This cohort study assesses risk of venous thromboembolism following COVID-19 infection in individuals with vs without immune-mediated inflammatory diseases in Ontario, Canada.
Key Points
Question
Are individuals with immune-mediated inflammatory diseases (IMIDs) more likely to experience a venous thromboembolism (VTE) following COVID-19 infection than individuals without IMIDs?
Findings
In a matched cohort study conducted using health administrative data from Ontario, Canada, the incidence of VTE among 28 440 individuals with an IMID was 2.64 per 100 000 person-days compared with 2.18 per 100 000 person-days among 126 437 individuals without an IMID. After adjusting for having 2 or more doses of a COVID-19 vaccine, history of VTE, and comorbidities, the risk of VTE was not statistically significantly different for people with IMIDs.
Meaning
Following a COVID-19 diagnosis, the overall risk of VTE is low among individuals with IMIDs.
Abstract
Importance
Immune-mediated inflammatory diseases (IMIDs) and COVID-19 are independently associated with venous thromboembolisms (VTEs).
Objective
To determine if individuals with IMIDs are at higher risk of VTE following COVID-19 infection compared with individuals without IMIDs.
Design, Setting, and Participants
Population-based matched cohort study using multiple deterministically linked health administrative databases from Ontario, Canada, and including patients testing positive for COVID-19 between January 1, 2020, and December 30, 2021, and followed up until March 31, 2022. Individuals with IMIDs (n = 28 440) who tested positive for COVID-19 were matched with up to 5 individuals without an IMID (n = 126 437) who tested positive for COVID-19. Matching was based on year of birth, sex, neighborhood income, and rural/urban residence. Data analysis was performed from August 6, 2022, to August 21, 2023.
Exposure
Diagnosis of an IMID, identified using algorithms based on diagnostic codes, procedures, and specialist visits.
Main Outcome and Measure
The main outcome was estimated age- and sex-standardized incidence of VTE. Proportional cause-specific hazard models compared the risk of VTE in people with and without IMIDs. Death was a competing risk. Models adjusted for history of VTE, 2 or more doses of a COVID-19 vaccine 14 or more days prior to COVID-19 diagnosis, and the Charlson Comorbidity Index. Routinely collected health data were used, so the hypothesis tested was formulated after data collection but prior to being granted access to data.
Results
The study included 28 440 individuals (16 741 [58.9%] female; 11 699 [41.1%] male) with an IMID diagnosed prior to first COVID-19 diagnosis, with a mean (SD) age of 52.1 (18.8) years at COVID-19 diagnosis. These individuals were matched to 126 437 controls without IMIDs. The incidence of VTE within 6 months of COVID-19 diagnosis among 28 440 individuals with an IMID was 2.64 (95% CI, 2.23-3.10) per 100 000 person-days compared with 2.18 (95% CI, 1.99-2.38) per 100 000 person-days among 126 437 matched individuals without IMIDs. The VTE risk was not statistically significantly different among those with vs without IMIDs (adjusted hazard ratio, 1.12; 95% CI, 0.95-1.32).
Conclusions and Relevance
In this retrospective population-based cohort study of individuals with IMIDs following COVID-19, individuals with IMIDs did not have a higher risk of VTE compared with individuals without an IMID. These data provide reassurance to clinicians caring for individuals with IMIDs and COVID-19.
Introduction
COVID-19 may cause widespread inflammation; hyperactivation of this systemic immune response may ultimately cause multiorgan failure and death.1,2,3 Venous thromboembolisms (VTEs) are known complications following COVID-194,5,6 and are associated with significant morbidity and mortality.
Immune-mediated inflammatory diseases (IMIDs) are a heterogeneous group of chronic diseases that result from abnormal activation of the immune system, affecting 5% to 7% of people in the Western world.7 Individuals with IMIDs are at a higher risk of VTE than the general population, particularly during disease flares,8,9 potentially due to inflammation causing endothelial dysfunction, platelet abnormalities, activation of the coagulation system, and impaired fibrinolysis. Inflammatory bowel disease (IBD),10,11,12,13 rheumatoid arthritis,14,15 psoriasis,16 vasculitis,17 and multiple sclerosis18 have all been reported to be associated with higher VTE risk.
The interplay between COVID-19, IMIDs, and VTE remains undefined. We hypothesized that individuals with IMIDs may have an elevated risk of VTE following COVID-19. We aimed to evaluate the incidence and risk of VTE following COVID-19 in individuals with IMIDs compared with those without IMIDs using population-based health administrative data from Ontario, Canada.
Methods
Data Sources
This study used health administrative data from Ontario, Canada, which has a publicly funded universal health care system. Data include all health care interactions of Ontario residents with valid health cards (>99% of the population). This includes hospitalizations (Canadian Institute for Health Information [CIHI] Discharge Abstract Database), procedures requiring single-day hospital admission (CIHI Same Day Surgery Database), emergency department visits (CIHI National Ambulatory Care Reporting System), and visits to the hospital for outpatient surgeries or other procedures (CIHI Same Day Surgery Database). Data also include physician billings for all patient-physician interactions, including outpatient visits; these data include physician specialty. Data were linked deterministically to the Registered Persons Database (demographic characteristics), Ontario Cancer Registry, C19INTGR (COVID-19 testing), and COVaxON (COVID-19 vaccinations). All data are held by ICES, an independent, nonprofit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. Data are available in uncleaned, unedited format to researchers and data analysts. The use of the data in this project is authorized under section 45 of Ontario’s Personal Health Information Protection Act (PHIPA) and does not require review by a research ethics board. This study was reported in accordance with the Reporting of Studies Conducted Using Observational Routinely Collected Health Data (RECORD), an extension to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for research using routinely collected health administrative data.19
Study Design and Eligibility
We conducted a retrospective matched cohort study. Individuals with an IMID who tested positive for COVID-19 were randomly matched with up to 5 individuals without any IMIDs who tested positive for COVID-19 based on year of birth (±1 year), sex, mean neighborhood income quintile, and rural/urban residence. Before matching, we excluded individuals without a valid Ontario health card number, date of birth, date of death (date of death preceded date of positive COVID-19 test result), or sex. We also excluded people diagnosed with a malignant neoplasm within 5 years prior to testing positive for COVID-19.
