Key Points
Question
Is the novel Kv7.2/Kv7.3 potassium channel opener XEN1101 effective in reducing the monthly seizure frequency in adults experiencing focal-onset seizures (FOSs) despite treatment with 1 to 3 baseline antiseizure medications (ASMs)?
Findings
In this phase 2b randomized clinical trial including 325 patients with FOSs, treatment with XEN1101 was associated with a statistically significant and robustly dose-dependent decrease in monthly seizure frequency and was generally well tolerated, with adverse effects similar to those of currently available ASMs.
Meaning
The findings of this trial support the continued development of XEN1101 for the treatment of FOSs.
Abstract
Importance
Many patients with focal epilepsy experience seizures despite treatment with currently available antiseizure medications (ASMs) and may benefit from novel therapeutics.
Objective
To evaluate the efficacy and safety of XEN1101, a novel small-molecule selective Kv7.2/Kv7.3 potassium channel opener, in the treatment of focal-onset seizures (FOSs).
Design, Setting, and Participants
This phase 2b, randomized, double-blind, placebo-controlled, parallel-group, dose-ranging adjunctive trial investigated XEN1101 over an 8-week treatment period from January 30, 2019, to September 2, 2021, and included a 6-week safety follow-up. Adults experiencing 4 or more monthly FOSs while receiving stable treatment (1-3 ASMs) were enrolled at 97 sites in North America and Europe.
Interventions
Patients were randomized 2:1:1:2 to receive XEN1101, 25, 20, or 10 mg, or placebo with food once daily for 8 weeks. Dosage titration was not used. On completion of the double-blind phase, patients were offered the option of entering an open-label extension (OLE). Patients not participating in the OLE had follow-up safety visits (1 and 6 weeks after the final dose).
Main Outcomes and Measures
The primary efficacy end point was the median percent change from baseline in monthly FOS frequency. Treatment-emergent adverse events (TEAEs) were recorded and comprehensive laboratory assessments were made. Modified intention-to-treat analysis was conducted.
Results
A total of 325 patients who were randomized and treated were included in the safety analysis; 285 completed the 8-week double-blind phase. In the 325 patients included, mean (SD) age was 40.8 (13.3) years, 168 (51.7%) were female, and 298 (91.7%) identified their race as White. Treatment with XEN1101 was associated with seizure reduction in a robust dose-response manner. The median (IQR) percent reduction from baseline in monthly FOS frequency was 52.8% (P < .001 vs placebo; IQR, −80.4% to −16.9%) for 25 mg, 46.4% (P < .001 vs placebo; IQR, −76.7% to −14.0%) for 20 mg, and 33.2% (P = .04 vs placebo; IQR, −61.8% to 0.0%) for 10 mg, compared with 18.2% (IQR, −37.3% to 7.0%) for placebo. XEN1101 was generally well tolerated and TEAEs were similar to those of commonly prescribed ASMs, and no TEAEs leading to death were reported.
Conclusions and Relevance
The efficacy and safety findings of this clinical trial support the further clinical development of XEN1101 for the treatment of FOSs.
Trial Registration
ClinicalTrials.gov Identifier: NCT03796962
This randomized clinical trial evaluates the efficacy and safety of XEN1101, a potassium channel opener, in the treatment of adults with intractable focal-onset seizures.
Introduction
Many individuals with epilepsy continue to experience seizures despite treatment with available antiseizure medications (ASMs). There is therefore a need for more efficacious ASMs, and particularly for ones that confer greater seizure reduction or seizure freedom.1 Antiseizure medications act through an interaction with diverse molecular targets in the brain.2 The Kv7.2/Kv7.3 voltage-gated potassium channels, which exhibit perisomatic and axonal expression in brain neurons, represent a particularly attractive target.3 These inhibitory ion channels oppose neuronal membrane depolarization near spike threshold, restraining epileptic hyperexcitability.4,5 Loss of function mutations in the KCNQ2 and KCNQ3 genes encoding these channels variously cause benign familial neonatal seizures and early-onset epileptic encephalopathy.5 Conversely, drugs that enhance the opening of Kv7.2/Kv7.3 channels have been shown to confer seizure reduction.6 The first-generation Kv7.2/Kv7.3 channel opener ezogabine demonstrated efficacy in the treatment of focal-onset seizures (FOSs), but its long-term use was found to be associated with tissue pigmentation, putatively through formation of chromophoric dimers, and it was voluntarily removed from the market.6,7
XEN1101 is a novel, small-molecule, selective Kv7.2/Kv7.3 potassium channel opener being developed for the treatment of FOSs, the most common type of seizures experienced by individuals with epilepsy.8 The novel structure differs from that of ezogabine. The pharmacokinetic properties of XEN1101, including a long terminal elimination half-life (approximately 10 days), support once-daily oral dosing without the need for titration at initiation of dosing or tapering at termination of dosing.9
XEN1101 was previously evaluated in phase I studies in healthy volunteers, including a pharmacodynamic crossover study using transcranial magnetic stimulation.9,10 Data from these studies demonstrated that dosing XEN1101 up to 25 mg is generally well tolerated and that following a single 20-mg oral dose cortical excitability is reduced to an extent that correlates well with the XEN1101 plasma concentration. We present herein the results of a phase 2b study of XEN1101, the X-TOLE study.
X-TOLE was a phase 2b randomized, double-blind placebo-controlled study of XEN1101 in adults with FOSs dosed orally with food. The primary objectives of X-TOLE were to assess the efficacy, safety, and tolerability of 3 doses of XEN1101 in comparison with placebo on FOS frequency in adults with focal epilepsy. Secondary objectives included an assessment of 50% responder rates and a characterization of the effects of each XEN1101 dose on seizure frequency in weekly intervals during the double-blind phase (DBP) to allow a determination of the speed of onset and durability of response. Additional exploratory analyses reported herein include a time-to-event analysis (time for seizure count to reach the baseline monthly count) and an assessment of the effects of XEN1101 on overall health status as reported by the patients and their caregivers.
