Abstract
Renal ischemia/reperfusion injury (RIRI) represents the principal factor underlying acute kidney injury (AKI), which primarily stems from cellular injuries and ferroptosis caused by reactive oxygen species (ROS). Salidroside (SA), an antioxidant natural ester, has been attributed with the potential to protect against RIRI. In the present study, rats received daily SA doses (1, 10, or 100 mg/kg) by gavage for 7 consecutive days before surgery. The results revealed aggravated renal injury in the RIRI group, which was effectively prevented by SA pretreatment (10 and 100 mg/kg), with the 1 mg/kg dosage demonstrating lesser efficacy. Additionally, the results indicated that SA pretreatment mitigated the RIRI-related upregulation of antioxidative superoxide dismutase. In vitro studies corroborated SA's ability to maintain hypoxia/reoxygenation-treated NRK cell viability, with the protective effect being observed at SA concentrations ≥1 µM and peaking at 100 µM. Furthermore, the results showed that SA safeguarded renal tubular epithelial cells from oxidative damage, reduced ROS accumulation, and inhibited ferroptosis via activation of the PI3K/AKT signaling pathway. Therefore, the results of the present study highlight the promising therapeutic potential of SA as an effective intervention for RIRI via targeting of PI3K/AKT signaling pathway-mediated anti-oxidative and anti-ferroptotic mechanisms.
Keywords: Salidroside, renal ischemia/reperfusion injury, oxidative stress, ferroptosis, PI3K/AKT
Introduction
Ischemia-reperfusion injury (IRI) is a frequent occurrence seen in various diseases and surgical procedures such as coronary heart disease, kidney transplants, and liver transplants (1-3). Interruption or reduction of blood flow leads to cellular ischemia and hypoxia, resulting in tissue and/or organ damage. The metabolites and mediators produced during ischemia promote further damage, as reflected by the serious dysfunction of the cell ultrastructure, metabolism, electrophysiology, and function (4). The primary causes of renal ischemia-reperfusion injury (RIRI) are cardiac arrest (systemic hypoperfusion) and renal surgical intervention (5). In the case of kidney transplants, kidney injury occurs following a short period of warm ischemia following the removal of the organ from the donor, a prolonged period of cold ischemia during cryopreservation, and a final stage of warm ischemia during recipient implantation. Post-revascularization, renal blood reperfusion triggers a series of events that can exacerbate renal injury (6), resulting in hemodynamic fluctuations, inflammation, and damage to tubular epithelial cells (7). The pathogenesis of RIRI may be attributed to structural and functional dysmetabolism of the kidneys induced by a range of factors, such as excessive production of oxygen-free radicals, intracellular calcium overload, inflammatory factors and transmitters, apoptosis, membrane lipid peroxidation, and alterations in nitric oxide content (8-10).
Salidroside (SA) is a natural active ingredient extracted from Rhodiola rosea (11), renowned for its diversified biological activities, including anti-inflammatory, anti-oxidative, anti-tumorigenic, and anti-radiation properties (11). As a result of its broad pharmacological effects, SA has attracted the attention both domestically and internationally. Numerous studies have documented the protective effects of SA against a range of organ injuries, primarily by reducing oxidative stress, inhibiting apoptosis, reducing intracellular calcium overload, and enhancing mitochondrial function (11-13). Prior studies have shown SA to have a protective effect on myocardial cell injury caused by hypoxia, as well as having anti-arrhythmic properties. Furthermore, it inhibits the proliferation and contraction of vascular smooth muscle cells. Its mechanisms possibly involve reducing creatine phosphate (CK) activity and malonaldehyde (MDA) content, increasing superoxide dismutase (SOD) activity, increasing sarcoplasmic reticulum Ca2+-ATPase (SERCA) activity, reducing Ca2+ overload, and suppressing apoptosis-related gene expression (14-16). Although numerous studies have confirmed that SA has a protective effect on IRI of myocardium, testis, and the brain, its effect on RIRI and the underlying mechanisms remain unknown.
Materials and methods
Animals
Male Sprague-Dawley rats (weighing 200-220 g) were purchased from the Animal Center of Wuhan University. The rats were housed in a temperature and humidity-controlled environment with a 12-h light/dark cycle and provided with standard food and water. This study was performed in accordance with the Helsinki Declaration II (17) and was approved by the Institutional Review Boards of Renmin Hospital of Wuhan University (approval no. K2021-08-012).
Establishment of the RIRI model
The rats were subjected to an 8 h fast prior to the surgical procedure. Thereafter, sodium pentobarbital (50 mg/kg, intraperitoneal injection) was injected into the abdominal cavity to anesthetize the rats, and the limbs were immobilized during the operation. An abdominal incision was used to reveal the renal pedicle, and the bilateral renal arteries were ligated for 45 min. During reperfusion, the hemostatic clamp was removed, and changes in kidney color were assessed. Blood flow to the kidney generally resumed to normal within a few min, which was then followed by suturing of the incision. The sham group underwent the same surgical procedure without clamping of the renal pedicle. After 24 h, rats were humanely sacrificed under anesthesia by intraperitoneal injection with 1% pentobarbital sodium (150 mg/kg). The kidneys were harvested, and serum samples were collected.
In vivo treatment protocols
All rats were randomly divided into three groups: Sham group (n=6), IR group (n=6), and IR+SA group (n=18). To explore the effects of SA on RIRI, SA group rats were given 1, 10, or 100 mg/kg SA (cat. no. 10338-51-9; MedChemExpress) daily by gavage for 7 days before the IR procedure (n=6 per group).
Cell culture
The rat renal tubular epithelial cell line, NRK cells, were cultured in DMEM (cat. no. 12430112; Sigma-Aldrich; Merck KGaA; Thermo Fisher Scientific, Inc.) with 1% Penicillin-Streptomycin solution (cat. no. 15140122; Sigma-Aldrich; Merck KGaA; Thermo Fisher Scientific, Inc.) and 10% FBS (cat. no. 16140089; Sigma-Aldrich; Merck KGaA; Thermo Fisher Scientific, Inc.) and maintained in a humidified incubator at 37˚C incubator supplied with 5% CO2.
Establishment of the hypoxia-reoxygenation (HR) model
NRK cells were starved in serum-free DMEM for 12 h, and then incubated in a thermostatic tri-gas incubator supplied with 95% N2 for 8 h to simulate a hypoxic environment. Subsequently, the cells were moved to a normoxic incubator for a period of 12 h, followed by the replacement of the media with supplemented with DMEM for 12 h of reoxygenation.
In vitro treatment protocols
To explore the effects of SA and the PI3K/AKT signaling pathway, NRK cells were incubated with SA (1-1,000 µM) or PI3K/AKT-IN-1 (2.62 µM; cat. no. HY-144806; MedChemExpress) at the onset of reoxygenation. Cells were randomly divided into one of four groups: Control group, HR group, HR+SA group, and HR+SA+PI3K/AKT-IN-1 group.
Cell viability assay
Cell viability was determined using a CCK-8 assay (cat. no. C0038; Sigma-Aldrich; Merck KGaA Institute of Biotechnology). Briefly, NRK cells were seeded into 96-well plates at a density of 1x104 cells/well and incubated for 12 h in a thermostatic incubator. Upon treating cells with SA and/or PI3K/AKT-IN-1 for 4 h, 10 µl CCK-8 solution was added to each well, and cells were cultured for an additional 1 h. Subsequently, the absorbance at 450 nm was measured using a microplate reader (PerkinElmer; Thermo Fisher Scientific, Inc.).
Renal function analysis and histopathological analysis
Renal function was evaluated by measuring serum creatinine (Scr) and blood urea nitrogen (BUN) levels in plasma at the Department of Clinical Laboratories of Renmin Hospital, Wuhan University. Kidney samples were procured, fixed with paraformaldehyde, and then embedded in paraffin. The samples were sectioned into 4-µm thick slices, and then stained with hematoxylin for 5 min and eosin for 3 min at room temperature. The severity of RIRI was assessed using the Paller scoring system (18), with histopathological changes graded as follows: i) 1 point, tubular epithelial smoothness or tubular expansion; ii) 1 or 2 points, loss of brush-like edge; iii) 1 or 2 points, obstruction of the tubular lumen; iv) 1 point, cytoplasmic vacuolization; and v) 1 point, cell necrosis. The histological data were analyzed and graded independently by two observers who were blinded to the experimental groups.
Western blotting
Proteins from kidney tissues or NRK cells were extracted and lysed in RIPA buffer supplemented with protease and phosphatase inhibitors. Protein content was measured using a BCA kit (cat no. A045-4-2, Nanjing Jiancheng Bioengineering Institute). Subsequently, 50 µg protein samples were loaded on 10% SDS gels, resolved using SDS-PAGE, and subsequently transferred to a PVDF membrane. The membranes were blocked with 5% BSA for 1 h at room temperature and incubated with one of the following primary antibodies: Rabbit anti-GPX4 (1:1,000; cat. no. 67763-1-Ig, Sigma-Aldrich; Merck KGaA Group, Inc.), rabbit anti-ACSL4 (1:1,000. cat. no.22401-1-AP; Sigma-Aldrich; Merck KGaA Group, Inc.), rabbit anti-PI3K antibody (1:1,000; cat. no. 4249; Cell Signaling Technology, Inc.), rabbit anti-p-PI3K antibody (1:1,000; cat. no. 17366; Cell Signaling Technology, Inc.), rabbit anti-AKT (1:1,000; cat. no. 9272; Cell Signaling Technology, Inc.), rabbit anti-p-AKT antibody (1:1,000; cat. no. 4060; Cell Signaling Technology, Inc.), rabbit anti-SOD1 antibody (1:1,000; cat. no. 37385; Cell Signaling Technology, Inc.), rabbit anti-SOD2 antibody (1:1,000; cat. no. 13141; Cell Signaling Technology, Inc.) and rabbit anti-GAPDH antibody (1:10,000; cat. no. 5174; Cell Signaling Technology, Inc.) overnight at 4˚C. The following day, the membranes were washed in TBST and incubated for 2 h at room temperature with the corresponding secondary antibodies. Signals were visualized using an ECL system and ECL kit (cat. no. W028-2-1; Nanjing Jiancheng Bioengineering Institute), and densitometry analysis was calculated using ImageJ version 1.8.0 (National Institutes of Health).
Detection of the oxidative stress indicators
The levels of malondialdehyde (MDA) and superoxide dismutase (SOD) in kidney tissues or NRK cells were detected using an MDA assay kit (cat. no. S0131S; Sigma-Aldrich; Merck KGaA Institute of Biotechnology) and SOD assay kit (cat. no. S0101S; Sigma-Aldrich; Merck KGaA Institute of Biotechnology) according to the manufacturer's protocol.
Measurement of ROS levels in the kidney tissues
The levels of ROS were analyzed in the tissues utilizing the dihydroethidium (DHE) fluorescent probe (cat. no. D7008; MilliporeSigma), according to the manufacturer's protocol. The sections were stained by incubation with 50 µM DHE for 1 h in the dark at room temperature, and then stained with 1 mg/ml DAPI for 10 min at 4˚C. The sections were rinsed three times with PBS. A fluorescent microscope (magnification, x200) was used to evaluate the fluorescence intensity at an excitation wavelength of 525 nm and an emission wavelength of 610 nm.
Evaluation of intracellular lipid hydroperoxide (LPO)
C11-BODIPY581/591 (cat. no. HY-D1301; MedChemExpress, Inc.) was used as a fluorescent probe to assess intracellular LPO levels. The probe integrates into the lipid membrane and is oxidized by intracellular LPO, fluorescing green once oxidized. A total of 5 µM C11-BODIPY581/591 was used, and kidney slices were incubated for 30 min at 4˚C. After washing with PBS, the slices were examined by flow cytometer BD FACSaria II (version 7.6.1, BD Biosciences).
Evaluation of ROS levels in NRK cells
The 2',7'-dichlorofluorescein diacetate (DCFH-DA) fluorescent probe (cat. no. D6883; MilliporeSigma-Aldrich; Merck KGaA) was used to detect ROS levels in NRK cells according to the manufacturer's protocols. After incubating with 10 µM DCFH-DA for 1 h at 4˚C in the dark, NRK cells were treated with 1 µg/ml DAPI for 10 min at 4˚C. The cells were observed using a fluorescent microscope at a wavelength of 485 nm.
Statistical analysis
GraphPad Prism version 8.0.1 (GraphPad Software, Inc.) was used to analyze the data. Data are presented as the mean ± SD of three repeats. Differences between groups were compared using one-way ANOVA followed by a post hoc Tukey's. P<0.05 was considered to indicate a statistically significant difference.
Results
SA protects against RIRI in a dose-dependent manner
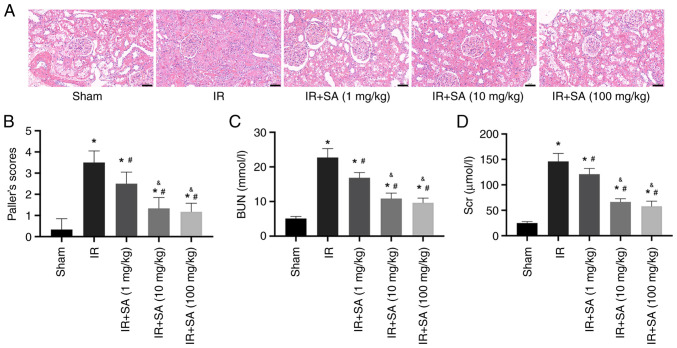
To assess the role of SA in RIRI, rats were subjected to different doses of SA (1, 10, or 100 mg/kg/day), administered via gavage for 7 days before undergoing the establishment of RIRI. Renal function was assessed following this treatment. The results of hematoxylin and eosin staining and renal injury score analysis demonstrated reduced IR-induced renal tubular injury in the rats pretreated with SA. In the RIRI rats, pretreatment with 1, 10, and 100 mg/kg of SA significantly decreased renal tubular injury, with the optimal effect apparent at 10 and 100 mg/kg, whereas the renal protective effect of 1 mg/kg SA pretreatment was less prominent (Fig. 1A and B). Accordingly, 10 mg/kg SA was used for all subsequent experiments. Moreover, the concentrations of Scr and BUN in the IR group were notably higher than those in the sham group. Compared to the IR group, pretreatment with SA significantly reduced Scr and BUN levels in the serum (Fig. 1C and D).
Figure 1.
SA attenuates IR-induced kidney injury. Rats underwent a sham operation or renal IR with or without pretreatment with SA (1, 10, or 100 mg/kg/day). (A) Representative images of HE staining (magnification, x400) of the kidney sections. (B) Paller scores of kidney injury. (C and D) Serum concentrations of BUN and Scr in the rats. *P<0.05 vs. sham group; #P<0.05 vs. IR, &P<0.05 vs. IR+SA (1 mg/kg) group. HE, hematoxylin and eosin; BUN, blood urea nitrogen; Scr, serum creatinine; SA, Salidroside; IR, ischemia/reperfusion.
SA inhibits IR-induced oxidative stress and ferroptosis in rats
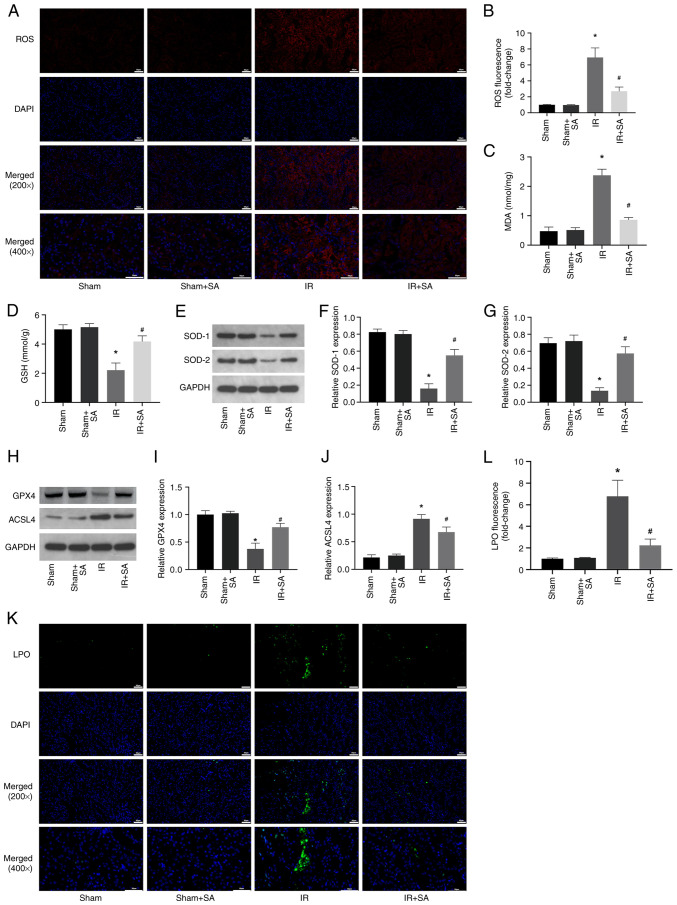
Next, the effect of SA pretreatment on the levels of ROS in the IR-induced kidneys was examined. The results showed that SA pretreatment inhibited IR-induced increases in ROS and MDA while increasing the levels of GSH, SOD1, and SOD2 (Fig. 2A-G). Numerous studies have shown that high levels of oxidative stress can induce ferroptosis (19-21). Therefore, subsequent investigations focused on the effect of SA on the ferroptosis of renal cells during IR. The results showed that SA pretreatment inhibited the decrease in the expression of GPX4 and the increase in the expression of ACSL4 induced by RIRI (Fig. 2H-J). Furthermore, the levels of LPO were detected using the lipid peroxidation probe C11-BODIPY581/591. The significant increase in LPO induced by IR was prevented by treatment with SA (Fig. 2K and L). Together, the results suggested that SA pretreatment conferred its renal protective effects through anti-oxidative and anti-ferroptotic means.
Figure 2.
SA inhibits IR-induced increases in oxidative stress and ferroptosis in rats. (A and B) Representative images of kidney sections stained with dihydroethidium and semi-quantitative analysis of fluorescence intensity indicated that SA pretreatment reduced the ROS levels in rats subjected to RIRI (magnification, x200 and x400). (C and D) The levels of MDA and GSH in kidney tissues were detected. (E-G) SOD1 and SOD2 levels were detected using western blotting. (H-J) The expression of GPX4 and ACSL4 was detected using western blotting. (K and L) The levels of LPO were detected using the lipid peroxidation probe C11-BODIPY581/591 (magnification, x200 and x400). *P<0.05 vs. sham group, #P<0.05 vs. IR. SA, Salidroside; IR, ischemia/reperfusion; ROS, reactive oxygen species; RIRI, renal ischemia/reperfusion injury.
SA reduces oxidative stress induced by HR injury in NRK cells
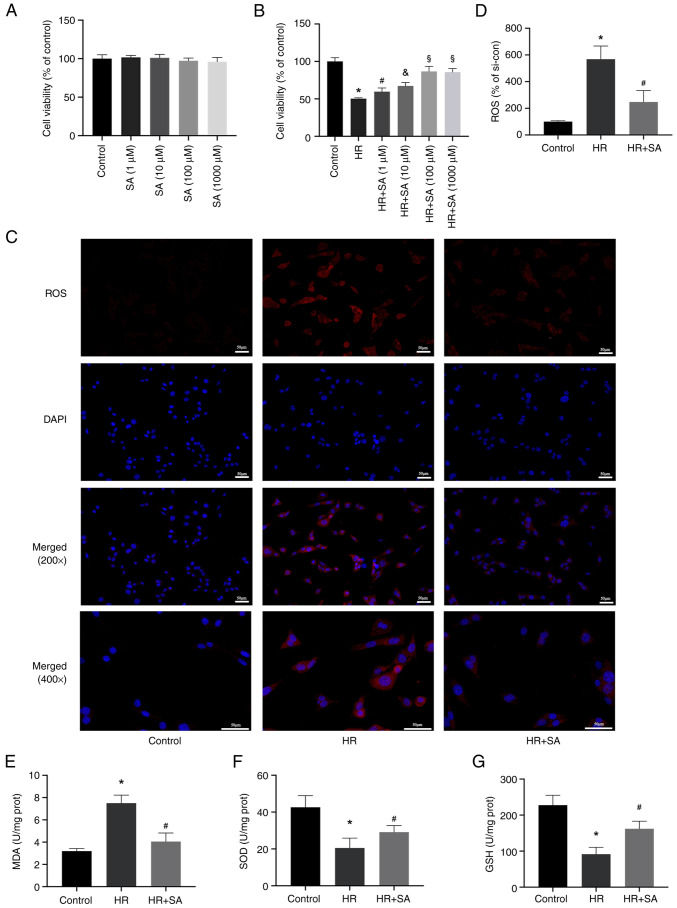
Subsequently, in vitro experiments were performed to evaluate the effect of SA on the human renal tubular epithelial cell line NRK. First, the toxicity of SA in NRK cells was evaluated and it was found that SA did not noticeably impact cell viability after 24 h (Fig. 3A). Additionally, we discovered that pretreatment with SA reduced HR-induced cell death. Treatment with 1-1,000 µM SA exerted a renal protective effect, with the optimal effect evident at 100 µM (Fig. 3B). Therefore, a concentration of 100 µM of SA was used for further experiments. DHE staining and MDA detection kits were used to assess the effects of SA on ROS levels induced by HR. The results showed that reduced ROS levels in NRK cells were associated with increased SOD and GSH activity and decreased activity of MDA (Fig. 3C-G). In agreement with the in vivo experiments, the outcomes of the in vitro experiments showed that SA protected renal tubular epithelial cells from oxidative damage through suppression of ROS buildup.
Figure 3.
SA reduces oxidative stress induced by HR injury in NRK cells. (A) SA had no notable toxic effects on NRK cell viability. (B) SA (1-1,000 µM) had notable renal-protective effects on cell viability. (C and D) Representative images and statistical analysis of dihydroethidium staining indicated that SA pretreatment reduced ROS content in HR-induced NRK cells (magnification, x200 and x400). (E-G) MDA levels were reduced while SOD and GSH activity increased in cells pretreated with SA in HR-induced NRK cells. *P<0.05 vs. control group; #P<0.05 vs. HR group; &P<0.05 vs. HR+SA (1µM) group; §P<0.05 vs HR+SA (10µM) group. SA, Salidroside; HR, hypoxia-reoxygenation.
PI3K/AKT signaling pathway is involved in the protective effects of SA on oxidative stress against HR injury
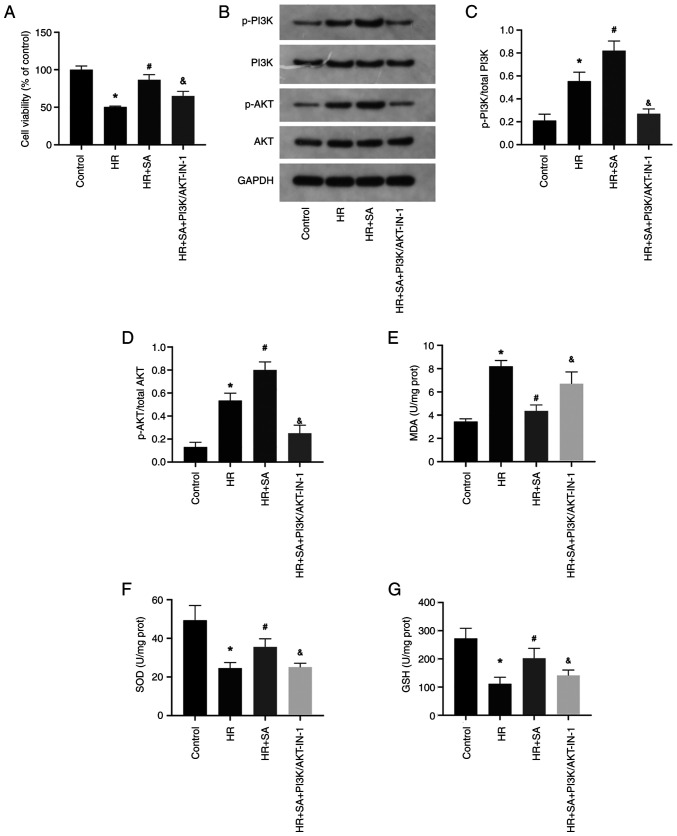
The PI3K/AKT signaling pathway is a critical player in the modulation of oxidative stress. Therefore, to investigate whether SA exerts a protective effect on HR injury by targeting the PI3K/AKT signaling pathway, a PI3K/AKT pathway inhibitor, PI3K/AKT-IN-1 (2.62 µM), was employed. The cell viability assay demonstrated that PI3K/AKT-IN-1 partially abrogated the protective effect of SA against HR-induced NRK cell injury (Fig. 4A). Moreover, compared to the sham group, there was an increase in the phosphorylation levels of PI3K and AKT during HR injury, and SA pretreatment further augmented the phosphorylation of PI3K and AKT. However, in the presence of PI3K/AKT-IN-1, the increased phosphorylation of PI3K and AKT was suppressed (Fig. 4B-D). Additionally, PI3K/AKT-IN-1 increased MDA levels while decreasing the levels of SOD and GSH (Fig. 4E-G). Accordingly, SA exerts its antioxidant effect on NRK cells by activating the PI3K/AKT signaling pathway.
Figure 4.
The PI3K/AKT signaling pathway is involved in the protective effects of SA on oxidative stress against HR injury. (A) PI3K/AKT-IN-1 partially reversed the protective effects of SA on HR-induced NRK cell injury. (B-D) HR-induced PI3K and AKT phosphorylation increased with SA pretreatment but was blocked by PI3K/AKT-IN-1. (E-G) Levels of MDA, SOD, and GSH were detected. Magnification, x200 and x400. *P<0.05 vs. control; #P<0.05 vs. HR group; &P<0.05 vs. SA+HR. SA, Salidroside; HR, hypoxia-reoxygenation.
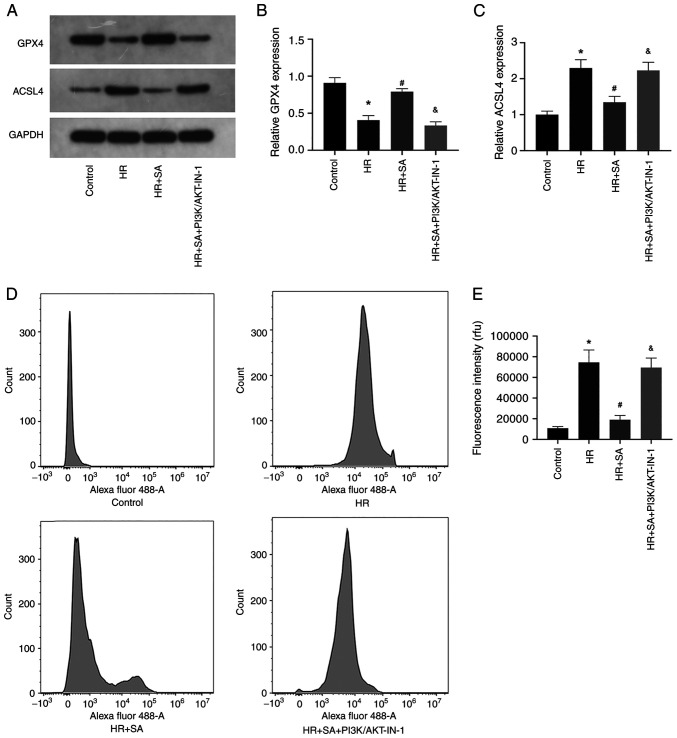
HR-induced ferroptosis can be aggravated by blocking the PI3K/AKT signaling pathway
Finally, the effects of PI3K/AKT signaling pathway inhibition on HR-induced ferroptosis of NRK cells were explored. The results of western blotting showed that PI3K/AKT-IN-1 prevented the increased expression of GPX4 and the decreased expression of ACSL4 induced by SA (Fig. 5A-C). Additionally, the levels of LPO were assessed using the lipid peroxidation probe C11-BODIPY581/591. The data confirmed that PI3K/AKT-IN-1 reversed the inhibitory effect of SA on LPO (Fig. 2D and E). Collectively, the outcomes indicated that the protective effects of SA pretreatment on renal tubular epithelial cells were brought about through an anti-ferroptotic effect; however, this effect could be counteracted by inhibition of the PI3K/AKT signaling pathway.
Figure 5.
HR-induced ferroptosis is aggravated by blocking the PI3K/AKT signaling pathway. (A-C) The expression of GPX4 and ACSL4 was detected using western blotting. (D and E) The levels of LPO were detected using flow cytometry. *P<0.05 vs. control group; #P<0.05 vs. HR group; &P<0.05 vs. SA+HR. SA, Salidroside; HR, hypoxia-reoxygenation.
Discussion
The results of the present study showed that SA treatment is an effective method of reducing oxidative stress and inhibiting ferroptosis during RIRI, leading to the alleviation of this condition. Therefore, the use of natural products can be considered a reference point for managing and preventing RIRI. Natural products have demonstrated good efficacy in preventing and treating AKI with minimal side effects. Among these, Rhodiola, a perennial herb belonging to the sedum genus in the Sedum family (22), is noteworthy due to the presence of SA, tyrosol, flavonoid, amino acid, trace volatile oil, and other constituents, which underlie its beneficial effects (23,24). Recent studies have demonstrated the potential of Rhodiola rosea to affect various biological pathways, including anti-hypoxic, anti-fatigue, anti-viral, immunity-enhancing, anti-aging, and anti-radiation pathways, and bidirectional regulation of bodily functions (25,26). Rhodiola can significantly increase the endurance of hypoxia-exposed human bodies, promote aerobic metabolism, reduce the concentration of lactic acid in the blood, muscles, and brain, increase the aerobic metabolism of the heart, brain, lung, and other critical organs, assist the body in adapting to an hypoxic environment, and deferring the onset of further fatigue (27-29). SA, the primary constituent of Rhodiola, has been found by previous studies to safeguard organs from multiple IRI-related pathway dysfunctions (30-32).
The process of IRI involves various factors and mechanisms, including reduced nitric oxide production, inflammation-induced cell damage, oxygen-derived oxidative stress formation, and lipid peroxidation (33). Prior work has identified oxidative stress as a key potential mechanism of tissue damage induced by IRI (34). Oxidative-free radicals and ROS scavengers are vital for maintaining the oxidant-antioxidant balance in vivo during IRI (35). Excessive generation of oxygen-free radicals by ischemia-reperfusion can surpass local tissue scavenging capacity and lead to damage (36). MDA serves as a stable metabolic intermediate indicative of lipid peroxidation levels and oxygen-free radical content in tissues. Prior research has shown that IRI elevates ROS and MDA levels (35). The present study also confirmed that RIRI led to a significant increase in oxidative stress and that pretreatment with SA mitigated this, demonstrating the protective effect of SA by inhibiting oxidative stress and ferroptosis.
SA is a widely studied natural antioxidant with potent antioxidant effects. AKT, which is mediated by phosphoinositol-dependent kinase-1 (PDK-1) (37), acts as a downstream factor of PI3K. Studies have suggested that natural antioxidants can activate PI3K by binding to GPCRs and EGFR (38,39). Therefore, it was hypothesized that SA, like other natural antioxidants, may activate the PI3K/AKT signaling pathway; prior work confirmed that SA indeed activates this pathway (11,40). In the present study, SOD1/2 expression and activity in IRI kidneys were initially evaluated, followed by verification of these findings using NRK cells. SA pretreatment increased SOD activity by further activating the PI3K/AKT signaling pathway during HR treatment. These results confirmed that the PI3K/AKT signaling pathway underlies the anti-oxidative stress effect of SA.
Ferroptosis is a form of cell death resulting from lethal lipid peroxidation and iron-dependent damage of membrane lipids. Ferroptosis is involved in various organ injuries induced by IRI, including the heart, liver, kidney, and brain (41-44). Nonetheless, it remains unclear whether SA can exert a protective effect by inhibiting ferroptosis during RIR. Inactivation of the lipid repair enzyme GPX4 leads to the accumulation of lipid peroxides, including lipid hydrogen peroxide, as GPX4 is the sole enzyme capable of reducing lipid peroxidation in biofilms, and GSH is a necessary co-factor for GPX4 enzyme activity. GPX4 inactivation results in the accumulation of high levels of ROS, ultimately leading to cell damage. The results of the present study revealed that SA enhanced the antioxidant capacity of the kidney by activating the PI3K/AKT signaling pathway, thereby inhibiting ferroptosis induced by high levels of oxidative stress. In short, SA exhibits a potent anti-ferroptotic effect on NRK cells, and this effect can be reversed by inhibiting PI3K/AKT. Consequently, ferroptosis induced by ROS is also suppressed. Additionally, treatment with the PI3K/AKT pathway inhibitor, PI3K/AKT-IN-1, inhibited the activation of the PI3K/AKT pathway and its downstream regulation of oxidative stress and ferroptosis. These findings support the notion that SA pretreatment can alleviate RIRI by activating the PI3K/AKT signaling pathway.
However, the present study has some limitations. First, SA may be used as a clinical agent to treat RIRI, thus the efficacy of SA in patients who already have RIRI should be explored. Secondly, it was confirmed that 10-100 mg/kg SA had a strong renal protective effect; however, the optimal dose of SA was determined. These factors should be considered in further studies.
In conclusion, the present study showed that SA increased the activity of SODs by activating the PI3K/AKT signaling pathway, thereby eliminating ROS, and inhibiting oxidative stress injury and ferroptosis, thus protecting renal function in RIRI.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
ZT and CL conceived and designed the study, analyzed the data, and wrote the manuscript. YW collected and analyzed the data. YL analyzed the data and wrote the manuscript. CL and ZT confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
This study was performed in accordance with the Helsinki Declaration II and was approved by the Institutional Review Boards of Renmin Hospital of Wuhan University (approval no. K2021-08-012).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.References Tian H, Xiong Y, Zhang Y, Leng Y, Tao J, Li L, Qiu Z, Xia Z. Activation of NRF2/FPN1 pathway attenuates myocardial ischemia-reperfusion injury in diabetic rats by regulating iron homeostasis and ferroptosis. Cell Stress Chaperones. 2021;27:149–164. doi: 10.1007/s12192-022-01257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei X, Deng W, Dong Z, Xie Z, Zhang J, Wang R, Zhang R, Na N, Zhou Y. Identification of subtypes and a delayed graft function predictive signature based on ferroptosis in renal ischemia-reperfusion injury. Front Cell Dev Biol. 2022;10(800650) doi: 10.3389/fcell.2022.800650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardallo RG, Panisello-Roselló A, Sanchez-Nuno S, Alva N, Roselló-Catafau J, Carbonell T. Nrf2 and oxidative stress in liver ischemia/reperfusion injury. FEBS J. 2022;289:5463–5479. doi: 10.1111/febs.16336. [DOI] [PubMed] [Google Scholar]
- 4.Patel PM, Connolly MR, Coe TM, Calhoun A, Pollok F, Markmann JF, Burdorf L, Azimzadeh A, Madsen JC, Pierson RN*III. Minimizing ischemia reperfusion injury in xenotransplantation. Front Immunol. 2021;12(681504) doi: 10.3389/fimmu.2021.681504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatauret N, Badet L, Barrou B, Hauet T. Ischemia-reperfusion: From cell biology to acute kidney injury. Prog Urol. 2014;24 (Suppl 1):S4–S12. doi: 10.1016/S1166-7087(14)70057-0. [DOI] [PubMed] [Google Scholar]
- 6.Zhao H, Alam A, Soo AP, George A, Ma D. Ischemia-reperfusion injury reduces long term renal graft survival: Mechanism and beyond. EBioMedicine. 2018;28:31–42. doi: 10.1016/j.ebiom.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen Y, Qiu T, Liu XH, Zhang L, Wang ZS, Zhou JQ. Renal ischemia-reperfusion injury attenuated by splenic ischemic preconditioning. Eur Rev Med Pharmacol Sci. 2018;22:2134–2142. doi: 10.26355/eurrev_201804_14747. [DOI] [PubMed] [Google Scholar]
- 9.Kinra M, Mudgal J, Arora D, Nampoothiri M. An insight into the role of cyclooxygenase and lipooxygenase pathway in renal ischemia. Eur Rev Med Pharmacol Sci. 2017;21:5017–5020. [PubMed] [Google Scholar]
- 10.Rodriguez F, Bonacasa B, Fenoy FJ, Salom MG. Reactive oxygen and nitrogen species in the renal ischemia/reperfusion injury. Curr Pharm Design. 2013;19:2776–2794. doi: 10.2174/1381612811319150014. [DOI] [PubMed] [Google Scholar]
- 11.Rong L, Li Z, Leng X, Li H, Ma Y, Chen Y, Song F. Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway. Biomed Pharmacother. 2020;122(109726) doi: 10.1016/j.biopha.2019.109726. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Y, Wang Z, Nan B, Yang C, Wang M, Ye H, Xi C, Zhang Y, Yan H. Salidroside alleviates liver inflammation in furan-induced mice by regulating oxidative stress and endoplasmic reticulum stress. Toxicology. 2021;461(152905) doi: 10.1016/j.tox.2021.152905. [DOI] [PubMed] [Google Scholar]
- 13.Zhang P, Xu J, Cui Q, Lin G, Wang F, Ding X, You S, Sang N, Tan J, Xu W, et al. Multi-pathway neuroprotective effects of a novel salidroside derivative SHPL-49 against acute cerebral ischemic injury. Eur J Pharmacol. 2023;949(175716) doi: 10.1016/j.ejphar.2023.175716. [DOI] [PubMed] [Google Scholar]
- 14.Jiang S, Fan F, Yang L, Chen K, Sun Z, Zhang Y, Cairang N, Wang X, Meng X. Salidroside attenuates high altitude hypobaric hypoxia-induced brain injury in mice via inhibiting NF-κB/NLRP3 pathway. Eur J Pharmacol. 2022;925(175015) doi: 10.1016/j.ejphar.2022.175015. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Tang Y, Xie N, Bai J, Jiang S, Zhang Y, Hou Y, Meng X. Salidroside, a phenyl ethanol glycoside from Rhodiola crenulata, orchestrates hypoxic mitochondrial dynamics homeostasis by stimulating Sirt1/p53/Drp1 signaling. J Ethnopharmacol. 2022;293(115278) doi: 10.1016/j.jep.2022.115278. [DOI] [PubMed] [Google Scholar]
- 16.Qi C, Zhang J, Chen X, Zhang J, Yang P, Jiao Q, Zhang P, Lu HX, Liu Y. Salidroside protects cultured rat subventricular zone neural stem cells against hypoxia injury by inhibiting Bax, Bcl-2 and caspase-3 expressions. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:962–966. (In Chinese) [PubMed] [Google Scholar]
- 17.Issue Information-Declaration of Helsinki. J Bone Miner Res. 2019;34:BMi–BMii. doi: 10.1002/jbmr.3492. [DOI] [PubMed] [Google Scholar]
- 18.Paller MS. Free radical-mediated postischemic injury in renal transplantation. Ren Fail. 1992;14:257–260. doi: 10.3109/08860229209106627. [DOI] [PubMed] [Google Scholar]
- 19.Mancardi D, Mezzanotte M, Arrigo E, Barinotti A, Roetto A. Iron overload, oxidative stress, and ferroptosis in the failing heart and liver. Antioxidants (Basel) 2021;10(1864) doi: 10.3390/antiox10121864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren JX, Li C, Yan XL, Qu Y, Yang Y, Guo ZN. Crosstalk between oxidative stress and ferroptosis/oxytosis in ischemic stroke: Possible targets and molecular mechanisms. Oxid Med Cell Longev. 2021;2021(6643382) doi: 10.1155/2021/6643382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G, Liu Y, Zhao X, Qian L, Liu P, Xiong Y. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021;7(193) doi: 10.1038/s41420-021-00579-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinckmann JA, Cunningham AB, Harter D. Running out of time to smell the roseroots: Reviewing threats and trade in wild Rhodiola rosea L. J Ethnopharmacol. 2021;269(113710) doi: 10.1016/j.jep.2020.113710. [DOI] [PubMed] [Google Scholar]
- 23.Langeder J, Grienke U, Döring K, Jafari M, Ehrhardt C, Schmidtke M, Rollinger JM. High-performance countercurrent chromatography to access Rhodiola rosea influenza virus inhibiting constituents. Planta Med. 2021;87:818–826. doi: 10.1055/a-1228-8473. [DOI] [PubMed] [Google Scholar]
- 24.Rattan S, Kumar A, Kumar D, Warghat AR. Enhanced production of phenylethanoids mediated through synergistic approach of precursor feeding and light regime in cell suspension culture of Rhodiola imbricata (Edgew.) Appl Biochem Biotech. 2022;194:3242–3260. doi: 10.1007/s12010-022-03914-8. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Pham V, Bui M, Song L, Wu C, Walia A, Uchio E, Smith-Liu F, Zi X. Rhodiola rosea L.: An herb with anti-stress, anti-aging, and immunostimulating properties for cancer chemoprevention. Curr Pharmacol Rep. 2017;3:384–395. doi: 10.1007/s40495-017-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labachyan KE, Kiani D, Sevrioukov EA, Schriner SE, Jafari M. The impact of Rhodiola rosea on the gut microbial community of Drosophila melanogaster. Gut Pathog. 2018;10(12) doi: 10.1186/s13099-018-0239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y, Wu Y, Xia Z, Li J, Li X, Xu P, Zhou X, Xue M. Anti-hypoxic molecular mechanisms of Rhodiola crenulata extract in zebrafish as revealed by metabonomics. Front Pharmacol. 2019;10(1356) doi: 10.3389/fphar.2019.01356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Hou Y, Li Q, Li X, Wang W, Ai X, Kuang T, Chen X, Zhang Y, Zhang J, et al. Rhodiola crenulata attenuates apoptosis and mitochondrial energy metabolism disorder in rats with hypobaric hypoxia-induced brain injury by regulating the HIF-1α/microRNA 210/ISCU1/2(COX10) signaling pathway. J Ethnopharmacol. 2019;241(111801) doi: 10.1016/j.jep.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 29.Xie N, Fan F, Jiang S, Hou Y, Zhang Y, Cairang N, Wang X, Meng X. Rhodiola crenulate alleviates hypobaric hypoxia-induced brain injury via adjusting NF-κB/NLRP3-mediated inflammation. Phytomedicine. 2022;103(154240) doi: 10.1016/j.phymed.2022.154240. [DOI] [PubMed] [Google Scholar]
- 30.Dong C, Wen S, Zhao S, Sun S, Zhao S, Dong W, Han P, Chen Q, Gong T, Chen W, et al. Salidroside inhibits reactive astrogliosis and glial scar formation in late cerebral ischemia via the Akt/GSK-3β pathway. Neurochem Res. 2021;46:755–769. doi: 10.1007/s11064-020-03207-8. [DOI] [PubMed] [Google Scholar]
- 31.Tian X, Huang Y, Zhang X, Fang R, Feng Y, Zhang W, Li L, Li T. Salidroside attenuates myocardial ischemia/reperfusion injury via AMPK-induced suppression of endoplasmic reticulum stress and mitochondrial fission. Toxicol Appl Pharm. 2022;448(116093) doi: 10.1016/j.taap.2022.116093. [DOI] [PubMed] [Google Scholar]
- 32.Yin L, Ouyang D, Lin L, Xin X, Ji Y. Salidroside regulates imbalance of Th17/Treg and promotes ischemic tolerance by targeting STAT-3 in cerebral ischemia-reperfusion injury. Arch Med Sci. 2021;17:523–534. doi: 10.5114/aoms.2019.85349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han SJ, Lee HT. Mechanisms and therapeutic targets of ischemic acute kidney injury. Kidney Res Clin Prac. 2019;38:427–440. doi: 10.23876/j.krcp.19.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baltaci AK, Gokbudak H, Baltaci SB, Mogulkoc R, Avunduk MC. The effects of resveratrol administration on lipid oxidation in experimental renal ischemia-reperfusion injury in rats. Biotech Histochem. 2019;94:592–599. doi: 10.1080/10520295.2019.1612091. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Zhong D, Lei L, Jia Y, Zhou H, Yang B. Propofol prevents renal ischemia-reperfusion injury via inhibiting the oxidative stress pathways. Cell Physiol Biochem. 2015;37:14–26. doi: 10.1159/000430329. [DOI] [PubMed] [Google Scholar]
- 36.Qiao X, Li RS, Li H, Zhu GZ, Huang XG, Shao S, Bai B. Intermedin protects against renal ischemia-reperfusion injury by inhibition of oxidative stress. Am J Physiol Renal Physiol. 2013;304:F112–F119. doi: 10.1152/ajprenal.00054.2012. [DOI] [PubMed] [Google Scholar]
- 37.Manning BD, Toker A. AKT/PKB signaling: Navigating the network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh CK, Chhabra G, Ndiaye MA, Siddiqui IA, Panackal JE, Mintie CA, Ahmad N. Quercetin-resveratrol combination for prostate cancer management in TRAMP mice. Cancers (Basel) 2020;12(2141) doi: 10.3390/cancers12082141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahsan A, Liu M, Zheng Y, Yan W, Pan L, Li Y, Ma S, Zhang X, Cao M, Wu Z, et al. Natural compounds modulate the autophagy with potential implication of stroke. Acta Pharm Sin B. 2021;11:1708–1720. doi: 10.1016/j.apsb.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu L, Liu Z, Ren Y, Wu X, Liu Y, Wang T, Li Y, Cong Y, Guo Y. Neuroprotective effects of salidroside on ageing hippocampal neurons and naturally ageing mice via the PI3K/Akt/TERT pathway. Phytother Res. 2021;35:5767–5780. doi: 10.1002/ptr.7235. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Jiao H, Yue Y, He K, Jin Y, Zhang J, Zhang J, Wei Y, Luo H, Hao Z, et al. Ubiquitin ligase E3 HUWE1/MULE targets transferrin receptor for degradation and suppresses ferroptosis in acute liver injury. Cell Death Differ. 2022;29:1705–1718. doi: 10.1038/s41418-022-00957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su L, Jiang X, Yang C, Zhang J, Chen B, Li Y, Yao S, Xie Q, Gomez H, Murugan R, Peng Z. Pannexin 1 mediates ferroptosis that contributes to renal ischemia/reperfusion injury. J Biol Chem. 2019;294:19395–19404. doi: 10.1074/jbc.RA119.010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C, Sun G, Chen B, Xu L, Ye Y, He J, Bao Z, Zhao P, Miao Z, Zhao L, et al. Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacol Res. 2021;174(105933) doi: 10.1016/j.phrs.2021.105933. [DOI] [PubMed] [Google Scholar]
- 44.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA. 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.