Abstract
Background
Evidence on sleep duration or quality and cognitive function in diverse older adults is limited. We examined prospective associations between subjective sleep measures and cognitive function, with modifying effects of sex and age (<65 vs ≥65 years).
Methods
Data are from the longitudinal Boston Puerto Rican Health Study, Waves 2 (n = 943) and 4 (n = 444), with mean follow-up of 10.5 years (range 7.2–12.8). Subjective measures of sleep duration (short <7, ref. 7, or long ≥8 hours) and insomnia symptoms (sum of difficulty falling asleep, waking up at night, and early morning awakening), were assessed at Wave 2. Linear regression models were used to assess changes in global cognition, executive function, memory, and Mini-Mental State Examination, and tested for modifying roles of sex and age.
Results
Significant 3-way interaction (sex × age × cognition) in fully adjusted models showed greater decline in global cognitive function in older men with short (β [95% confidence interval]: −0.67 [−1.24, −0.10]) or long sleep duration (−0.92 [−1.55, −0.30]), compared to women, younger men, and older men with 7 hours of sleep. Insomnia symptoms were associated with a greater decline in memory (−0.54, [−0.85, −0.22]) among older men, compared to women and younger men.
Conclusion
Sleep duration showed a U-shaped association with cognitive decline, and insomnia symptoms were associated with memory decline in fully adjusted models. Older men, versus women and younger men, were at relatively greater risk for cognitive decline associated with sleep factors. These findings are important for personalizing sleep interventions to support cognitive health.
Keywords: Cognition, Cognitive aging, Minority aging, Sleep
Cognitive health is an important public health concern for America’s aging population (1). The increasing proportion of persons with dementia and cognitive decline (2) requires focus on factors to prevent or slow cognitive impairment and Alzheimer’s disease (3). The United States is becoming increasingly diverse and, by 2060, ethnic minority groups will represent 45% of the older (≥65 years) population (vs 22% in 2014) (4). The largest increase in the older population will be among Latinx/Hispanic (L/H) persons, who are projected to grow by 391%, compared to 75% in non-L/H White, and 172% in non-L/H Black groups (4). Cumulative disadvantage exposes older L/H to greater risk of cognitive impairment, compared to non-L/H White older adults (5). Within L/H groups, it is documented that Caribbean L/H, that is, of Puerto Rican and Dominican descent, have higher risk for cognitive impairment than other L/H heritages (6). The social and cultural heterogeneity among U.S. L/H heritages points to the need for disaggregated epidemiological examinations to identify unique strengths and difficulties experienced within each group (7,8).
Poor sleep has been identified as a risk factor for cognitive decline (9). A recent meta-analysis estimated that sleep-related problems may account for 15% of Alzheimer’s disease (AD) in the population; individuals with sleep problems were shown to have 1.68 higher risk for cognitive impairment or AD (10). Systematic reviews and meta-analyses report significant cross-sectional (11) and prospective associations between sleep and cognitive function (10,12). Cross-sectionally, sleep disturbances (ie, fewer hours of sleep, long onset latency, waking up in the middle of the night) were associated with poorer cognitive function and greater odds of having dementia (10) Prospectively, sleep characteristics, including daytime sleepiness and longer hours of sleep, predicted cognitive decline (10,12). Mixed results about the effect of short and long sleep exist; however, a recent meta-analysis provided support for an inverted U-association between sleep duration and cognitive decline, suggesting that ~7-hour sleep duration provides the greatest benefit against cognitive decline and/or impairment and dementia (13).
Sleep is a multidimensional construct defined by several factors including total hours of sleep, quality of sleep, regularity, efficiency, timing, and alertness (14). Multidimensional quality of sleep may interact with individual characteristics, such as sex, age, and ancestry, and environmental factors (14). Consequently, the examination of age and sex effects of sleep among diverse populations may provide empirically driven explanations for identifying mechanisms to improve sleep health (14). Few studies have examined the role of sex and age as modifiers of the sleep–cognition association among racial/ethnically diverse groups, specifically L/H (15). Therefore, sex- and age-related differences in the association between sleep and cognition are unclear in these groups (15). To address this gap, we examined the modifying effect of sex and age in the sleep-cognition association in a cohort of older Puerto Rican adults residing in the Northeast region of the United States. Identifying L/H-heritage-specific and modifiable factors may potentially inform the development of tailored interventions to improve both sleep and cognition.
Method
Data and Participants
We used data from the Boston Puerto Rican Health Study (BPRHS), an ongoing longitudinal cohort study of Puerto Rican adults, which included a total of 1 500 self-identified Puerto Rican adults aged 45 to 75 years at baseline (2004–2009). The BPRHS was designed to examine psycho-sociological, genetic, environmental, and nutritional factors associated with the health and well-being of Puerto Rican adults residing in the greater Boston, Massachusetts, metropolitan area (16). Households with at least one person from the target population were identified using the 2000 census data, exclusion criteria included inability to answer questions due to serious health conditions, plans to move from the Greater Boston area within 2 years and/or a Mini-Mental State Examination (MMSE) score <10. Recruitment efforts included door-to-door and community-based activities. Additional details of the study and recruitment methodology are described elsewhere (16). The study was approved by the Institutional Review Boards at Tufts Medical Center, Northeastern University, and the University of Massachusetts Lowell. All participants provided signed informed consent in their language of choice (Spanish or English). A 2-year (Wave 2), 5-year (Wave 3), and approximately 10-year (Wave 4) of follow-up data have been collected. At the completion of the 2-year follow-up (Wave 2), participants were invited to participate in the Boston Puerto Rican Osteoporosis Study (BPROS), an ancillary study of the BPRHS. Cognitive data for this study were extracted from Waves 2 and 4, and sleep data were extracted from the BPROS (detailed recruitment chart and sample size at each wave provided in Supplementary Figure 1). For the current analyses, we use cognitive data from Wave 2 (n = 1 258) and Wave 4 (n = 573) follow-up interviews, and sleep data from the BPROS (n = 958) ancillary study. This study included 952 participants (268 men and 684 women) in the cross-sectional, and 444 (110 men and 334 women) in the longitudinal analysis. The mean follow-up time between Waves 2 and 4 was 10.5 years (range 7.2–12.8).
Measures
Sleep
Sleep duration was measured using one question, “please indicate the total number of hours that you sleep, typically, during a 24-hour period?,” with 6 possible responses: 5 hours or less, 6, 7, 8, 9 and 10 hours or more. We categorized this variable into <7 hours (short sleep), 7 hours (reference category), and ≥8 hours (long sleep). We used a cutoff of 8 hours or greater for long sleep informed by research with older adults reporting long sleep-cognition associations using a cutoff of 8 hours or greater (17). To assess insomnia symptoms, we created a score by summing 3 Likert items (0—never/almost never; 1—sometimes; 2—most of the time), including: “How frequently do you have difficulty falling asleep? (onset latency),” “How frequently do you have trouble with waking up at night? (wake after sleep onset [WASO]),” and “How frequently do you have trouble with waking up too early in the morning and not being able to fall asleep again? (early-morning awakening).” The summed score ranged from 0 to 6, with higher scores indicating higher insomnia symptomatology.
