Abstract
Background
Older men with the worse alignment of activity and light may have lower levels of cognition and increased rates of cognitive decline.
Methods
This cohort consisted of 1 036 older men (81.1 ± 4.6 years) from the MrOS Sleep Study (2009–2012). Light and activity levels were gathered by wrist actigraphy. Phasor analysis was used to quantify the alignment of light–dark and rest-activity patterns (magnitude) and their temporal relationship (angle). Global cognitive function (Modified Mini-Mental State examination [3MS]) and executive function (Trails B test) were measured, then repeated 4.2 ± 0.8 years later. Linear regression models examined the associations of phasor magnitude and angle with cognition and cognitive decline. Models were adjusted for age, clinic, race, education, and season.
Results
Smaller phasor magnitude (worse aligned light and activity patterns) was associated with lower initial level and increased decline in executive function. Compared to those with higher phasor magnitude, those with lower magnitude took an average of 11.1 seconds longer to complete the Trails B test (quartile 1 vs quartile 4, p = .02). After follow-up, Trails B completion time increased an average of 5.5 seconds per standard deviation decrease in phasor magnitude (95% confidence interval [CI] 0.7–10.4, p = .03). There were no associations with phasor angle, and none with magnitude and global cognition (3MS).
Conclusion
Among older men, worse alignment of light and activity patterns was associated with worse initial performance and increased decline in executive function, but not related to global cognition. Interventions that improve the alignment of light and activity may slow cognitive decline in older adults.
Keywords: Circadian rhythms, Cognitive function, Light exposure, Phasor analysis
Sleep problems are common in older adults with dementia, and we and others have shown that these sleep–wake disturbances are related to cognitive impairment and cognitive decline (1–6). Specifically, studies have shown a relationship of circadian rest-activity rhythm disturbances to cognitive decline (7–12). One factor that could contribute to the disruption of rest-activity rhythms and nighttime sleep in older adults is their 24-hour pattern of light exposure. Daily patterns of light–dark exposure affect the timing and amplitude of circadian rhythms. Poor alignment between 24-hour light and activity patterns can contribute to sleep disturbances as well as impairments in mood, metabolism, and other health outcomes (13). The effect of poor alignment of the light–dark exposure with rest-activity patterns on cognition is understudied and could be an important pathway in understanding the relationship of poor sleep, wake activity, and cognition.
Circadian responses to light can be diminished in otherwise healthy older adults (14). Furthermore, the difference between daytime and nighttime light exposure can be dampened in older adults as they may spend more time in relatively dim lighting during the daytime hours and be exposed to excessive light during the sleeping period (eg, leaving the light on to safely navigate to the restroom). Studies demonstrate improved sleep and daily rhythms with bright light therapy in adults with dementia (15,16), and a modest benefit for improving some cognitive symptoms of dementia (17). While lighting interventions may be more easily operationalized in older adults, an intervention with moderate exercise at specified times has been shown to have circadian phase-shifting effects (18).
Circadian disruption can be measured by salivary melatonin concentration or core body temperature under highly controlled circumstances, but these measurements can be expensive and difficult to obtain and are less reliable outside the laboratory setting (19). Wearable technology allows for continuous measurement of both activity and light exposure for a number of sequential 24-hour periods. Specifically, actigraphy allows the study of sleep–wake patterns occurring over many days; it is therefore well suited to the study of circadian rest-activity rhythms. Activity is a valid marker of entrained polysomnography (PSG) sleep phase and correlates strongly with entrained endogenous circadian phase (20). Actigraphy has been used to study circadian rhythms in a variety of populations, including demented older persons (6,15).
Light patterns are the main determinant for the timing and amplitude of the human circadian clock, and to a lesser extent timing of exercise influences the human circadian system (21). The relationship between light exposure patterns and activity patterns, which are under the strong influence of the circadian clock, can be used to impute the relationship between the input (light) and output (activity) of the circadian system. Phasor analysis can be used to quantitate this relationship in terms of both the magnitude (strength of coupling) and angle (relative difference in timing) (19).
Using actigraphy data from a prospective study of older men (the Osteoporotic Fractures in Men [MrOS] Sleep Study), which provided 24-hour patterns of light and activity over an average of 4.7 consecutive 24-hour periods, we calculated phasor magnitude and phasor angle. We hypothesized that men with less aligned patterns of activity and light, delineated by smaller phasor magnitudes and larger phasor angles, would have a greater risk of having a worse cognitive function (cross-sectional) and cognitive decline (longitudinal) over approximately 4 years of follow-up.
Method
Participants
During the MrOS baseline examination (March 2000 to April 2002), 5 994 community-dwelling men 65 years or older were enrolled at 6 clinical centers in the United States: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; the Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California (22,23). In order to participate, men needed to be able to walk without assistance and could not have had a bilateral hip replacement.