Methods
Study Design
This was a multicenter, randomized, double-blind, parallel-group, dose-ranging, placebo-controlled adjunctive-therapy clinical trial in which XEN1101 was administered once daily with food as a 10-, 20-, or 25-mg oral capsule. The study design, which was reviewed and approved by the appropriate ethics committees and/or institutional review boards at all 97 trial sites and conducted in accordance with the Declaration of Helsinki11 and the International Council for Harmonisation Guidelines for Good Clinical Practice, is outlined in Figure 1A. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. Adults aged 18 to 75 years with FOSs provided written informed consent and were enrolled; participants did not receive financial compensation. The sample size of 300 participants in the DBP was chosen to provide at least 80% power at a 2-sided .05-level for detecting a monotonic dose response. Key eligibility criteria included experiencing 4 or more countable FOSs on average per month, recorded from home in an e-diary during a prospective 8-week baseline period, while receiving stable treatment of between 1 and 3 ASMs. The e-diary stored daily seizure and treatment adherence information that was electronically transmitted to the database. Central surveillance of e-diary functionality and patient adherence, as well as clinical status, was used in addition to patient monitoring by participating sites. The types of countable focal seizures assessed included focal aware seizures with motor signs, focal seizures with impaired awareness, and focal seizures evolving to bilateral tonic-clonic seizures.
Figure 1. X-TOLE Trial Design and Patient Disposition.
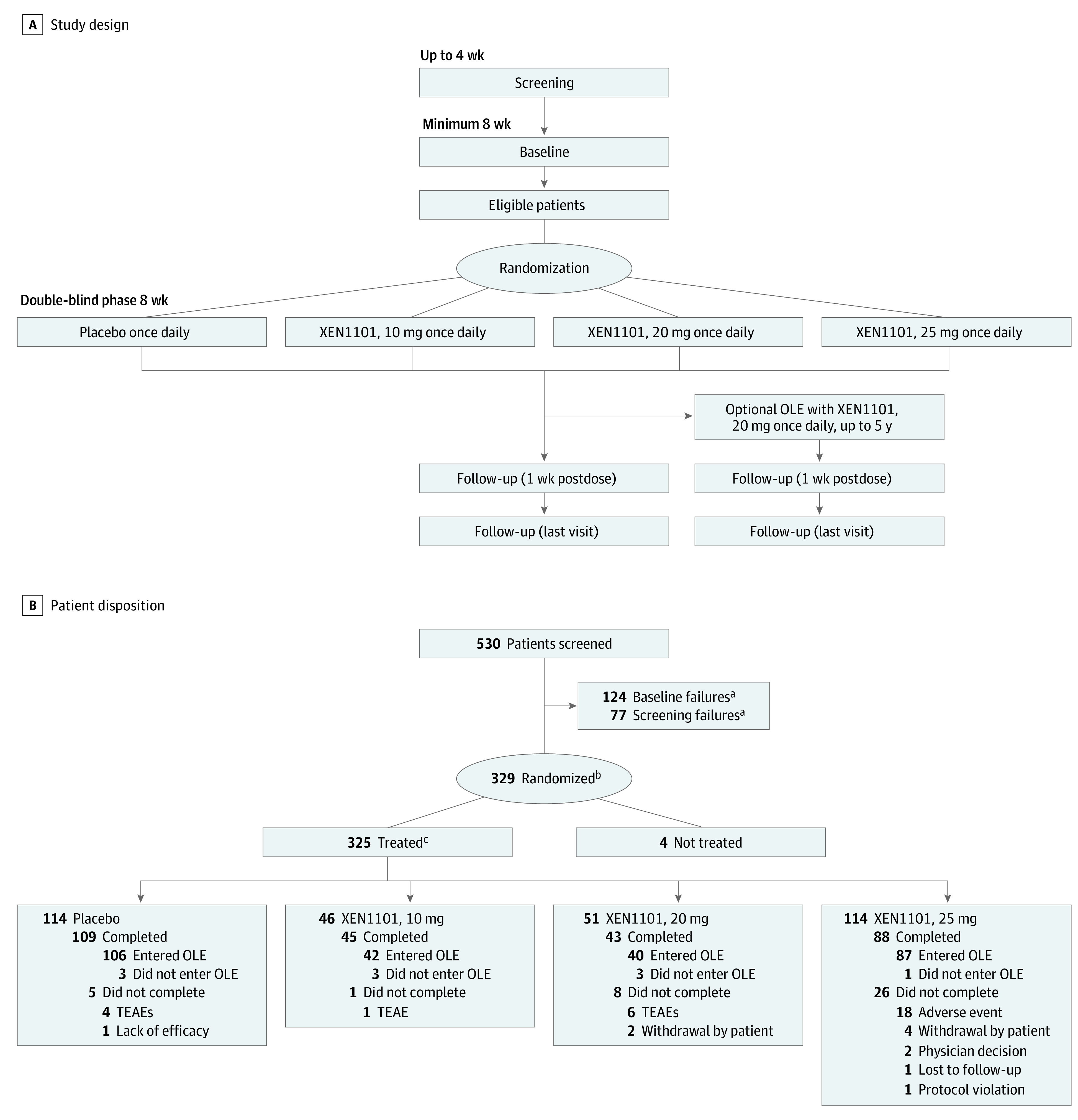
A, X-TOLE trial design, with 2:1:1:2 randomization. B, Patient disposition. Screening included all patients who signed informed consent and were entered into the clinical database. All doses were administered as a once-daily capsule with food, and titration was not required. OLE indicates open-label extension; TEAE, treatment-emergent adverse event.
aScreening failures and patients who did not enter baseline for any other reason.
bAll patients who were provided a treatment assignment and recorded in the interactive response technology database, regardless of whether treatment was used.
cPatients in the safety population.