Cognitive function
Participants completed a battery of 7 cognitive tests to assess cognitive function. The cognitive assessments were administered during in-home visits by trained bilingual and native Spanish-speaking interviewers, at baseline, Wave 2, and Wave 4 follow-up. Participants completed the tests in their language of preference, either Spanish (96% at Wave 2 follow-up and 97% at Wave 4) or English. Participants with MMSE <10 were not recruited at baseline, in follow-up interviews participants with MMSE <10 could use a proxy if requested, except for completion of the cognitive assessments. Assessments included: (i) the MMSE (18), a test of general cognition; 22 (2.3%) and 9 (2.0%) participants from Waves 2 and 4 had an MMSE between 10 and 16; (ii) a word list learning test to assess verbal memory, recognition, and percentage retention (number of words recalled after a delay by the number of words recalled from the fifth learning attempt) (19); (iii) digit span forward and backward (19), a test of working memory and attention; (iv) Stroop test (19), a test of executive function; (v) verbal fluency (19), naming as many words as possible starting with a specified letter; (vi) clock drawing (20); and (vii) figure copying (21), to assess executive function, including visual and spatial function and organization. From these 7 assessments, 10 cognitive scores were generated (MMSE, word list learning, word list recognition, percent retention, Stroop, letter fluency, digit span forward, digit span backward, clock drawing, and weighted figure copying), and their Z-scores were averaged to calculate a global cognitive function score (GCS), described in detail previously (22,23). Missing scores in any of the 7 assessments used to compute the GCS were imputed using the minimum Z-score of the missing assessment in the cohort, unless the missing value was due to a participant’s inability, including illiteracy, hearing impairment, or poor vision. Missing values due to a participant’s inability were imputed with the averaged values of the participant’s existing assessments. The number of participants missing at least 1 cognitive score at Wave 2 (n = 1 258) and Wave 4 (n = 573) were 186 (14.8%) and 54 (9.4%), respectively. Principal components analysis (PCA) with varimax rotation, using the PROC FACTOR procedure in SAS (version 9.4; SAS Institute, Cary, NC), was used to reduce redundancy of the 10 cognitive scores and to detect structures in the relationships between variables (22). Two factors (executive function and memory) were identified (23). Change in cognitive function was calculated by subtracting Wave 4 cognitive scores from Wave 2 cognitive scores; lower values indicate greater decline in cognitive function over time.
Covariates
Demographics included age (continuous), sex, and education. Language acculturation was measured with 7 items from the Acculturation Scale for Hispanics (24) and modified for the Puerto Rican population (25). These items assess language use in various activities (eg, watching TV, listening to the radio, reading newspapers, talking with family and friends) with a 5-point Likert scale (only Spanish, more Spanish than English, both equally, more English than Spanish, only English). Summed scores (α = 0.90) ranged from 0 to 100, with higher scores indicating higher level of English language use.
We adjusted for Wave 2 health behaviors and health conditions associated with cognition, including smoking (current vs past or never); body mass index (BMI, kg/m2); and physical activity, assessed with a modified Paffenbarger questionnaire of the Harvard Alumni Activity Survey (26,27). Weight (kg) was measured using a regularly calibrated clinical scale (Toledo Weight Plate, Model I5S, Bay State and Systems), and standing height (m) was measured with a stadiometer (HM200P Portable Stadiometer) in duplicate by interviewers during in-home visits. Health conditions included depressive symptomatology, measured with the CES-D-20 (28), a scale to evaluate frequency of symptoms associated with depression (α = 0.90); diabetes was defined as fasting glucose ≥126 mg/dL or use of diabetes medication; heart disease (self-reported, yes = if a doctor ever diagnosed them with a heart attack, heart disease other than heart attack, or a stroke); and medication use, including prescription antidepressants and prescription sleep aids (anxiolytics, sedatives, and hypnotics). Interviewers requested medication bottles to record the name from the bottle’s label.
Data Analysis
We conducted analyses in 3 different steps using Stata, version 14 (StataCorp, 2015). First, we estimated descriptive statistics and used cross-tabulation with Chi-square tests for categorical variables and t tests for continuous variables to examine associations with participants’ characteristics (Table 1), cognitive function and sleep, sex, and age (Table 2). Next, we used linear regressions to evaluate cross-sectional and longitudinal associations between sleep duration and insomnia symptoms, with cognitive function measures, that is, GCS, executive factor, memory factor, and MMSE, controlling for covariates. We run linear regression diagnostics to assess model fit. We checked for influential data, normality of residuals, heteroscedasticity, multicollinearity, and linearity. Three extreme and influential outliers located at 200% or more of the interquartile range (IQR) in the GCS change models, and one extreme outlier located at 150% of the IQR in the Memory change model were identified and removed in longitudinal models. Other assumptions were met. We excluded participants with missing sleep data and covariate data in cross-sectional analyses (n = 311 and n = 45, respectively) and longitudinal analyses (n = 136 and n = 17, respectively). Participants with missing data at Wave 2 were older (mean = 60.9 ± 8.1 vs mean = 58.6 ± 7.4; t = 4.8; p < .0001), had lower CES-D scores (mean = 16.9 ± 11.9 vs mean = 18.6 ± 12.7; t = −2.1; p = .0320), and had greater diabetes rates (49.2% vs 39.7%; χ = 8.9; p = .003) than participants with complete data. Participants with missing data at Wave 4 had higher physical activity levels (mean = 33.0 ± 5.5 vs mean = 31.8 ± 4.1; t = 2.3; p = .0218) compared to participants with complete data. Our primary analyses employed the complete-case approach. Additionally, due to the substantial loss to follow-up in the BPHRS cohort in Wave 4, we conducted sensitivity analyses using the multiple imputation method. We use the iterative chained equations routine in Stata that accounts for the longitudinal data design in the estimation and supports factor variables to test the interactions included in our final models. We controlled for sociodemographic and cultural factors, including age, sex, education, and language acculturation; and potential behavioral and health confounders including smoking, physical activity, BMI, depressive symptoms, diabetes, heart disease, and the use of antidepressants and sleep aids medication in cross-sectional models. Additionally, we adjusted for number of days between the Wave 2 and Wave 4 interviews, and for the Wave 2 cognitive measure in longitudinal models. Third, we included multiplicative terms of sex, age, and cognitive measure, sex × age × cognitive score, to examine whether the associations between sleep duration and insomnia symptoms, and cognitive function were modified by sex (male vs female) and/or age (<65 vs ≥65 years). Significance of interaction terms (sex × age × sleep duration) were tested by the Wald test. Significance (2-tailed) was set at p <.05. It is possible that cognitive impairment and related pathologies may alter sleep before the onset of clear symptomatology; therefore, we conducted additional sensitivity analysis restricted to participants with Wave 2 MMSE scores ≥22.