Identifying Individuals Testing Positive for COVID-19
Individuals testing positive for COVID-19 were identified from C19INTGR, which includes the results of all polymerase chain reaction (PCR) tests conducted in Ontario from January 1, 2020, to December 30, 2021, after which time PCR testing was limited to selected groups. Participants were followed up from the date of the first positive COVID-19 test result until VTE, death, migration out of Ontario, or end of follow-up (March 31, 2022).
Identifying Individuals With IMIDs
Individuals with at least 1 diagnosis of ankylosing spondylitis, IBD, multiple sclerosis, polymyalgia rheumatica, psoriasis, psoriatic arthritis, rheumatoid arthritis, systemic autoimmune rheumatic disease (encompassing connective tissues disorders, such as lupus and scleroderma), uveitis, or vasculitis were identified using algorithms based on multiple health care encounters with disease-specific diagnostic codes (for hospitalizations, International Classification of Diseases, Ninth Revision [ICD-9] before April 1, 2002, and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] on or after April 1, 2002), the specialty of the physician submitting the billing claim for physician claims, procedure codes for endoscopy (IBD only), and indication-specific medications. Validated algorithms were available for IBD,20,21 multiple sclerosis,22 psoriasis,23 psoriatic arthritis,23 and rheumatoid arthritis.24,25 See eTable 1 in Supplement 1 for a description of the algorithms used to identify each of the IMIDs. We looked back until 1991 (the start of Ontario’s health administrative data) for incident or prevalent IMID diagnoses, except for vasculitis (April 1, 2002, due to improvements with ICD-10 coding system introduction). Only individuals for whom the first diagnostic code for an IMID occurred before the date of COVID-19 diagnosis were included.
Identifying Individuals With VTE
Venous thromboembolisms, including pulmonary embolism (PE) and deep vein thrombosis (DVT), were identified from emergency department and hospitalization data using ICD-10 codes (eTable 2 in Supplement 1). Diagnostic codes used to identify VTE were previously validated in Alberta, Canada (sensitivity, 75.0%; specificity, 93.8%; positive predictive value, 73.3%; negative predictive value, 94.3%, when combined with radiology codes).26 Radiology codes used in the validation study were not widely used in patients with VTE in Ontario and were excluded from the identification algorithm. Our primary outcome was the first VTE of any type. Secondary outcomes were PE and DVT.
Covariates and Matching Characteristics
Comorbidities
We used the Deyo adaptation of the Charlson Comorbidity Index (CCI), converted from ICD-9 to ICD-10 coding.27,28,29,30 We adapted the index to (1) include comorbidities treated in an outpatient setting and (2) remove codes used in our IMID-identification algorithms to avoid inflation of the CCI in people with IMIDs (see eTable 3 in Supplement 1 for list of excluded codes). We calculated CCI using data from the 5 years prior to COVID-19 diagnosis.
We report (1) the total number of IMIDs (among those with IMIDs; categorical: 1, 2, or ≥3); (2) the number of IMIDs in addition to the IMID of interest (among those with IMIDs; categorical: 0, 1, or ≥2); (3) IMID duration at the time of COVID-19 diagnosis; and (4) history of VTE in the 5 years prior to COVID-19 diagnosis. Patients with any recorded diagnosis of chronic obstructive pulmonary disease,31 congestive heart failure,32 or diabetes33,34 prior to their COVID-19 diagnosis were identified using previously validated algorithms (eTable 4 in Supplement 1).
Duration of IMID was calculated as the difference between the date of IMID diagnosis (date of the first code in the identification algorithm) and the date of COVID-19 diagnosis and categorized (<1, 1 to <3, 3 to <5, or ≥5 years). When the exact date of diagnosis was unknown (eg, a person did not meet a minimum lookback period), cases were assumed to be prevalent on the date of their first diagnostic code and assigned a disease duration of 5 or more years. When individuals had multiple IMIDs, IMID duration was defined as the time from the first IMID diagnosis to date of COVID-19 diagnosis.
Vaccination
COVaxON includes information on all COVID-19 vaccines administered in Ontario, Canada, and any out-of-province vaccines reported to public health. Anyone with 2 or more vaccinations 14 or more days before their positive COVID-19 test result was considered vaccinated.
Sociodemographic Characteristics
Sex, rural/urban residence, death prior to end of follow-up, and mean neighborhood income quintile (a validated proxy for individual-level socioeconomic status35) at the time of COVID-19 diagnosis were identified from the Registered Persons Database.
Statistical Analysis
We summarized the characteristics of individuals with and without IMIDs using percentages for categorical variables. Continuous variables such as age were summarized using means with SDs. The CCI scores were summarized using medians with first and third quartiles.
The incidence of VTE (overall and stratified by type) was calculated by dividing the number of VTEs by the total person-time and reported as events per 100 000 person-days (PDs). We reported the crude and age- and sex-standardized incidence rates within 6 and 12 months of COVID-19 diagnosis using the 2017 Canadian population as the standard. Confidence intervals for both the crude and standardized incidence rates were calculated using the gamma method.36 Proportional cause-specific hazards models compared the risk of VTEs in people with and without IMIDs, treating death as a competing risk. We conducted a series of 3 models: (1) unadjusted, (2) adjusted for COVID-19 vaccination and history of VTE, and (3) adjusted for COVID-19 vaccination, history of VTE, and the CCI (continuous). We selected these variables as important confounders because of their impact on the magnitude of the association between IMIDs and VTE, with the number of events limiting the number of variables that could be included in a regression model.
