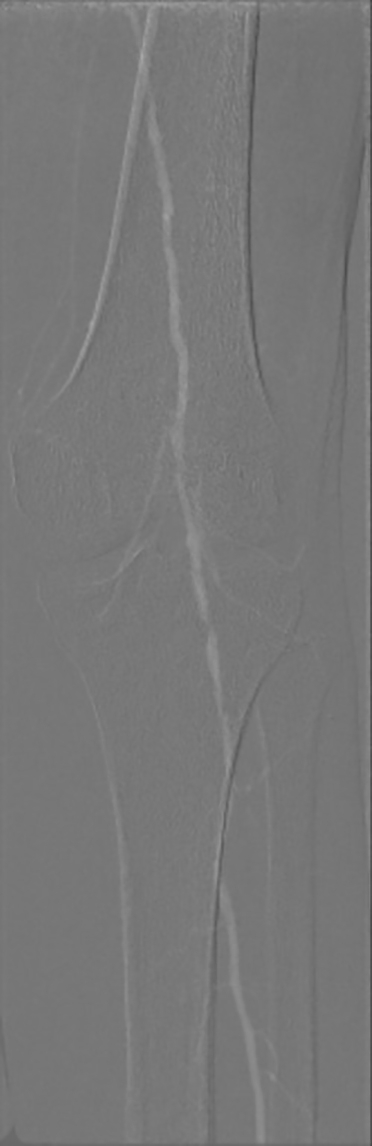
77-year-old woman with DM and CKD. CO2 angiography showing a crucial popliteal artery disease with 1 vessel runoff. Below the knee disease is typical in diabetic patients. DM, diabetes mellitus; CKD, chronic kidney disease.
Diagnosis
Peripheral artery disease is diagnosed by the ankle-brachial index (ABI), which compares the systolic pressure in each leg to the highest systolic pressure in the arms. The normal range is 1-1.4, where 0.9-1 is borderline abnormal, below 0.9 is diagnostic of PAD, and above 1.4 suggests arterial calcification with noncompressible vessels. Patients with chronic limb-threatening ischemia (CLTI) generally have an ABI of 0.4 or below. In patients with PAD symptoms and a normal ABI, an exercise ABI can be performed to diagnose occult PAD (Figure 4).
Figure 4.
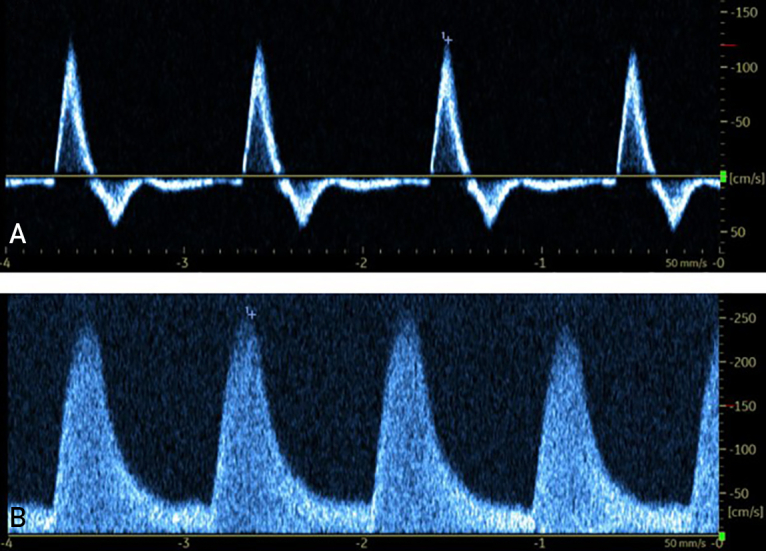
A, Doppler ultrasound of a healthy femoral artery in a 67-year-old woman. Waveform reports rapid upstroke with a sharp peak, multiphasic flow, and minimal spectral broadening with normal velocity. B, Doppler ultrasound of a diseased posterior tibial artery in a 68-year-old man with moderate PAD. This study revealed multiple signs of stenosis, such as elevated velocity, spectral broadening, monophasic flow, and low resistance, which is characterized by slow downstroke and diastolic flow. PAD, peripheral artery disease.
Treatment
The treatment of PAD involves risk factor modification, guideline-directed medical therapy, ET, and, in advanced cases, revascularization. Smoking cessation improves claudication symptoms with longer pain-free walking times, maximal walking times, exercise physiology, limb-related outcomes, and overall mortality.21 Controlling hypertension, DM, and dyslipidemia in PAD patients has been shown to considerably reduce the risk of major adverse cardiovascular events.22, 23, 24, 25
Medications
The current recommendations from the ACC/AHA Guidelines 2016 recommend that all symptomatic patients take either aspirin or clopidogrel.26 Statins are recommended for all patients with PAD, regardless of the presence of dyslipidemia.27 Cilostazol is effective in symptomatic improvement and pain-free walking distance for patients who experience claudication but has not been shown to decrease cardiovascular disease (CVD) mortality.28 The addition of a 2.5-mg rivaroxaban twice daily dose after lower extremity revascularization for symptomatic PAD has also been shown to significantly decrease adverse outcomes.29 Although the cost of rivaroxaban may be prohibitive for some patients, multiple cost-effectiveness analyses have shown that rivaroxaban plus aspirin is more cost effective than aspirin alone.30, 31, 32
Revascularization
Revascularization therapy aims to restore blood flow to the limb and is recommended for patients with lifestyle-limiting claudication and an inadequate response to medical therapy and ET, or for patients with CLTI and in emergent acute limb ischemia. Revascularization techniques include endovascular and surgical procedures.
Future Therapies
Potential future therapies for PAD include stem cell therapy and angiogenic growth factors, with the aim of increasing blood supply to the distal limbs.33, 34, 35, 36 More data on potential therapeutic effect and long-term safety profile of both stem cells and gene therapy is necessary before becoming routine clinical therapy.