Table 1.
Descriptive Characteristics of the Boston Puerto Rican Health Study at Wave 2
| All | Men | Women | Younger, <65 y | Older, ≥65 y | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||||
| N = 952† | n = 268 | n = 684 | n = 211 | n = 516 | n = 57 | n = 168 | ||||
| Age, y, mean ± SD (range: 46–78) | 58.7 ± 7.5 | 58.2 ± 7.8 | 58.9 ± 7.3 | 55.1 ± 5.3 | 55.6 ± 4.9 | 69.8 ± 3.4 | 69.0 ± 3.2 | |||
| Education, n (%) | * | * | ||||||||
| No schooling or <5th grade (ref.) | 214 (22.5) | 47 (17.7) | 167 (24.4) | 32 (15.3) | 87 (16.9) | 15 (26.3) | 80 (47.6) | |||
| 5th–8th grade | 240 (25.3) | 73 (27.4) | 167 (24.4) | 55 (26.3) | 131 (25.4) | 18 (31.6) | 36 (21.4) | |||
| 9th–12th grade | 353 (37.2) | 112 (42.1) | 241 (35.2) | 93 (44.5) | 204 (39.5) | 19 (33.3) | 37 (22.0) | |||
| College/graduate | 143 (15.1) | 34 (12.8) | 109 (15.9) | 29 (13.9) | 94 (18.2) | 5 (8.8) | 15 (8.9) | |||
| Acculturation, mean ± SD (range: 0–96) | 23.3 ± 22.2 | 28.0 ± 23.4 | 21.5 ± 21.5 | *** | 30.1 ± 23.7 | 24.9 ± 22.1 | ** | 20.2 ± 20.3 | 11.1 ± 15.5 | *** |
| Currently smoking, n (% yes) | 198 (20.1) | 80 (30.1) | 118 (17.3) | *** | 68 (32.5) | 99 (19.2) | *** | 12 (21.1) | 19 (11.3) | + |
| Physical activity, mean ± SD (range: 24–61) | 31.8 ± 4.6 | 33.0 ± 5.8 | 31.3 ± 4.0 | *** | 33.3 ± 5.8 | 31.5 ± 4.1 | *** | 32.0 ± 5.9 | 30.7 ± 3.7 | * |
| BMI, categories, kg/m2 (%) | *** | *** | ||||||||
| <25 | 107 (11.4) | 40 (15.3) | 67 (9.9) | 33 (16.2) | 51 (10.0) | 7 (12.7) | 16 (9.7) | |||
| 25–29.9 | 266 (28.4) | 102 (39.1) | 164 (24.3) | 83 (40.3) | 122 (23.9) | 19 (34.6) | 42 (25.5) | |||
| >30 | 563 (60.2) | 119 (45.6) | 444 (65.8) | 90 (43.7) | 337 (66.1) | 29 (52.7) | 107 (64.9) | |||
| Diabetes, n (% yes) | 375 (40.2) | 106 (40.6) | 269 (40.1) | 76 (36.9) | 180 (35.4) | 30 (54.6) | 89 (54.9) | |||
| Heart condition, n (% yes) | 219 (23.0) | 64 (23.9) | 155 (22.7) | 44 (20.9) | 105 (20.4) | 20 (35.1) | 50 (29.8) | |||
| Depressive symptoms, CES-D mean ± SD | 18.6 ± 12.6 | 14.6 ± 11.6 | 20.1 ± 12.7 | *** | 5.9 ± 11.6 | 20.9 ± 12.8 | *** | 9.7 ± 10.5 | 17.7 ± 12.0 | *** |
| Medication use, n (% yes) | ||||||||||
| Antidepressants | 357 (37.6) | 65 (24.3) | 929 (42.8) | *** | 58 (27.5) | 236 (45.7) | *** | 7 (12.3) | 56 (33.7) | ** |
| Sleep aids: anxiolytics or benzodiazepines | 233 (24.6) | 37 (13.8) | 196 (28.8) | *** | 30 (14.2) | 165 (32.0) | *** | 7 (12.3) | 31 (18.8) | |
Notes: CES-D = Center for Epidemiological Studies—Depression scale; SD = standard deviation. Frequencies, percentages, and Chi-square tests performed for categorical variables; and mean, standard deviation, and t tests performed for continuous variables.
+ p < .10. *p < .05. **p < .01. ***p < .001.
†Not all categories add to n = 952 due to missing data in some covariates, a total of 45 participants had at least one covariate value missing.
Table 2.
Cognitive Function and Sleep Characteristics of the Boston Puerto Rican Health Study at Wave 2 and Wave 4
| All | Men | Women | Younger, <65 y | Older, ≥65 y | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||||
| Wave 2 | ||||||||||
| N = 952 | n = 268 | n = 684 | n = 211 | n = 516 | n = 57 | n = 168 | ||||
| Wave 2 cognitive function scores, mean ± SD | ||||||||||
| GCS | −0.03 ± 0.6 | −0.05 ± 0.6 | −0.02 ± 0.6 | −0.003 ± 0.6 | 0.07 ± 0.5 | + | −0.23 ± 0.7 | −0.30 ± 0.6 | ||
| Executive factor | 0.07 ± 1.0 | 0.23 ± 1.0 | 0.01 ± 1.2 | ** | 0.26 ± 1.0 | 0.17 ± 1.0 | 0.05 ± 0.9 | −0.55 ± 0.9 | *** | |
| Memory factor | 0.21 ± 0.9 | −0.09 ± 0.9 | 0.32 ± 0.04 | *** | −0.03 ± 0.9 | 0.39 ± 0.9 | *** | −0.37 ± 0.9 | 0.09 ± 0.9 | ** |
| MMSE | 23.2 ± 3.4 | 23.7 ± 3.7 | 23.1 ± 3.2 | ** | 23.7 ± 3.6 | 23.44 ± 3.0 | 23.5 ± 3.9 | 21.9 ± 3.5 | ** | |
| Sleep measures from BPROS | ||||||||||
| Insomnia symptoms, mean ± SD | 3.26 ± 1.9 | 2.88 ± .0 | 3.41 ± 1.8 | *** | 3.03 ± 2.0 | 3.50 ± 1.8 | ** | 2.39 ± 2.0 | 3.10 ± 1.9 | * |
| Sleep duration, n (%) | ||||||||||
| 7 h | 181 (19.1) | 50 (18.7) | 131 (19.3) | 39 (18.8) | 101 (19.7) | 11 (19.3) | 30 (18.1) | |||
| <7 h | 452 (47.8) | 126 (47.2) | 326 (48.0) | 100 (48.1) | 245 (47.9) | 25 (43.9) | 80 (48.2) | |||
| ≥8 h | 313 (33.1) | 91 (34.1) | 222 (32.7) | 69 (33.1) | 166 (32.4) | 21 (36.8) | 56 (33.7) | |||
| Wave 4 (~10 y after Wave 2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 444 | n = 110 | n = 334 | n = 90 | n = 260 | n = 20 | n = 74 | ||||
| Wave 4 cognitive function scores, mean ± SD | ||||||||||
| GCS | −0.06 ± 0.6 | −0.08 ± 0.7 | −0.06 ± 0.6 | 0.01 ± 0.5 | 0.07 ± 0.6 | −0.53 ± 1.1 | −0.48 ± 0.7 | |||
| Executive factor | −0.49 ± 1.2 | −0.38 ± 1.3 | −0.53 ± 1.2 | −0.25 ± 1.2 | −0.28 ± 1.1 | −0.98 ± 1.5 | −1.42 ± 1.0 | |||
| Memory factor | 0.14 ± 1.1 | −0.09 ± 1.1 | 0.21 ± 1.1 | * | 0.06 ± 1.0 | 0.31 ± 1.1 | + | −0.79 ± 1.3 | −0.15 ± 1.2 | * |