Our primary analysis included individuals with any IMID. When a person had multiple IMIDs, we included them once in the analysis of all IMIDs and in disease-specific analyses. We conducted a sensitivity analysis censoring participants with a second COVID-19 diagnosis, applying a minimum look-forward period of 60 days to minimize the risk of censoring individuals following a second positive test result for the same infection. We also conducted a sensitivity analysis excluding patients with any history of VTE (after 2002).
All statistical tests were 2-sided with a 5% level of significance. Analyses were conducted using SAS, version 9.4 (SAS Institute Inc).
Results
We identified 28 440 individuals with an IMID diagnosed prior to their first COVID-19 diagnosis, who were matched to 126 437 controls. Cases and controls were comparable for matched characteristics (Table 1). Patients with IMIDs had a mean (SD) age of 52.1 (18.8) years at COVID-19 diagnosis, and 58.9% were female, compared with the cohort without IMIDs, which had a mean (SD) age of 50.1 (18.0) years, and 58.4% were female. Psoriasis was the most common IMID (37.8%). The characteristics of included participants stratified by type of IMID are provided in eTable 5 in Supplement 1. We reported disease-specific analyses for all IMIDs except vasculitis, which was suppressed due to small cell sizes. Overall, we excluded 1332 individuals with IMIDs: 97 due to missing rural/urban status, and 1235 due to unsuccessful matching with any person without an IMID.
Table 1. Characteristics of Individuals With IMIDs and Matched People Without an IMID on the Date of Positive COVID-19 Test Result.
| Characteristic | No. (%) | |
|---|---|---|
| People with any IMID (n = 28 440) | Matched people without an IMID (n = 126 437) | |
| Age, mean (SD), y | 52.1 (18.8) | 50.1 (18.0) |
| Sex | ||
| Female | 16 741 (58.9) | 73 793 (58.4) |
| Male | 11 699 (41.1) | 52 644 (41.6) |
| Rural | 936 (3.3) | 2014 (1.6) |
| Mean neighborhood income quintile | ||
| 1 (Lowest) | 6095 (21.4) | 27 179 (21.5) |
| 2 | 5794 (20.4) | 25 792 (20.4) |
| 3 | 5876 (20.7) | 26 410 (20.9) |
| 4 | 5507 (19.4) | 24 466 (19.4) |
| 5 (Highest) | 5168 (18.2) | 22 590 (17.9) |
| Previous VTE | 427 (1.5) | 866 (0.7) |
| Chronic obstructive pulmonary disease | 1286 (4.5) | 3033 (2.4) |
| Congestive heart failure | 1549 (5.4) | 3907 (3.1) |
| Diabetes | 5868 (20.6) | 21 700 (17.2) |
| Charlson Comorbidity Index, median (IQR) | 1 (0-2) | 0 (0-1) |
| Type of IMID | ||
| Ankylosing spondylitis | 1453 (5.1) | NA |
| Inflammatory bowel disease | 4331 (15.2) | NA |
| Multiple sclerosis | 1954 (6.9) | NA |
| Psoriatic arthritis | 1134 (4.0) | NA |
| Polymyalgia rheumatica | 535 (1.9) | NA |
| Psoriasis | 10 739 (37.8) | NA |
| Rheumatoid arthritis | 5883 (20.7) | NA |
| Systemic autoimmune rheumatic disease | 2506 (8.8) | NA |
| Uveitis | 3000 (10.5) | NA |
| Vasculitis | 512 (1.8) | NA |
| IMID duration | ||
| <1 y | 1115 (3.9) | NA |
| 1 to <3 y | 2762 (9.7) | NA |
| 3 to <5 y | 2695 (9.5) | NA |
| ≥5 y | 21 868 (76.9) | NA |
| No. of IMIDs | ||
| 1 | 25 479 (89.6) | NA |
| 2 | 2411(8.5) | NA |
| ≥3 | 550 (2.0) | NA |
| COVID-19 vaccination | 7704 (27.1) | 34 303 (27.1) |
| Deaths | 15 661 (5.5) | 4403 (3.5) |
Abbreviations: IMID, immune-mediated inflammatory disease; NA, not applicable; VTE, venous thromboembolism.
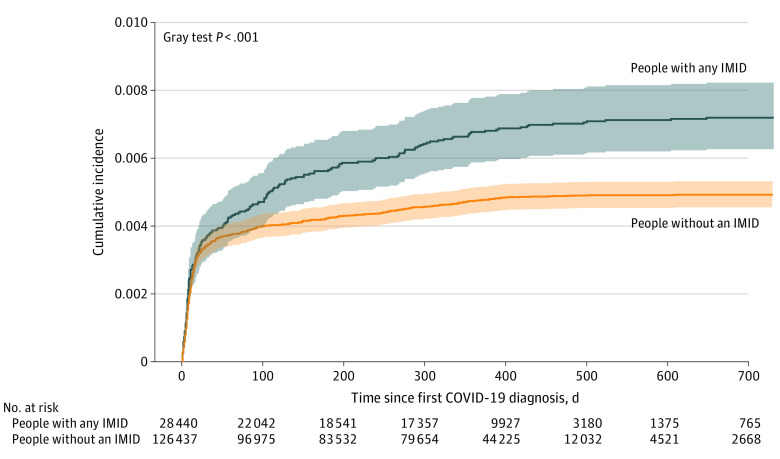
Within 6 months of COVID-19 diagnosis, the standardized incidence rate of VTE was 2.64 (95% CI, 2.23-3.10) per 100 000 PDs among people with IMIDs compared with 2.18 (95% CI, 1.99-2.38) among matched individuals without IMIDs (Figure 1; Table 2). Within 12 months of COVID-19 diagnosis, the standardized incidence rates were 1.82 (95% CI, 1.56-2.11) per 100 000 PDs among individuals with an IMID and 1.37 (95% CI, 1.25-1.49) among individuals without IMIDs. The highest standardized incidence of VTEs occurred among individuals with systemic autoimmune rheumatic disease (6.73 per 100 000 PDs; 95% CI, 3.13-12.62); the lowest, ulcerative colitis (0.79 per 100 000 PDs; 95% CI, 0.21-2.04).
