Abstract
Multisystem inflammatory syndrome in children (MIS-C) is a complication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection; in the United States, reporting of MIS-C after coronavirus disease 2019 (COVID-19) vaccination is required for vaccine safety monitoring. Pfizer-BioNTech COVID-19 vaccine was authorized for children aged 5−11 years on 29 October 2021. Covering a period when approximately 7 million children received vaccine, surveillance for MIS-C ≤ 90 days postvaccination using passive systems identified 58 children with MIS-C and laboratory evidence of past/recent SARS-CoV-2 infection, and 4 without evidence. During a period with extensive SARS-CoV-2 circulation, MIS-C illness in children after COVID-19 vaccination who lacked evidence of SARS-CoV-2 infection was rare (<1 per million vaccinated children).
Keywords: COVID-19, COVID-19 vaccine, MIS-C, SARS-CoV-2, multisystem inflammatory syndrome in children
Multisystem inflammatory syndrome in children (MIS-C) is a serious complication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection characterized by a hyper-inflammatory response, with illness onset typically 2−6 weeks after infection [1]. Features include fever, multiorgan system involvement, and laboratory evidence of inflammation. A definitive MIS-C diagnosis can be challenging in clinical care and for surveillance because there is no specific diagnostic biomarker and it can resemble other illnesses, including acute coronavirus disease 2019 (COVID-19), toxic shock syndrome, and Kawasaki disease (KD). Over 9000 cases and 74 deaths meeting Centers for Disease Control and Prevention (CDC) case definition have been reported in the United States through 28 November 2022, with 45% of cases among children aged 5–11 years [2]. COVID-19 vaccination has been shown to protect against MIS-C in US children aged 5−11 years and adolescents aged 12−18 years [3]. Other countries have also demonstrated vaccine effectiveness against MIS-C [4, 5].
Given the known association with SARS-CoV-2 infection, CDC and Food and Drug Administration (FDA) included MIS-C as an adverse event of special interest for COVID-19 vaccine safety monitoring. The emergency use authorizations (EUA) for COVID-19 vaccines required reporting of MIS after COVID-19 vaccination to the Vaccine Adverse Event Reporting System (VAERS). We previously reported results of surveillance for MIS-C after COVID-19 vaccination in people aged 12−20 years [6]. Following FDA’s EUA for Pfizer-BioNTech COVID-19 vaccine in children aged 5−11 years on 29 October 2021, we conducted surveillance in this age group for MIS-C illness occurring ≤90 days after COVID-19 vaccination to assess clinical characteristics and information on SARS-CoV-2 infection and vaccination.
METHODS
Surveillance methods were similar to those previously published [6]. We used CDC’s MIS-C national surveillance system, VAERS, and CDC’s Clinical Immunization Safety Assessment (CISA) Project to identify potential MIS-C illnesses in children aged 5−11 years who had illness onset 1 November 2021 through 31 March 2022 and ≤90 days after a COVID-19 vaccine dose, with receipt of first COVID-19 vaccine dose ≤ 31 December 2021. CDC’s MIS-C national surveillance system, which receives case reports from health departments, was queried weekly. VAERS was searched weekly for reports with coding or free-text mention of possible multisystem inflammation, MIS-C, or KD (Supplementary Material); CDC and FDA physicians reviewing VAERS reports also referred potential MIS-C to our team. CISA receives vaccine safety consultation requests from health care providers. For children first identified through national MIS-C surveillance or CISA, we encouraged reporting to VAERS by our close date, 13 May 2022. For this age, only Pfizer-BioNTech vaccine was authorized by 31 December 2021.
CDC physicians reviewed medical records and adjudicated cases with CISA academic site physicians. First, we determined if identified children met clinical and inflammatory criteria of the 2020 CDC MIS-C case definition and whether there was a plausible alternative etiology (Figure 1).
Figure 1.