Prognosis or Outcomes
Disability and Quality of Life
Disability because of PAD is rising globally with the development of functional limitations and increased cardiovascular risk.37 Many patients with PAD will be asymptomatic owing to their limited mobility for other reasons or will unknowingly limit their mobility to avoid claudication. This functional limitation, in turn, leads to poor clinical outcomes, including reduced QoL and activity restriction, which in turn are associated with higher cardiovascular events, limb events, and mortality.38, 39 In addition, over half of PAD patients have been shown in registry data to have co-existing coronary artery disease (CAD).40
Major Adverse Limb Events (MALE) or Major Adverse Cardiovascular Events
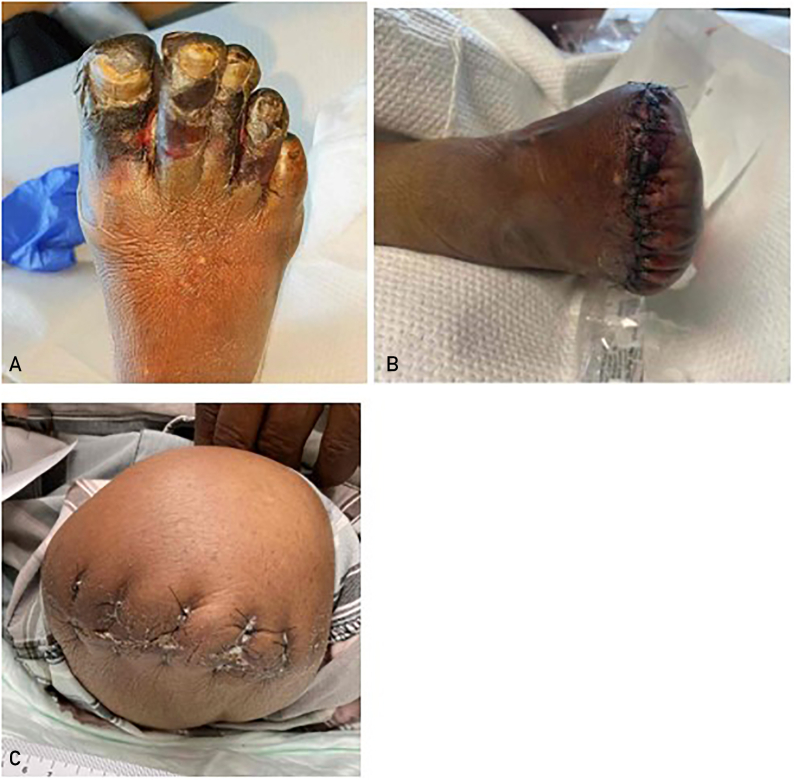
One of the most important goals of treating PAD is to prevent the development of MALE, which is defined as progression to CLTI, development of acute limb ischemia, or amputation (Figure 5). The estimated 5-year outcomes for patients with PAD with intermittent claudication include progression to CLTI in 1%-2% and development of nonfatal myocardial infarction or stroke in 20%.9 In a meta-analysis of observational studies, it was found that at a follow-up of up to 13 years, ∼7% of asymptomatic patients with PAD progressed to claudication, 21% of patients with claudication were diagnosed as having CLTI, and anywhere between 4% and 27% of patients with CLTI underwent amputations.41
Figure 5.
A, Dry gangrene of the first 4 toes of the right foot in a 74-year-old man, characteristic of CLTI. B, After transmetatarsal amputation. C, Contralateral above knee amputation in the same patient, the ultimate outcome of CLTI. CLTI, chronic limb-threatening ischemia.
Mortality
Estimates of the mortality rate of patients with PAD differ widely depending on their current stage of PAD. The ACC/AHA guidelines estimate a 5-year mortality rate of about 15%-30% for patients with claudication. A study of the California Office of Statewide Planning and Development hospital database of over 26,000 patients with major amputations revealed a 5-year mortality rate of 18%.42
Economic Burden
Analysis of the 2014 National Inpatient Sample revealed over 286,000 hospitalizations for patients with PAD.4 The median hospitalization cost was $15,755, resulting in a total annual cost burden of approximately $6.31 billion for PAD alone. Taking into consideration other costs related to PAD management, such as medications, office visits, laboratory tests, and other expenses, it is clear that PAD is associated with a marked economic burden.
Cardiac Rehabilitation and Exercise Therapy
“If we had a pill that conferred all the proven health benefits of exercise, physicians would widely prescribe it to their patients and our health care system would see to it that every patient had access to this wonder drug.”—Dr. Robert Sellis, past-president of the American College of Sports Medicine.
History
In 2007, the American College of Sports Medicine, with endorsement from the American Medical Association and the Office of the Surgeon General, launched a global initiative to mobilize physicians, health care professionals and providers, and educators to promote exercise in their practice or activities to prevent, reduce, manage, or treat diseases that effect health and the QoL.43,44 Historically, exercise was prescribed as early as 600 BC.45 Evidence of the clinical benefit of ET for patients with PAD was first published in 1966, in Denmark, in which 14 patients were randomized to ET vs placebo, and the 7 patients who underwent exercise therapy had an increase in walking distance until the onset of claudication and total walking distance.46 The 2016 ACC/AHA Guidelines give a Class 1A recommendation for a supervised exercise program in patients with claudication.9
Description
A structured exercise program begins by evaluating multiple patient factors, such as current activity level, age, sex, and potential barriers to activity. This is followed by exercise testing, assessing parameters of hemodynamics, signs and symptoms, ST-segment changes, perceived exertion, and exercise capacity. This allows for the risk stratification of patients to determine the level of necessary supervision and monitoring during exercise. This is followed by an individualized exercise prescription considering all the aforementioned factors.47
Mode of Exercise
Treadmill walking has the strongest evidence of benefit for PAD and is currently the preferred modality.48 Cycling or arm-cranking are alternative methods with the studied benefit of maximal walking time.49 Aerobic training is preferred over resistance training, although there is evidence to support resistance training in patients with PAD.50 Other exercise methods have been described, such as total body recumbent stepping exercise training, descending stair walking, and nonweight-bearing exercises. These exercises may provide similar efficacy to treadmill walking and improve adherence for patients with barriers to treadmill walking, such as diabetic foot ulcers.51, 52, 53
Frequency and Duration of Session
Current ACC/AHA recommendations are at least 3 sessions per week, which has shown superior benefit to less than 3 sessions per week, although greater than 3 sessions per week have not shown any added benefit.54,55 The same meta-analysis recommended at least 30 and up to 60 minutes of exercise per session. More recent data suggest that improvement appears to peak at 45 minutes.55 The duration of ET has not been standardized, and benefits have been seen in as little as 2 months.56 The Centers for Medicare & Medicaid Services have approved coverage for up to 36 sessions over a 12-week period for symptomatic patients with PAD, and this is the minimum recommended duration from the ACC/AHA guidelines.9
Intensity
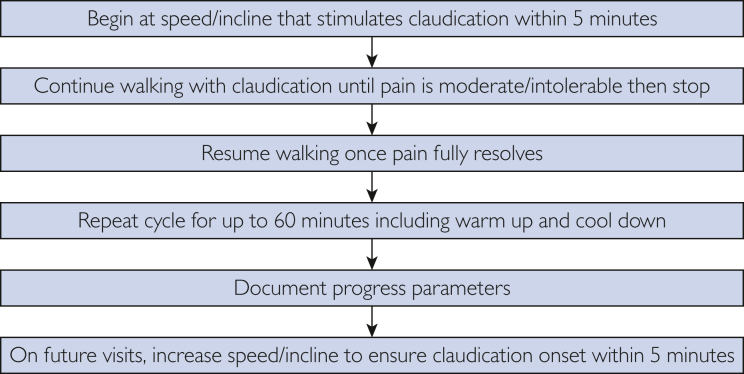
Currently, it is unclear whether high-intensity exercise or low-intensity exercise is superior in improving claudication symptoms; however, low-intensity exercise may have better adherence. Walking should be performed at a safe pace with the incline adjusted to an intensity of exercise that causes the onset of claudication within 3-5 minutes.57 The intensity may be further increased as exercise tolerance improves. The patient should continue walking until the claudication pain is unbearable and then rest until the claudication resolves. This cycle should continue for the full duration of therapy (Figures 6 and 7).