Figure 1. Cumulative Incidence of Venous Thromboembolism Among Individuals With and Without an Immune-Mediated Inflammatory Disease (IMID) Following COVID-19 Diagnosis.
Table 2. Incidence of Venous Thromboembolism Among Individuals With IMIDs and Matched Controls Within 6 Months and 12 Months of COVID-19 Diagnosis.
| IMID | People with an IMID | Matched people without an IMID | ||
|---|---|---|---|---|
| Crude IR per 100 000 PD (95% CI) | Standardized IR per 100 000 PD (95% CI) | Crude IR per 100 000 PD (95% CI) | Standardized IR per 100 000 PD (95% CI) | |
| Events within 6 mo of COVID-19 diagnosis | ||||
| Any IMID | 3.72 (3.17-4.35) | 2.64 (2.23-3.10) | 2.77 (2.54-3.02) | 2.18 (1.99-2.38) |
| Ankylosing spondylitis | 2.72 (1.00-5.93) | 2.34 (0.81-5.27) | 3.38 (2.34-4.72) | 2.84 (1.88-4.10) |
| Inflammatory bowel disease | 1.84 (0.95-3.21) | 1.70 (0.78-3.21) | 2.21 (1.71-2.81) | 2.17 (1.58-2.91) |
| Crohn disease | 2.86 (1.23-5.63) | 3.54 (0.99-8.96) | 1.94 (1.25-2.86) | 2.69 (1.24-5.08) |
| Ulcerative colitis | 1.16 (0.32-2.98) | 0.79 (0.21-2.04) | 2.55 (1.82-3.47) | 2.20 (1.46-3.19) |
| Multiple sclerosis | 3.03 (1.38-5.75) | 3.03 (1.06-6.77) | 2.14 (1.43-3.07) | 1.77 (1.12-2.67) |
| Psoriasis | 2.93 (2.16-3.88) | 2.24 (1.63-3.00) | 2.94 (2.57-3.36) | 2.24 (1.94-2.58) |
| Psoriatic arthritis | 4.06 (1.63-8.36) | 5.71 (2.57-10.96) | 2.19 (1.28-3.51) | 1.29 (0.74-2.10) |
| Polymyalgia rheumatica | 6.48 (2.10-15.12) | 1.13 (0.26-3.14) | 3.94 (1.97-7.06) | 0.85 (0.36-1.68) |
| Rheumatoid arthritis | 6.47 (4.91-8.36) | 2.89 (0.91-6.85) | 3.29 (2.75-3.92) | 2.20 (1.76-2.71) |
| Systemic autoimmune rheumatic disease | 6.06 (3.84-9.10) | 6.73 (3.13-12.62) | 2.07 (1.45-2.86) | 1.75 (1.05-2.74) |
| Uveitis | 4.20 (2.53-6.56) | 2.91 (1.65-4.75) | 2.97 (2.25-3.84) | 1.78 (1.34-2.33) |
| Events within 12 mo of COVID-19 diagnosis | ||||
| Any IMID | 2.60 (2.24-2.99) | 1.82 (1.56-2.11) | 1.79 (1.65-1.94) | 1.37 (1.25-1.49) |
| Ankylosing spondylitis | 2.14 (0.92-4.21) | 1.79 (0.71-3.70) | 2.03 (1.41-2.82) | 1.66 (1.11-2.38) |
| Inflammatory bowel disease | 1.56 (0.91-2.50) | 1.48 (0.80-2.50) | 1.47 (1.16-1.85) | 1.43 (1.06-1.89) |
| Crohn disease | 2.19 (1.05-4.02) | 2.56 (0.82-6.02) | 1.36 (0.91-1.96) | 1.95 (0.87-3.74) |
| Ulcerative colitis | 1.21 (0.48-2.48) | 0.99 (0.38-2.10) | 1.65 (1.20-2.22) | 1.42 (0.96-2.02) |
| Multiple sclerosis | 2.57 (1.37-4.39) | 2.96 (1.22-6.00) | 1.42 (0.98-1.99) | 1.13 (0.74-1.66) |
| Psoriasis | 2.17 (1.66-2.79) | 1.61 (1.21-2.09) | 1.91 (1.68-2.17) | 1.41 (1.23-1.61) |
| Psoriatic arthritis | 3.06 (1.40-5.81) | 4.33 (1.47-9.84) | 1.50 (0.92-2.32) | 1.02 (0.59-1.64) |
| Polymyalgia rheumatica | 5.07 (2.04-10.45) | 4.29 (0.38-17.19) | 2.38 (1.23-4.15) | 0.53 (0.24-1.01) |
| Rheumatoid arthritis | 4.06 (3.13-5.18) | 2.02 (0.80-4.21) | 2.09 (1.76-2.46) | 1.37 (1.11-1.67) |
| Systemic autoimmune rheumatic disease | 3.66 (2.35-5.45) | 4.10 (1.82-7.92) | 1.38 (1.00-1.87) | 1.16 (0.73-1.75) |
| Uveitis | 2.80 (1.75-4.23) | 1.90 (1.12-3.01) | 1.96 (1.52-2.48) | 1.11 (0.85-1.43) |
Abbreviations: IMID, immune-mediated inflammatory disease; IR, incidence rate; PD, person-days.