| MMSE | 22.9 ± 3.7 | 23.4 ± 4.1 | 22.8 ± 3.6 | 23.8 ± 3.8 | 23.4 ± 3.1 | 21.7 ± 4.9 | 20.6 ± 4.3 | |||
| Sleep measures from BPROS | ||||||||||
| Insomnia symptoms, mean ± SD | 3.41 ± 1.9 | 2.99 ± 2.0 | 3.55 ± 1.8 | ** | 3.22 ± 1.99 | 3.58 ± 1.8 | 1.95 ± 1.9 | 3.46 ± 1.9 | ** | |
| Sleep duration, n (%) | ||||||||||
| 7 h | 86 (19.6) | 22 (20.4) | 64 (19.3) | 15 (17.1) | 50 (19.4) | 7 (35.0) | 14 (18.9) | |||
| <7 h | 214 (48.6) | 48 (44.4) | 166 (50.0) | 41 (46.6) | 126 (48.8) | 7 (35.0) | 40 (54.1) | |||
| ≥8 h | 140 (31.8) | 38 (35.2) | 102 (30.7) | 32 (36.4) | 82 (31.8) | 6 (30.0) | 20 (27.0) | |||
Notes: BPROS = Boston Puerto Rican Osteoporosis Study, an ancillary study of the BPRHS that collected data at one point in time after Wave 2; GCF = global cognitive function; MMSE = Mini-Mental State Examination; SD = standard deviation. Frequencies, percentages, and Chi-square tests performed for categorical variables; and mean, standard deviation, and t tests performed for continuous variables.
+ p < .10.
* p < .05.
** p < .01.
*** p < .001.
Results
Sample Descriptive Statistics
Wave 1 sample (Table 1) included 952 participants, 71.8% (n = 684) women. The mean age was 58.7 ± 7.4 years, and 52.2% had ninth grade or higher level of education. Mean language acculturation was low (23.3 ± 22.2, range 0–96), approximately 1 in 5 participants were current smokers, 40.2% had diabetes, 23% had a heart condition diagnosis, and more than half were classified as obese. Mean scores for physical activity and depressive symptomatology were 31.8 (SD 4.6, range 0–54) and 18.6 (SD 12.6, range 24–61), respectively. More than one-third were taking antidepressants and almost a quarter reported using sleep aids. Compared to women, men had significantly higher levels of education (p = .041) and acculturation (p < .0001), were current smokers in higher proportions (p < .0001), used antidepressants (p < .0001) and sleep aid (p < .0001) medications in lower proportions, and reported higher physical activity (p < .0001) and lower depressive symptomatology (p < .0001). A similar pattern of differences was observed between men and women in the younger (<65 years) and older (≥65 years) groups.
Sex and Age Differences in Cognitive Function and Sleep Characteristics
Compared to women, men had significantly higher executive function (p = .007) and MMSE (p = .006) scores, and lower reported symptoms of insomnia (p = .0001) at Wave 2. Memory scores were significantly lower in men, both at Wave 2 (p < .0001) and Wave 4 (p = .017), compared to women. Similar patterns were observed between men and women in comparison by age group (see details in Table 2). Insomnia symptoms were highest among men (p < .0001) and women (p < .0001) with short sleep duration (<7 hours) compared to 7 and ≥8 hours of sleep.
Cross-Sectional Models
Sleep duration
In fully adjusted models (n = 902), compared to 7 hours of sleep, long sleep duration (≥8 hours) became non significantly associated with lower global cognitive function (β [95% CI]: −0.08, [−0.17, 0.001], p = .054), executive function (−0.16, [−0.33, 0.01], p = .058), and MMSE (−0.53, [−1.06, 0.008], p = .053) scores (Table 3). We found no significant sex-, age-, or sex × age-sleep duration interactions with cognitive scores (Supplementary Tables 1 and 1A).
Table 3.
Cross-Sectional Associations Between Sleep Measurements and Cognitive Scores at Wave 2
| Global Cognition Score | Executive Function | Memory | Mini-Mental State Examination | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | ||||
| Sleep duration | |||||||||||
| Sleep duration, h† | |||||||||||
| 7 h (ref.) | |||||||||||
| <7 h | −0.04 | (−0.12, 0.04) | −0.07 | (−0.23, 0.08) | 0.11 | (−0.05, 0.28) | −0.14 | (−0.64, 0.36) | |||
| ≥8 h | −0.08 | (−0.17, 0.001) | * | −0.16 | (−0.33, 0.01) | * | 0.03 | (−0.14, 0.21) | −0.53 | (−1.06, 0.008) | * |
| n | 902 | 786 | 786 | 903 | |||||||
| Insomnia symptoms | |||||||||||
| Insomnia symptoms, mean† | 0.003 | (−0.01, 0.02) | 0.0001 | (−0.03, 0.03) | −0.02 | (−0.06, 0.01) | 0.05 | (−0.05, 0.15) | |||
| n | 911 | 792 | 792 | 912 | |||||||
Notes: CI = confidence interval.
* p < .10.
†Models adjusted for sex, education, smoking, acculturation, physical activity, BMI, depressive symptomatology, diabetes, heart disease, antidepressant use, and sleep aids use.
Insomnia symptoms
Insomnia symptomatology was not significantly associated with global cognitive function, MMSE, or the 2 domain-specific cognitive outcomes, that is, executive and memory function, after adjusting for potential confounders (Table 3). No significant sex-, age-, or sex × age-insomnia symptom interactions were observed (Supplementary Tables 2 and 2A).