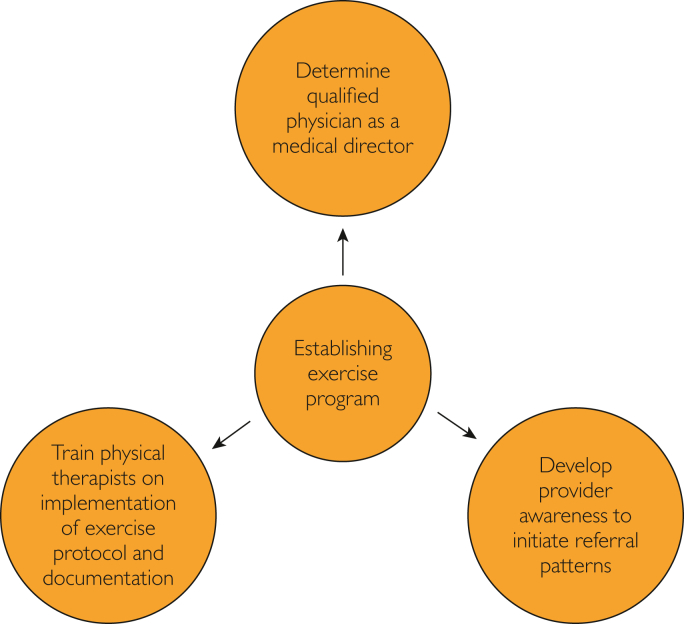
Figure 6.
Establishing supervised exercise program.
Figure 7.
Typical exercise program.
Physiologic Mechanism
There is an abundance of evidence that ET is beneficial for patients with PAD. Many physiologic mechanisms are thought to play a role, which include improved skeletal muscle oxidative metabolism, enhanced arterial collateralization, improved endothelial function, increased lipid metabolism, and reduced inflammatory activation (Figure 8).58,59
Figure 8.
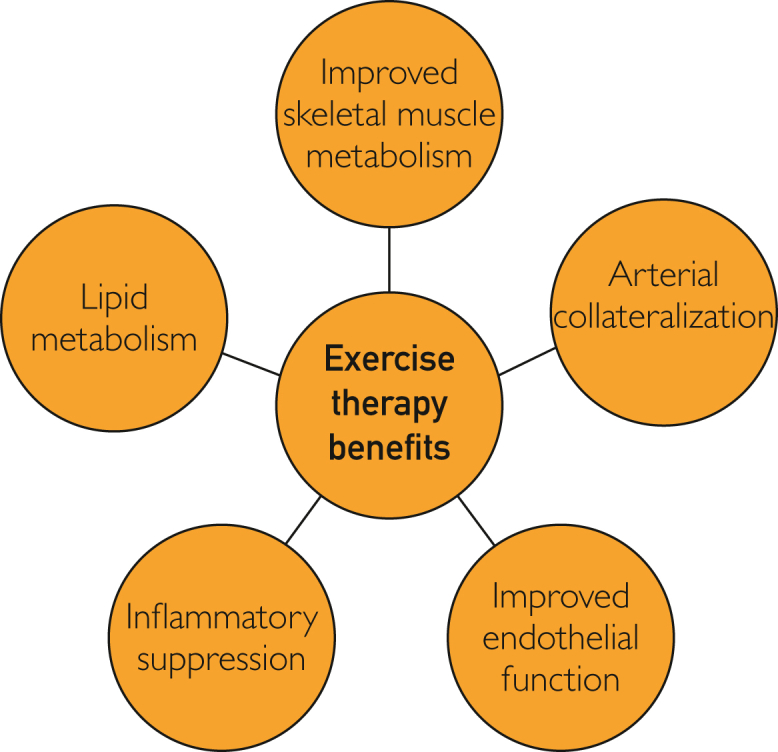
Physiologic benefits of exercise therapy.
Improved Skeletal Muscle Oxidative Metabolism
Acylcarnitine is a transport form of fatty acids and can be used for energy production in mitochondria or for the synthesis of endogenous molecules. The accumulation of acylcarnitine can contribute to muscle oxidative stress and insulin resistance.60 In patients with PAD, acylcarnitine levels have been found to be consistently elevated in plasma because of chronic, repeated episodes of ischemia; this has been associated with the functional impairment observed in these patients.61 Improved metabolism in skeletal muscles facilitates the extraction of oxygen and substrate and is associated with a reduction in plasma concentrations of short-chain acylcarnitine.59 These changes contribute to improved functionality in PAD.
Arterial Collateralization
Claudication pain occurs when there is limited blood flow supplying muscles during physical exertion, thus depriving cells of oxygen and their metabolic needs. Exercise has been hypothesized to enhance performance in PAD by improving distal collateral circulation. Although this has been proven in some animal studies, such as that done by Prior BM et al,62 which reported a gain in collateral blood flow in exercise-trained rodents, research done on patients with PAD has not been able to yield substantial evidence demonstrating relevant gains in peripheral blood flow.46,63 The discrepancy observed between animal and human studies may be attributable to the multilevel complexity of disease in patients with PAD and concomitant endothelial dysfunction that would impair vascular remodeling and sufficient collateral growth in these patients.64 Although there is insufficient data to prove the beneficial role of exercise in improving peripheral circulation in patients with PAD, it cannot be fully excluded.
Improved Endothelial Function
A vicious cycle exists between endothelial dysfunction and PAD. Oxidative stress and endothelial dysfunction can predispose susceptible patients to developing PAD, and this may be because of the impairment of the endothelial vasodilator response. Simultaneously, PAD contributes to further endothelial dysfunction by increased oxidative stress.65 Some studies have shown that endothelial function can be improved with ET. A study published in 2009 involving 111 patients with PAD concluded that a higher level of physical activity was independently and significantly associated with better endothelial function as measured by brachial flow–mediated dilation in response to reactive hyperemia.66 In another study on the effect of different types of exercise training on the functional effect of PAD, treadmill exercise enhanced flow-mediated dilation, consistent with an improvement in endothelial health. On contrary resistance training exercise was associated with improved functional performance measured by treadmill walking, QoL, and stair climbing ability, but there was no significant improvement in endothelial function.67 Another study of endothelial function in patients with CAD and post-myocardial infarction revealed improved endothelial function after ET, regardless of the type of training. However, the improvement noticed disappeared after 1 month of detraining.68 Several studies have been published regarding the prognostic use of endothelial function, and in a systematic review and meta-analysis, peripheral endothelial function was determined to be a significant predictor of future CVD events.69 Therefore, it can be deduced that exercise may decrease CVD risk in patients with PAD by improvement in endothelial function.
