Abstract
The mode of action of sulfanilyl fluoroquinolones (NSFQs) was investigated with NSFQ-104, NSFQ-105, and some structurally related compounds. Evidence arising from interactions with p-aminobenzoic acid and trimethoprim suggested that a sulfonamidelike mechanism of action makes little or no contribution to the in vitro activity of NSFQs. NSFQ-105 showed an activity that inhibits gyrase-catalyzed DNA supercoiling that is similar to the activity of other fluoroquinolones. Also, NSFQ-105 uptake was decreased by the presence of Mg2+ and increased by a lower pH. These results indicate that NSFQs having only one ionizable group could exhibit more favorable kinetics of access to the bacterial cell than zwitterionic fluoroquinolones.
Sulfanilyl fluoroquinolones (NSFQs) are a new class of antibacterial fluoroquinolones (FQs) with especially high in vitro activity against gram-positive bacteria (1). From a structural point of view, NSFQs could be considered hybrid drugs since they incorporate moieties of both sulfonamides and FQs. FQs act by inhibiting the bacterial DNA synthesis that results from inhibition of gyrase-catalyzed DNA supercoiling (4, 21). On the other hand, sulfa drugs are well-known antibacterial agents that exert their action by a competitive inhibition of p-aminobenzoic acid (PABA) utilization in the synthesis of dihydrofolate that is catalyzed by dihydropteroate synthase (DHPS) (4).
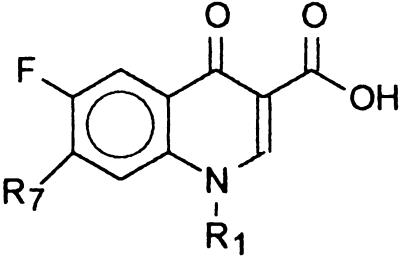
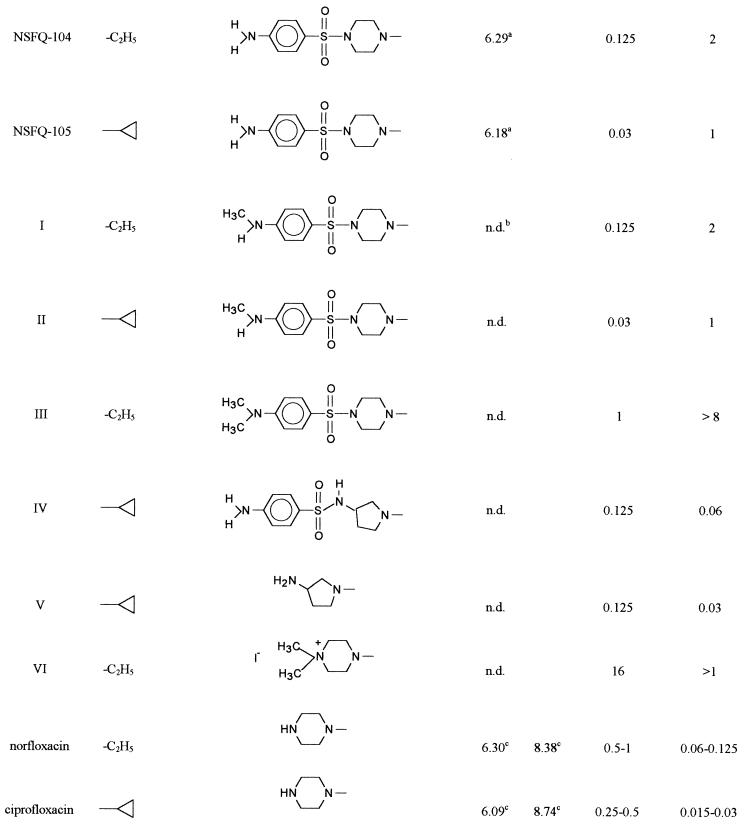
The present study aimed to clarify the question of whether NSFQs are truly hybrid drugs, i.e., if the antibacterial activity is the result of a quinolonelike and sulfalike action. In addition, the mode of action of NSFQ-104, NSFQ-105, and some structurally related derivatives was compared with that of their zwitterionic FQ (ZFQ) analogs ciprofloxacin and norfloxacin. For this purpose, compounds I to IV were synthesized (Table 1). Compounds I to III are useful for evaluation of the role played by the fragment 4-NH2-C6H4-SO2-Y, which is considered an essential molecular fragment for recognition and binding of the DHPS active site (5). Besides, in compound IV, the 3-aminopyrrolidinyl group replaces the piperacinyl group; the former is a well-known biological mimic of the second in ZFQs (17). The resulting NSFQ analog (IV), having the acidic SO2-NH group, has the same structure as the antibacterial sulfa drugs currently used in therapy. Finally, compound VI was designed because of its permanently ionized ammonium group.