Patients meeting eligibility criteria were randomized for an 8-week DBP to 1 of 3 active treatment groups or placebo in a 2:1:1:2 ratio: 25, 20, or 10 mg of XEN1101 or placebo. XEN1101 is primarily metabolized by cytochrome P450 3A4 isozyme (CYP3A4) without substantial involvement of other CYPs, and plasma levels of XEN1101 may decrease in the presence of CYP3A4 inducers. To ensure balanced distribution across groups, randomization was stratified by background use of CYP3A4 inducer medications (eg, carbamazepine, eslicarbazepine acetate, oxcarbazepine, phenytoin, topiramate, or phenobarbital). Randomization was implemented centrally in the Medidata Rave Randomization and Trial Supply Management system, using a dynamic allocation randomization algorithm (nondeterministic).
XEN1101 was administered as a once-daily adjunctive treatment in capsule formulation by mouth. Because the oral bioavailability of XEN1101 is enhanced by coadministration with food, patients were instructed to take the study medication with the evening meal. Patients who did not participate in the open-label extension (OLE) had a follow-up visit 1 week posttreatment and a final follow-up visit 6 weeks following the last dose. The total study duration per randomized patient (for those not entering the OLE) was approximately 26 weeks (6 months) from screening to follow-up. Extensions of the baseline period up to 20 weeks were allowed if COVID-19 pandemic-related site access restrictions prevented patients from attending site visits. The study duration was from January 30, 2019, through September 2, 2021. Safety was assessed as severity and frequency of treatment-emergent adverse events (TEAEs) and serious AEs, clinically significant changes in laboratory findings, and other measures including electrocardiography.
Statistical Analysis
The primary study objective was to assess the dose-response activity of XEN1101 in reducing monthly (28-day) FOS frequency, and the primary end point assessed the monotonic dose-response relationship between the XEN1101 active dose groups compared with placebo, based on a ranked analysis of covariance model. All efficacy analyses were performed using the modified intention-to-treat population (randomized and treated patients with at least 1 seizure diary entry recorded post treatment). The analysis of percent change from baseline was calculated based on monthly (28 days) FOS frequency. Both 1-sided and 2-sided tests are reported, with significance threshold set at P < .05. The Hodges-Lehmann estimate for each dose vs placebo was calculated accompanied by the associated 2-sided 90% and 95% CIs. Statistical analysis was conducted with SAS, version 9.4 or higher (SAS Institute Inc).
Results
Patient Disposition and Demographic Characteristics
A total of 325 patients who were randomized and treated were included in the safety population (Figure 1B) and 323 patients in the modified intention-to-treat population were included in the efficacy analyses. A total of 285 patients completed the DBP of the trial. Of these, 275 individuals (96.5%) entered the OLE to evaluate the long-term safety, tolerability, and effectiveness of XEN1101. The present article reports findings from the completed DBP of the study, including the first patient randomized in June 2019 through the last patient treated in August 2021.
Patient baseline demographic and other characteristics are presented in Table 1 and were generally comparable across groups at study entry. Mean (SD) patient age was 40.8 (13.3) years, 168 (51.7%) were female, and 298 (91.7%) identified their race as White. The US Food and Drug Administration expects race to be collected as a covariate across the development program. With less than 4% representation in any other race category, analysis by race would not contribute to a meaningful analysis in this single study. Patients were enrolled in North America (39.1%) or Europe (60.9%). The mean (SD) body mass index was 26.8 (5.2) (calculated as weight in kilograms divided by height in meters squared). Patient mean (SD) age at the time of disease onset was 17.1 (13.6) years, with a range from 0 to 61 years.
Table 1. Patient Baseline Demographic Characteristics.
| Characteristic | Patients, No. (%) | ||||
|---|---|---|---|---|---|
| Placebo (n = 114) | XEN1101, 10 mg (n = 46) | XEN1101, 20 mg (n = 51) | XEN1101, 25 mg (n = 114) | Total (N = 325) | |
| Age, mean (SD), y | 42.9 (13.7) | 40.0 (12.1) | 41.7 (13.6) | 38.7 (13.1) | 40.8 (13.3) |
| Age at study entry category | |||||
| ≥65 y | 5 (4.4) | 2 (4.3) | 4 (7.8) | 1 (0.9) | 12 (3.7) |
| <65 y | 109 (95.6) | 44 (95.7) | 47 (92.2) | 113 (99.1) | 313 (96.3) |
| Sex | |||||
| Female | 61 (53.5) | 27 (58.7) | 26 (51.0) | 54 (47.4) | 168 (51.7) |
| Male | 53 (46.5) | 19 (41.3) | 25 (49.0) | 60 (52.6) | 157 (48.3) |
| Region | |||||
| Europe | 67 (58.8) | 31 (67.4) | 32 (62.7) | 68 (59.6) | 198 (60.9) |
| North America | 47 (41.2) | 15 (32.6) | 19 (37.3) | 46 (40.4) | 127 (39.1) |
| BMI, mean (SD) | 27.3 (5.4) | 26.6 (5.1) | 26.7 (5.0) | 26.5 (5.1) | 26.8 (5.2) |
| Age at disease onset, mean (SD), y | 19.2 (14.7) | 19.8 (14.8) | 14.1 (12.1) | 15.3 (12.1) | 17.1 (13.6) |
| CYP3A4 inducer use | |||||
| No | 45 (39.5) | 21 (45.7) | 22 (43.1) | 49 (43.0) | 137 (42.2) |
| Yes | 69 (60.5) | 25 (54.3) | 29 (56.9) | 65 (57.0) | 188 (57.8) |
| Background ASM use | |||||
| 1 | 12 (10.5) | 4 (8.7) | 2 (3.9) | 11 (9.6) | 29 (8.9) |
| 2 | 46 (40.4) | 18 (39.1) | 20 (39.2) | 48 (42.1) | 132 (40.6) |
| 3 | 56 (49.1) | 24 (52.2) | 29 (56.9) | 55 (48.2) | 164 (50.5) |
| No. of prestudy ASMs failed, median (IQR) | 6.0 (4.0-8.0) | 5.0 (4.0-9.0) | 6.0 (4.0-9.0) | 6.0 (3.0-9.0) | 6.0 (4.0-9.0) |
Abbreviations: ASM, antiseizure medication; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
A total of 8.9% of the patients received 1 stable ASM at baseline and concomitantly throughout the DBP of the study, 40.6% of the patients received 2, and 50.5% of the patients received 3. Patients had tried and stopped a median of 6 previous ASMs before study entry. More than half of the patients (57.8%) were taking a CYP3A4 inducer at baseline. The median baseline FOS frequency was 13.5 (IQR, 7.9-30.3) per month.