Inflammatory Activation
Several inflammatory mediators have been found to play a role in the development of PAD. These include C-reactive protein, interleukin-6, soluble intercellular adhesion molecule-1, D-dimer, and homocysteine.70, 71, 72, 73 Not only are they associated with disease initiation, but they also play a predictive role in determining the progression and severity of the condition as well as complication development.16,74,75 Exercise has a therapeutic advantage in patients with PAD by decreasing or suppressing inflammatory activation, therefore potentially reducing the severity of the disease.58,72
Lipid Metabolism
Both aerobic exercise and resistance training at moderate intensity significantly increase high density lipoprotein-C, and higher intensity exercise adds to reductions in low density lipoprotein-C and triglyceride levels in the general population.76 A systematic review and meta-analysis of patients with intermittent claudication undergoing ET reported significantly decreased total cholesterol and low density lippoprotein.77 The same study also revealed a significant decrease in systolic blood pressure, suggesting ET has a considerable effect on controlling risk factors for PAD.
Outcomes
Walking Distance
The benefits of ET on walking distance have been evaluated in multiple studies. In the PROPEL trial, the exercise alone group improved the 6-minute walk distance (6MWD) by 33.6 m.78 In another randomized controlled trial, the ET group improved 6MWD by 35.9 m, although this study included all patients with an ABI below 0.95, both with and without claudication.67 In the CLEVER trial comparing stenting to ET, it was found that ET improved peak walking time, even though stenting improved ABI more than ET.79 Another study revealed that ET improved walking distance in initial claudication and absolute claudication without improving the ABI.80 The EXITPAD trial revealed that supervised ET significantly improved walking distance and QoL as compared with unsupervised ET.81 A systemic review of multiple modes of ET revealed that most modes and intensities of exercise resulted in significantly improved walking capability.82
Mortality
Patients with PAD with higher baseline functional performance measured with a 6MWD test and a 4-meter walk speed have lower mortality than patients with lower baseline functional performance.83,84 A decline in total walking distance has also been shown to be an indicator of higher mortality.85 Although we were not able to find any randomized control trials to evaluate mortality in patients with PAD for ET vs control, retrospective data has provided some insight into the mortality benefit of ET for PAD. For example, a large retrospective study including patients with PAD with claudication found that patients who underwent primary ET had lower mortality and fewer revascularizations compared with patients who underwent endovascular or surgical revascularizations.86 It is important to note that this study excluded patients with CLTI, and there is no evidence currently to recommend delaying revascularization for patients with CLTI; rather, this data supports the use of ET for primary treatment of PAD without CLTI. Another 10-year retrospective study has shown that patients who completed a home-based ET program had a 27% significantly lower mortality rate than those who were unable to complete for medical reasons and those who did not complete for other reasons.87
Nonhealing Ulcers
Although ET may improve outcomes for patients with PAD in general, its use and utilization in patients with DM foot ulcers are questionable. This is because repetitive stress to an area that is subject to shear stress or high vertical stress may result in the development of new ulcers. Currently, there is not conclusive evidence of the benefits and harms of ET in this specific population.88 ET is not contraindicated for DM without foot ulcers.89 A meta-analysis of 139 patients from 3 studies concluded that although there is insufficient evidence to support ET as an intervention to improve healing of DM foot ulcers, the results did report some degree of wound size reduction and no negative consequences of the intervention.90 Nonweight-bearing exercises are encouraged for patients with nonhealing ulcers because of PAD. Further studies are necessary to confirm these findings.
Amputation
Although limited evidence is available, in advanced cases, ET should not be intended to delay revascularization; rather, it should be an adjunct. This is evidenced by a recent meta-analysis that revealed lower amputation rates in patients who underwent revascularization in addition to ET (3.5%) as compared with ET alone (17.3%).91 This data, however, should not discourage the utilization of ET; on the contrary, the same meta-analysis found that revascularization alone was not significantly different than ET in reducing the risk of amputations or improving maximum walking distance, even though there was a considerable improvement in ABI in the revascularization group.
Cost-Effectiveness
A cost-effectiveness analysis using data from the EXITPAD trial found that compared with “go home and walk” advice, supervised ET resulted in an incremental cost-effectiveness ratio of about $29,874 per quality-adjusted life year (QALY).92 This is below the current willingness-to-pay threshold in the United States, which ranges between $50,000 and $100,000, although this value was established in 1982 and has never been adjusted for inflation.93 Another study using data from the EXITPAD trial and the CETAC trial revealed that supervised ET is associated with an approximate $6800 cost as compared with endovascular revascularization with no significant difference in QALY.94 An older study published in 2004 revealed that angioplasty had superior effectiveness and an additional $123 per additional meter walked before the onset of claudication pain compared with ET; however, after 6 months, ET was more effective and cost less than angioplasty.95 These results conflict with those of another old study published in 2002, which revealed that angioplasty had an incremental cost-effectiveness ratio of $38,000 per QALY compared with ET with an improved effectiveness of 33-61 quality-adjusted life days.96 With advances in medical and percutaneous therapies over the past 20 years, the latter results may be outdated.