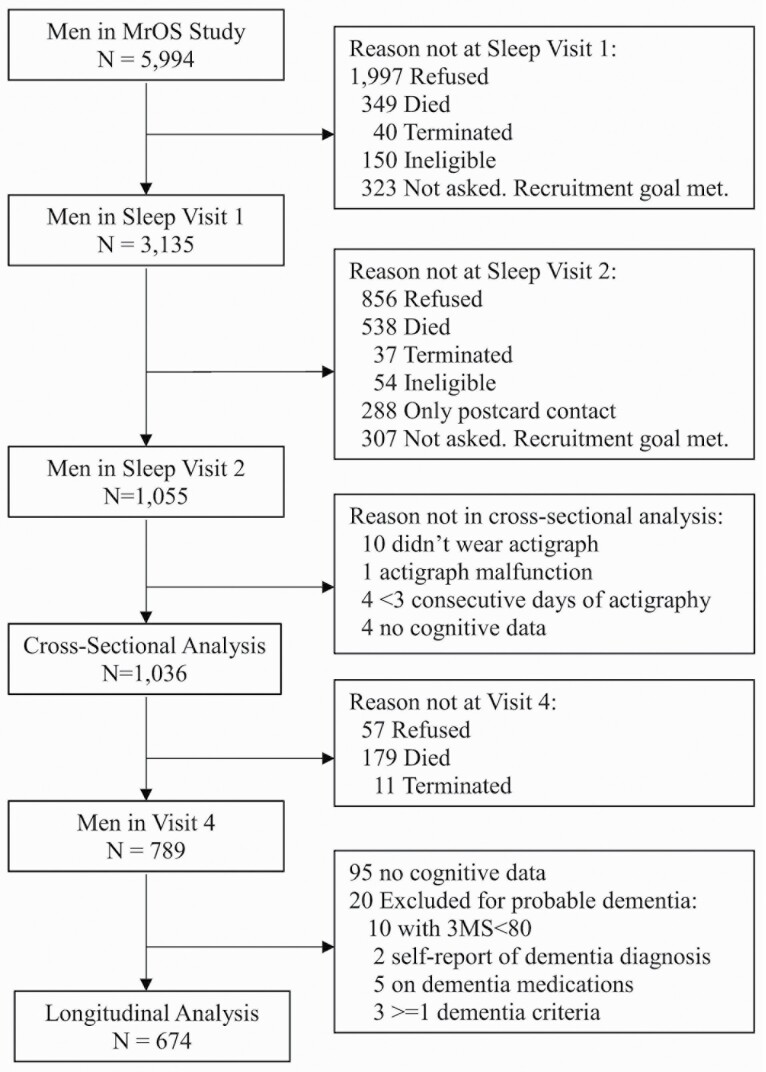
The ancillary MrOS Sleep Study recruited 3 135 participants from the parent cohort from December 2003 to March 2005 (Sleep Visit 1) for comprehensive sleep assessment. At that initial sleep visit, men being treated for sleep apnea or using nocturnal oxygen were excluded if they could not forgo use during a PSG recording. All active MrOS Sleep Study participants with objective sleep data at Sleep Visit 1 were eligible to participate in Sleep Visit 2 (November 2009 to March 2012), when the measurement of light was added to the battery of data collected. The present analyses use light and activity data gathered via actigraphy and covariate data from Sleep Visit 2, which is considered the initial timepoint for the analyses. Actigraph data were considered technically adequate for measurement of activity and light patterns if there were ≥72 consecutive hours of data collection, which is the minimum current requirement for actigraphy monitoring for the Centers for Medicare Services (Current Procedural Technology [CPT® code 95803]) (24). Cross-sectional analyses were performed on the subset of men with both data for technically adequate actigraph data for light and activity along with cognitive data at Sleep Visit 2 (N = 1 036). Longitudinal analyses on change in cognition used data gathered at Sleep Visit 2 and repeated at the MrOS Visit 4 (May 2014 to May 2016). Longitudinal analyses excluded those without a follow-up measure of cognitive function (n = 342). The goal of the longitudinal analyses was to assess if there was an association of circadian misalignment with incident cognitive decline, so those with probable dementia at the Sleep Visit 2 (initial visit for this analysis) were excluded (Modified Mini-Mental State examination [3MS] score <80, self-report of a dementia diagnosis or on a medication for dementia, n = 20). There were 674 men in the longitudinal analytic cohort (Figure 1).
Figure 1.
Progression of participants. MrOS = the Osteoporotic Fractures in Men Sleep study; 3MS = Modified Mini-Mental State examination.
All men provided written informed consent; the study was approved by the Institutional Review Board at each participating study site.
Actigraphy and Phasor Analysis
At Sleep Visit 2, activity and light were measured using an actigraph (Actiwatch® 2, Philips Respironics Inc, Murrysville, PA). The actigraph, which is similar in size to a wristwatch, digitally records an integrated measure of gross motor activity using a solid-state piezoelectric accelerometer with a sensitivity of 0.025 G and a sampling rate of 23 Hz. The actigraph was also equipped with an ambient light sensor that gathered data on light illuminance (lux). The light sensor was a silicon photodiode with a wavelength range of 400–900 nanometers and an illuminance range up to 100 000 lux. This light sensor has been shown to provide good performance in monitoring the temporal patterning of light, whereas the absolute illuminance values tend to underestimate illuminance levels (25). Data were stored as sum (activity) or average (light) in 1-minute epochs. Men were asked to wear the actigraphs continuously and did so for an average of 4.7 24-hour periods (range 3.0–13.5). Protocols did not require the light meter remain uncovered.
Light and activity data were used to estimate circadian entrainment and disruption with phasor analysis (see for example Figures in Supplementary Materials) (19,26). Epochs in which the actigraph was removed were not used in the calculation. Any light value less than 1 lux was replaced with 1 in the calculation, as it is likely the light sensor did not have a resolution of less than 1 lux. The human circadian system responds to light intensity with a rough approximation of a log-compression (27). Therefore, illuminance values were converted to the log scale (log-light). The preprocessed activity and log-light data were used to compute phasor magnitude and phasor angle for each participant. A cosine wave with a 24-hour period was fit to both activity and log-light. Acrophase was calculated for both activity and log-light as the time of the peak of the fitted cosine wave. The difference in acrophase between the fitted cosine wave forms is the phasor angle, reported here in hours. Phasor angle is, therefore, a measure of the offset of time between the fitted peak of the 24-hour rest-activity pattern and the fitted peak of the 24-hour light exposure pattern. A positive phasor angle means that the activity pattern is delayed with respect to light exposure pattern, and a negative phasor angle means that the activity pattern is advanced with respect to light exposure pattern (28). Phasor angle estimates the temporal relationship between light–dark and rest-activity patterns (29). People who are considered to be entrained have a positive phasor angle of approximately 1 hour (28). The phasor magnitude is the strength of correlation between the 2 signals once the phase difference is removed; this is computed as the normalized cross covariance, by dividing by the number of minutes sampled, subtracting the product of the individual signal means, and dividing by the product of the standard deviations (SDs) of each signal (26). Smaller phasor magnitude may imply weaker circadian entrainment because the correlation between light–dark and rest-activity patterns is lower.
Total sleep time during the in-bed interval and sleep efficiency (the percentage of time in bed after “lights off” spent sleeping) were also assessed with actigraphy, calculated using an algorithm in the manufacturer-supplied software (30). Values for sleep efficiency and total sleep time were averaged over all nights the participant wore the device.
Assessment of Cognitive Function
Two tests of cognitive function were administered at both timepoints by trained staff: the Trail Making Test―Part B (Trails B) and the 3MS. These 2 cognitive tests were selected for this examination of the relationship of the alignment of activity and light with cognition because they were the only measures of cognition at both timepoints, and parametric and nonparametric circadian rhythm parameters with these 2 cognitive tests have been examined previously in this cohort (7,8).
The Trails B is a timed test of processing speed that measures attention, sequencing, visual scanning, and executive function. Executive function is a measure of the ability for planning or decision making, error correction or trouble shooting, and abstract thinking. The Trails B test requires the participant to continuously scan a page to identify numbers and letters in a specified sequence while shifting from number to letter sets (31). The participant is given 300 seconds to complete the test. A lower time for completion (in seconds) represents better executive functioning. A positive increase in completion between visits time represents cognitive decline (took longer to complete the test at the follow-up timepoint).