Longitudinal Models
Sleep duration
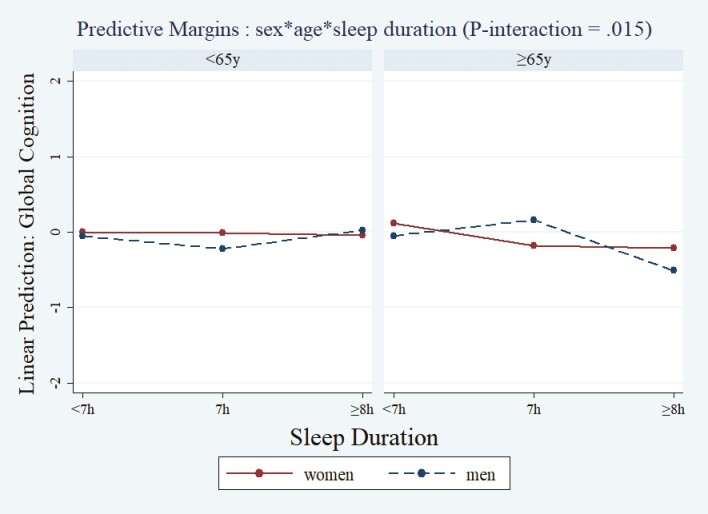
After adjusting for covariates (n = 420), we did not observe a significant main effect of sleep duration on ~10-year change in cognitive scores. We observed a significant sex × age × sleep duration interaction (p-interaction = .015) indicating that older men with short sleep duration (β [95% CI]: −0.67, [−1.24, −0.10]) or long sleep duration (−0.92, [−1.55, −0.30]) had greater declines in global cognition scores, compared to women, younger men, and older men with 7 hours of sleep (Figure 1; Supplementary Tables 3 and 3A).
Figure 1.
Marginal effects of sleep duration at Wave 2 on ~10 years global cognitive score (GCS) change by sex and by age. Predicted values of GCS (y-axis) were plotted against the sleep duration (x-axis) by sex and age based on the adjusted models (models adjusted for: Wave 2 cognitive score + days between interviews + age + sex + education + smoking + acculturation + physical activity + BMI + depressive symptomatology + diabetes + heart disease + antidepressant use + sleep aids use). The significance of the interaction term (sex × age × sleep duration) was tested by the Wald test (p-interaction = .015), n = 420. BMI = body mass index.
Insomnia symptoms
Parallel to sleep duration models, we did not observe a significant main effect of insomnia symptomatology on ~10-year change in cognitive scores after adjusting for covariates. In interaction analyses, a 1-point increase in insomnia symptomatology at Wave 2 was significantly associated with greater declines in memory at Wave 4 in older men (sex × age × insomnia symptoms, (β [95% CI]: −0.54, [−0.85, −0.22], p-interaction = .004), compared to younger and older women and younger men (Figure 2; Supplementary Tables 4 and 4A).
Figure 2.
Marginal effects of insomnia symptoms at Wave 2 on ~10 years memory factor change by sex and by age. Predicted values of memory factor (y-axis) were plotted against the insomnia symptoms (x-axis) by sex and age based on the adjusted models (models adjusted for: Wave 2 cognitive score + days between interviews + age + sex + education + smoking + acculturation + physical activity + BMI + depressive symptomatology + diabetes + heart disease + antidepressant use + sleep aids use). The significance of the interaction term (sex × age × insomnia symptoms) was tested by the Wald test (p-interaction = .004), n = 366. BMI = body mass index
Results of sensitivity analysis including only participants with MMSE score of 22 points or higher at Wave 2 were analogous to results from the main analyses. Results of sensitivity analyses using imputed data were equivalent to the main results (Imputation Method results and longitudinal models using the imputed data are described in detail in Supplementary Tables 5–7).
Discussion
The current analysis was designed to examine associations between sleep duration and insomnia symptoms with an ~10-year change in several cognitive function assessments in a cohort of older Puerto Rican adults residing in the Northeast region of the United States. We did not observe significant cross-sectional associations between sleep duration or insomnia symptoms and cognitive function scores, or between sleep duration or insomnia symptoms and ~10-year change in cognitive function. Further interaction analyses indicated that the association between sleep and cognition may be partly dependent on age and sex. In longitudinal models, the significant age × sex × sleep duration interaction on ~10-year change in global cognitive function had an inverted U-shaped association in older men, indicating that older men with either short or long sleep duration had greater decline in 10-year global cognitive function compared to older men with average 7 hours of sleep, younger men, and women. Moreover, the significant age × sex × insomnia symptoms interaction on 10-year memory function change indicated that older men had greater ~10-year memory loss, compared to younger men and women. We discuss these findings and implications for future research and sleep interventions for cognitive health in older Puerto Rican adults.
The prospective link between sleep duration and global cognitive function, and the inverted U-shape, shown in our study with older Puerto Rican adults align with findings from studies with older White adults in the United States (29) and prospective studies with participants from other Western countries (9,12). The significant sleep duration and global cognitive function association observed only in older men in our study parallels recent findings with other Latino groups. In cross-sectional analysis, a curvilinear association between sleep duration and cognition was found in men, but not women in participants from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) (15). In longitudinal analysis with HCHS/SOL participants, a significant longitudinal (7 years) decline in episodic learning and memory was also observed in long sleepers (>9 hours) (30). The mechanism underlying the effect of sleep duration on cognition remains unknown. Self-reported short and long sleep durations may both be indicators of poor sleep quality, including insufficient duration of the different stages of sleep. Short sleep durations in the N3 stage of NREM sleep may reduce the process of removing toxic metabolites in the brain, such as amyloid-β peptides (31), and sleep deprivation may increase Aβ deposition (31). Moreover, it is plausible that sleep loss may lead to neural damage associated with cognitive decline as suggested by a recent study with older adults with poorer sleep quality and lower sleep efficiency (32).
In line with previous studies that show a greater risk for insomnia symptoms and insomnia diagnosis in women compared to men (33), younger and older women in our sample reported significantly higher symptoms of insomnia compared to younger and older men. Nevertheless, this study showed that insomnia symptoms were associated with a significant decline in memory over approximately 10 years only in older men. This finding contrasts with previous studies showing midlife or late-life insomnia and poor sleep quality are associated with cognitive impairment in women but not in men (34). Our findings suggest that insomnia symptoms may be a marker for memory decline in older men, but not women, in this sample. Insomnia symptoms such as frequent WASO are negatively associated with memory in older adults (35). Frequent WASO, in turn, may shorten the length of non-rapid eye movement (NREM) sleep stages and affect memory. Older adults with short N2 and N3 report memory problem consolidation and reduced episodic memory (36,37). Whereas, a study with older (58–91 years) adults without sleep problems, higher spindle density, characteristic of N2 sleep stage, reported better verbal learning, visual attention, and verbal fluency (36). It is suggested that spindle density and verbal learning performance may be mediated by restoration of hippocampus encoding capacity, in part due to disruptions in cyclic adenosine monophosphate (cAMP) signaling (38). Our finding that greater insomnia symptoms were associated with prospective memory decline in older, but not young men could be related to the additive effect of poor sleep and aging-related changes in the brain, particularly medial prefrontal cortex atrophy that may reduce periods of slow wave sleep leading to less effective sleep-dependent memory consolidation (39), Moreover, the older Puerto Rican men in our sample may have longer exposures to psychosocial stressors, including ethnic discrimination, that overtime accumulates and interact with other health problems.