Challenges
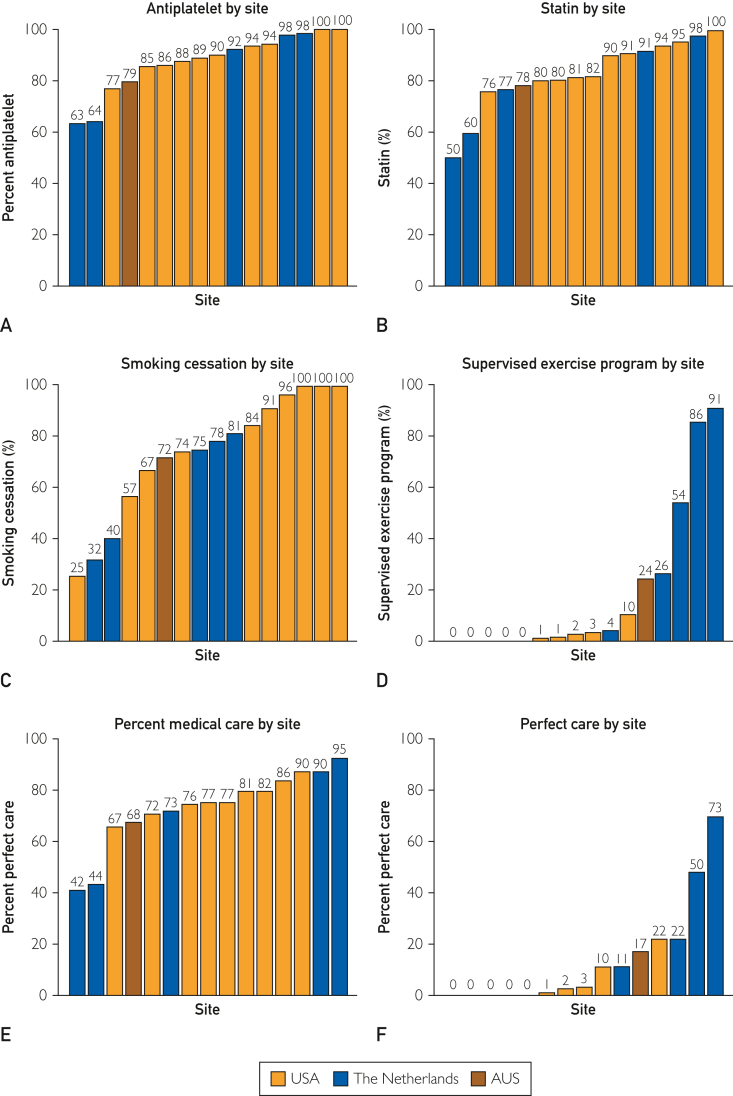
Several barriers are present that limit successful widespread utilization, such as the availability of supervised exercise therapy (SET), awareness among physicians of CMS coverage for SET, physician referral, access to SET facilities for patients, and patient adherence to SET programs. In an international survey published in 2012, which included 378 responses, only 30% of the respondents had access to a SET program.97 In a survey of American vascular specialists published in 2020 with 135 responses, 54% of respondents stated there was no SET program in their facilities.98 In the same survey, 26% of physicians were not aware that CMS covers SET programs. The barriers discovered in this survey included travel distance for patients in 50%, lack of available SET centers in 33%, lack of patient interest in 30%, and cost or copay in 29%. More research is needed to identify and overcome barriers to the widespread utilization of ET for PAD (Figure 9).99
Figure 9.
Graphs depicting adherence of 16 different centers to quality measures related to PAD management. Although medical therapy is employed at most of the centers, exercise therapy is rarely used. PAD, peripheral artery disease.
Discussion
Despite marked advancements in the treatment of PAD, including the increasing evidence of the benefits of ET and its determination to be a first-line treatment, ET remains underutilized. Data surrounding ET lags behind data for cardiac rehabilitation for CAD and even for heart failure, despite the high morbidity and mortality associated with PAD. Advocation for the development of SET programs is necessary for improving outcomes in patients with PAD, nationwide. Physicians and public health leaders should encourage patients and the public to increase their physical activity to attain the associated health benefits and improve global health.
Conclusion
Peripheral artery disease is an increasingly prevalent and potentially preventable disease. Uncontrolled PAD can lead to decreased QoL, debility, amputation, and death. Exercise therapy is a first-line treatment for PAD with proven benefit and minimal risk. Utilization of ET for PAD remains suboptimal. Research surrounding ET for PAD is limited. The constellation of data available currently supports ET therapy in the primary and secondary prevention of PAD, and improving symptoms, walking distance, QoL, and major CVD events for these patients. Future directions for ET include the development of guidelines to tailor exercise interventions on the basis of patient-specific factors, particularly for patients unable to participate in standard treadmill exercise for various reasons. The investigation of applying wearable technology to direct and monitor exercise interventions may also improve adherence and the success of SET. Exploring barriers to the integration of SET into routine PAD at each individual center may improve implementation strategies.
Potential Competing Interests
Given his role as Editorial Board Member, Dr Carl Lavie had no involvement in the peer-review of this article and has no access to information regarding its peer-review. All other authors have reported no conflicts of interest.
Supplemental Online Material
References
- 1.Sharma A.M., Norton P.T., Zhu D. Conditions presenting with symptoms of peripheral arterial disease. Semin Intervent Radiol. 2014;31(4):281–291. doi: 10.1055/s-0034-1393963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDermott M.M. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circ Res. 2015;116(9):1540–1550. doi: 10.1161/CIRCRESAHA.114.303517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aday A.W., Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ Res. 2021;128(12):1818–1832. doi: 10.1161/CIRCRESAHA.121.318535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohn C.G., Alberts M.J., Peacock W.F., Bunz T.J., Coleman C.I. Cost and inpatient burden of peripheral artery disease: findings from the National Inpatient Sample. Atherosclerosis. 2019;286:142–146. doi: 10.1016/j.atherosclerosis.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Lane R., Harwood A., Watson L., Leng G.C. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;12(12):CD000990. doi: 10.1002/14651858.CD000990.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vemulapalli S., Dolor R.J., Hasselblad V., et al. Supervised vs unsupervised exercise for intermittent claudication: a systematic review and meta-analysis. Am Heart J. 2015;169(6):924–937.e3. doi: 10.1016/j.ahj.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Fakhry F., Rouwet E.V., Den Hoed P.T., Hunink M.G.M., Spronk S. Long-term clinical effectiveness of supervised exercise therapy versus endovascular revascularization for intermittent claudication from a randomized clinical trial. Br J Surg. 2013;100(9):1164–1171. doi: 10.1002/bjs.9207. [DOI] [PubMed] [Google Scholar]
- 8.Ehrman J.K., Lui K., Treat-Jacobson D. Supervised exercise training for symptomatic peripheral artery disease. Clin Exer Physiol. 2017;6(4):78–83. [Google Scholar]
- 9.Gerhard-Herman M.D., Gornik H.L., Barrett C., et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69(11):e71–e126. doi: 10.1016/j.jacc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Divakaran S., Carroll B.J., Chen S., Shen C., Bonaca M.P., Secemsky E.A. Supervised exercise therapy for symptomatic peripheral artery disease among Medicare beneficiaries between 2017 and 2018: participation rates and outcomes. Circ Cardiovasc Qual Outcomes. 2021;14(8) doi: 10.1161/CIRCOUTCOMES.121.007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allison M.A., Ho E., Denenberg J.O., et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32(4):328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Song P., Rudan D., Zhu Y., et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7(8):e1020–e1030. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 13.Gordon M.B., Jain R., Beckman J.A., Creager M.A. The contribution of nitric oxide to exercise hyperemia in the human forearm. Vasc Med. 2002;7(3):163–168. doi: 10.1191/1358863x02vm439oa. [DOI] [PubMed] [Google Scholar]
- 14.McDermott M.M., Hoff F., Ferrucci L., et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55(3):400–406. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell R.G., Duscha B.D., Robbins J.L., et al. Increased levels of apoptosis in gastrocnemius skeletal muscle in patients with peripheral arterial disease. Vasc Med. 2007;12(4):285–290. doi: 10.1177/1358863X07084858. [DOI] [PubMed] [Google Scholar]