The 3MS is a global measurement of cognitive function, with components for orientation, concentration, language, praxis, and immediate and delayed memory. The 3MS test is a broad sampling of cognitive domains. Scores range from 0 to 100, with higher scores representing better cognitive functioning (32). A decrease in 3MS score between visits represents cognitive decline (score was lower at the follow-up timepoint).
Other Measurements
All participants completed questionnaires at the initial timepoint, including questions about demographics, self-reported medical history, self-reported health status, living arrangement, physical activity, smoking, daily caffeine intake, and alcohol use. The Geriatric Depression Scale (GDS) was used to assess depressive symptoms, with depression defined as GDS≥6 (33). Physical activity was measured using the Physical Activity Scale for the Elderly (PASE) (34). All prescription and nonprescription medications used within the preceding 30 days were entered into an electronic database; each medication was matched to its ingredient(s) based on the Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) (35). Functional status was assessed based on 5 instrumental activities of daily living (IADL) (36,37). Self-reported caffeine intake was calculated based on the number of ingested drinks of caffeinated coffee, tea, or soda (38). A comprehensive examination included measurements of body weight and height, which were used to calculate body mass index (BMI).
Sleep-disordered breathing and sleep staging were measured at the initial timepoint using in-home overnight PSG, as described previously (2,3). Nocturnal hypoxemia was defined as the percentage of time during sleep in which arterial oxygen saturation was less than 90% (SaO2 < 90%). The apnea–hypopnea index (AHI) was defined as the total number of apneas and hypopneas per hour of sleep at 3% or greater desaturation, with apneas defined as complete or near complete cessation of airflow for longer than 10 seconds, and hypopneas scored if clear reductions in breathing amplitude (≥30% below baseline breathing) occurred and lasted longer than 10 seconds (39). Sleep stages (rapid eye movement [REM], stage 1, stage 2, and slow wave sleep) were scored using standard criteria (40,41).
Statistical Analysis
The phasor parameters were expressed as quartiles. Analyses were also performed to evaluate the linear relationship of the phasor parameters and cognitive outcomes, with the phasor parameters expressed as continuous variables.
Characteristics of participants were compared by quartile of phasor magnitude using chi-square tests for homogeneity for categorical variables, ANOVA for normally distributed continuous variables, and Kruskal–Wallis tests for continuous variables with skewed distributions. Similar comparisons were performed across quartiles of the phasor angle (data not shown).
Linear regression models were used to assess the relationships of phasor angle and phasor magnitude and the cognitive outcomes. Results are presented as adjusted means or beta coefficients and their 95% confidence intervals (CIs). The cross-sectional cognitive scores were transformed to meet model requirements of normality (log transformation for Trails B, squared transformation for 3MS) and back-transformed for the display of results. The change in cognition outcomes were normally distributed. Quartile 1 of phasor angle and quartile 4 of phasor magnitude served as the reference groups in models with the predictor expressed as quartiles. Tests for a linear trend across quartiles were performed by including each predictor (ordinal variable, 4 levels) as an independent variable in models. Covariates for model adjustment were selected based on potential biological plausibility. All models were minimally adjusted for age, clinic, race, education level, and season of activity and light assessment. These models were further adjusted by potentially confounding factors (alcohol use, daily caffeine use, living alone, depressive symptoms, physical activity, BMI, history of cataracts that were not corrected, and number of comorbid conditions [diabetes mellitus, stroke/transient ischemic attack, Parkinson’s disease, chronic obstructive pulmonary disease, high blood pressure, and coronary heart disease]).
A number of secondary analyses were performed to determine if the associations of phasor parameters and cognition were independent of other characteristics of sleep that have been shown to be associated to cognition in this cohort (1–4). In particular, individual multivariable models were further adjusted to include total sleep time, sleep efficiency, sleep staging, the AHI, and nocturnal hypoxemia.
A limitation of this analysis is that participant instructions on wearing the actigraph did not ask that the light meter remain uncovered, so it may have been blocked by clothing or bedding. Sensitivity analyses were performed, excluding those men in the highest quartile of percent time with probable covering of the light sensor. A detailed description of these sensitivity analyses can be found in Supplementary Materials.
Finally, although all analyses were prespecified, a Bonferroni correction was applied to all significant p values in the primary analysis to examine whether the associations observed held after correction for multiple comparisons.
All significance levels reported were 2-sided, and all analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Participant Characteristics
The analysis cohort was comprised of 1 036 primarily White (87%) men with an average age of 81.1 ± 4.6 years at the initial timepoint. The average phasor angle was −0.29 ± 1.30 hours. The negative value for phasor angle implies that, on average, the activity patterns of the men in this cohort were slightly advanced with respect to light exposure patterns. The average phasor magnitude was 0.24 ± 0.09. Participants slept on average 6.6 ± 1.1 hours per night with a sleep efficiency of 79.3 ± 8.2%.
Many covariates differed significantly across quartile of phasor magnitude (Table 1). Compared to men with larger phasor magnitude, those men with smaller values on average were older, had more depressive symptoms, consumed less alcohol, had lower levels of physical activity, and slept less. Men with smaller phasor magnitude were more likely to have at least one IADL impairment. Education level also differed by phasor magnitude level. Of the characteristics examined, only the level of physical activity differed across quartiles of phasor angle, with those in the lowest and highest quartiles having lower average activity level than those in quartiles 2 and 3 (p < .01).
Table 1.