Sleep is considered essential for memory consolidation (35). Sleep deprivation may act as a chronic stressor that may lead to memory deficits and impairment by altering the brain plasticity, reducing neurogenesis in the hippocampus, and reducing tau levels as observed in sleep-deprived mice models with significant shrinkage of the hippocampus (40). In our study, approximately half of the participants reported short sleep duration (≤6 hours) and participants in this category had the highest insomnia symptomatology, suggesting that sleep deprivation may underlie the changes observed in memory function associated with insomnia symptoms. Insomnia symptoms are higher in Latinx compared to White groups (41) and Puerto Rican adults are more likely to report shorter sleep. In line with previous studies, men in this study self-report lower symptoms of insomnia compared to women suggesting that the association of insomnia symptoms with decline in memory observed only in older men may indicate the effect of a third variable affecting sleep behaviors and cognition. Given the amount of obesity and diabetes in the sample, it is possible that sleep apnea may be a confounding factor in these analyses, although controlling for snoring in post hoc analysis did not change the findings. Additionally, it is plausible that sleep is mediating the effect of stress on cognition as has been observed in the Survey of Health, Aging, and Retirement in Europe (42). Racial and ethnic disparities in poor sleep are well documented (43), chronic stress and perceived ethnic discrimination are found to be significantly associated with insomnia symptoms in Latinos (44), and partly explained the differences in objective and subjective sleep between midlife African American and White participants (45). Moreover, perceived discrimination has been shown to be associated with poorer episodic memory and information processing speed in African American persons (46), and with worse cognitive function in adult Latinos of Mexican origin (47). The experiences of perceived ethnic discrimination in older men versus the younger men and women in this sample may be a significant psychosocial stressor given their distinct migratory experiences to the mainland during the 1950s and 1960s characterized by discriminatory and exploitative employment practices that were common, overt, and unsanctioned (48). The effect of stressful circumstances earlier in life may interact with other stressors, including lower levels of education and higher poverty levels that characterized older men vs younger men in this study, and/or cumulate to render them more sensitive to the effects of poor sleep on cognition later in life. Like older men, older women in this study have overall lower levels of education and higher levels of poverty compared to younger women; however, a great majority of them migrated to the mainland for family reunification purposes and stayed at home thus it is possible that they were exposed to lower levels of discrimination upon arrival.
In sum, sleep may mediate the prospective association between stressors such as lifetime racial/ethnic discrimination and chronic stress, and memory decline in this sample of older Puerto Rican men. Sleep difficulties are common in racial and ethnic minorities and older adults, however, the effects on memory and the neural mechanisms linking the 2 need further investigation. Our study only had subjective measures of sleep, future studies with objective measures of sleep architecture could investigate the influence of NREM sleep oscillation on stress, memory, and changes that occur with aging.
Taken together, our findings suggest that insomnia symptoms may be a marker for memory decline in older men, indicating that sleep evaluation in older adults may provide important clinical information for the early detection and prevention of sleep-related cognitive impairment. Thus, it may be effective to integrate sleep health strategies into interventions to prevent cognitive impairment. Psychobehavioral interventions have proven to be effective in improving sleep health; however, the great majority of individuals from racial/ethnic diverse groups with sleep problems are not benefiting from these interventions. In a recent review, of the 56 behavioral randomized controlled trials found to test psychological interventions for sleep disorders, only 6.97% targeted racial/ethnic minorities and/or other disadvantaged groups, and no intervention targeted linguistic minorities or immigrants (49). Additionally, only 4 interventions incorporated cultural adaptations such as involving other family members or incorporation of cultural values, or adapted the material to match the literacy level of participants; 3 tested the effectiveness of a culturally adapted behavioral intervention such as the CBT-I, and no interventions tested cultural adaptations of Cognitive Processing Therapy and Problem Solving Therapy, proven to be effective for improving sleep health (49). Hence, increased qualitative and mixed-methods research that investigate diverse participants’ perceptions of the causes of disrupted sleep patterns and preferred treatment strategies are needed to inform the development of tailored interventions. For example, this specific population may benefit from increased sleep health literacy and access to sleep-related services targeted at diverse racial/ethnic and older individuals. Future research should then also increase the diversity of participants in randomized control trials that test for the effectiveness of culturally adapted interventions that match with participant’s cultural and literacy levels.
Strengths and Limitations
A strength of our study is the inclusion of a representative sample of middle-aged and older Latinos of Puerto Rican descent, who are underrepresented in studies of sleep risk factors for neurocognitive decline. Additionally, the study addressed multiple potential confounding factors, and the findings were robust even after adjusting for demographic, socioeconomic, cultural, health behavioral, and vascular risk factors. Controlling for clinical risk conditions at baseline such as heart disease, depressive symptomatology and use of antidepressants, diabetes, obesity, and use of sleep aid medication, previously identified as potential causes of cognitive decline as well as associated with sleep disorders supports the validity of findings.
This study also has several limitations. Our findings suggest that insomnia symptoms and hours of sleep are associated with a decline in memory and overall cognitive function in older men, however, our study included a low percentage of male participants. Future studies are needed to confirm these prospective associations and should include an adequate number of male participants. The BPRHS was not originally designed to test hypotheses about the association between sleep duration or sleep quality with specific cognitive domains, and thus, it did not necessarily assess the most relevant objective and subjective sleep parameters, or potential confounds. Sleep duration and quality were assessed with a general retrospective self-report measure. Objective measures of sleep through polysomnography (PSG) can provide detailed sleep architecture information and more precise information on time spent in sleep stages. Sleep actigraphy can also provide more accurate data on duration and additional dimensions of sleep, such as efficiency and timing. However, self-report measures of sleep have been shown to have good reproducibility, are convenient, and to correlate with objective measures of sleep. These measures are sometimes preferred to objective measures that may not detect, for example, symptoms of insomnia, a diagnosis that relies on subjective perception of sleep difficulty. Sleep quality is often measured as a subjective assessment of satisfaction with sleep, whereas the variables used to assess quality in our study captured elements of insomnia symptoms. Additionally, objective measures such as actigraphy or PSG may not capture generalized patterns of sleep. Future studies may benefit from the use of complementary methods combining self-report with objective assessments of sleep that may allow for the assessment of all relevant domains of sleep (14).
Lastly, although our findings remained robust after potential behavioral and clinical factors were included, we cannot rule out the possibility of unmeasured confounding or alternative explanations. The associations between extreme sleep patterns and subjective sleep quality with global cognition and memory function observed may be related to lifetime exposure to individual- and context-level stressors predicting neurocognitive damage through alternative mechanisms other than through the role of clinical risk factors or health behaviors. For example, stress related to pressures to assimilate, exposure to racial/ethnic discrimination, and/or socioeconomic status discrimination may underlie sleep patterns in minoritized communities (45−47). Therefore, it is possible that sleep disruptions share common mechanisms with or are prodromal symptoms of later cognitive impairment.
