Abstract
We evaluated the effect of roxithromycin on cytokine production and neutrophil attachment to human airway epithelial cells. Roxithromycin suppressed production of interleukin 8 (IL-8), IL-6, and granulocyte-macrophage colony-stimulating factor. It inhibited neutrophil adhesion to epithelial cells. Roxithromycin modulates local recruitment and activation of inflammatory cells, which may have relevance to its efficacy in airway diseases.
Roxithromycin is a 14-member macrolide antibiotic effective for the treatment of upper and lower respiratory tract infections (10). Recent reports showed that roxithromycin is also effective for the treatment of chronic airway diseases such as diffuse panbronchiolitis, bronchial asthma, and chronic sinusitis (6, 7, 13), although its precise actions remain unclear. In the present study, we investigated if roxithromycin had any effect on the production of cytokines, especially interleukin 8 (IL-8), by human bronchial epithelial cells (8) and if it had any effect on the process of neutrophil adhesion onto these cells in vitro.
Normal human bronchial epithelial cells were prepared by the proteolytic digestion of bronchi as reported previously (9, 14, 15). The cells were plated onto collagen-coated 24-well flat-bottom tissue culture plates (Koken, Tokyo, Japan) and cultured in hormonally defined Ham’s F-12 medium (HD-F12) as reported previously (9, 14) until confluence. The cells were keratin positive but vimentin negative, showing that they were epithelial cells (9, 14). A human bronchial epithelial cell line, Bet-1A (11), was cultured in HD-F12.
Roxithromycin was dissolved in methanol as stock solutions and further diluted in sterile physiological saline. The methanol at the final concentrations in each experiment showed no cytotoxicity or effect on IL-8 release by bronchial epithelial cells (data not shown). Various concentrations of roxithromycin were added to the cells simultaneously with and without stimulation of IL-1 α. After 24 h the supernatants were harvested for the measurement of cytokines. Specific immunoreactivity for IL-8 in culture supernatants was measured with enzyme-linked immunosorbent assay (ELISA) kits (R & D Systems, Inc., Minneapolis, Minn.) (15).
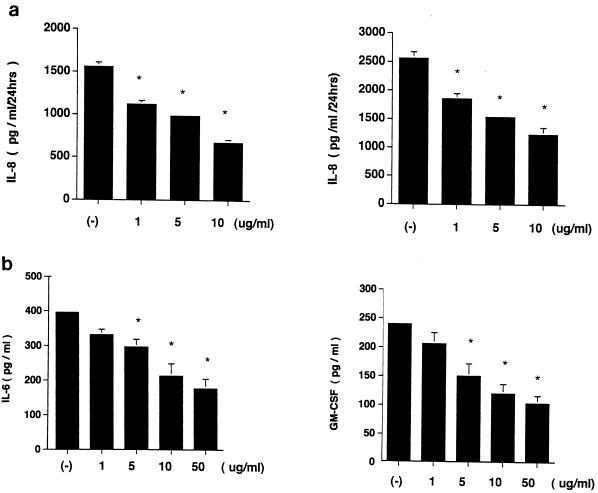
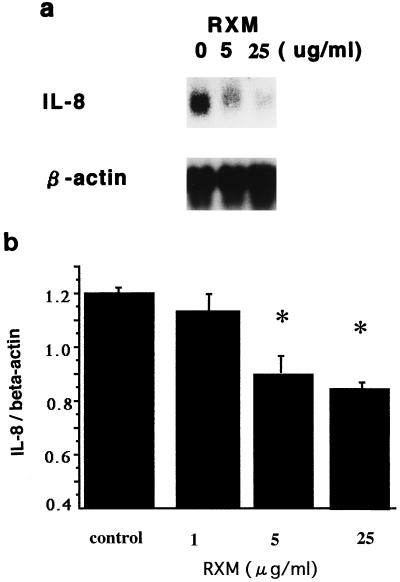
Roxithromycin showed an inhibitory action on IL-8 release by unstimulated and IL-1 α-stimulated human primary bronchial epithelial cells in a concentration-dependent fashion (Fig. 1a). A lactic dehydrogenase release assay, trypan blue dye exclusion test, and a colormetric 3-4, 5-dimethyl-thiazol-2-yl-2, 5-diphenyltetrazolium bromide assay (17) showed that this effect was not due to cytotoxicity (data not shown). This antibiotic also showed an inhibitory action on IL-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF) release by IL-1 α-stimulated human bronchial epithelial cells in a concentration-dependent fashion as assessed by ELISA kits specific for each cytokine (R & D Systems) (Fig. 1b). Northern blot analysis for IL-8 mRNA was performed by the method described previously (1, 3, 14, 19). Roxithromycin decreased the steady-state levels of IL-8 mRNA in IL-1 α (10 ng/ml)-stimulated Bet-1A cells in a concentration-dependent fashion (Fig. 2).