TABLE 1.
Structures and some properties of the FQs assayed
The bacterial strains used in this study were Staphylococcus aureus ATCC 29213 and Escherichia coli ATCC 25922. NSFQ-104; NSFQ-105; compound IV (10); compounds I, II, and III (11), and compound V (17) were synthesized in our laboratory as described previously. Compound VI was obtained by exhaustive alkylation of norfloxacin with methyl iodide (19). Spectroscopic data of purified compound VI confirmed its proposed structure.
Other agents were obtained from the following sources: ciprofloxacin, Amifarma; norfloxacin, Marsing & Co., Ltd.; MgCl2 · 6H2O, Anedra; PABA, Merck; NaH2PO4 and Na2HPO4 · 2H2O, Anedra; ofloxacin, fleroxacin, novobiocin, trimethoprim, and sulfamethoxazole, Hoffmann-La Roche Ltd., Basel, Switzerland.
MICs were determined by broth macrodilution techniques (Mueller-Hinton broth; Merck) (13). The inoculum size was approximately 5 × 105 CFU/ml. The MIC was defined as the lowest concentration of a drug that completely inhibited visible growth of the organisms after 18 to 20 h at 37°C.
Broth with PABA at 10 μg/ml was used to test the effect of PABA on the MICs. Combinations of trimethoprim and NSFQs were evaluated by the checkerboard technique; twofold dilutions of each drug were tested in combination. The fractional inhibitory concentration (FIC) index was calculated as the minimum concentration of each of the two antimicrobial agents that had an inhibitory effect when acting together divided by the MIC of that drug alone. The sum of the FICs of both drugs was the FIC index (15).
NSFQ-105 (fixed concentration, 50 μg/ml) was tested for inhibition of the E. coli and S. aureus DHPSs. Sulfamethoxazole (50 μg/ml) was assayed simultaneously; 10 mM 3H-PABA (20 Ci/mol), 20 mM H2-pterin-CH2O-PP, and the appropriate inhibitor were incubated with the purified enzyme at 0.3 to 1 μg/ml in 50 mM Tris-HCl (pH 8.3) containing 5 mM MgCl2 and 1 mM dithiothreitol for 20 min at 25°C. Detection of 3H-pteroate was done by paper chromatography (20).
The supercoiling assay with purified E. coli DNA gyrase was carried out as previously described (8). The maximal noneffective concentration in this assay was determined by testing a range of inhibitor concentrations.
The accumulation assays were carried out by the fluorometric method described by Chapman and Georgopapadakou with modifications (3, 12) essentially as follows. Mid-log-phase bacterial cells were harvested, washed once, and suspended to 40 mg (wet weight) per ml with 50 mM sodium phosphate buffer (pH 7.0 or 5.5, as appropriate). Samples were dispensed into 50-ml flasks and incubated in a shaking water bath at 37°C for 10 min. A quinolone was added to a final concentration of 10 μg/ml. At timed intervals of up to 1 h, 0.5 ml was transferred to microcapped centrifuge tubes chilled on ice and cells were pelleted (5,600 × g for 1 min) without prior addition of buffer. They were washed with 1.5 ml of quinolone-free buffer (pH 7.0) at 4°C and immediately centrifuged. The pellet was resuspended in 1.5 ml of 0.1 M glycine hydrochloride (pH 3.0) and incubated at room temperature overnight. Samples were centrifuged for 5 min at 7,000 × g, and the amount of drug in the supernatant was determined by fluorometry. Accumulation assays were also carried out in the presence of 10 mM MgCl2.
The following results clarify the contribution of the sulfonamide moiety to the in vitro activity. (i) Concentrations of PABA as high as 10 μg/ml did not change the MICs against S. aureus ATCC 29213 of derivatives NSFQ-104, NSFQ-105, and compound IV. (ii) Results from combination studies revealed only addition of the effects of trimethoprim and either NSFQ-105 or compound IV since in both experiments a FIC index of 0.75 was found. (iii) Although N,N-dimethyl derivative III was less active than NSFQ-104, N-methyl derivatives I and II exhibited the same activity as their respective analogs, NSFQ-104 and NSFQ-105, against reference strains (Table 1).