Efficacy
A robust dose response for seizure reduction was present (primary dose-response test: P < .001) (Table 2). There was a greater reduction in monthly FOS frequency from baseline during the treatment period for patients receiving XEN1101 compared with those receiving placebo, and the effect was statistically significant for each dose. The median percent reduction and associated 2-sided P values from baseline in monthly FOS frequency was 52.8% (P < .001 vs placebo; IQR, −80.4% to −16.9%) in the 25-mg group, 46.4% (P < .001 vs placebo; IQR, −76.7% to −14.0%) in the 20-mg group, and 33.2% (P = .04 vs placebo; IQR, −61.8% to 0.0%) in the 10-mg group, compared with 18.2% (IQR, −37.3% to 7.0%) in the placebo group (Table 2 and Figure 2A).
Table 2. Efficacy of XEN1101a.
| Variable | Treatment group | |||
|---|---|---|---|---|
| Placebo (n = 114) | XEN1101, 10 mg (n = 46) | XEN1101, 20 mg (n = 51) | XEN1101, 25 mg (n = 112) | |
| Focal-onset seizure frequency | ||||
| Monthly seizure frequency in baseline, median (IQR) | 13.4 (8.0 to 30.1) | 17.4 (8.0 to 55.6) | 14.5 (7.5 to 36.4) | 12.8 (8.4 to 24.6) |
| Monthly seizure frequency in the double-blind phase, median (IQR) | 10.5 (5.4 to 25.1) | 10.9 (3.5 to 41.2) | 5.2 (3.0 to 24.9) | 5.3 (2.5 to 13.6) |
| Percent change from baseline to the double-blind phase, median (IQR) | −18.2 (−37.3 to 7.0) | −33.2 (−61.8 to 0.0) | −46.4 (−76.7 to −14.0) | −52.8 (−80.4 to −16.9) |
| P value from ranked ANCOVA model | NA | NA | NA | NA |
| P value for pairwise comparison vs placebo (2-sided) | NA | .04 | <.001 | <.001 |
| P value for primary dose response (2-sided) | <.001 | NA | NA | NA |
| Responder ratesb | ||||
| OR vs placebo (95% CI) | NA | 2.48 (1.05 to 5.84) | 4.78 (2.18 to 10.52) | 7.30 (3.77 to 14.10) |
| CGI-C | ||||
| At least much improved, % of patients | 22.8 | 23.9 | 33.3 | 46.4 |
| Difference vs placebo | NA | 1.1 | 10.5 | 23.6 |
| OR vs placebo | NA | 1.06 | 1.69 | 2.93 |
| 95% CI for OR | NA | 0.47 to 2.38 | 0.82 to 3.51 | 1.65 to 5.21 |
| P value (2-sided) | NA | .96 | .17 | <.001 |
| PGI-C | ||||
| At least much improved, % of patients | 21.9 | 34.8 | 37.3 | 42.9 |
| Difference vs placebo | NA | 12.9 | 15.3 | 20.9 |
| OR vs placebo | NA | 1.90 | 2.11 | 2.67 |
| 95% CI for OR | NA | 0.90 to 4.03 | 1.03 to 4.34 | 1.49 to 4.77 |
| P value (2-sided) | NA | .10 | .04 | .001 |
Abbreviations: ANCOVA, analysis of covariance; CGI-C, Clinical Global Impression–Change; NA, not applicable; OR, odds ratio; PGI-C, Patient Global Impression–Change.
All doses were administered with food.
Responders were defined as those who achieved a 50% or more reduction from baseline in monthly seizure frequency, and responder rates as the percentage of patients in each treatment group who reached that benchmark.
Figure 2. Dose-Dependent Efficacy of XEN1101.