FIG. 1.
Effect of roxithromycin on IL-8, IL-6, and GM-CSF release by human primary bronchial epithelial cells. (a) Roxithromycin was added to primary bronchial epithelial cells simultaneously with (left panel) and without (right panel) IL-1 α (10 ng/ml). The supernatants were harvested after 24 h for IL-8 measurement. (b) Effect of roxithromycin on IL-6 and GM-CSF release by IL-1 α (10 ng/ml)-stimulated primary human bronchial epithelial cells. Simultaneous treatment with roxithromycin significantly inhibited IL-6 and GM-CSF release after 24 h. For all panels, an asterisk represents a P of <0.01 compared to controls (ANOVA; n = 5). Error bars, standard deviations.
FIG. 2.
Northern blot analysis showing effect of roxithromycin (RXM) on IL-8 mRNA expression by IL-1 α (10 ng/ml)-stimulated Bet-1A cells in culture. Roxithromycin was added to the cells with IL-1 α, and total cellular RNA was extracted after 12 h. (a) Roxithromycin showed a concentration-dependent inhibition on the steady-state levels of IL-8 mRNA. (b) Measurement of densitometric signals of IL-8 corrected by β-actin transcripts showed a significant inhibition by roxithromycin. ∗, P < 0.01 compared to controls (ANOVA; n = 4). Error bars, standard deviations.
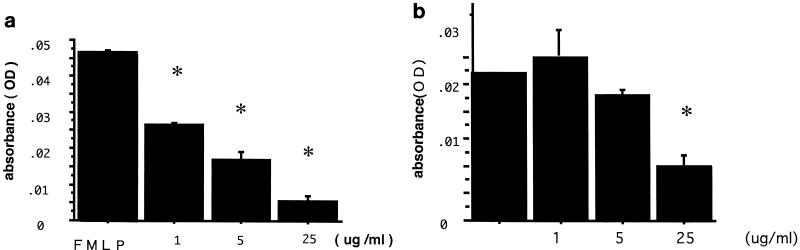
Next, neutrophil adhesion to human bronchial epithelial Bet-1A cells was studied in vitro. Briefly, Bet-1A cells were cultured on collagen-coated 96-chamber flat-bottom culture plates. Neutrophils were isolated by density gradient Percoll centrifugation (>97% pure) (5). When the epithelial cells reached confluency, the cells were rinsed and purified neutrophils (2.0 × 105/ml) were applied to culture plates. After 30 min of incubation, each well was rinsed twice, and 0.01% Triton ×-100 was added for cell lysis. Myeloperoxidase (MPO) activity was measured by spectrophotometric assay as reported previously (5). There was a significant positive correlation between the actual number of the attached neutrophils per three randomized high-power fields and MPO activity (r = 0.976; P < 0.001 [Pearson’s correlation test]). Human peripheral neutrophils adhered to epithelial cells, and the pretreatment of neutrophils with N-formyl-methionyl-leucyl-phenylalanine (FMLP) (10−7 M) for 30 min, but not with lipopolysaccharide (10 ng/ml), IL-8 (5 ng/ml), or C5a (10−8 M), significantly enhanced adhesion (by 245% ± 33.4%, 116% ± 14.7%, 92.8% ± 20.1%, and 71.2% ± 12.4%, respectively [for the first value, P < 0.01]; baseline MPO activity = 100 [analysis of variance {ANOVA}; n = 4]. As for the stimulation of epithelial cells, gamma interferon (IFN-γ) (100 ng/ml) and tumor