- 16.Tzoulaki I., Murray G.D., Lee A.J., Rumley A., Lowe G.D., Fowkes F.G.R. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh artery Study. Circulation. 2005;112(7):976–983. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- 17.Anda R.F., Remington P.L., Sienko D.G., Davis R.M. Are physicians advising smokers to quit? The patient’s perspective. JAMA. 1987;257(14):1916–1919. [PubMed] [Google Scholar]
- 18.Criqui M.H., Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 19.Hypertension prevalence in the U.S. Million Hearts®. Centers for Disease Control and Prevention. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html
- 20.Assimes T.L., Roberts R. Genetics: implications for prevention and management of coronary artery disease. J Am Coll Cardiol. 2016;68(25):2797–2818. doi: 10.1016/j.jacc.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 21.Bhatt D.L., Steg P.G., Ohman E.M., et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180–189. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 22.Gornik H.L., Creager M.A. In: Vascular Medicine: A Companion to Braunwald’s Heart Disease. 2nd ed. Creager M.A., Beckman J.A., Loscalzo J., editors. Elsevier; 2013. Medical treatment of peripheral artery disease; pp. 242–248. [Google Scholar]
- 23.Mehler P.S., Coll J.R., Estacio R., Esler A., Schrier R.W., Hiatt W.R. Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation. 2003;107(5):753–756. doi: 10.1161/01.cir.0000049640.46039.52. [DOI] [PubMed] [Google Scholar]
- 24.Bavry A.A., Anderson R.D., Gong Y., et al. Outcomes among hypertensive patients with concomitant peripheral and coronary artery disease: findings from the INternational verapamil-SR/trandolapril STudy. Hypertension. 2010;55(1):48–53. doi: 10.1161/HYPERTENSIONAHA.109.142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathan D.M., Cleary P.A., Backlund J.Y., et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebocontrolled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 28.Bedenis R., Stewart M., Cleanthis M., Robless P., Mikhailidis D.P., Stansby G. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2014;2014(10):CD003748. doi: 10.1002/14651858.CD003748.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonaca M.P., Bauersachs R.M., Anand S.S., et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382(21):1994–2004. doi: 10.1056/NEJMoa2000052. [DOI] [PubMed] [Google Scholar]
- 30.Feng T., Zheng Z., Gao S., et al. Cost-effectiveness analysis of rivaroxaban in Chinese patients with stable cardiovascular disease. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.921387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrara P., Cortesi P.A., Di Laura D., Maggioni A.P., Mantovani L.G. Cost-effectiveness analysis of rivaroxaban plus aspirin compared with aspirin alone in patients with coronary and peripheral artery diseases in Italy. Clin Drug Investig. 2021;41(5):459–468. doi: 10.1007/s40261-021-01023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowie M.R., Lamy A., Levy P., et al. Health economic evaluation of rivaroxaban in the treatment of patients with chronic coronary artery disease or peripheral artery disease. Cardiovasc Res. 2020;116(11):1918–1924. doi: 10.1093/cvr/cvz278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie B., Luo H., Zhang Y., Wang Q., Zhou C., Xu D. Autologous stem cell therapy in critical limb ischemia: a meta-analysis of randomized controlled trials. Stem Cells Int. 2018;2018:7528464. doi: 10.1155/2018/7528464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morishita R., Aoki M., Hashiya N., et al. Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. Hypertension. 2004;44(2):203–209. doi: 10.1161/01.HYP.0000136394.08900.ed. [DOI] [PubMed] [Google Scholar]
- 35.Sanada F., Taniyama Y., Muratsu J., et al. Gene-therapeutic strategies targeting angiogenesis in peripheral artery disease. Medicines (Basel) 2018;5(2):31. doi: 10.3390/medicines5020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barć P., Antkiewicz M., Śliwa B., et al. Double VEGF/HGF gene therapy in critical limb ischemia complicated by diabetes mellitus. J Cardiovasc Transl Res. 2021;14(3):409–415. doi: 10.1007/s12265-020-10066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fowkes F.G.R., Rudan D., Rudan I., et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 38.Ramirez J.L., Drudi L.M., Grenon S.M. Review of biologic and behavioral risk factors linking depression and peripheral artery disease. Vasc Med. 2018;23(5):478–488. doi: 10.1177/1358863X18773161. [DOI] [PubMed] [Google Scholar]
- 39.Nead K.T., Zhou M., Diaz Caceres R., Olin J.W., Cooke J.P., Leeper N.J. Walking impairment questionnaire improves mortality risk prediction models in a high-risk cohort independent of peripheral arterial disease status. Circ Cardiovasc Qual Outcomes. 2013;6(3):255–261. doi: 10.1161/CIRCOUTCOMES.111.000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDermott M.M., Guralnik J.M., Ferrucci L., et al. Community walking speed, sedentary or lying down time, and mortality in peripheral artery disease. Vasc Med. 2016;21(2):120–129. doi: 10.1177/1358863X15626521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhatt D.L., Eagle K.A., Ohman E.M., et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304(12):1350–1357. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 42.Jones W.S., Patel M.R., Dai D., et al. High mortality risks after major lower extremity amputation in Medicare patients with peripheral artery disease. Am Heart J. 2013;165(5):809–815. doi: 10.1016/j.ahj.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Canela M., Estruch R., Corella D., Salas-Salvadó J., Martínez-González M.A. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311(4):415–417. doi: 10.1001/jama.2013.280618. [DOI] [PubMed] [Google Scholar]
- 44.Berryman J.W. Exercise is medicine: a historical perspective. Curr Sports Med Rep. 2010;9(4):195–201. doi: 10.1249/JSR.0b013e3181e7d86d. [DOI] [PubMed] [Google Scholar]
- 45.Tipton C.M. The history of “Exercise Is Medicine” in ancient civilizations. Adv Physiol Educ. 2014;38(2):109–117. doi: 10.1152/advan.00136.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsen O.A., Lassen N.A. Effect of daily muscular exercise in patients with intermittent claudication. Lancet. 1966;2(7473):1093–1096. doi: 10.1016/s0140-6736(66)92191-x. [DOI] [PubMed] [Google Scholar]
- 47.Balady G.J., Williams M.A., Ades P.A., et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart association exercise, cardiac rehabilitation, and prevention committee, the council on clinical cardiology; the councils on cardiovascular nursing, epidemiology and prevention, and nutrition, physical activity, and metabolism; and the American association of cardiovascular and pulmonary rehabilitation. Circulation. 2007;115(20):2675–2682. doi: 10.1161/CIRCULATIONAHA.106.180945. [DOI] [PubMed] [Google Scholar]