Although determinations with the DHPS enzymes from S. aureus and E. coli using sulfamethoxazole as the reference showed that the inhibitory activities of NSFQ-105 were 33 and 40%, respectively, results i to iii suggest that a sulfonamidelike mode of action makes little or no contribution to the in vitro activity of NSFQs.
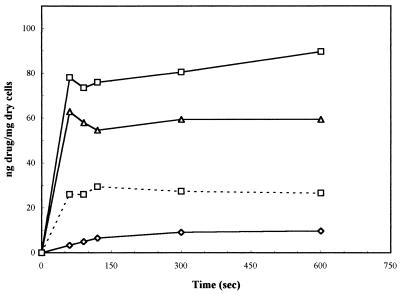
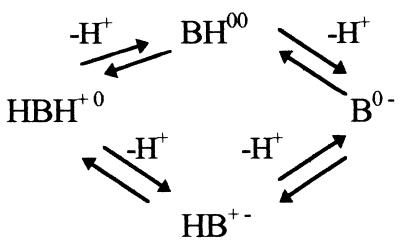
The antibacterial activity of an FQ is determined by at least two factors: its rate of penetration of the bacterial envelope and its ability to inhibit the DNA gyrase-catalyzed supercoiling reaction (7, 9). The following results would agree with an FQ-like mechanism. (i) A maximal noneffective concentration of 0.2 μg/ml was found for NSFQ-105, which is a value similar to those exhibited by other FQs, such as ciprofloxacin (0.05), ofloxacin (0.25), and fleroxacin (0.50). (ii) Profiles of NSFQ-105 and ciprofloxacin accumulation in S. aureus obtained at pH 7.0 and an external concentration of 10 μg/ml were similar (Fig. 1); the uptake of NSFQ-105 was 1.4 ± 0.2 times that of ciprofloxacin (Table 2). The uptake of NSFQ-105 after 10 min of contact was linearly related to the external concentration. This behavior suggests a passive-diffusion uptake process similar to that reported for ZFQs (7, 22). (iii) The accumulation of NSFQ-105 decreased approximately threefold with a high external concentration of Mg2+ (Fig. 1), and the MIC was also affected in the same way as observed with ZFQs (1, 2, 18) (data not shown). (iv) As previously reported, the higher the acidity of the growth medium, the lower the MICs of NSFQs (1). On the other hand, it is also well known that the MICs of ciprofloxacin and other ZFQs become higher as the external pH is shifted away from their isoelectric pHs (1, 14, 18). These results correlate with the fact that at pH 5.5, the uptake of NSFQ-105 rises to 1.7 ± 0.3 times that of ciprofloxacin. The behavior observed is consistent with the acid-base characteristics of both classes of molecules. In fact, on the one hand, acid pHs repress the dissociation of the carboxylic group, yielding a high proportion of the uncharged form (AH) of an NSFQ, which is believed to penetrate more easily than the ionized form (A−) (14). On the other hand, it produces a lower availability of the neutral species BH00 and HB+− of ZFQs (Fig. 2).
FIG. 1.
Accumulation of some FQs, at an external concentration of 10 μg/ml, in S. aureus ATCC 29213 over 10 min. Each point shown is the mean of at least three experiments. Symbols: □——□, NSFQ-105; □- - -□, NSFQ-105 with added 10 mM MgCl2; ▵, ciprofloxacin; ◊, compound VI.
TABLE 2.
Uptake of some FQs by S. aureus
| Compound | pH | Drug concn (ng/mg of cell dry wt)a |
|---|---|---|
| NSFQ-105 | 7.0 | 79.5 ± 6.3 |
| NSFQ-105 | 5.5 | 88.0 ± 6.5 |
| NSFQ-105 + Mg2+ | 7.0 | 27.1 ± 1.4 |
| Ciprofloxacin | 7.0 | 57.3 ± 5.0 |
| Ciprofloxacin | 5.5 | 51.7 ± 7.5 |
| VI | 7.0 | 9.36 ± 0.42 |
Each value is the average (± the standard error) of the set of samples taken at steady state (60, 90, 120, 300, and 600 s after addition of FQ). For compound VI, only the last two samples were averaged.