Conclusion
We observed that sleep duration was significantly associated with global cognitive function, longitudinally, and that insomnia symptoms were associated with longitudinal memory function scores in older men. These observed adverse cognitive effects remained robust after adjustment for demographic, socioeconomic, and cultural factors, relevant health behaviors, and several clinical risk factors for dementia. Additional research is warranted to examine whether different sleep architecture factors are associated with longitudinal changes in specific cognitive functions. The findings of this study support the important role of sleep duration and quality in determining cognitive decline and have important implications for personalizing sleep monitoring and sleep interventions for cognitive health in older Puerto Rican adults. Notably, multidisciplinary research with more diverse racial/ethnic participants that examines the modifying role of age, sex, and ancestry is needed to better understand pathways for optimal sleep and healthy brain aging.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Contributor Information
Sandra P Arévalo, Department of Human Development, California State University, Long Beach, California, USA.
Selena T Nguyen-Rodriguez, Department of Health Science, California State University, Long Beach, California, USA.
Tammy M Scott, Neuroscience and Aging Laboratory, Jean Mayer U.S. Department of Agriculture Human Nutrition Research Center on Aging, Boston, Massachusetts, USA.
Xiang Gao, Department of Nutrition and Food Hygiene, School of Public Health, Fudan University, Shanghai, China.
Luis M Falcón, College of Fine Arts, Humanities and Social Sciences, University of Massachusetts, Lowell, Massachusetts, USA.
Katherine L Tucker, Department of Biomedical and Nutritional Sciences, University of Massachusetts Lowell, Massachusetts, USA.
Funding
This work was supported by the National Institutes of Health under grants P01 AG023394, P50 HL105185, and R01 AG055948. Sandra P. Arévalo receives funding from the National Institutes of Health under grant P30 AG059300.
Conflict of Interest
None.
References
- 1. Kivipelto M, Mangialasche F, Snyder HM, et al. World-Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimer Dement. 2020;16(7):1078–1094. doi: 10.1002/alz.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–e125. doi: 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alzheimer’s Association and Centers for Disease Control and Prevention. Healthy Brain Initiative, State and Local Public Health Partnerships to Address Dementia: The 2018–2023 Road Map. Alzheimer’s Association; 2018. https://www.cdc.gov/aging/pdf/2018-2023-Road-Map-508.pdf. Accessed June 26, 2023. [Google Scholar]
- 4. Matthews KA, Wei X, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer Dement. 2019;15(1):17–24. doi: 10.1016/j.jalz.2018.06.3063. https://www.ncbi.nlm.nih.gov/pubmed/30243772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta KM, Yeo GW.. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimer Dement. 2017;13(1):72–83. doi: 10.1016/j.jalz.2016.06.2360 [DOI] [PubMed] [Google Scholar]
- 6. González HM, Tarraf W, Schneiderman N, et al. Prevalence and correlates of mild cognitive impairment among diverse Hispanics/Latinos: study of Latinos: investigation of neurocognitive aging results. Alzheimer Dement. 2019;15(12):1507–1515. doi: 10.1016/j.jalz.2019.08.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alegría M, Canino G, Shrout PE, et al. Prevalence of mental illness in immigrant and non-immigrant U.S. Latino groups. Am J Psychiatry. 2008;165(3):359–369. doi: 10.1176/appi.ajp.2007.07040704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jimenez DE, Alegría M, Chen CN, Chan D, Laderman M.. Prevalence of psychiatric illnesses in older ethnic minority adults. J Am Geriatr Soc. 2010;58(2):256–264. doi: 10.1111/j.1532-5415.2009.02685.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Virta JJ, Heikkilä K, Perola M, et al. Midlife sleep characteristics associated with late life cognitive function. Sleep. 2013;36(10):1533–1541A. Published 2013 Oct 1. doi: 10.5665/sleep.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bubu OM, Brannick M, Mortimer J, et al. Sleep, cognitive impairment, and Alzheimer's disease: a systematic review and meta-analysis. Sleep. 2017;40(1):10. doi: 10.1093/sleep/zsw032 [DOI] [PubMed] [Google Scholar]
- 11. Devore EE, Grodstein F, Schernhammer ES.. Sleep duration in relation to cognitive function among older adults: a systematic review of observational studies. Neuroepidemiology. 2016;46(1):57–78. doi: 10.1159/000442418 [DOI] [PubMed] [Google Scholar]
- 12. Lo JC, Groeger JA, Cheng GH, Dijk DJ, Chee MW.. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med. 2016;17:87–98. doi: 10.1016/j.sleep.2015.08.021 [DOI] [PubMed] [Google Scholar]
- 13. Liang Y, Qu LB, Liu H.. Non-linear associations between sleep duration and the risks of mild cognitive impairment/dementia and cognitive decline: a dose-response meta-analysis of observational studies. Aging Clin Exp Res. 2019;31(3):309–320. doi: 10.1007/s40520-018-1005-y [DOI] [PubMed] [Google Scholar]
- 14. van de Langenberg SCN, Kocevska D, Luik AI.. The multidimensionality of sleep in population-based samples: a narrative review. J Sleep Res. 2022;31(4):e13608. doi: 10.1111/jsr.13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramos AR, Tarraf W, Daviglus M, et al. Sleep duration and neurocognitive function in the Hispanic Community Health Study/Study of Latinos. Sleep. 2016;39(10):1843–1851. Published 2016 Oct 1. doi: 10.5665/sleep.6166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tucker KL, Mattei J, Noel SE, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. Published 2010 Mar 1. doi: 10.1186/1471-2458-10-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumari M, Green R, Nazroo J.. Sleep duration and sleep disturbance. In: Banks J, Lessof C, Nazroo J, et al. , eds. Financial Circumstances, Health and Well-Being of the Older Population in England: The 2008 English Longitudinal Study of Ageing (Wave 4). Institute for Fiscal Studies. 2010:178–226 [Google Scholar]
- 18. Folstein MF, Folstein SE, McHugh PR.. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 19. Artiola Fortuny L, Hermosillo D, Heaton R, Pardee RI.. Manual de Normas y Procedimientos Para la Bateria Neuropsicologica en Español; Manual of Norms and Procedures for a Neuropsychological Battery in Spanish. M Press; 1999. [Google Scholar]
- 20. Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS.. Screening for Alzheimer's disease by clock drawing. J Am Geriatr Soc. 1989;37(8):730–734. doi: 10.1111/j.1532-5415.1989.tb02234.x [DOI] [PubMed] [Google Scholar]
- 21. Beery K. The Developmental Test of Visual-Motor Integration Manual. Revised ed. Modern Curriculum Press;1989. [Google Scholar]