- 48.Norgren L., Hiatt W.R., Dormandy J.A., et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(1 suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 49.Zwierska I., Walker R.D., Choksy S.A., Male J.S., Pockley A.G., Saxton J.M. Upper- vs lower-limb aerobic exercise rehabilitation in patients with symptomatic peripheral arterial disease: a randomized controlled trial. J Vasc Surg. 2005;42(6):1122–1130. doi: 10.1016/j.jvs.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 50.Parmenter B.J., Mavros Y., Ritti Dias R., King S., Fiatarone Singh M. Resistance training as a treatment for older persons with peripheral artery disease: a systematic review and meta-analysis. Br J Sports Med. 2020;54(8):452–461. doi: 10.1136/bjsports-2018-100205. [DOI] [PubMed] [Google Scholar]
- 51.Lanzi S., Nussbaumer P., Calanca L., Mazzolai L., Malatesta D. Descending stair walking in patients with symptomatic lower extremity peripheral artery disease: a pilot study. Vasc Med. 2022;27(2):171–173. doi: 10.1177/1358863X211058784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salisbury D.L., Swanson K., Brown R.J., Treat-Jacobson D. Total body recumbent stepping vs treadmill walking in supervised exercise therapy: a pilot study. Vasc Med. 2022;27(2):150–157. doi: 10.1177/1358863X211068888. [DOI] [PubMed] [Google Scholar]
- 53.Eraydin Ş., Avşar G. The effect of foot exercises on wound healing in type 2 diabetic patients with a foot ulcer: a randomized control study. J Wound Ostomy Continence Nurs. 2018;45(2):123–130. doi: 10.1097/WON.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 54.Gardner A.W., Poehlman E.T. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274(12):975–980. [PubMed] [Google Scholar]
- 55.Bulmer A.C., Coombes J.S. Optimising exercise training in peripheral arterial disease. Sports Med. 2004;34(14):983–1003. doi: 10.2165/00007256-200434140-00004. [DOI] [PubMed] [Google Scholar]
- 56.Gardner A.W., Montgomery P.S., Parker D.E. Optimal exercise program length for patients with claudication. J Vasc Surg. 2012;55(5):1346–1354. doi: 10.1016/j.jvs.2011.11.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Treat-Jacobson D., McDermott M.M., Bronas U.G., et al. Optimal exercise programs for patients with peripheral artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139(4):e10–e33. doi: 10.1161/CIR.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 58.Hamburg N.M., Balady G.J. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation. 2011;123(1):87–97. doi: 10.1161/CIRCULATIONAHA.109.881888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hiatt W.R., Regensteiner J.G., Hargarten M.E., Wolfel E.E., Brass E.P. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation. 1990;81(2):602–609. doi: 10.1161/01.cir.81.2.602. [DOI] [PubMed] [Google Scholar]
- 60.Aguer C., McCoin C.S., Knotts T.A., et al. Acylcarnitines: potential implications for skeletal muscle insulin resistance. FASEB J. 2015;29(1):336–345. doi: 10.1096/fj.14-255901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hiatt W.R., Nawaz D., Brass E.P. Carnitine metabolism during exercise in patients with peripheral vascular disease. JAppl Physiol (1985) 1987;62(6):2383–2387. doi: 10.1152/jappl.1987.62.6.2383. [DOI] [PubMed] [Google Scholar]
- 62.Prior B.M., Lloyd P.G., Ren J., et al. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol. 2004;287(6):H2434–H2447. doi: 10.1152/ajpheart.00398.2004. [DOI] [PubMed] [Google Scholar]
- 63.Dahllöf A.G., Holm J., Scherstén T., Sivertsson R. Peripheral arterial insufficiency, effect of physical training on walking tolerance, calf blood flow, and blood flow resistance. Scand J Rehabil Med. 1976;8(1) [PubMed] [Google Scholar]
- 64.Vita J.A., Holbrook M., Palmisano J., et al. Flow-induced arterial remodeling relates to endothelial function in the human forearm. Circulation. 2008;117(24):3126–3133. doi: 10.1161/CIRCULATIONAHA.108.778472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brevetti G., Silvestro A., Di Giacomo S., et al. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg. 2003;38(2):374–379. doi: 10.1016/s0741-5214(03)00124-1. [DOI] [PubMed] [Google Scholar]
- 66.Payvandi L., Dyer A., McPherson D., et al. Physical activity during daily life and brachial artery flow-mediated dilation in peripheral arterial disease. Vasc Med. 2009;14(3):193–201. doi: 10.1177/1358863X08101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McDermott M.M., Ades P., Guralnik J.M., et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301(2):165–174. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vona M., Codeluppi G.M., Iannino T., Ferrari E., Bogousslavsky J., von Segesser L.K. Effects of different types of exercise training followed by detraining on endothelium-dependent dilation in patients with recent myocardial infarction. Circulation. 2009;119(12):1601–1608. doi: 10.1161/CIRCULATIONAHA.108.821736. [DOI] [PubMed] [Google Scholar]
- 69.Matsuzawa Y., Kwon T.G., Lennon R.J., Lerman L.O., Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4(11) doi: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li J.J., Fang C.H. C-reactive protein is not only an inflammatory marker but also a direct cause of cardiovascular diseases. Med Hypotheses. 2004;62(4):499–506. doi: 10.1016/j.mehy.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 71.Pradhan A.D., Rifai N., Ridker P.M. Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral arterial disease in men. Circulation. 2002;106(7):820–825. doi: 10.1161/01.cir.0000025636.03561.ee. [DOI] [PubMed] [Google Scholar]
- 72.Craft L.L., Guralnik J.M., Ferrucci L., et al. Physical activity during daily life and circulating biomarker levels in patients with peripheral arterial disease. Am J Cardiol. 2008;102(9):1263–1268. doi: 10.1016/j.amjcard.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McDermott M.M., Liu K., Ferrucci L., et al. Circulating blood markers and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2008;56(8):1504–1510. doi: 10.1111/j.1532-5415.2008.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rossi E., Biasucci L.M., Citterio F., et al. Risk of myocardial infarction and angina in patients with severe peripheral vascular disease: predictive role of C-reactive protein. Circulation. 2002;105(7):800–803. doi: 10.1161/hc0702.104126. [DOI] [PubMed] [Google Scholar]