FIG. 2.
Scheme for protonation of ZFQs.
Since the BH00/HB+− ratio is not pH dependent, evaluation of the ability to permeate the cells of each species was performed by measuring the uptake of compound VI (Table 1); this quaternary ammonium derivative, almost completely in its zwitterionic form at pH 7.0, should mimic the behavior of HB+−. Its uptake was slow and considerably lower than that of ciprofloxacin under the same conditions (Fig. 1). This result confirms the statement (7, 14) that B00 is the ZFQ species that more easily penetrates bacterial cells. The corresponding un-ionized molecular fraction (UMF) is
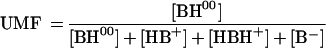 |
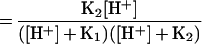 |
where K1 and K2 are the first and second apparent dissociation constants.
Although the intracellular distribution of the species depicted in Fig. 2 is not well known, there are a number of ways in which each species can interact with the different intracellular constituents, such as association, adsorption, etc. (14). Also, the pH gradient affects the internal speciation (9). In the same way, it is not known which species is in equilibrium with the target.
Nevertheless, it could be considered that an NSFQ (or a ZFQ) reaches a plateau when the influx of its un-ionized species AH (or BH00) is counterbalanced by a retrodiffusion process also governed by Fick’s law. Under such conditions, the intracellular concentration of free AH (or BH00) should not be far from its external concentration.
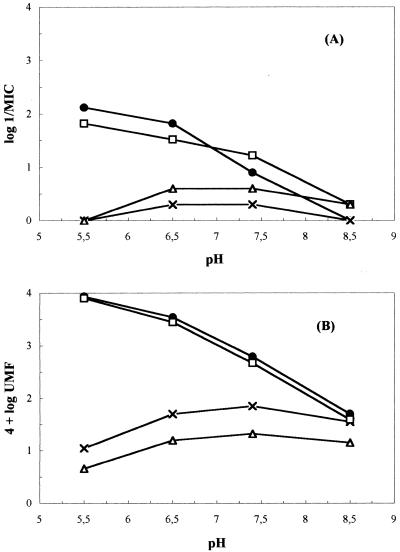
Figure 3 shows the parallelism between the variations of both 1/MIC and UMF as a consequence of modification of the external pH. Therefore, the external availability of AH (or BH00) should be recognized as one of the main factors determining its intracellular concentration, which, in turn, directly or indirectly determines the interaction with the target.
FIG. 3.
Effect of pH on the MIC (A) and the UMF (B) for S. aureus ATCC 29213. Symbols: •, NSFQ-104; □, NSFQ-105; ×, norfloxacin; ▵, ciprofloxacin.
In conclusion, the structural change introduced into the ZFQs ciprofloxacin and norfloxacin to get the corresponding NSFQs yields compounds having only one ionizable group in the biological pH range of interest. Such compounds, like WIN 57273 (and nalidixic acid), exhibit a more favorable kinetics of access to the bacterial cell than do ZFQs (14). This factor appears to be of primary importance in determining their high in vitro activity against gram-positive cocci since ZFQs and NSFQs inhibit DNA gyrase similarly.
Acknowledgments
Financial support was received from CONICOR and SECyT (UNC). F. Alovero thanks CONICOR for a fellowship.
We thank H. Gmünder and J. Nielsen for performing the enzyme tests.
