Abstract
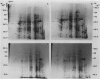
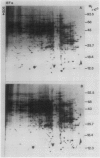
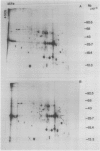
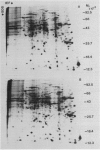
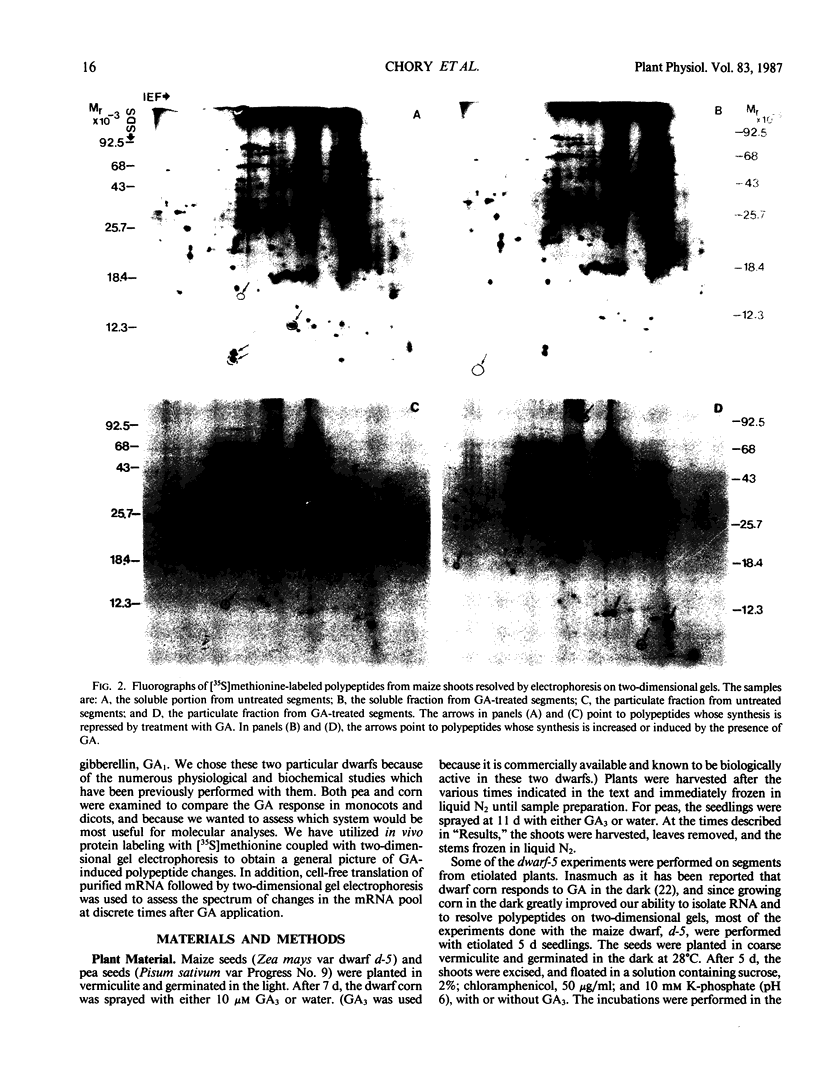
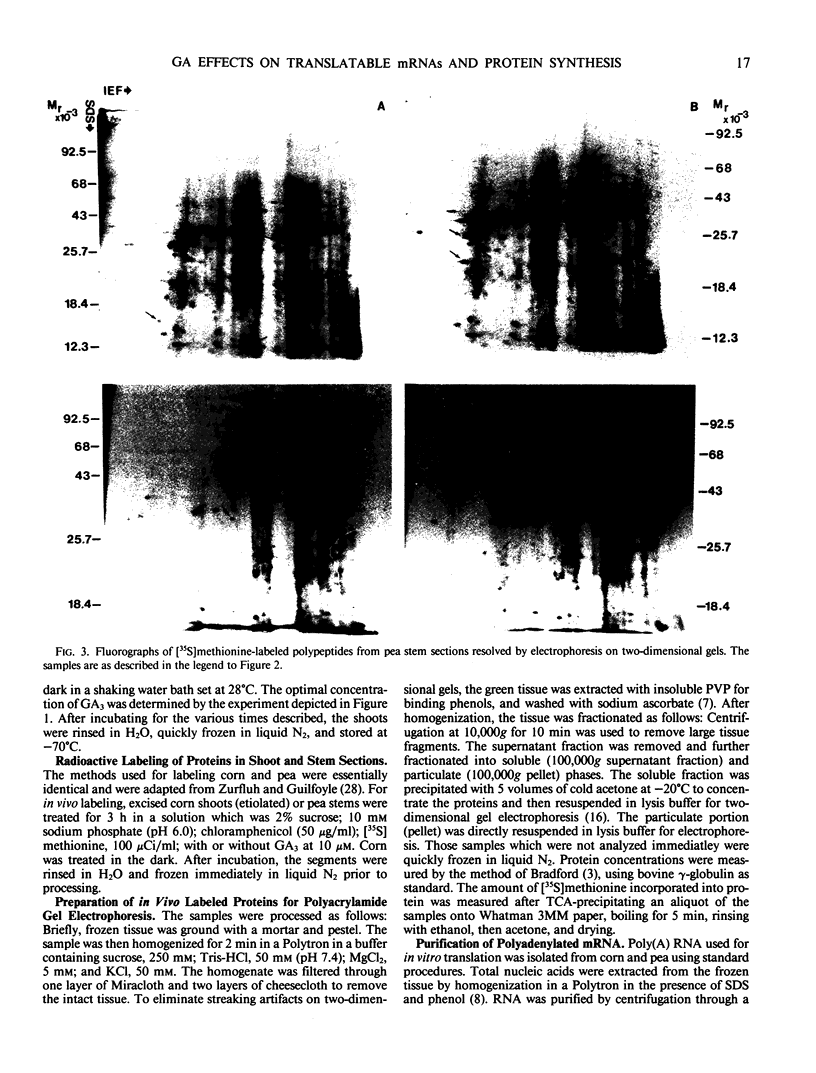
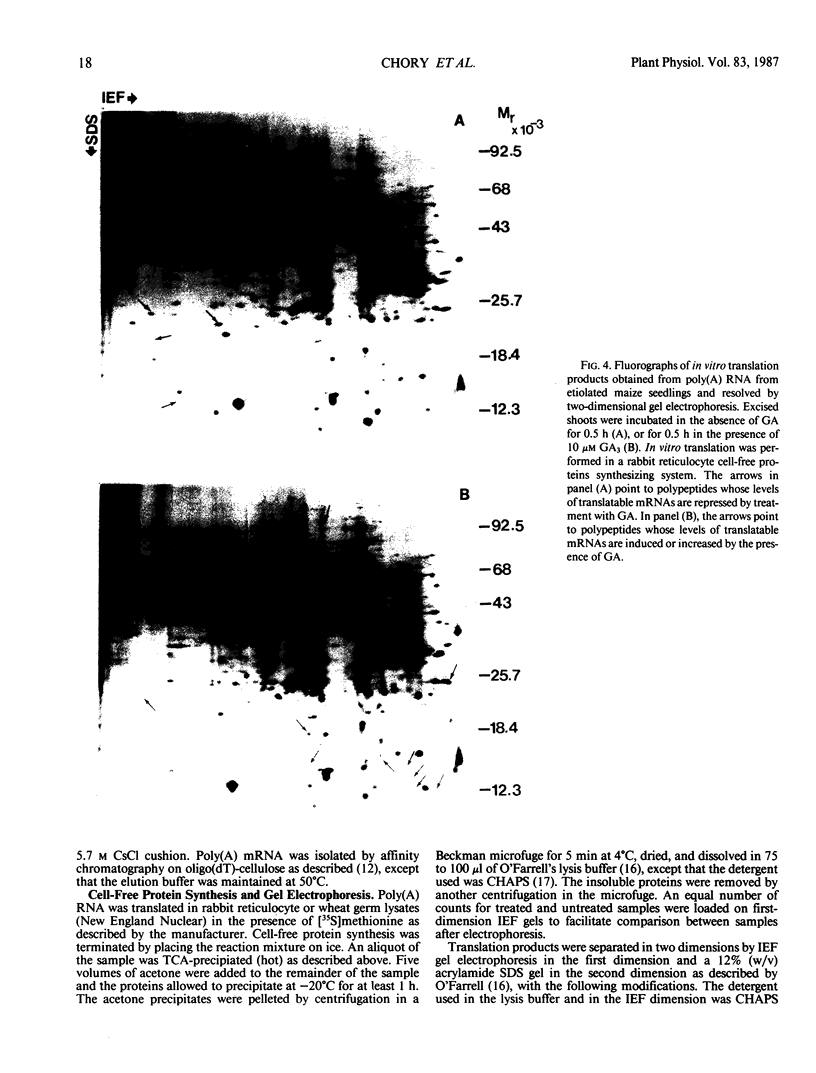
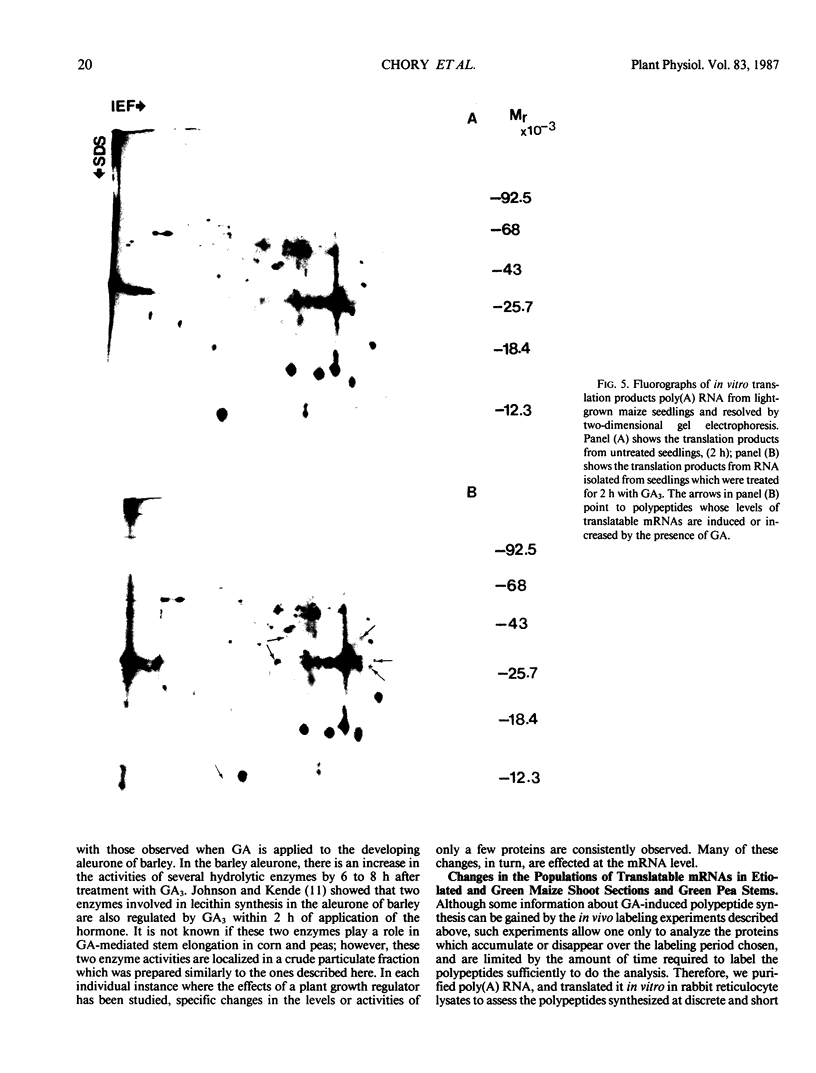
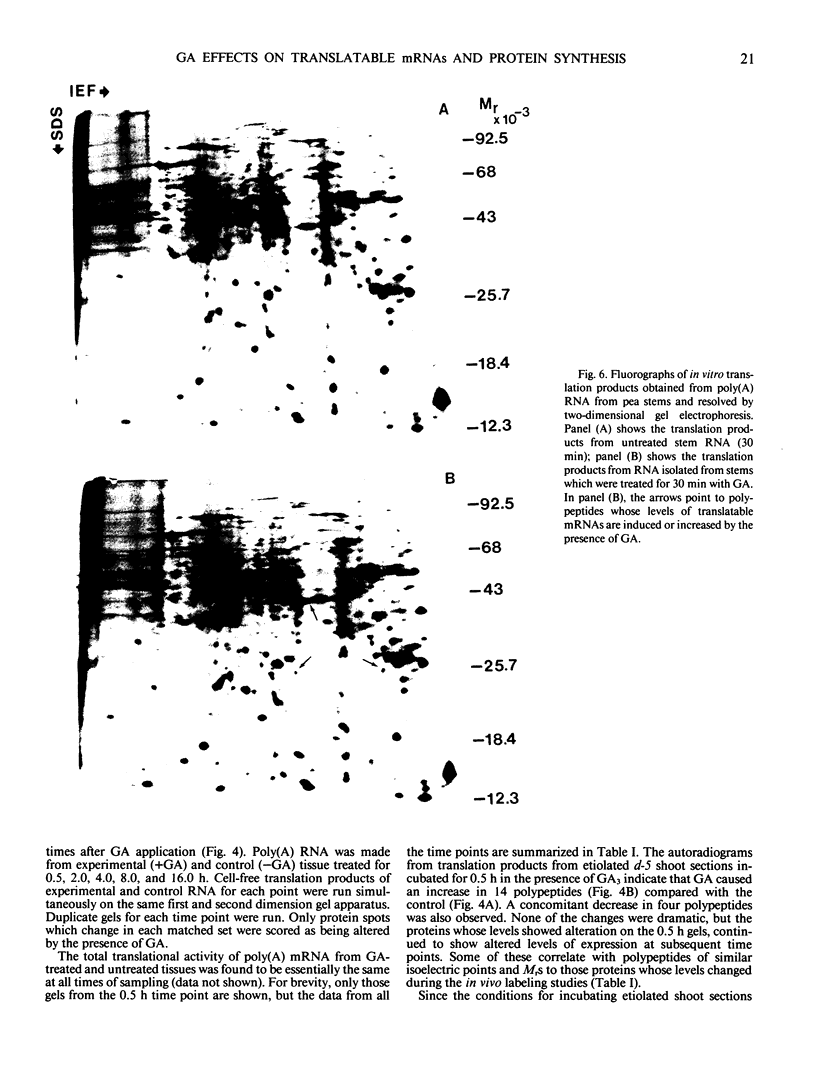
Two-dimensional gel electrophoresis was used to characterize the molecular mechanism of gibberellin-induced stem elongation in maize and pea. Dwarf mutants of maize (d-5) and pea (Progress No. 9) lack endogenous gibberellin (GA1) but become phenotypically normal with exogenous applications of this hormone. Sections from either etiolated maize or green pea seedlings were incubated in the presence of [35S] methionine for 3 hours with or without gibberellin. Labeled proteins from soluble and particulate fractions were analyzed by two-dimensional gel electrophoresis and specific changes in the patterns of protein synthesis were observed upon treatment with gibberellin. Polyadenylated mRNAs from etiolated or green maize shoots and green pea epicotyls treated or not with gibberellin (a 0.5 to 16 hour time course) were assayed by translation in a rabbit reticulocyte extract and separation of products by two-dimensional gel electrophoresis. Both increases and decreases in the levels of specific polypeptides were seen for pea and corn, and these changes were observed within 30 minutes of treatment with gibberellin. Together, these data indicate that gibberellin induces changes in the expression of a subset of gene products within elongating dwarfs. This may be due to changes in transcription rate, mRNA stability, or increased efficiency of translation of certain mRNAs.