necrosis factor alpha (10 ng/ml) significantly upregulated neutrophil adhesion to the epithelial cells after 18 h (by 155% ± 10.5% and 191% ± 12.2%, respectively [P < 0.01]; baseline MPO activity = 100 [ANOVA; n = 4]). Confluent monolayers of Bet-1A cells were treated with IFN-γ (100 ng/ml) with different concentrations of roxithromycin for 18 h. Then, the purified neutrophils with and without pretreatment of FMLP were added to each well and incubated for 30 min. The adherence of neutrophils onto epithelium was evaluated by MPO assay. As shown in Fig. 3a, roxithromycin (1 to 25 μg/ml) inhibited neutrophil adhesion to epithelial cells in a concentration-dependent fashion when the neutrophils were pretreated with 10−7 M FMLP for 30 min. Roxithromycin also showed an inhibitory effect on adhesion of naive neutrophils, but it was significant only at 25 μg/ml (Fig. 3b).
FIG. 3.
Effect of roxithromycin on neutrophil adhesion onto IFN-γ (100 ng/ml)-treated Bet-1A in vitro. Different concentrations of roxithromycin were added to Bet-1A epithelial cells simultaneously with IFN-γ (100 ng/ml) for 18 h. The purified neutrophils were then added with and without 30 min of pretreatment with FMLP (10−7 M). Roxithromycin showed a concentration-dependent inhibitory effect on neutrophil-epithelium adhesion at a concentration of no less than 1 μg/ml in FMLP-treated neutrophils (MPO assay) (a) and at a concentration of 25 μg/ml in naive neutrophils (b). For both panels, an asterisk represents a P of <0.05 compared to controls (ANOVA; n = 4). Error bars, standard deviations; OD, optical density.
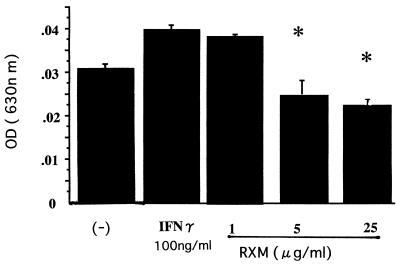
To elucidate the key role of intercellular adhesion molecule-1 (ICAM-1) on human bronchial epithelial cells (18), its expression on Bet-1A was evaluated by a cell ELISA method. Briefly, Bet-1A cells were cultured until confluency on 96-well plates, and the cells were treated with IFN-γ (100 ng/ml) with and without roxithromycin for 18 h. Then, the cells were rinsed and anti-human ICAM-1 monoclonal antibody conjugated with horseradish peroxidase (British Biotechnology Products, Ltd., Abingdon, United Kingdom) was added to each well and incubated for 1 h at room temperature. After the wells were washed twice, the substrate solution (tetramethylbenzidine) was added to quantify the magnitude of ICAM-1 expression on the surfaces of the cells. The epithelial cells expressed ICAM-1 molecules on their surfaces in a manner that was upregulated by IFN-γ (100 ng/ml) (Fig. 4). Roxithromycin was shown to decrease the magnitude of ICAM-1 expression on IFN-γ (100 ng/ml)-treated epithelial cells (Fig. 4).
FIG. 4.