Participant Characteristics at the Initial Visit by Quartile of Phasor Magnitude
| Characteristic | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p Value |
|---|---|---|---|---|---|
| <0.175 | 0.175 to <0.238 | 0.238 to <0.303 | ≥0.303 | ||
| (N = 259) | (N = 259) | (N = 259) | (N = 259) | ||
| Age, years | 81.66 ± 4.85 | 81.44 ± 4.55 | 80.93 ± 4.44 | 80.37 ± 4.64 | .007 |
| Race/ethnicity | .20 | ||||
| African American or Black | 12 (4.63) | 13 (5.02) | 8 (3.09) | 7 (2.70) | |
| Asian | 16 (6.18) | 15 (5.79) | 13 (5.02) | 6 (2.32) | |
| Hispanic | 5 (1.93) | 7 (2.70) | 5 (1.93) | 10 (3.86) | |
| White | 220 (84.94) | 221 (85.33) | 232 (89.58) | 229 (88.42) | |
| Other | 6 (2.32) | 3 (1.16) | 1 (0.39) | 7 (2.70) | |
| Education | <.001 | ||||
| Less than high school | 15 (5.79) | 5 (1.93) | 5 (1.93) | 10 (3.86) | |
| High school | 20 (7.72) | 42 (16.22) | 40 (15.44) | 41 (15.83) | |
| Some college or graduate school | 224 (86.49) | 212 (81.85) | 214 (82.63) | 208 (80.31) | |
| Body mass index, kg/m2 | 27.13 ± 4.04 | 26.98 ± 4.17 | 26.85 ± 3.98 | 26.87 ± 3.19 | .83 |
| Geriatric Depression Scale (GDS) score (0–15) | 1.97 ± 2.20 | 1.75 ± 2.05 | 1.93 ± 2.28 | 1.59 ± 2.17 | .03 |
| Depression (GDS score ≥6) | 13 (5.04) | 15 (5.81) | 12 (4.63) | 13 (5.04) | .94 |
| Smoking status | .29 | ||||
| Never | 136 (52.71) | 132 (50.97) | 114 (44.02) | 113 (43.63) | |
| Past | 118 (45.74) | 124 (47.88) | 142 (54.83) | 143 (55.21) | |
| Current | 4 (1.55) | 3 (1.16) | 3 (1.16) | 3 (1.16) | |
| Alcohol consumption (average drinks/week) | .001 | ||||
| <1 | 129 (50.39) | 125 (48.64) | 118 (45.56) | 122 (47.29) | |
| 1–13 | 119 (46.48) | 123 (47.86) | 132 (50.97) | 109 (42.25) | |
| ≥14 | 8 (3.13) | 9 (3.50) | 9 (3.47) | 27 (10.47) | |
| Caffeine use, mg/day | 249.77 ± 270.90 | 249.55 ± 245.11 | 236.57 ± 215.55 | 245.89 ± 247.41 | .99 |
| Physical activity score (PASE) | 100.92 ± 62.28 | 120.84 ± 67.74 | 126.68 ± 61.83 | 151.72 ± 71.32 | <.001 |
| Any impairment of instrumental activities of daily living (of 5) | 97 (37.45) | 81 (31.27) | 78 (30.12) | 56 (21.71) | .002 |
| Self-reported health status good or excellent | 208 (80.62) | 225 (86.87) | 215 (83.01) | 227 (87.64) | .09 |
| Lives alone | 60 (23.62) | 43 (16.67) | 41 (15.89) | 43 (16.67) | .08 |
| Current antidepressant use | 34 (13.13) | 27 (10.42) | 24 (9.27) | 21 (8.11) | .27 |
| Current benzodiazepine use | 11 (4.25) | 10 (3.86) | 7 (2.70) | 11 (4.25) | .77 |
| Current prescription sleep medication use | 13 (5.02) | 11 (4.25) | 10 (3.86) | 6 (2.32) | .44 |
| Number of select comorbid conditions (0–6)* | 1.28 ± 1.06 | 1.34 ± 1.04 | 1.22 ± 0.94 | 1.10 ± 0.91 | .09 |
| History of diabetes mellitus | 53 (20.54) | 46 (17.76) | 33 (12.74) | 37 (14.29) | .07 |
| History of stroke or transient ischemic attack | 31 (12.02) | 35 (13.51) | 45 (17.37) | 24 (9.27) | .049 |
| History of Parkinson’s disease | 5 (1.94) | 5 (1.93) | 5 (1.93) | 2 (0.77) | .65 |
| History of chronic obstructive pulmonary disease | 13 (5.33) | 13 (5.26) | 14 (5.74) | 5 (2.00) | .16 |
| History of high blood pressure | 143 (55.43) | 154 (59.46) | 125 (48.26) | 126 (48.65) | .03 |
| History of coronary heart disease† | 96 (36.21) | 94 (36.58) | 103 (40.08) | 91 (35.14) | .70 |
| History of cataracts that were not corrected | 56 (21.71) | 54 (20.85) | 46 (17.76) | 65 (25.10) | .24 |
| Total sleep time, min | 381.02 ± 74.30 | 398.60 ± 59.85 | 397.38 ± 60.41 | 404.29 ± 60.90 | <.001 |
| Sleep efficiency, % | 78.15 ± 9.80 | 79.79 ± 7.80 | 79.71 ± 7.53 | 79.26 ± 7.24 | .08 |
| Apnea–hypopnea index | 18.44 ± 16.63 | 18.91 ± 15.55 | 19.77 ± 17.86 | 19.29 ± 16.11 | .50 |
| Nocturnal hypoxemia (% of sleep time with SaO2 <90%) | 4.64 ± 10.72 | 5.46 ± 11.48 | 6.04 ± 14.23 | 5.61 ± 11.40 | .33 |
| % of sleep time in Stage 1 | 12.11 ± 8.45 | 11.97 ± 8.58 | 13.64 ± 10.43 | 11.94 ± 8.09 | .38 |
| % of sleep time in Stage 2 | 63.16 ± 11.00 | 60.71 ± 10.51 | 61.03 ± 11.50 | 61.75 ± 9.50 | .05 |
| % of sleep time in Slow Wave Sleep | 23.35 ± 26.24 | 24.17 ± 24.35 | 24.09 ± 25.45 | 22.54 ± 22.66 | .91 |
| % of sleep time in REM | 17.97 ± 6.71 | 20.36 ± 7.25 | 18.53 ± 7.35 | 19.98 ± 6.65 | <.001 |
| Season of light and activity assessment | <.001 | ||||
| Winter (January–March) | 109 (42.08) | 78 (39.73) | 58 (22.39) | 39 (15.06) | |
| Spring (April–June) | 44 (16.99) | 56 (21.62) | 76 (29.34) | 81 (31.27) | |
| Summer (July–September) | 21 (8.11) | 48 (18.53) | 73 (28.19) | 92 (35.52) | |
| Fall (October–December) | 85 (32.82) | 78 (30.12) | 52 (20.08) | 47 (18.15) |
Notes: p Values for continuous data from an ANOVA for normally distributed variables, a Kruskal–Wallis test for skewed data. p Values for categorical data from a chi-square test for homogeneity. REM = rapid eye movement.
*Comorbid conditions include diabetes mellitus, stroke or transient ischemic attack, Parkinson’s disease, chronic obstructive pulmonary disease, high blood pressure, and cardiovascular disease.
†Coronary heart disease includes myocardial infarction, angina, congestive heart failure, bypass surgery, angioplasty, and pacemaker placement.
Compared to those 674 men with longitudinal data, those 141 surviving men without probable dementia who did not provide data at follow-up for cognitive assessment were on average 2 years older and less active, were more likely to be non-White, had more depressive symptoms and were more likely to take antidepressants, had more comorbidities and IADL impairments, had lower self-reported health status, had lower levels of cognitive function and shorter phasor magnitude (p < .05 for all comparisons).