- 75.Vainas T., Stassen F.R., de Graaf R., et al. C-reactive protein in peripheral arterial disease: relation to severity of the disease and to future cardiovascular events. J Vasc Surg. 2005;42(2):243–251. doi: 10.1016/j.jvs.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 76.Mann S., Beedie C., Jimenez A. Differential effects of aerobic exercise, resistance training, and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations [review] Sports Med. 2014;44(2):211–221. doi: 10.1007/s40279-013-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jansen S.C.P., Hoorweg B.B.N., Hoeks S.E., et al. A systematic review and meta-analysis of the effects of supervised exercise therapy on modifiable cardiovascular risk factors in intermittent claudication. J Vasc Surg. 2019;69(4):1293–1308.e2. doi: 10.1016/j.jvs.2018.10.069. [DOI] [PubMed] [Google Scholar]
- 78.McDermott M.M., Ferrucci L., Tian L., et al. Effect of granulocyte-macrophage colony-stimulating factor with or without supervised exercise on walking performance in patients with peripheral artery disease: the PROPEL randomized clinical trial. JAMA. 2017;318(21):2089–2098. doi: 10.1001/jama.2017.17437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy T.P., Cutlip D.E., Regensteiner J.G., et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125(1):130–139. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schieber M.N., Pipinos, Johanning J.M., et al. Supervised walking exercise therapy improves gait biomechanics in patients with peripheral artery disease. J Vasc Surg. 2020;71(2):575–583. doi: 10.1016/j.jvs.2019.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nicolaï S.P., Teijink J.A., Prins M.H. Exercise Therapy in Peripheral Arterial Disease Study Group. Multicenter randomized clinical trial of supervised exercise therapy with or without feedback versus walking advice for intermittent claudication. J Vasc Surg. 2010;52(2):348–355. doi: 10.1016/j.jvs.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 82.Parmenter B.J., Raymond J., Dinnen P., Singh M.A.F. A systematic review of randomized controlled trials: walking versus alternative exercise prescription as treatment for intermittent claudication. Atherosclerosis. 2011;218(1):1–12. doi: 10.1016/j.atherosclerosis.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 83.McDermott M.M., Tian L., Liu K., et al. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2008;51(15):1482–1489. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Liefde, Hoeks S.E., van Gestel Y.R., et al. The prognostic value of impaired walking distance on long-term outcome in patients with known or suspected peripheral arterial disease. Eur J Vasc Endovasc Surg. 2009;38(4):482–487. doi: 10.1016/j.ejvs.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 85.de Liefde, van Domburg R.T., Bax J.J., Klein J., Verhagen H.J., Poldermans D. A decline in walking distance predicts long-term outcome in patients with known or suspected peripheral artery disease. Eur J Cardiovasc Prev Rehabil. 2010;17(3):321–328. doi: 10.1097/HJR.0b013e32833254ce. [DOI] [PubMed] [Google Scholar]
- 86.Jansen S.C.P., van Nistelrooij L.P.J., Scheltinga M.R.M., Rouwet E.V., Teijink J.A.W., Vahl A.C. Successful implementation of the exercise first approach for intermittent claudication in the Netherlands is associated with few lower limb revascularisations. Eur J Vasc Endovasc Surg. 2020;60(6):881–887. doi: 10.1016/j.ejvs.2020.07.074. [DOI] [PubMed] [Google Scholar]
- 87.Lamberti N., López-Soto P.J., Guerzoni F., et al. Changes in exercise capacity and risk of all-cause mortality in patients with peripheral artery disease: a 10-year retrospective cohort study. Intern Emerg Med. 2020;15(2):289–298. doi: 10.1007/s11739-019-02176-3. [DOI] [PubMed] [Google Scholar]
- 88.Aagaard T.V., Moeini S., Skou S.T., Madsen U.R., Brorson S. Benefits and harms of exercise therapy for patients with diabetic foot ulcers: a systematic review. Int J Low Extrem Wounds. 2022;21(3):219–233. doi: 10.1177/1534734620954066. [DOI] [PubMed] [Google Scholar]
- 89.Colberg S.R., Sigal R.J., Fernhall B., et al. Exercise and type 2 diabetes. Diabetes Care. 2010;33(12):e147–e167. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tran M.M., Haley M.N. Does exercise improve healing of diabetic foot ulcers? A systematic review. J Foot Ankle Res. 2021;14(1):19. doi: 10.1186/s13047-021-00456-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pandey A., Banerjee S., Ngo C., et al. Comparative efficacy of endovascular revascularization versus supervised exercise training in patients with intermittent claudication: meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2017;10(7):712–724. doi: 10.1016/j.jcin.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 92.Van Asselt A.D.I., Nicolaï S.P.A., Joore M.A., Prins M.H., Teijink J.A.W. Exercise therapy in peripheral arterial disease study group. cost-effectiveness of exercise therapy in patients with intermittent claudication: supervised exercise therapy versus a ‘go home and walk’ advice. Eur J Vasc Endovasc Surg. 2011;41(1):97–103. doi: 10.1016/j.ejvs.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 93.Ubel P.A., Hirth R.A., Chernew M.E., Fendrick A.M. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163(14):1637–1641. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 94.Van Den Houten M.M.L., Lauret G.J., Fakhry F., et al. Cost-effectiveness of supervised exercise therapy compared with endovascular revascularization for intermittent claudication. Br J Surg. 2016;103(12):1616–1625. doi: 10.1002/bjs.10247. [DOI] [PubMed] [Google Scholar]
- 95.Treesak C., Kasemsup V., Treat-Jacobson D., Nyman J.A., Hirsch A.T. Cost-effectiveness of exercise training to improve claudication symptoms in patients with peripheral arterial disease. Vasc Med. 2004;9(4):279–285. doi: 10.1191/1358863x04vm570oa. [DOI] [PubMed] [Google Scholar]
- 96.de Vries S.O., Visser K., de Vries J.A., Wong J.B., Donaldson M.C., Hunink M.G. Intermittent claudication: cost-effectiveness of revascularization versus exercise therapy. Radiology. 2002;222(1):25–36. doi: 10.1148/radiol.2221001743. [DOI] [PubMed] [Google Scholar]
- 97.Makris G.C., Lattimer C.R., Lavida A., Geroulakos G. Availability of supervised exercise programs and the role of structured home-based exercise in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2012;44(6):569–575. doi: 10.1016/j.ejvs.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 98.Dua A., Gologorsky R., Savage D., et al. National assessment of availability, awareness, and utilization of supervised exercise therapy for peripheral artery disease patients with intermittent claudication. J Vasc Surg. 2020;71(5):1702–1707. doi: 10.1016/j.jvs.2019.08.238. [DOI] [PubMed] [Google Scholar]
- 99.Saxon J.T., Safley D.M., Mena-Hurtado C., et al. Adherence to guideline-recommended therapy-including supervised exercise therapy referral-across peripheral artery disease specialty clinics: insights from the international PORTRAIT registry. J Am Heart Assoc. 2020;9(3) doi: 10.1161/JAHA.119.012541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.