REFERENCES
- 1.Allemandi D A, Alovero F L, Manzo R H. In vitro activity of new sulphanilyl fluoroquinolones against Staphylococcus aureus. J Antimicrob Chemother. 1994;34:261–264. doi: 10.1093/jac/34.2.261. [DOI] [PubMed] [Google Scholar]
- 2.Alovero F L, Allemandi D A, Nieto M J, Mazzieri M R, Manzo R H. Program and abstracts of the II Congreso Federación Farmacéutica Sudamericana. Santiago, Chile. 1995. Efecto del Mg2+ sobre la actividad de NSFQ-104 y NSFQ-105 a distintos pHs, abstr. 95. [Google Scholar]
- 3.Chapman J S, Georgopapadakou N H. Fluorometric assay for fleroxacin uptake by bacterial cells. Antimicrob Agents Chemother. 1989;33:27–29. doi: 10.1128/aac.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dámaso D, editor. Antibacterianos. Madrid, Spain: Marketing Pharm, S.A.; 1990. [Google Scholar]
- 5.De Benedetti P G. Molecular modeling and quantitative structure-activity analysis of antibacterial sulfanilamides and sulfones. Prog Drug Res. 1991;36:361–417. doi: 10.1007/978-3-0348-7136-5_5. [DOI] [PubMed] [Google Scholar]
- 6.Fallati C S. Ph.D. thesis. Córdoba, Argentina: Universidad Nacional de Córdoba; 1994. [Google Scholar]
- 7.Furet Y X, Deshusses J, Pechére J-C. Transport of pefloxacin across the bacterial cytoplasmic membrane in quinolone-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:2506–2511. doi: 10.1128/aac.36.11.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gmünder H, Kuratli K, Keck W. Effect of pyrimido[1,6-a]benzimidazoles, quinolones, and Ca2+ on the DNA gyrase-mediated cleavage reaction. Antimicrob Agents Chemother. 1995;39:163–169. doi: 10.1128/aac.39.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lecomte S, Baron M H, Chenon M T, Coupry C, Moreau N J. Effect of magnesium complexation by fluoroquinolones on their antibacterial properties. Antimicrob Agents Chemother. 1994;38:2810–2816. doi: 10.1128/aac.38.12.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manzo, R. H., D. A. Allemandi, and J. D. Perez. March 1992. Argentine patent application 322,011. March 1995. U.S. patent 5,395,936.
- 11.Manzo, R. H., M. R. Mazzieri, M. J. Nieto, and F. L. Alovero. February 1997. Argentine patent application P970106669.
- 12.McCaffrey C, Bertasso A, Pace J, Georgopapadakou N H. Quinolone accumulation in Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:1601–1605. doi: 10.1128/aac.36.8.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 2nd ed. Approved standard M7-A2. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1990. [Google Scholar]
- 14.Nikaido H, Thanassi D. Penetration of lipophilic agents with multiple protonation sites into bacterial cells: tetracyclines and fluoroquinolones as examples. Antimicrob Agents Chemother. 1993;37:1393–1399. doi: 10.1128/aac.37.7.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohner P, Herter C, Auckenthaler R, Pechére J-C, Waldvogel F A, Lew D P. Synergistic effect of quinolones and oxacillin on methicillin-resistant Staphylococcus species. Antimicrob Agents Chemother. 1989;33:2037–2041. doi: 10.1128/aac.33.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross D L, Riley C M. Physicochemical properties of the fluoroquinolone antibacterials. II. Acid ionization constants and their relationship to structure. Int J Pharm. 1992;83:267–272. [Google Scholar]
- 17.Sanchez J, Domagala J M, Hagen S E, Heifetz C L, Marland P H, Nichols J B, Trehan A. Quinolone antibacterial agents. Synthesis and structure-activity relationships of 8-substituted quinoline-3-carboxylic acids and 1,8-naphthyridine-3-carboxylic acids. J Med Chem. 1988;31:983–991. doi: 10.1021/jm00400a016. [DOI] [PubMed] [Google Scholar]
- 18.Smith J T, Ratcliffe N T. Effect of pH and magnesium on the in-vitro activity of ciprofloxacin. Excerpta Med Curr Clin Pract Ser. 1986;34:12–16. [Google Scholar]
- 19.Sommer H Z, Hayden J L, Jackson L L. Alkylation of amines. A general exhaustive alkylation method for the synthesis of quaternary ammonium compounds. J Org Chem. 1971;36:824–828. [Google Scholar]
- 20.Swedberg G, Castensson S, Sköld O. Characterization of mutationally altered dihydropteroate synthase and its ability to form a sulfonamide-containing dihydrofolate analog. J Bacteriol. 1979;137:129–136. doi: 10.1128/jb.137.1.129-136.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfson J S, Hooper D C, Swartz M N. Mechanism of action of and resistance to quinolone antimicrobial agents. In: Wolfson J S, Hooper D, editors. Quinolone antimicrobial agents. Washington, D.C: American Society for Microbiology; 1989. pp. 5–34. [Google Scholar]
- 22.Yoshida S, Kojima T, Matsuhisa I, Mitsuhashi S. Uptake of sparfloxacin and norfloxacin by clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:368–370. doi: 10.1128/aac.35.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]