Cross-Sectional Associations of Phasor Analysis With Cognition
Overall, this cohort of relatively healthy community-dwelling older men had high levels of global cognition at the initial visit (92.5 ± 6.3 points on the 3MS), with similar Trails B completion times to published norms for community-dwelling adults of similar age and education level (130.1 ± 65.8 seconds) (42).
There were no significant cross-sectional associations observed between phasor angle and cognition (Table 2). Phasor magnitude was not associated with 3MS score, but was associated with executive function. After minimal adjustment, those with smaller phasor magnitude took longer to complete the Trails B test at the initial visit (Q1 vs Q4 122.5 vs 111.4 sec, p = .02; Table 2). In the fully adjusted model, the association of phasor magnitude and Trails B test time was no longer statistically significant.
Table 2.
Cross-Sectional Association of Phasor Angle and Phasor Magnitude With Cognition. Adjusted Means or Beta Coefficient (95% Confidence Interval)
| Phasor Predictor | Trails B Test Completion Time, sec | 3MS Score | ||
|---|---|---|---|---|
| Minimally* | Multivariable† | Minimally* | Multivariable† | |
| Adjusted | Adjusted | Adjusted | Adjusted | |
| Phasor angle, hours | ||||
| Continuous, per SD increase (1.30), beta coefficient | 1.9 (−1.6, 5.5) | 2.0 (−1.5, 5.7) | −0.1 (−0.5, 0.2) | −0.17 (−0.5, 0.2) |
| Q1: <−0.967 | 114.8 (108.9, 121.0) | 113.4 (107.5, 119.7) | 92.7 (92.1, 93.4) | 93.0 (92.3, 93.6) |
| Q2: −0.967 to <−0.305 | 119.7 (113.6, 126.1) | 117.4 (111.3, 123.9) | 92.6 (91.9, 93.2) | 92.7 (92.1, 93.4) |
| Q3: −0.305 to <0.403 | 114.9 (109.0, 121.2) | 112.9 (106.9, 119.2) | 92.9 (92.2, 93.5) | 92.9 (92.2, 93.6) |
| Q4: ≥0.403 | 116.5 (110.5, 122.9) | 116.0 (109.7, 122.6) | 92.6 (91.9, 93.2) | 92.6 (91.9, 93.3) |
| p-trend | .97 | .82 | .97 | .59 |
| Phasor magnitude | ||||
| Continuous, per SD decrease (−0.09), beta coefficient | 3.7 (−0.2, 7.7) | 2.3 (−1.8, 6.6) | −0.1 (−0.5, 0.2) | 0.06 (−0.3, 0.4) |
| Q1: <0.175 | 122.5 (116.0, 129.5)‡ | 120.3 (113.5, 127.5) | 92.6 (91.9, 93.2) | 92.9 (91.2, 93.6) |
| Q2: 0.175 to <0.238 | 116.7 (110.7, 123.1) | 114.4 (108.4, 120.8) | 92.7 (92.1, 93.4) | 92.8 (92.2, 93.5) |
| Q3: 0.238 to <0.303 | 115.8 (109.9, 122.0) | 113.4 (107.4, 119.6) | 92.6 (91.9, 93.2) | 92.8 (92.1, 93.4) |
| Q4: ≥0.303 | 111.4 (105.5, 117.5) | 112.1 (106.0, 118.6) | 92.9 (92.2, 93.6) | 92.8 (92.1, 93.4) |
| p-trend | .02 | .12 | .57 | .80 |
Notes: A higher value for Trails B completion time denotes worse cognitive function. A lower value for 3MS score denotes worse cognitive function. SD = standard deviation; 3MS = Modified Mini-Mental State examination.
*Minimally adjusted: clinic, age, race, education level, and season.
†Multivariable adjusted: clinic, age, race, education level, season, alcohol use, living alone, caffeine intake, depressive symptoms, physical activity, body mass index, history of cataracts that were not corrected, and number of comorbid conditions.
‡ p < .05.
Longitudinal Associations of Phasor Analysis With Cognitive Decline
After 4.2 ± 0.8 years of follow-up, on average, the 3MS scores for the men declined by 1.3 ± 5.6 points, which is considered a detectable change (43). The men took 23.1 ± 57.0 seconds longer to complete the Trails B test at the follow-up time point.
There were no significant associations observed between phasor angle and cognitive decline (Table 3). There was, however, a significant association between phasor magnitude and subsequent decline in executive function. After multivariable adjustment, lower phasor magnitude was associated with an average 6.9 second increase in test completion time per SD decrease in phasor magnitude (Table 3). Examination of the association of phasor magnitude with the decline in 3MS score was not significant. (Table 3).
Table 3.
Association of Phasor Angle and Phasor Magnitude With Change in Cognition. Adjusted Means or Beta Coefficient (95% Confidence Interval)
| Phasor Predictor | Change in Trails B Test Completion Time, Sec | Change in 3MS Score | ||
|---|---|---|---|---|
| Minimally* | Multivariable† | Minimally* | Multivariable† | |
| Adjusted | Adjusted | Adjusted | Adjusted | |
| Phasor angle, hours | ||||
| Continuous, per SD increase (1.24), beta coefficient | −0.9 (−5.2, 3.5) | 0.3 (−4.1, 4.8) | 0.09 (−0.3, 0.5) | 0.1 (−0.3, 0.6) |
| Q1:<−0.967 | 22.9 (14.0, 31.8) | 21.0 (12.0, 30.0) | −1.3 (−2.1, −0.4) | −1.3 (−2.2, −0.4) |
| Q2: −0.967 to <−0.305 | 20.2 (11.8, 28.7) | 18.5 (9.8, 27.2) | −1.9 (−2.7, −1.1) | −1.8 (−2.6, −0.9) |
| Q3: −0.305 to <0.403 | 29.9 (21.3, 38.5) | 31.8 (23.0, 40.6) | −1.1 (−1.9, −0.3) | −1.1 (−1.9, −0.2) |
| Q4: ≥0.403 | 19.7 (10.8, 28.5) | 20.4 (11.4, 29.5) | −1.1 (−2.0, −0.3) | −1.0 (−1.9, −0.2) |
| p-trend | .97 | .55 | .49 | .44 |
| Phasor magnitude | ||||
| Continuous, per SD decrease (−0.09), beta coefficient | 5.5 (0.7, 10.4)‡ | 6.9 (1.7, 12.0)* | −0.4 (−0.9, 0.06) | −0.4 (−0.9, 0.1) |
| Q1: <0.175 | 30.9 (21.0, 40.9) | 32.5 (22.1, 42.8) | −1.4 (−2.3, −0.4) | −1.5 (−2.4, −0.5) |
| Q2: 0.175 to <0.238 | 28.7 (20.1, 37.3) | 29.0 (20.2, 37.8) | −2.1 (−2.9, −1.2)* | −1.9 (−2.8, −1.1) |
| Q3: 0.238 to <0.303 | 15.0 (6.3, 23.7) | 13.8 (4.9, 22.7) | −1.3 (−2.2, −0.4) | −1.2 (−2.1, −0.4) |
| Q4: ≥0.303 | 19.7 (11.3, 28.2) | 18.8 (10.0, 27.6) | −0.7 (−1.5, 0.1) | −0.6 (−1.5, 0.2) |
| p-trend | .04 | .02 | .14 | .12 |
Notes: Change is calculated as follow-up time point―initial timepoint. A positive value for change in Trails B completion time denotes cognitive decline. A negative value for change in 3MS score denotes cognitive decline. SD = standard deviation; 3MS = Modified Mini-Mental State examination.