Effect of roxithromycin (RXM) on ICAM-1 expression on Bet-1A cells. Confluent Bet-1A cells were treated with IFN-γ and roxithromycin. After 18 h, the expression of ICAM-1 molecules was evaluated by cell ELISA. Roxithromycin inhibited the magnitude of ICAM-1 expression in a concentration-dependent fashion. ∗, P < 0.05 (ANOVA; n = 4); OD, optical density.
Neutrophils play an important role in the pathogenesis of various airway inflammatory disorders (2). Their local recruitment is induced by chemokines such as IL-8 (20) and increased expression of adhesion molecules such as ICAM-1 on airway epithelial cells (18). Roxithromycin clearly decreased the number of neutrophils in bronchoalveolar lavage fluids from patients with airway inflammation (12). Its actions seem to be apart from its antibiotic activity, since it was effective even when the pathogens were absent or resistant to this antibiotic (12). Therefore, it is reasonable to assume that roxithromycin suppresses expression of chemokines relevant to cell recruitment into the airways. It has been reported that erythromcin and/or clarithromycin inhibited IL-6 and IL-8 production by normal bronchial epithelial cells (4, 16). Neutrophil adhesion to epithelium inhibits the access of the antiprotease system to neutrophil-derived enzymes and superoxides to epithelium. Therefore, this process is important for the prolongation of airway inflammation. In the present report, we have showed that roxithromycin, another 14-member macrolide antibiotic, has an inhibitory effect on IL-8 and ICAM-1 expression in human bronchial epithelial cells. These findings may explain, at least in part, the attenuating effect of this drug on local neutrophil recruitment and activation. Further study is necessary to elucidate the molecular mechanisms of this drug.
Acknowledgments
We are grateful to J. F. Lechner and C. C. Harris for supplying Bet-1A cells. We also thank Eisai Pharmaceutical Company for supplying roxithromycin.
This work was supported in part by a grant from the Diffuse Lung Disease Research Committee, Japan Ministry of Health and Welfare, and Manabe Medical Foundation.
REFERENCES
- 1.Chomczynski D, Sacchi N. Single-step method of RNA isolation by guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 2.Kadota J, Sakito O, Kohno S, Sawa H, Mukae H, Oda H, Kawakami K, Fukushima K, Hiratani K, Hara K. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am Rev Respir Dis. 1993;147:153–159. doi: 10.1164/ajrccm/147.1.153. [DOI] [PubMed] [Google Scholar]
- 3.Kasama T, Strieter R M, Lukacs N W, Burdick M D, Kunkel S L. Regulation of neutrophil-derived chemokine expression by IL-10. J Immunol. 1994;152:3559–3569. [PubMed] [Google Scholar]
- 4.Khair O A, Devalia J L, Abdelaziz M M, Sapsford R J, Davis R J. Effect of erythromycin on Haemophilus influenzae endotoxin-induced release of IL-6, IL-8 and sICAM-1 by cultured human bronchial epithelial cells. Eur Respir J. 1995;8:1451–1457. [PubMed] [Google Scholar]
- 5.King C C, Jefferson M M, Thomas E L. Secretion and inactivation of myeloperoxidase by isolated neutrophils. J Leukocyte Biol. 1997;61:293–302. doi: 10.1002/jlb.61.3.293. [DOI] [PubMed] [Google Scholar]
- 6.Konno S, Kurokawa M, Ikeda K, Okamoto K, Adachi M. Antiasthmatic activity of a macrolide antibiotic, roxithromycin: analysis of possible mechanisms in vitro and in vivo. Int Arch Allergy Immunol. 1994;105:308–316. doi: 10.1159/000236773. [DOI] [PubMed] [Google Scholar]