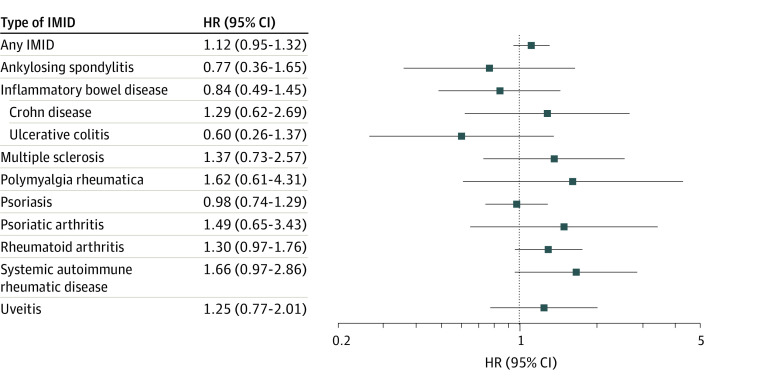
In unadjusted analysis, people with IMIDs were significantly more likely to have a VTE compared with matched controls (hazard ratio [HR], 1.46; 95% CI, 1.24-1.71; eFigure 1 in Supplement 1). The association persisted when adjusted for previous VTE and COVID-19 vaccination (HR, 1.40; 95% CI, 1.20-1.64; eFigure 2 in Supplement 1). There was no significant association when additionally adjusting for comorbidities (adjusted HR [aHR], 1.12; 95% CI, 0.95-1.32; Figure 2). Results were consistent when stratifying by type of IMID and in sensitivity analysis censoring the 1584 individuals (1%) with a second COVID-19 diagnosis (aHR, 1.122 vs 1.121). In the sensitivity analysis excluding all people with a history of VTE after 2002 (n = 2566), there remained no statistically significant difference in risk of VTE in patients with IMIDs after adjusting for comorbidities and vaccination status (aHR, 1.07; 95% CI, 0.90-1.27).
Figure 2. Forest Plot Depicting the Risk of Venous Thromboembolism Among Individuals With and Without (Reference) an IMID Following COVID-19 Diagnosis, Stratified by Type of IMID.
HR indicates hazard ratio; IMID, immune-mediated inflammatory disease.
Within 6 months of COVID-19 diagnosis, the standardized incidence rate of DVTs was 0.27 (95% CI, 0.14-0.46) per 100 000 PDs among individuals with IMIDs compared with 0.17 (95% CI, 0.12-0.23) among people without IMIDs (Table 3). Extending follow-up to 12 months, the standardized incidence rate of DVTs was 0.21 (95% CI, 0.12-0.33) per 100 000 PDs among individuals with IMIDs, compared with 0.12 (95% CI, 0.09-0.16) per 100 000 PDs among individuals without IMIDs. There was no difference in the risk of DVTs among those with and without an IMID (aHR, 0.99; 95% CI, 0.58-1.69).
Table 3. Incidence of Deep Vein Thrombosis and Pulmonary Embolism Among Individuals With IMIDs and Matched Controls Within 6 Months and 12 Months of COVID-19 Diagnosis.
| People with an IMID | Matched people without an IMID | |||
|---|---|---|---|---|
| Crude IR per 100 000 PD (95% CI) | Standardized IR per 100 000 PD (95% CI) | Crude IR per 100 000 PD (95% CI) | Standardized IR per 100 000 PD (95% CI) | |
| Deep vein thrombosis | ||||
| 6 mo | 0.32 (0.18-0.54) | 0.27 (0.14-0.46) | 0.23 (0.17-0.31) | 0.17 (0.12-0.23) |
| 12 mo | 0.25 (0.15-0.40) | 0.21 (0.12-0.33) | 0.16 (0.12-0.21) | 0.12 (0.09-0.16) |
| Pulmonary embolism | ||||
| 6 mo | 1.22 (0.92-1.60) | 0.85 (0.62-1.12) | 0.66 (0.55-0.79) | 0.53 (0.44-0.64) |
| 12 mo | 1.46 (1.20-1.76) | 0.99 (0.81-1.21) | 1.01 (0.90-1.12) | 0.79 (0.70-0.88) |
Abbreviations: IMID, immune-mediated inflammatory disease; IR, incidence rate; PD, person-days.
The standardized incidence rates of PEs within 6 months were 0.85 (95% CI, 0.62-1.12) per 100 000 PDs among individuals with IMIDs and 0.54 (95% CI, 0.44-0.64) among individuals without IMIDs (Table 3). The standardized incidence rates of PEs within 12 months were 0.99 (95% CI, 0.81-1.21) per 100 000 PDs among individuals with IMIDs and 0.79 (95% CI, 0.70-0.88) among individuals without IMIDs. There was no difference in the risk of PEs among those with and without an IMID (aHR, 1.10; 95% CI, 0.93-1.31).
Discussion
While IMIDs and COVID-19 are independently associated with VTEs, we observed no additional risk of VTEs among individuals with IMIDs relative to matched individuals without IMIDs following COVID-19 diagnosis after adjusting for COVID-19 vaccination, history of VTE, and comorbidities. However, individuals with IMIDs had a higher risk of VTEs when not adjusting for comorbidities. These findings were consistent in IMID-specific analyses and when separately investigating the risks of DVT and PE.