*Minimally adjusted: clinic, age, race, education level, and season.
†Multivariable adjusted: clinic, age, race, education level, season, alcohol use, living alone, caffeine intake, depressive symptoms, physical activity, body mass index, history of cataracts that were not corrected, and number of comorbid conditions.
‡ p < .05.
Further adjustment for other sleep characteristics did not alter the association of phasor magnitude and decline in executive function. Adjustment for total sleep time, sleep efficiency, AHI, nocturnal hypoxemia, and sleep staging had little impact on the effect size. Further adjustment for a percentage of time spent in REM sleep had the greatest effect, reducing the association to a 6.7 second increase in Trails B test time per SD decrease in phasor magnitude (p = .01). Results were similar after dropping the subset of men from the analyses who had the highest levels of time where the light sensor may have been covered (see Supplementary Materials).
Tables 2 and 3 each show the results of 6 models per phasor predictor per cognitive test, a total of 12 models per outcome. Adjusting the significance level of 0.05 with a Bonferroni correction would lead to a significance cutpoint of p < .004 (=.05/12). After applying this more-stringent cutpoint for significance, the associations between phasor magnitude and Trails B test time were no longer significant. Therefore, it is essential that results are replicated in future studies.
Discussion
In this cohort of community-dwelling older men, smaller phasor magnitude (ie, weaker circadian entrainment because the alignment between light–dark and rest-activity patterns is lower) was associated with lower initial level and subsequent decline in executive function, as measured by time to complete the Trails B test. The association of smaller phasor magnitude with the decline in executive function (but not initial level) was robust to further adjustment for other sleep characteristics. There were no associations of phasor magnitude with global cognition or decline in global cognition.
There were no associations observed between phasor angle (ie, the temporal relationship between light–dark and rest-activity patterns) and cognition or cognitive decline. Phasor angle reflects the timing of activity with respect to light, but not necessarily circadian entrainment, which is what we hypothesized would be associated with cognitive function.
The consistent relationship observed with phasor magnitude and executive function, while no association seen with global cognition may be due to variations of associations across specific domains of cognitive function. The Trails B test, a measure of attention and executive function, can be considered a test of prefrontal cortical function (44). It has been suggested that microstimulation of the prefrontal cortex modulates the pupil light reflex, which is a mechanism for light adaptation (45). A meta-analysis of trials of phototherapy for improvement of cognitive function among those with dementia found that the cognitive improvements seen with phototherapy were specific to the domains of attention, executive function, and working memory (17).
Studies in cohorts of older adults and older women have reported the association of rest-activity rhythm disruption and development of cognitive decline (10–12). In this MrOS cohort of older men, it has been shown previously that disrupted rest-activity rhythms, as measured by parametric and nonparametric patterns of daily activity were associated with cognitive decline (7–9). The parametric rest-activity rhythm parameters examined in prior work include the amplitude (strength of the activity rhythm) and the F-statistic (overall rhythm robustness). While these parameters are correlated to the phasor magnitude (amplitude rho = 0.57, F-statistic rho = 0.64) the effect size of their association to decline in executive function in this cohort is smaller than that of phasor magnitude (multivariable adjusted, per SD decrease: amplitude 5.9 [95% CI 0.9, 11.0]; F-statistic 5.4 [95% CI 0.7, 10.3], phasor magnitude 6.9 [95% CI 1.7, 12.0]; all p < .05). This current work builds on these previous findings to suggest a mechanism for the disruption in circadian rhythms, poor alignment of the light–dark, and rest-activity patterns.
The electric lighting present in today’s modern environment allows for incorrect timing of light exposure and insufficient brightness. The light and activity data in this MrOS study was measured approximately 10 years ago, and it is likely that the nighttime use of light-emitting electronic media has increased because that time. If similar data were measured today, there may be an even stronger misalignment of light–dark and rest-activity patterns, leading to higher levels of cognitive decline. It has been shown that this misalignment can be altered nonpharmacologically with lighting interventions (29,46,47). Light treatment is noninvasive, and interventions of light therapy have little attrition (48). Bright light treatment can be delivered with lightboxes, and light visors or glasses can be used to deliver or block light. Other light interventions target indoor lighting, or an increase in natural light exposure (48). An estimate of the global prevalence of dementia was 35.6 million in 2010, which is expected to double every 20 years (49). Therefore, a nonpharmacological lighting or exercise intervention that could slow the progression of cognitive decline would have a great public health impact.
This study has several strengths. The study had a large population of cognitively intact community-dwelling older men who were not selected for inclusion based on any sleep-related criteria. Adjustments for multiple potential confounding factors were made, suggesting these associations were not explained by other covariates, including depression, comorbidities, education, or lifestyle.
This study also has limitations. The findings may not be generalizable to populations other than community-dwelling primarily White older men. Adjustment for numerous covariates was performed, but there may be unmeasured confounders that could affect the results. Surviving men not returning to the clinic for repeat assessment of cognition had smaller phasor magnitude and poorer health at the initial visit. It is possible that this missing data may have biased the longitudinal findings toward the null hypothesis of no association. The cognitive battery of tests was somewhat limited and only included measures of global cognition and executive function. Our measure of light was imprecise as it was derived from wrist actigraphy. The light sensor used has been shown to provide good temporal patterning of light but less accurate values for absolute illuminance. Therefore, the phasor magnitude estimate would be most affected by the imprecision of the sensor (25). Protocols did not require the light meter remain uncovered, so participants may have blocked the meter with clothing or bedding. Measurement of light is preferred near the cornea, but the light meters in the current study were wrist-worn (50). It has been shown that estimating corneal light exposure from a wrist-worn light meter may introduce bias due to the covering of the light meter by clothing, particularly in winter months (50). Thus, imprecise light measurement may have biased findings towards the null hypothesis of no association (see Supplementary Materials). Adjustment for the season of data collection was an attempt to limit this bias. The follow-up time for assessment of cognitive decline was somewhat limited (4.2 ± 0.8 years) as we were unable to use the actigraphy data collected at the initial Sleep Visit 1 6 years earlier because the actigraph used at that visit did not have a light meter.