Full text
PDF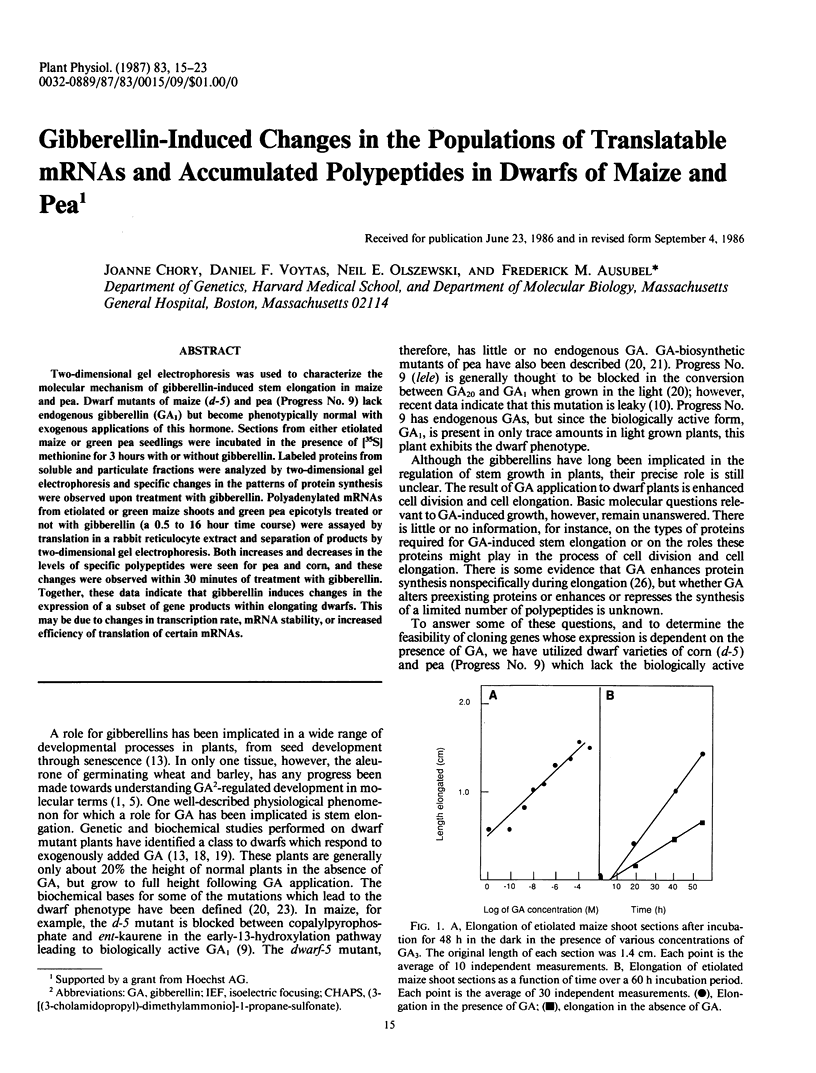



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen C. M., Leisner S. M. Cytokinin-modulated gene expression in excised pumpkin cotyledons. Plant Physiol. 1985 Jan;77(1):99–103. doi: 10.1104/pp.77.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer F., Van de Walle C. Method for extraction of proteins from green plant tissues for two-dimensional polyacrylamide gel electrophoresis. Anal Biochem. 1985 May 15;147(1):22–26. doi: 10.1016/0003-2697(85)90004-1. [DOI] [PubMed] [Google Scholar]
- Johnson K. D., Kende H. Hormonal Control of Lecithin Synthesis in Barley Aleurone Cells: Regulation of the CDP-Choline Pathway by Gibberellin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2674–2677. doi: 10.1073/pnas.68.11.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozer T. J. Control of protein synthesis in barley aleurone layers by the plant hormones gibberellic acid and abscisic acid. Cell. 1980 Jun;20(2):479–485. doi: 10.1016/0092-8674(80)90634-0. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Chandra G. R., Maxwell E. S. Hormonal control of alpha-amylase gene expression in barley. Studies using a cloned CDNA probe. J Biol Chem. 1983 Feb 25;258(4):2370–2375. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Perdew G. H., Schaup H. W., Selivonchick D. P. The use of a zwitterionic detergent in two-dimensional gel electrophoresis of trout liver microsomes. Anal Biochem. 1983 Dec;135(2):453–455. doi: 10.1016/0003-2697(83)90711-x. [DOI] [PubMed] [Google Scholar]
- Phinney B. O. GROWTH RESPONSE OF SINGLE-GENE DWARF MUTANTS IN MAIZE TO GIBBERELLIC ACID. Proc Natl Acad Sci U S A. 1956 Apr;42(4):185–189. doi: 10.1073/pnas.42.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J. B. Internode length in pisum: do the internode length genes effect growth in dark-grown plants? Plant Physiol. 1983 Jul;72(3):759–763. doi: 10.1104/pp.72.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Ray P. M. Early auxin-regulated polyadenylylated mRNA sequences in pea stem tissue. Proc Natl Acad Sci U S A. 1982 Jan;79(2):418–421. doi: 10.1073/pnas.79.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. C., Key J. L. Isolation of cloned cDNAs to auxin-responsive poly(A)RNAs of elongating soybean hypocotyl. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7185–7189. doi: 10.1073/pnas.79.23.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the patterns of protein synthesis in soybean hypocotyl. Proc Natl Acad Sci U S A. 1980 Jan;77(1):357–361. doi: 10.1073/pnas.77.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the population of translatable messenger RNA in elongating sections of soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):332–337. doi: 10.1104/pp.69.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]