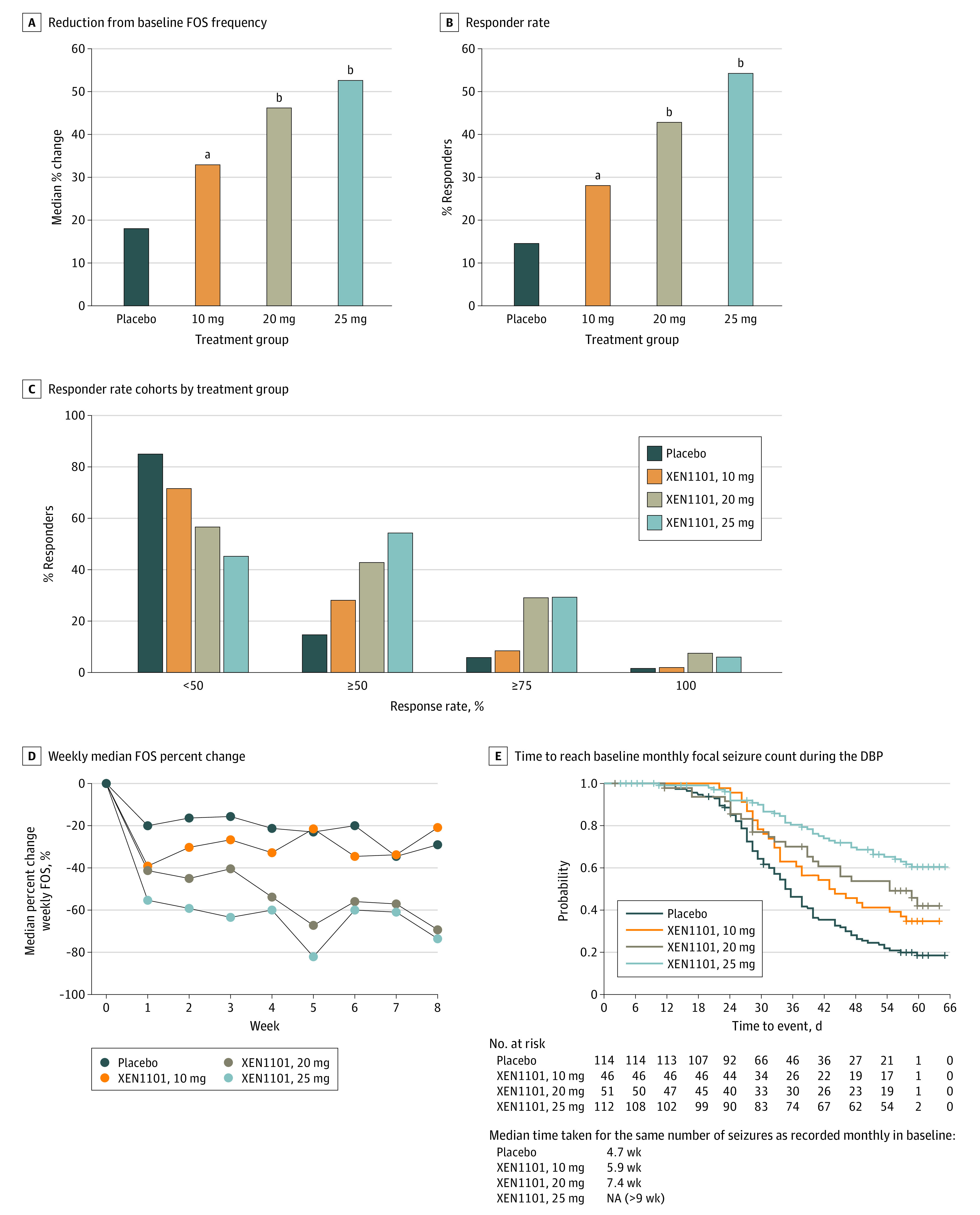
All doses were administered with food. For B, responder rate indicates greater than or equal to 50% reduction in FOS frequency from baseline. For C, responder rate indicates less than 50%, greater than or equal to 50%, greater than or equal to 75%. and 100% thresholds. DBP indicates double-blind phase; FOS, focal-onset seizure; NA, not applicable.
aP = .04.
bP < .001.
Responders were defined as those who achieved a 50% or greater reduction from baseline in monthly FOS frequency. There was a dose-dependent increase in the number of responders (Figure 2B). The percentage and associated 2-sided P values vs placebo of patients who were responders was 54.5% (P < .001 vs placebo) in the 25-mg group, 43.1% (P < .001 vs placebo) in the 20-mg group, and 28.3% (P = .04 vs placebo) in the 10-mg group, compared with 14.9% in the placebo group (Figure 2B). Seizure freedom rates, derived from the median percent change at 100% reduction during the 8-week treatment period, in all observed patients were 6.3% in the 25-mg group, 7.8% in the 20-mg group, 2.2% in the 10-mg group, and 1.8% in the placebo group. In the completers, the rates of seizure freedom were 4.5% in the 25-mg group, 3.9% in the 20-mg group, 2.2% in the 10-mg group, and 1.8% in the placebo group (Figure 2C).
The onset of response was rapid as assessed by post hoc analysis of the percentage of patients who were responders at the end of the first week. The percentages were 53.6% (P < .001 vs placebo) in the 25-mg group, 47.1% (P < .05 vs placebo) in the 20-mg group, and 43.5% (P < .05 vs placebo) in the 10-mg group, compared with 28.1% of patients receiving placebo. Analysis of the reductions in median weekly FOS frequencies at the end of the first week also demonstrated rapid onset: there was a reduction of 55.4% (P < .001 vs placebo) in the 25-mg group, 41.5% (P = .04 vs placebo) in the 20-mg group, and 39.1% (P < .01 vs placebo) in the 10-mg group, compared with 20.2% in the placebo group. The response in the 20- and 25-mg groups was sustained throughout the study (Figure 2D).
The exploratory end point of time to reach baseline monthly FOS count was also assessed (Figure 2E). Patients in the placebo group reached their baseline monthly FOS count by a median of 4.7 weeks, whereas patients in the 10-mg XEN1101 group did not reach their monthly baseline FOS count until approximately week 6, and those in the 20-mg group until a median of 7.4 weeks. The median time to baseline FOS count was more than 8 weeks in the 25-mg group, because most patients did not reach this end point at the end of the 8-week treatment period. Together, these changes support a dose-response relationship between XEN1101 and the reduction in FOS frequency.