Conclusion
In conclusion, among older community-dwelling men, we found some evidence to suggest that smaller phasor magnitude was associated with impairment and subsequent decline in executive function. Further study is needed to replicate these findings, as well as to examine if these associations hold after longer follow-up, are present in other populations, and vary by specific cognitive domains. Future studies might also investigate whether behavioral interventions designed to improve the alignment of light and activity patterns can slow cognitive decline in older adults, including among those who have not yet progressed to dementia.
Supplementary Material
Contributor Information
Terri L Blackwell, Research Institute, California Pacific Medical Center, San Francisco, California, USA.
Mariana G Figueiro, Department of Population Health Science and Policy, Light and Health Research Center, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Gregory J Tranah, Research Institute, California Pacific Medical Center, San Francisco, California, USA.
Jamie M Zeitzer, Department of Psychiatry and Behavioral Sciences, Center for Sleep and Circadian Sciences, Stanford University, Palo Alto, California, USA; Mental Illness Research, Education, and Clinical Center, VA Palo Alto Health Care System, Palo Alto, California, USA.
Kristine Yaffe, Departments of Psychiatry, Neurology, and Epidemiology, University of California, San Francisco, California, USA; the San Francisco VA Medical Center.
Sonia Ancoli-Israel, Department of Psychiatry, University of California, San Diego, La Jolla, California, USA.
Deborah M Kado, Department of Medicine, Stanford University, Stanford, California and VA Palo Alto, Palo Alto, California, USA; Geriatric Research Education and Clinical Center (GRECC), VA Palo Alto, Palo Alto, California, USA.
Kristine E Ensrud, Center for Chronic Disease Outcomes Research, Veterans Affairs Medical Center, Minneapolis, Minnesota, USA; Division of Epidemiology and Community Health, Department of Medicine, University of Minnesota, Minneapolis, Minnesota, USA.
Nancy E Lane, Department of Medicine, Center for Musculoskeletal Health, University of California at Davis School of Medicine, Sacramento, California, USA; Department of Epidemiology, University of California at San Francisco, San Francisco, California, USA.
Yue Leng, Department of Psychiatry, University of California, San Francisco, California, USA.
Katie L Stone, Research Institute, California Pacific Medical Center, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA.
Funding
The MrOS Study is supported by National Institutes of Health (NIH) funding through the National Institute on Aging (NIA) and the National Center for Advancing Translational Sciences (NCATS) under the grant numbers R01 AG066671 and UL1 TR000128.
The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. The NIA provided additional funding for data analysis (grant numbers R21 AG051380 and R01 AG034157). Y.L. is supported by NIH award R00AG056598.
Conflict of Interest
T.L.B. and K.L.S. receive partial salary support from a grant from Merck Sharp & Dohme Corp as well as NIH funding during the conduct of the study. M.G.F., G.J.T., J.M.Z., K.Y., N.E.L., and D.M.K. declare no conflict. S.A.-I. is a consultant for Eisai, Biogen, Idorsia, and Merck. K.E.E. reports grant funding from the NIA during the conduct of the study. Y.L. is supported by NIH award R00AG056598.
Author Contributions
T.L.B. conducted the analyses, interpreted the results, and wrote the manuscript; M.G.F. informed analyses, interpreted the results, and revised the manuscript; G.J.T. informed analyses, interpreted the results, and revised the manuscript; J.M.Z. informed analyses, interpreted the results, and revised the manuscript; K.Y. interpreted the results and revised the manuscript; S.A.-I. informed analyses, interpreted the results, and revised the manuscript; D.M.K. interpreted the results and revised the manuscript; K.E.E. conceived and designed the study, informed analyses, interpreted the results, and revised the manuscript; N.E.L. interpreted the results and revised the manuscript; Y.L. interpreted the results and revised the manuscript; K.L.S. conceived and designed the study, informed analyses, interpreted the results, and revised the manuscript.
References
- 1. Blackwell T, Yaffe K, Laffan A, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep. 2014;37(4):655–663. doi: 10.5665/sleep.3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blackwell T, Yaffe K, Laffan A, et al. ; Osteoporotic Fractures in Men Study Group. Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2015;63(3):453–461. doi: 10.1111/jgs.13321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song Y, Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Stone KL.; Osteoporotic Fractures in Men (MrOS) Study Group. Relationships between sleep stages and changes in cognitive function in older men: the MrOS sleep study. Sleep. 2015;38(3):411–421. doi: 10.5665/sleep.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blackwell T, Yaffe K, Ancoli-Israel S, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34(10):1347–1356. doi: 10.5665/SLEEP.1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diem SJ, Blackwell TL, Stone KL, et al. . Measures of sleep–wake patterns and risk of mild cognitive impairment or dementia in older women. Am J Geriatr Psychiatry. 2016;24(3):248–258. doi: 10.1016/j.jagp.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ancoli-Israel S, Klauber MR, Jones DW, et al. . Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20(1):18–23. doi: 10.1093/sleep/20.1.18 [DOI] [PubMed] [Google Scholar]
- 7. Xiao Q, Sampson JN, LaCroix AZ, et al. . Nonparametric parameters of 24-hour rest-activity rhythms and long-term cognitive decline and incident cognitive impairment in older men. J Gerontol A Biol Sci Med Sci. 2022;77(2):250–258. doi: 10.1093/gerona/glab275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rogers-Soeder TS, Blackwell T, Yaffe K, et al. ; Osteoporotic Fractures in Men Study Research Group. Rest-activity rhythms and cognitive decline in older men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2018;66(11):2136–2143. doi: 10.1111/jgs.15555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeitzer JM, Blackwell T, Hoffman AR, Cummings S, Ancoli-Israel S, Stone K.; Osteoporotic Fractures in Men (MrOS) Study Research Group. Daily patterns of accelerometer activity predict changes in sleep, cognition, and mortality in older men. J Gerontol A Biol Sci Med Sci. 2018;73(5):682–687. doi: 10.1093/gerona/glw250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xiao Q, Shadyab AH, Rapp SR, et al. . Rest-activity rhythms and cognitive impairment and dementia in older women: results from the Women’s Health Initiative. J Am Geriatr Soc. 2022. doi: 10.1111/jgs.17926. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tranah GJ, Blackwell T, Stone KL, et al. . Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70(5):722–732. doi: 10.1002/ana.22468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li P, Gao L, Gaba A, et al. . Circadian disturbances in Alzheimer’s disease progression: a prospective observational cohort study of community-based older adults. Lancet Healthy Longev. 2020;1(3):e96–e105. doi: 10.1016/s2666-7568(20)30015-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mason IC, Boubekri M, Figueiro MG, et al. . Circadian health and light: a report on the National Heart, Lung, and Blood Institute’s Workshop. J Biol Rhythms. 2018;33(5):451–457. doi: 10.1177/0748730418789506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28(5):799–807. doi: 10.1016/j.neurobiolaging.2006.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ancoli-Israel S, Gehrman P, Martin JL, et al. . Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1(1):22–36. doi: 10.1207/S15402010BSM0101_4 [DOI] [PubMed] [Google Scholar]