Both IMIDs and COVID-19 are associated with an increased risk of VTE.4,5,6,8,9,10,11,12,13,14,15,16,17,18 Studies examining the additive risk of having an IMID on the post–COVID-19 risk of VTE are sparse. A case-crossover study using health administrative data from a US Veterans Affairs health care system cohort reported that individuals with IBD had higher odds of a VTE following COVID-19 (odds ratio, 8.15; 95% CI, 4.34-15.30) relative to the pre–COVID-19 period.37 However, this study did not compare individuals with and without IBD. Another study using Kaiser Permanente data from California also reported elevated risk in patients with IBD and COVID-19 (aHR, 2.43 [95% CI, 1.02-5.80] overall; aHR, 3.46 [95% CI, 1.31-9.13] within 30 days of COVID-19 diagnosis; aHR, 1.05 [95% CI, 0.13-8.46] >30 days from COVID-19 diagnosis).38 The wide confidence intervals may indicate imprecision due to smaller sample size of patients with IBD (n = 2006). In addition, this study did not control for vaccination status. Reassuringly, the overall risk of VTEs after COVID-19 in this study (0.26 per 100 person-years [PYs]) was similar to that of the current study (2.18 per 100 000 PDs = 0.8 per 100 PYs).38 In a study from the United Kingdom, the risk of thrombosis was similar among those with autoimmune conditions and propensity-matched controls who were hospitalized for COVID-19.39 Findings were similar when restricting the analysis to individuals with rheumatologic conditions. In addition, VTE incidence in patients with COVID-19 in the current study was higher than prepandemic population-based VTE rates in Canada (1.29 [95% CI, 1.06-1.53] per 1000 PYs).40
The association between IMIDs and VTE following COVID-19 was substantially modified when controlling for comorbidities in the regression analysis. This suggested that comorbidities confounded the association between IMIDs and VTE in the presence of COVID-19. This is a noteworthy finding for clinicians caring for individuals with IMIDs with COVID-19. When treating patients with an IMID and COVID-19, physicians should consider both the underlying COVID-19 severity, individual risk factors, and the presence of comorbidities in determining the need for VTE prophylaxis. In the absence of severe IMID-related inflammation, VTE risk factors, and other comorbid conditions, the IMID itself should not determine the need for VTE prophylaxis.
Our study is strengthened by the use of health administrative data from Canada’s most populous province, including an ethnically diverse population from a large geographic region. Since data include information for all residents eligible for provincial health care coverage (>99%), our study is representative of the Ontario population.
Limitations
Our study has some limitations. As with all studies using health administrative data, it is subject to misclassification bias. However, we minimized this risk by using validated algorithms for most of the included IMIDs. Our study preceded the Omicron era, and therefore repeated SARS-CoV-2 infections were less common.41 Reassuringly, the results of a sensitivity analysis censoring individuals at the time of a second infection were nearly identical to our primary analysis. We also relied on PCR testing for SARS-CoV-2 to identify patients with COVID-19. We may have missed asymptomatic or mild cases, especially during times when the need for testing and/or contact tracing exceeded demand. While all Ontario residents had universal access to PCR testing at no expense, we could not assess whether patients with IMIDs were more likely to be tested than patients without IMIDs. However, all patients admitted to the hospital were routinely tested with PCR. The structure of our data does not allow us to differentiate among individuals admitted to the hospital because of COVID-19, individuals admitted for other reasons but had an incidental positive SARS-CoV-2 test result, or individuals admitted later due to longer-term complications following infection. We therefore could not account for COVID-19 severity. Two-thirds of individuals included in our study had not received 2 or more doses of a COVID-19 vaccine at the time of their infection; a substantial proportion of the individuals included in our study tested positive before vaccines were widely available. We removed IMID-related codes from the CCI to avoid inflation of the CCI in the IMID cohort. In addition, we removed people with cancer in the preceding 5 years from the cohort due to increased risk of VTE. Many other diseases that form parts of the CCI may increase the risk of VTE (eg, myocardial infarction, hemiplegia, peripheral vascular disease). However, removing patients from the cohort with these illnesses would have severely restricted the sample size. In addition, there is no reason to believe that these act as confounders, increasing the VTE risk more in patients with IMIDs than in patients without IMIDs. Our study surprisingly found that the incidence of PE was higher than that of DVT. This may be due to differences in accuracy of the codes/algorithms used to identify these subgroups of VTE. Alternatively, patients with PEs were likely hospitalized more frequently than those with DVTs, so we may have underestimated the risk of DVTs. Our study may also be at risk of residual confounding. For example, Canadian hospitalization data do not include medications. Use of anticoagulants in patients with COVID-19 and/or corticosteroid use could be unmeasured confounders that increase the risk of both severe COVID-1942,43 and VTEs9,44 in patients with IMIDs. Administrative data do not include clinical characteristics, such as whether there was active inflammation in patients with IMIDs, which may have increased the risk of VTEs.
Conclusions
In this matched cohort study, we found that the absolute risk of VTE was low in individuals with IMIDs, and the risk was not statistically significantly different from that of the general population after adjusting for comorbidities and vaccination status. Therefore, the presence of an IMID alone should not dictate the use of VTE prophylaxis in patients hospitalized with COVID-19.
eTable 1. Algorithms used to identify individuals with immune-mediated inflammatory diseases
eTable 2. International Classification of Diseases (ICD)-10 codes used to identify individuals developing a venous thromboembolism
eTable 3. International Classification of Disease (ICD)-10 codes for immune-mediated inflammatory diseases that were excluded from the adapted version of the Charlson Comorbidity Index used in this study
eTable 4. Previously validated algorithms used to identify individuals with selected comorbidities
eTable 5. Demographics and clinical characteristics of individuals with immune-mediated inflammatory diseases (IMIDs) and matched people without an IMID on the date of positive COVID-19 test, stratified by type of IMID
eFigure 1. Forest plot depicting the unadjusted hazard ratio of venous thromboembolism among individuals with and without (reference) an immune-mediated inflammatory disease (IMID) following COVID-19 infection, stratified by type of IMID
eFigure 2. Forest plot depicting the hazard ratio of venous thromboembolism among individuals with and without (reference) an immune-mediated inflammatory disease (IMID) following COVID-19 infection, adjusted for previous VTE and COVID-19 vaccination stratified by type of IMID
eReferences.