The significant reductions in FOS frequency in patients receiving XEN1101 were associated with improvement in overall health status as assessed by both investigators, using the Clinical Global Impression–Change (CGI-C) scale, and by patients, using the Patient Global Impression–Change (PGI-C) scale. The difference in the percentage of patients who were much improved or very much improved between the placebo vs the 25-mg XEN1101 treatment groups was statistically significant for both the CGI-C (46.4% vs 22.8%; P < .001) and PGI-C (42.9% vs 21.9%; P = .001) (Table 2). In addition, the PGI-C results for the 20-mg dose, although underpowered, were statistically significant (37.3% vs 21.9%; P = .04), and numeric improvements over placebo were noted in the CGI-C for the 20-mg dose and the CGI-C and PGI-C for the 10-mg dose.
Safety
The TEAEs are summarized in Table 3. XEN1101 was generally well tolerated in this study, with TEAEs being consistent with other commonly prescribed ASMs. The most common (>10%) TEAEs across all XEN1101 dose groups were dizziness (24.6%), somnolence (15.6%), and fatigue (10.9%). The incidence of TEAEs appeared to be dose related (Table 3). Weight increase as a TEAE was reported in 1 patient (0.9%) receiving placebo, 1 patient (2.2%) at 10 mg, 2 patients (3.9%) at 20 mg, and 3 patients (2.6%) at 25 mg. No TEAEs of tissue discoloration were reported. Two nonserious TEAEs of urinary retention were reported in the active treatment groups, 1 of which required a dose reduction; both patients continued to receive the study medication with no other changes or interventions, including no need for catheterization. The adverse event of confusional state was reported in 4.7% of the treated groups (2.2% receiving 10 mg, 5.9% receiving 20 mg, and 5.3% receiving 25 mg) and in 0.9% of the placebo group. No allergic rashes occurred.
Table 3. Summary of Adverse Eventsa.
| Variable | Treatment groupb | ||||
|---|---|---|---|---|---|
| Placebo (n = 114) | XEN1101, 10 mg (n = 46) | XEN1101, 20 mg (n = 51) | XEN1101, 25 mg (n = 114) | XEN1101, any dose (n = 211) | |
| Summary of all TEAEs in the double-blind period within the safety population | |||||
| At least 1 TEAE, No. (%) | 71 (62.3) | 31 (67.4) | 35 (68.6) | 97 (85.1) | 163 (77.3) |
| At least 1 serious TEAE, No. (%) | 3 (2.6) | 2 (4.3) | 2 (3.9) | 3 (2.6) | 7 (3.3) |
| At least 1 TEAE leading to permanent treatment discontinuation, No. (%) | 4 (3.5) | 1 (2.2) | 7 (13.7) | 18 (15.8) | 26 (12.3) |
| At least 1 serious TEAE leading to death, No. (%) | 0 | 0 | 0 | 0 | 0 |
| Most common TEAEs ≥5% in any arm | |||||
| Nervous system disorders | 35 (30.7) | 20 (43.5) | 28 (54.9) | 83 (72.8) | 131 (62.1) |
| Dizziness | 8 (7.0) | 3 (6.5) | 13 (25.5) | 36 (31.6) | 52 (24.6) |
| Somnolence | 8 (7.0) | 5 (10.9) | 11 (21.6) | 17 (14.9) | 33 (15.6) |
| Headache | 9 (7.9) | 6 (13.0) | 6 (11.8) | 9 (7.9) | 21 (10.0) |
| Balance disorder | 2 (1.8) | 2 (4.3) | 4 (7.8) | 13 (11.4) | 19 (9.0) |
| Tremor | 2 (1.8) | 3 (6.5) | 3 (5.9) | 12 (10.5) | 18 (8.5) |
| Aphasia | 1 (0.9) | 1 (2.2) | 1 (2.0) | 8 (7.0) | 10 (4.7) |
| Ataxia | 1 (0.9) | 3 (6.5) | 1 (2.0) | 5 (4.4) | 9 (4.3) |
| Dysarthria | 0 | 1 (2.2) | 0 | 8 (7.0) | 9 (4.3) |
| Memory impairment | 1 (0.9) | 1 (2.2) | 2 (3.9) | 6 (5.3) | 9 (4.3) |
| Disturbance in attention | 1 (0.9) | 0 | 3 (5.9) | 5 (4.4) | 8 (3.8) |
| Psychiatric disorders | 18 (15.8) | 7 (15.2) | 13 (25.5) | 31 (27.2) | 51 (24.4) |
| Confusional state | 1 (0.9) | 1 (2.2) | 3 (5.9) | 6 (5.3) | 10 (4.7) |
| Anxiety | 6 (5.3) | 0 | 5 (9.8) | 2 (1.8) | 7 (3.3) |
| Hallucination | 0 | 0 | 3 (5.9) | 0 | 3 (1.4) |
| General disorders and administration site conditions | 12 (10.5) | 10 (21.7) | 9 (17.6) | 30 (26.3) | 49 (23.2) |
| Fatigue | 6 (5.3) | 5 (10.9) | 4 (7.8) | 14 (12.3) | 23 (10.9) |
| Gait disturbance | 1 (0.9) | 2 (4.3) | 2 (3.9) | 8 (7.0) | 12 (5.7) |
| Gastrointestinal disorders | 10 (8.8) | 10 (21.7) | 5 (9.8) | 19 (16.7) | 34 (16.1) |
| Nausea | 3 (2.6) | 1 (2.2) | 1 (2.0) | 7 (6.1) | 9 (4.3) |
| Constipation | 1 (0.9) | 2 (4.3) | 3 (5.9) | 3 (2.6) | 8 (3.8) |
| Eye disorders | 6 (5.3) | 3 (6.5) | 5 (9.8) | 18 (15.8) | 26 (12.3) |
| Vision blurred | 1 (0.9) | 0 | 1 (2.0) | 7 (6.1) | 8 (3.8) |
| Infections and infestations | 13 (11.4) | 6 (13.0) | 6 (11.8) | 6 (5.3) | 18 (8.5) |
| Urinary tract infection | 4 (3.5) | 4 (8.7) | 3 (5.9) | 2 (1.8) | 9 (4.3) |
| Serious TEAEs in the double-blind period | |||||
| Overall | 3 (2.6) | 2 (4.3) | 2 (3.9) | 3 (2.6) | 7 (3.3) |
| Psychiatric disorders | 0 | 1 (2.2) | 2 (3.9) | 1 (0.9) | 4 (1.9) |
| Confusional state | 0 | 1 (2.2) | 0 | 0 | 1 (0.5) |