- 7.Kusano S, Kadota J, Kohno S. Effect of roxithromycin on peripheral neutrophil adhesion molecules in patients with chronic lower respiratory tract disease. Respiration. 1995;62:217–222. doi: 10.1159/000196450. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura H, Yoshimura K, Jaffe H A, Crystal R G. Interleukin-8 gene expression in human bronchial epithelial cells. J Biol Chem. 1991;266:19611–19617. [PubMed] [Google Scholar]
- 9.Ohtoshi T, Vancheri C, Cox G, Gauldie J, Denburg J A, Jordana M. Monocyte-macrophage differentiation induced by human upper airway epithelial cells. Am J Respir Cell Mol Biol. 1991;4:255–263. doi: 10.1165/ajrcmb/4.3.255. [DOI] [PubMed] [Google Scholar]
- 10.Puri, S. K., and H. B. Lassman. 1987. Roxithromycin: a pharmacokinetic review of a macrolide. J. Antimicrob. Chemother. 20(Suppl. B):89–100. [DOI] [PubMed]
- 11.Reddel R R, Ke Y, Gerwin B I, McMenamin M, Lechner J F, Su R T, Brash D E, Park J B, Rhim J S, Harris C C. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12/SV 40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988;48:1904–1909. [PubMed] [Google Scholar]
- 12.Sakito O, Kadota J, Kohno S. Interleukin lβ, tumor necrosis factor alpha, and interleukin 8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis. Respiration. 1996;63:42–48. doi: 10.1159/000196514. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu T, Kato M, Mochizuki H, Tokuyama K, Morikawa A, Kuroume T. Roxithromycin reduces the degree of bronchial hyperresponsiveness in children with asthma. Chest. 1994;106:458–461. doi: 10.1378/chest.106.2.458. [DOI] [PubMed] [Google Scholar]
- 14.Takizawa H, Ohtoshi T, Ohta K, Hirohata S, Yamaguchi M, Suzuki N, Ueda T, Ishii A, Shindoh G, Oka T, Hiramatsu K, Ito K. Interleukin 6/B cell stimulatory factor-2 is expressed and released by normal and transformed human bronchial epithelial cells. Biochem Biophys Res Commun. 1992;187:569–602. doi: 10.1016/0006-291x(92)91236-j. [DOI] [PubMed] [Google Scholar]
- 15.Takizawa H, Ohtoshi T, Kikutani T, Okazaki H, Akiyama N, Sato M, Shoji S, Ito K. Histamine activates bronchial epithelial cells to release inflammatory cytokines in vitro. Int Arch Allergy Immunol. 1995;108:260–267. doi: 10.1159/000237162. [DOI] [PubMed] [Google Scholar]
- 16.Takizawa H, Desaki M, Ohtoshi T, Kikutani T, Okazaki H, Sato M, Akiyama N, Shoji S, Hiramatsu K, Ito K. Erythromycin suppresses interleukin 6 expression by human bronchial epithelial cells. Biochem Biophys Res Commun. 1995;210:781–786. doi: 10.1006/bbrc.1995.1727. [DOI] [PubMed] [Google Scholar]
- 17.Takizawa H, Ohtoshi T, Ohta K, Yamashita N, Hirohata S, Hirai K, Hiramatsu K, Ito K. Growth inhibition of human lung cancer cell lines by interleukin 6 in vitro: a possible role in tumor growth via an autocrine mechanism. Cancer Res. 1993;53:4175–4181. [PubMed] [Google Scholar]
- 18.Tosi M F, Stark J M, Smith C W, Hamedani A, Gruenert D C, Infeld M D. Induction of ICAM-1 expression on human airway epithelial cells by inflammatory cytokines: effects on neutrophil-epithelial cell adhesion. Am J Respir Cell Mol Biol. 1992;7:214–221. doi: 10.1165/ajrcmb/7.2.214. [DOI] [PubMed] [Google Scholar]
- 19.Weiss S J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura T, Matsushima K, Oppenheim J J, Leonard E J. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin-1 (IL 1) J Immunol. 1987;139:3474–3483. [PubMed] [Google Scholar]