- 22. Gao X, Scott T, Falcon LM, Wilde PE, Tucker KL.. Food insecurity and cognitive function in Puerto Rican adults. Am J Clin Nutr. 2009;89(4):1197–1203. doi: 10.3945/ajcn.2008.26941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bigornia SJ, Scott TM, Harris WS, Tucker KL.. Prospective associations of erythrocyte composition and dietary intake of n-3 and n-6 PUFA with measures of cognitive function. Nutrients. 2018;10(9):1253. Published 2018 Sep 6. doi: 10.3390/nu10091253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marin G, Sabogal F, Marin BV, Otero-Sabogal R, Perez-Stable EJ.. Development of a short acculturation scale for Hispanics. Hisp J Behav Sci. 1987;9(2):183–205. doi: 10.1177/07399863870092005 [DOI] [Google Scholar]
- 25. Falcón LM, Tucker KL.. Prevalence and correlates of depressive symptoms among Hispanic elders in Massachusetts. J Gerontol B Psychol Sci Soc Sci. 2000;55(2):S108–S116. doi: 10.1093/geronb/55.2.s108 [DOI] [PubMed] [Google Scholar]
- 26. Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB.. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328(8):538–545. doi: 10.1056/NEJM199302253280804 [DOI] [PubMed] [Google Scholar]
- 27. Caspersen CJ, Powell KE, Christenson GM.. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 28. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 29. Lucey BP, Wisch J, Boerwinkle AH, et al. Sleep and longitudinal cognitive performance in preclinical and early symptomatic Alzheimer's disease. Brain. 2021;144(9):2852–2862. doi: 10.1093/brain/awab272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramos AR, Tarraf W, Wu B, et al. Sleep and neurocognitive decline in the Hispanic Community Health Study/Study of Latinos. Alzheimers Dement. 2020;16(2):305–315. doi: 10.1016/j.jalz.2019.08.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cross NE, Lagopoulos J, Duffy SL, et al. Sleep quality in healthy older people: relationship with (1)H magnetic resonance spectroscopy markers of glial and neuronal integrity. Behav Neurosci. 2013;127(5):803–810. doi: 10.1037/a0034154 [DOI] [PubMed] [Google Scholar]
- 33. Jaussent I, Dauvilliers Y, Ancelin ML, et al. Insomnia symptoms in older adults: associated factors and gender differences. Am J Geriatr Psychiatry. 2011;19(1):88–97. doi: 10.1097/JGP.0b013e3181e049b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miyata S, Noda A, Iwamoto K, Kawano N, Okuda M, Ozaki N.. Poor sleep quality impairs cognitive performance in older adults. J Sleep Res. 2013;22(5):535–541. doi: 10.1111/jsr.12054 [DOI] [PubMed] [Google Scholar]
- 35. Hokett E, Arunmozhi A, Campbell J, Verhaeghen P, Duarte A.. A systematic review and meta-analysis of individual differences in naturalistic sleep quality and episodic memory performance in young and older adults. Neurosci Biobehav Rev. 2021;127:675–688. doi: 10.1016/j.neubiorev.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lafortune M, Gagnon JF, Martin N, et al. Sleep spindles and rapid eye movement sleep as predictors of next morning cognitive performance in healthy middle-aged and older participants. J Sleep Res. 2014;23(2):159–167. doi: 10.1111/jsr.12108 [DOI] [PubMed] [Google Scholar]
- 37. Yeh A-Y, Pressler SJ, Giordani BJ, Pozehl BJ, Berger AM.. Integrative review of the relationship between sleep disturbances and episodic memory in older adults. Biol Res Nurs. 2018;20(4):440–451. doi: 10.1177/1099800418768070 [DOI] [PubMed] [Google Scholar]
- 38. Raven F, Van der Zee EA, Meerlo P, Havekes R.. The role of sleep in regulating structural plasticity and synaptic strength: implications for memory and cognitive function. Sleep Med Rev. 2018;39:3–11. doi: 10.1016/j.smrv.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 39. Mander B, Rao V, Lu B, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–364. doi: 10.1038/nn.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Di Meco A, Joshi YB, Praticò D.. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer's disease with plaques and tangles. Neurobiol Aging. 2014;35(8):1813–1820. doi: 10.1016/j.neurobiolaging.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 41. Gaston SA, Martinez-Miller EE, McGrath J, et al. Disparities in multiple sleep characteristics among non-Hispanic White and Hispanic/Latino adults by birthplace and language preference: cross-sectional results from the US National Health Interview Survey. BMJ Open. 2021;11(9):e047834. Published 2021 Sep 2. doi: 10.1136/bmjopen-2020-047834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan X, Lebedeva A, Åkerstedt T, Wang HX.. Sleep mediates the association between stress at work and incident dementia: study from the Survey of Health, Ageing and Retirement in Europe. J Gerontol A Biol Sci Med Sci. 2023;78(3):447–453. doi: 10.1093/gerona/glac104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tomfohr L, Pung MA, Edwards KM, Dimsdale JE.. Racial differences in sleep architecture: the role of ethnic discrimination. Biol Psychol. 2012;89(1):34–38. doi: 10.1016/j.biopsycho.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alcántara C, Patel SR, Carnethon M, et al. Stress and sleep: results from the Hispanic Community Health Study/Study of Latinos Sociocultural Ancillary Study. SSM Popul Health. 2017;3:713–721. doi: 10.1016/j.ssmph.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Dyke ME, Vaccarino V, Quyyumi AA, Lewis TT.. Socioeconomic status discrimination is associated with poor sleep in African-Americans, but not Whites. Soc Sci Med. 2016;153:141–147. doi: 10.1016/j.socscimed.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Owens SL, Hunte HER, Sterkel A, Johnson DA, Johnson-Lawrence V.. Association between discrimination and objective and subjective sleep measures in the midlife in the United States Study Adult Sample. Psychosom Med. 2017;79(4):469–478. doi: 10.1097/PSY.0000000000000428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Muñoz E, Robins RW, Sutin AR.. Perceived ethnic discrimination and cognitive function: a 12-year longitudinal study of Mexican-origin adults. Soc Sci Med. 2022;311:115296. doi: 10.1016/j.socscimed.2022.115296 [DOI] [PubMed] [Google Scholar]
- 48. Rodríguez FVM. Saving the Parcela: a short history of Boston’s Puerto Rican Community. In: Whalen CT, Vázquez-Hernández V, eds. Puerto Rican Diaspora: Historical Perspectives. Temple University Press;2005:200–226. http://www.jstor.org/stable/j.ctt14bt09b.12 [accessed December 4, 2022]. [Google Scholar]
- 49. Alcántara C, Giorgio Cosenzo L, McCullough E, Vogt T, Falzon AL, Perez Ibarra I.. Cultural adaptations of psychological interventions for prevalent sleep disorders and sleep disturbances: a systematic review of randomized controlled trials in the United States. Sleep Med Rev. 2021;56:101455. doi: 10.1016/j.smrv.2021.101455 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.