Data Sharing Statement
References
- 1.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529-539. doi: 10.1007/s00281-017-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortaz E, Tabarsi P, Varahram M, Folkerts G, Adcock IM. The immune response and immunopathology of COVID-19. Front Immunol. 2020;11:2037. doi: 10.3389/fimmu.2020.02037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jing H, Wu X, Xiang M, Liu L, Novakovic VA, Shi J. Pathophysiological mechanisms of thrombosis in acute and long COVID-19. Front Immunol. 2022;13:992384. doi: 10.3389/fimmu.2022.992384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunutsor SK, Laukkanen JA. Incidence of venous and arterial thromboembolic complications in COVID-19: a systematic review and meta-analysis. Thromb Res. 2020;196:27-30. doi: 10.1016/j.thromres.2020.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. doi: 10.1016/j.eclinm.2020.100639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hippisley-Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. doi: 10.1136/bmj.n1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Gabalawy H, Guenther LC, Bernstein CN. Epidemiology of immune-mediated inflammatory diseases: incidence, prevalence, natural history, and comorbidities. J Rheumatol Suppl. 2010;85:2-10. doi: 10.3899/jrheum.091461 [DOI] [PubMed] [Google Scholar]
- 8.Zöller B, Li X, Sundquist J, Sundquist K. Autoimmune diseases and venous thromboembolism: a review of the literature. Am J Cardiovasc Dis. 2012;2(3):171-183. [PMC free article] [PubMed] [Google Scholar]
- 9.Galloway J, Barrett K, Irving P, et al. Risk of venous thromboembolism in immune-mediated inflammatory diseases: a UK matched cohort study. RMD Open. 2020;6(3):e001392. doi: 10.1136/rmdopen-2020-001392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fumery M, Xiaocang C, Dauchet L, Gower-Rousseau C, Peyrin-Biroulet L, Colombel JF. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: a meta-analysis of observational studies. J Crohns Colitis. 2014;8(6):469-479. doi: 10.1016/j.crohns.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 11.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375(9715):657-663. doi: 10.1016/S0140-6736(09)61963-2 [DOI] [PubMed] [Google Scholar]
- 12.McCurdy JD, Kuenzig ME, Smith G, et al. Risk of venous thromboembolism after hospital discharge in patients with inflammatory bowel disease: a population-based study. Inflamm Bowel Dis. 2020;26(11):1761-1768. doi: 10.1093/ibd/izaa002 [DOI] [PubMed] [Google Scholar]
- 13.Kuenzig ME, Bitton A, Carroll MW, et al. Inflammatory bowel disease increases the risk of venous thromboembolism in children: a population-based matched cohort study. J Crohns Colitis. 2021;15(12):2031-2040. doi: 10.1093/ecco-jcc/jjab113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Lu N, Avina-Galindo AM, et al. The risk and trend of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a general population-based study. Rheumatology (Oxford). 2021;60(1):188-195. doi: 10.1093/rheumatology/keaa262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ungprasert P, Srivali N, Spanuchart I, Thongprayoon C, Knight EL. Risk of venous thromboembolism in patients with rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol. 2014;33(3):297-304. doi: 10.1007/s10067-014-2492-7 [DOI] [PubMed] [Google Scholar]
- 16.Ungprasert P, Sanguankeo A, Upala S, Suksaranjit P. Psoriasis and risk of venous thromboembolism: a systematic review and meta-analysis. QJM. 2014;107(10):793-797. doi: 10.1093/qjmed/hcu073 [DOI] [PubMed] [Google Scholar]
- 17.Marozoff S, Mai A, Dehghan N, Sayre EC, Choi HK, Aviña-Zubieta JA. Increased risk of venous thromboembolism in patients with granulomatosis with polyangiitis: a population-based study. PLoS One. 2022;17(6):e0270142. doi: 10.1371/journal.pone.0270142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peeters PJ, Bazelier MT, Uitdehaag BM, Leufkens HG, De Bruin ML, de Vries F. The risk of venous thromboembolism in patients with multiple sclerosis: the Clinical Practice Research Datalink. J Thromb Haemost. 2014;12(4):444-451. doi: 10.1111/jth.12523 [DOI] [PubMed] [Google Scholar]
- 19.Benchimol EI, Smeeth L, Guttmann A, et al. ; RECORD Working Committee . The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benchimol EI, Guttmann A, Griffiths AM, et al. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut. 2009;58(11):1490-1497. doi: 10.1136/gut.2009.188383 [DOI] [PubMed] [Google Scholar]
- 21.Benchimol EI, Guttmann A, Mack DR, et al. Validation of international algorithms to identify adults with inflammatory bowel disease in health administrative data from Ontario, Canada. J Clin Epidemiol. 2014;67(8):887-896. doi: 10.1016/j.jclinepi.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 22.Widdifield J, Ivers NM, Young J, et al. Development and validation of an administrative data algorithm to estimate the disease burden and epidemiology of multiple sclerosis in Ontario, Canada. Mult Scler. 2015;21(8):1045-1054. doi: 10.1177/1352458514556303 [DOI] [PubMed] [Google Scholar]
- 23.Eder L, Widdifield J, Rosen CF, et al. Identifying and characterizing psoriasis and psoriatic arthritis patients in Ontario administrative data: a population-based study from 1991 to 2015. J Rheumatol. 2020;47(11):1644-1651. doi: 10.3899/jrheum.190659 [DOI] [PubMed] [Google Scholar]
- 24.Widdifield J, Bernatsky S, Paterson JM, et al. Accuracy of Canadian health administrative databases in identifying patients with rheumatoid arthritis: a validation study using the medical records of rheumatologists. Arthritis Care Res (Hoboken). 2013;65(10):1582-1591. doi: 10.1002/acr.22031 [DOI] [PubMed] [Google Scholar]