| Psychogenic seizure | 0 | 0 | 0 | 1 (0.9) | 1 (0.5) |
| Psychotic disorder | 0 | 0 | 1 (2.0) | 0 | 1 (0.5) |
| Somatic delusion | 0 | 0 | 1 (2.0) | 0 | 1 (0.5) |
| Nervous system disorders | 2 (1.8) | 1 (2.2) | 0 | 2 (1.8) | 3 (1.4) |
| Dizziness | 0 | 0 | 0 | 1 (0.9) | 1 (0.5) |
| Muscle spasticity | 0 | 0 | 0 | 1 (0.9) | 1 (0.5) |
| Seizure | 0 | 1 (2.2) | 0 | 0 | 1 (0.5) |
| Partial seizures | 1 (0.9) | 0 | 0 | 0 | 0 |
| Presyncope | 1 (0.9) | 0 | 0 | 0 | 0 |
| Metabolism and nutrition disorders | 0 | 1 (2.2) | 0 | 0 | 1 (0.5) |
| Hyponatremia | 0 | 1 (2.2) | 0 | 0 | 1 (0.5) |
| Infections and infestations | 1 (0.9) | 0 | 0 | 0 | 0 |
| Coronavirus infection | 1 (0.9) | 0 | 0 | 0 | 0 |
| Injury, poisoning and procedural complications | 1 (0.9) | 0 | 0 | 0 | 0 |
| Pneumothorax traumatic | 1 (0.9) | 0 | 0 | 0 | 0 |
| Rib fracture | 1 (0.9) | 0 | 0 | 0 | 0 |
Abbreviation: TEAEs, treatment-emergent adverse events.
The TEAEs listed here are only the most common ones (≥5% in any arm). Because not all TEAEs in each cohort occurred at this level, the sum may not equal the total.
All doses were administered with food.
Discontinuation associated with TEAEs occurred in 3.5% of patients receiving placebo, 2.2% of patients receiving 10 mg, 13.7% of patients receiving 20 mg, and 15.8% of patients receiving 25 mg. The most common TEAEs leading to discontinuation across XEN1101 groups were dizziness (4.7%), balance disorder (2.4%), dysarthria (1.9%), and gait disturbance (1.9%). The incidence of serious AEs was low and balanced across treatment groups, occurring in 4.3% of patients receiving 10 mg, 3.9% of patients receiving 20 mg, and 2.6% of patients receiving 25 mg. The incidence of serious AEs in patients receiving placebo (2.6%) was the same as that recorded at the highest dose of XEN1101. There were no deaths in the study.
There were no cardiovascular signals of concern based on vital signs including orthostatic tests, and no safety signals of concern from physical or neurologic examinations. Mean (SD) body weight changes from baseline were 0.2 (2.4) kg in patients receiving placebo, 0.6 (2.3) kg in patients receiving 10 mg, 1.6 (2.2) kg in patients receiving 20 mg, and 1.9 (2.9) kg in patients receiving 25 mg. There were no signals of concern from electrocardiograms, laboratory tests evaluating safety, or urinalysis.
Discussion
To our knowledge, this is the first report of a controlled clinical trial of XEN1101, a high-potency, highly selective Kv7.2/Kv7.3 potassium channel opener, in patients with FOSs. XEN1101 treatment was associated with a dose-dependent reduction in seizure frequency, providing validation of Kv7 voltage-activated potassium channels as a therapeutic target in the treatment of FOSs.4
Despite the introduction into clinical practice in recent decades of several ASMs for the treatment of FOSs, clinicians continue to seek new treatment options that have the potential to confer seizure freedom for patients who continue to have uncontrolled seizures and are well tolerated.12 The results of this study suggest that XEN1101 may be such an option. In an interim analysis of the open-label long-term extension study, seizure freedom for patients receiving consecutive durations of XEN1101 for 6 months or more was achieved in 17.5% of the patients, and for 12 months or more, 10.5% of the patients, suggesting that this effect is sustained.13 Treatment with XEN1101, 25 mg, once daily was associated with a significant reduction in seizure frequency with a large effect size compared with placebo and was adjudicated by 42.9% of patients to be clinically meaningful. At the highest dose used, 25 mg per day, the median percent change in FOS frequency decreased by 52.8%, compared with 18.2% with placebo; the 50% or more responder rate was 54.5%, compared with 14.9% with placebo. The 25-mg dose was also well tolerated, with only 15.8% of patients in this treatment arm discontinuing the study due to AEs, of which the most frequent was dizziness; patient discontinuations, mainly due to AEs, occurred at a higher rate early in the study. Seizure reductions were statistically significant across all 3 doses of XEN1101 in a dose-related manner compared with placebo for both efficacy outcomes (median percent change in FOS frequency and ≥50% responder rate). These results are supported by the finding that the study was carried out in a highly treatment-resistant patient population, characterized by having previously tried and stopped a median of 6 ASMs; 50.5% of the participants were using 3 concomitant ASMs, and there was a high median baseline seizure frequency of 13.5 per month.