- 16. Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. J Am Geriatr Soc. 2002;50(2):282–289. doi: 10.1046/j.1532-5415.2002.50060.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu G, Tong Q, Ye X, et al. . Phototherapy for cognitive function in patients with dementia: a systematic review and meta-analysis. Front Aging Neurosci. 2022;14:936489. doi: 10.3389/fnagi.2022.936489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Youngstedt SD, Elliott JA, Kripke DF. Human circadian phase-response curves for exercise. J Physiol. 2019;597(8):2253–2268. doi: 10.1113/JP276943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rea MS, Figueiro MG. Quantifying light-dependent circadian disruption in humans and animal models. Chronobiol Int. 2014;31(10):1239–1246. doi: 10.3109/07420528.2014.957302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342 [DOI] [PubMed] [Google Scholar]
- 21. Lewis P, Korf HW, Kuffer L, Groß JV, Erren TC. Exercise time cues (zeitgebers) for human circadian systems can foster health and improve performance: a systematic review. BMJ Open Sport Exerc Med. 2018;4(1):e000443. doi: 10.1136/bmjsem-2018-000443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Orwoll E, Blank JB, Barrett-Connor E, et al. . Design and baseline characteristics of the Osteoporotic Fractures in Men (MrOS) Study―a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 23. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. . Overview of recruitment for the Osteoporotic Fractures in Men Study (MrOS). Contemp Clin Trials. 2005;26(5):557–568. doi: 10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 24. Coding FAQ. American Academy of Sleep Medicine. http://www.aasmnet.org/codingfaq.Aspx. Accessed December 23, 2021.
- 25. Joyce DS, Zele AJ, Feigl B, Adhikari P. The accuracy of artificial and natural light measurements by actigraphs. J Sleep Res. 2020;29(5):e12963. doi: 10.1111/jsr.12963 [DOI] [PubMed] [Google Scholar]
- 26. Rea MS, Bierman A, Figueiro MG, Bullough JD. A new approach to understanding the impact of circadian disruption on human health. J Circadian Rhythms. 2008;6:7. doi: 10.1186/1740-3391-6-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526 Pt 3:695–702. doi: 10.1111/j.1469-7793.2000.00695.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Figueiro M, Steverson B, Heerwagen J, et al. . The impact of daytime light exposures on sleep and mood in office workers. Sleep Health. 2017;3(3):204–215. doi: 10.1016/j.sleh.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 29. Figueiro MG, Hunter CM, Higgins P, et al. . Tailored lighting intervention for persons with dementia and caregivers living at home. Sleep Health. 2015;1(4):322–330. doi: 10.1016/j.sleh.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Actiware and Actiware CT Software Manual, Respironics, Inc. Bend, OR. [Google Scholar]
- 31. Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 32. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 33. Sheikh JY. Geriatric Depression Scale: recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. doi: 10.1300/J018v05n01_09 [DOI] [Google Scholar]
- 34. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4 [DOI] [PubMed] [Google Scholar]
- 35. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. doi: 10.1007/BF01719664 [DOI] [PubMed] [Google Scholar]
- 36. Fitti JE, Kovar MG. The supplement on aging to the 1984 National Health Interview Survey. Vital Health Stat 1. 1987(21):1–115. [PubMed] [Google Scholar]
- 37. Pincus T, Summey JA, SoraciSA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26(11):1346–1353. doi: 10.1002/art.1780261107 [DOI] [PubMed] [Google Scholar]
- 38. Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34(1):119–129. doi: 10.1016/0278-6915(95)00093-3 [DOI] [PubMed] [Google Scholar]
- 39. Quan SF, Howard BV, Iber C, et al. . The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. doi: 10.1093/sleep/20.12.1077 [DOI] [PubMed] [Google Scholar]
- 40. Rechtschaffen A, Kales A, eds. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Washington, DC: National Institutes of Health; 1968. NIH publication 204. [Google Scholar]
- 41. American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. doi: 10.1093/sleep/15.2.173 [DOI] [PubMed] [Google Scholar]
- 42. Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- 43. Andrew MK, Rockwood K. A five-point change in Modified Mini-Mental State Examination was clinically meaningful in community-dwelling elderly people. J Clin Epidemiol. 2008;61(8):827–831. doi: 10.1016/j.jclinepi.2007.10.022 [DOI] [PubMed] [Google Scholar]
- 44. Andrew MK, Fisk JD, Rockwood K. Social vulnerability and prefrontal cortical function in elderly people: a report from the Canadian Study of Health and Aging. Int Psychogeriatr. 2011;23(3):450–458. doi: 10.1017/S1041610210001195 [DOI] [PubMed] [Google Scholar]
- 45. Ebitz RB, Moore T. Selective modulation of the pupil light reflex by microstimulation of prefrontal cortex. J Neurosci. 2017;37(19):5008–5018. doi: 10.1523/JNEUROSCI.2433-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Figueiro MG, Plitnick BA, Lok A, et al. . Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527–1537. doi: 10.2147/CIA.S68557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sloane PD, Figueiro M, Garg S, et al. . Effect of home-based light treatment on persons with dementia and their caregivers. Light Res Technol. 2015;47(2):161–176. doi: 10.1177/1477153513517255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Faulkner SM, Dijk DJ, Drake RJ, Bee PE. Adherence and acceptability of light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuropsychiatric illness: a systematic review. Sleep Health. 2020;6(5):690–701. doi: 10.1016/j.sleh.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 49. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):6375.e2. doi: 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 50. Figueiro MG, Hamner R, Bierman A, Rea MS. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Light Res Technol. 2013;45(4):421–434. doi: 10.1177/1477153512450453 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.