- 25.Widdifield J, Bombardier C, Bernatsky S, et al. An administrative data validation study of the accuracy of algorithms for identifying rheumatoid arthritis: the influence of the reference standard on algorithm performance. BMC Musculoskelet Disord. 2014;15:216. doi: 10.1186/1471-2474-15-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS. The validity of ICD codes coupled with imaging procedure codes for identifying acute venous thromboembolism using administrative data. Vasc Med. 2015;20(4):364-368. doi: 10.1177/1358863X15573839 [DOI] [PubMed] [Google Scholar]
- 27.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 29.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288-1294. doi: 10.1016/j.jclinepi.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 30.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 31.Lee TM, Tu K, Wing LL, Gershon AS. Identifying individuals with physician-diagnosed chronic obstructive pulmonary disease in primary care electronic medical records: a retrospective chart abstraction study. NPJ Prim Care Respir Med. 2017;27(1):34. doi: 10.1038/s41533-017-0035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33(3):160-166. doi: 10.24095/hpcdp.33.3.06 [DOI] [PubMed] [Google Scholar]
- 33.Weisman A, Tu K, Young J, et al. Validation of a type 1 diabetes algorithm using electronic medical records and administrative healthcare data to study the population incidence and prevalence of type 1 diabetes in Ontario, Canada. BMJ Open Diabetes Res Care. 2020;8(1):e001224. doi: 10.1136/bmjdrc-2020-001224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512-516. doi: 10.2337/diacare.25.3.512 [DOI] [PubMed] [Google Scholar]
- 35.Glazier RH, Creatore MI, Agha MM, Steele LS; Inner City Toronto Time Trends Working Group . Socioeconomic misclassification in Ontario’s health care registry. Can J Public Health. 2003;94(2):140-143. doi: 10.1007/BF03404588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16(7):791-801. doi: [DOI] [PubMed] [Google Scholar]
- 37.Mahmud N, Weiss A, Trivedi C, Yang YX, Lewis J, Khan N. Risk of venous thromboembolism among patients with inflammatory bowel disease who contract severe acute respiratory syndrome coronavirus 2. Gastroenterology. 2021;161(5):1709-1711.e1. doi: 10.1053/j.gastro.2021.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang MC, Reynolds K, Tabada GH, et al. Assessment of the risk of venous thromboembolism in nonhospitalized patients with COVID-19. JAMA Netw Open. 2023;6(3):e232338. doi: 10.1001/jamanetworkopen.2023.2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arachchillage DJ, Rajakaruna I, Pericleous C, Nicolson PLR, Makris M, Laffan M; CA-COVID-19 Study Group . Autoimmune disease and COVID-19: a multicentre observational study in the United Kingdom. Rheumatology (Oxford). 2022;61(12):4643-4655. doi: 10.1093/rheumatology/keac209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Payne JG, Tagalakis V, Wu C, Lazo-Langner A; CanVECTOR Network . Current estimates of the incidence of acute venous thromboembolic disease in Canada: a meta-analysis. Thromb Res. 2021;197:8-12. doi: 10.1016/j.thromres.2020.10.030 [DOI] [PubMed] [Google Scholar]
- 41.Buonsenso D, Cusenza F, Passadore L, Bonanno F, De Guido C, Esposito S. Duration of immunity to SARS-CoV-2 in children after natural infection or vaccination in the omicron and pre-omicron era: a systematic review of clinical and immunological studies. Front Immunol. 2023;13:1024924. doi: 10.3389/fimmu.2022.1024924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Silva KM, Wallace ZS. COVID-19 and rheumatoid arthritis. Curr Opin Rheumatol. 2021;33(3):255-261. doi: 10.1097/BOR.0000000000000786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ungaro RC, Brenner EJ, Agrawal M, Zhang X, Kappelman MD, Colombel JF; Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) Research Group . Impact of medications on COVID-19 outcomes in inflammatory bowel disease: analysis of more than 6000 patients from an international registry. Gastroenterology. 2022;162(1):316-319.e5. doi: 10.1053/j.gastro.2021.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silvestri E, Scalera A, Emmi G, et al. Thrombosis in autoimmune diseases: a role for immunosuppressive treatments? Semin Thromb Hemost. 2016;42(6):650-661. doi: 10.1055/s-0036-1579642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Algorithms used to identify individuals with immune-mediated inflammatory diseases
eTable 2. International Classification of Diseases (ICD)-10 codes used to identify individuals developing a venous thromboembolism
eTable 3. International Classification of Disease (ICD)-10 codes for immune-mediated inflammatory diseases that were excluded from the adapted version of the Charlson Comorbidity Index used in this study
eTable 4. Previously validated algorithms used to identify individuals with selected comorbidities
eTable 5. Demographics and clinical characteristics of individuals with immune-mediated inflammatory diseases (IMIDs) and matched people without an IMID on the date of positive COVID-19 test, stratified by type of IMID
eFigure 1. Forest plot depicting the unadjusted hazard ratio of venous thromboembolism among individuals with and without (reference) an immune-mediated inflammatory disease (IMID) following COVID-19 infection, stratified by type of IMID
eFigure 2. Forest plot depicting the hazard ratio of venous thromboembolism among individuals with and without (reference) an immune-mediated inflammatory disease (IMID) following COVID-19 infection, adjusted for previous VTE and COVID-19 vaccination stratified by type of IMID
eReferences.
Data Sharing Statement