Many ASMs require titration, and there is a need for new efficacious therapies that have a rapid onset of action. XEN1101 does not require titration due to its long half-life of approximately 10 days,9 which enables gradual self-titration associated with a rapid onset of effect, with nearly the maximum seizure reduction achieved in each arm by the first week of treatment, which was sustained throughout the 8-week DBP in the 20- and 25-mg groups.
XEN1101 was generally well tolerated. The most frequent TEAEs (dizziness, balance disorder, dysarthria, and gait disturbance) were related to the central nervous system and were similar to those associated with other commonly used ASMs. Some of these TEAEs were dose related and occurred at the highest dose. The adverse event of confusional state occurred at an overall rate of 4.7% in the treated group, which was 3.8% higher than placebo. These rates are similar to the frequencies of the rates observed in placebo-controlled phase 3 trials of lacosamide and oxcarbazepine, and less than the rates in trials of eslicarbazepine, pregabalin, perampanel, and topiramate.14 There were no cardiovascular signals of concern in electrocardiograms or vital signs, no allergic skin rashes, and no deaths in the study. Urinary problems were infrequent and mild; no catheterization was required. Moreover, tissue discoloration was not observed in this limited duration trial nor in an interim analysis of the ongoing 5-year, open-label extension study.13
Limitations
Study limitations include the relatively short 8-week treatment duration; however, analysis of participants in the ongoing open-label extension phase is expected to provide insight into the long-term safety and efficacy of XEN1101. There were fewer individuals in the 10- and 20-mg groups compared with the placebo and 25-mg groups; nevertheless, a clear dose response was demonstrated. It is unknown what the impact of COVID-19 was on study outcomes. It is possible that general pandemic restrictions on site access may have contributed to enrollment of a more refractory population (ie, medians of 13.5 seizures/month and 6 previously stopped ASMs).
Conclusions
The results of this study support the further clinical development of XEN1101 for the treatment of FOSs. The findings of this study suggest that XEN1101 has the potential to address the unmet need for a treatment with a novel mechanism of action for patients for FOSs.
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement
References
- 1.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319. doi: 10.1056/NEJM200002033420503 [DOI] [PubMed] [Google Scholar]
- 2.Sills GJ, Rogawski MA. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology. 2020;168:107966. doi: 10.1016/j.neuropharm.2020.107966 [DOI] [PubMed] [Google Scholar]
- 3.Barrese V, Stott JB, Greenwood IA. KCNQ-encoded potassium channels as therapeutic targets. Annu Rev Pharmacol Toxicol. 2018;58:625-648. doi: 10.1146/annurev-pharmtox-010617-052912 [DOI] [PubMed] [Google Scholar]
- 4.Rogawski MA, Bazil CW. New molecular targets for antiepileptic drugs: α(2)δ, SV2A, and K(v)7/KCNQ/M potassium channels. Curr Neurol Neurosci Rep. 2008;8(4):345-352. doi: 10.1007/s11910-008-0053-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gribkoff VK, Winquist RJ. Potassium channelopathies associated with epilepsy-related syndromes and directions for therapeutic intervention. Biochem Pharmacol. 2023;208:115413. doi: 10.1016/j.bcp.2023.115413 [DOI] [PubMed] [Google Scholar]
- 6.Brickel N, Hewett K, Rayner K, et al. Safety of retigabine in adults with partial-onset seizures after long-term exposure: focus on unexpected ophthalmological and dermatological events. Epilepsy Behav. 2020;102:106580. doi: 10.1016/j.yebeh.2019.106580 [DOI] [PubMed] [Google Scholar]
- 7.Groseclose MR, Castellino S. An investigation into retigabine (ezogabine) associated dyspigmentation in rat eyes by MALDI imaging mass spectrometry. Chem Res Toxicol. 2019;32(2):294-303. doi: 10.1021/acs.chemrestox.8b00313 [DOI] [PubMed] [Google Scholar]
- 8.National Institute of Neurological Disorders and Stroke. Epilepsy and seizures. Accessed March 7, 2023. https://www.ninds.nih.gov/health-information/disorders/epilepsy-and-seizures
- 9.Bialer M, Johannessen SI, Koepp MJ, et al. Progress report on new antiepileptic drugs—a summary of the Sixteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XVI): II, drugs in more advanced clinical development. [XEN1101 section: Beatch GN, Premoli I, Namdari R, Cadieux JA, Man Y, Leung C, Kato HS, Crean C, Aycardi E, Sherrington R, Richardson MP, Pimstone SN, Goldberg YP.]. Epilepsia. 2022;63(11):2883-2910. doi: 10.1111/epi.17376 [DOI] [PubMed] [Google Scholar]
- 10.Premoli I, Rossini PG, Goldberg PY, et al. TMS as a pharmacodynamic indicator of cortical activity of a novel anti-epileptic drug, XEN1101. Ann Clin Transl Neurol. 2019;6(11):2164-2174. doi: 10.1002/acn3.50896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 12.French JA. Cenobamate for focal seizures—a game changer? Nat Rev Neurol. 2020;16(3):133-134. doi: 10.1038/s41582-019-0309-7 [DOI] [PubMed] [Google Scholar]
- 13.French J, Porter R, Perucca E, et al. XEN1101, a novel potassium channel modulator: interim data from an ongoing, long-term, open-label extension of a phase 2B study (X-TOLE) in adults with focal epilepsy [abstract 2.235]. Presented at: American Epilepsy Society 2022; Nashville, TN, December 4, 2022. [Google Scholar]
- 14.Sarkis RA, Goksen Y, Mu Y, Rosner B, Lee JW. Cognitive and fatigue side effects of anti-epileptic drugs: an analysis of phase III add-on trials. J Neurol. 2018;265(9):2137-2142. doi: 10.1007/s00415-018-8971-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement