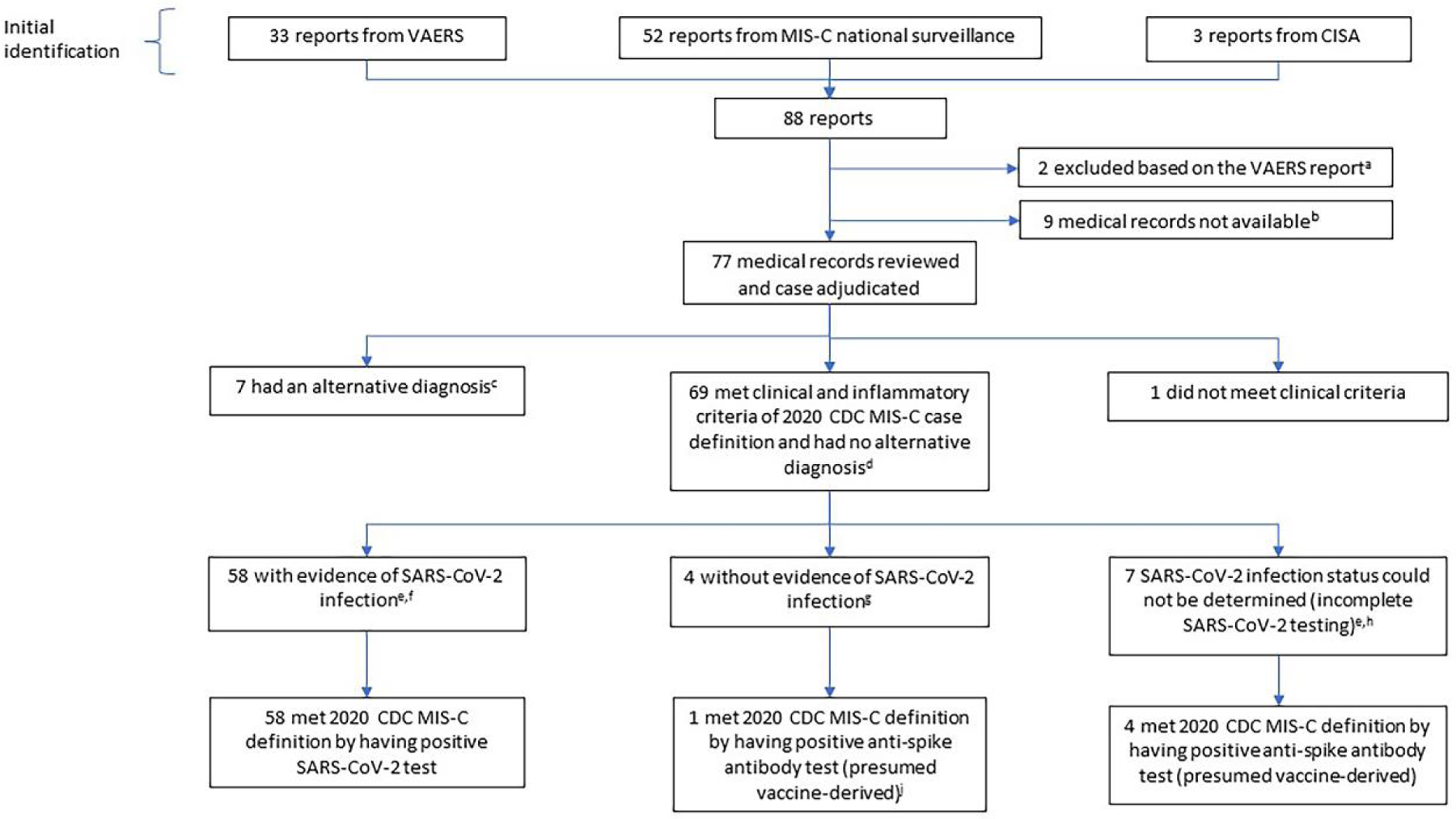
Investigation of potential MIS-C in children who had received COVID-19 vaccine. Abbreviations: CDC, US Centers for Disease Control and Prevention; CISA, Clinical Immunization Safety Assessment Project; IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children; NAAT/Ag, SARS-CoV-2 nucleic acid amplification test or antigen test; VAERS, Vaccine Adverse Event Reporting System. aVAERS report indicated the child was not hospitalized and there was no clinical suspicion of possible MIS-C. bIn 2 instances, the VAERS report was incomplete and patient information needed to request medical records was not provided; there was no information on SARS-CoV-2 testing. In 7 instances, a report was made to CDC’s MIS-C surveillance unit but not to VAERS and medical records were not available. Four of 7 were described to be NAAT/Ag positive in the past or during MIS-C evaluation, the 3 others were described to be NAAT negative/serology positive with no further details on testing. cAlternative diagnoses: adenovirus infection (n = 2), murine typhus (n = 2), and 1 each of monoarticular arthritis, pericarditis, adrenal insufficiency. dOne child who tested NAAT positive at admission died; the cause of death as assessed by the state medical examiner was MIS-C associated with COVID-19. This child was included as meeting clinical and inflammatory criteria and without alternative diagnosis based on that information. eWe used only positive serology results from specimens collected before any IVIG receipt (positive serology results from only post-IVIG specimens are considered as “test not performed”). fHistory of positive NAAT/Ag test in the past, or positive NAAT/Ag test during the MIS-C illness evaluation, or positive anti-nucleocapsid antibody test during the evaluation. gNo history of positive NAAT/Ag test in the past, negative NAAT/Ag test during the MIS-C illness evaluation, and negative anti-nucleocapsid antibody test during the evaluation. hNo history of positive NAAT/Ag test in the past, negative NAAT/Ag test during the MIS-C illness evaluation, and anti-nucleocapsid antibody test not performed. jAn anti-spike antibody test was obtained in only 1 of the 4 children in the box above, and the result was positive.
According to the 2020 US CDC case definition for MIS-C [6] cases must meet all the following clinical and laboratory criteria:
Age <21 years with subjective or objective (>38.0°C) fever for 24 hours or longer
Clinically severe illness requiring hospitalization
-
Multisystem (2 or more) organ system involvement
-
•
Cardiac: eg, elevated troponin, elevated B-type natriuretic peptide or N-terminal prohormone BNP, arrythmia, coronary artery aneurysm, cardiac dysfunction, or shock
-
•
Renal: eg, acute kidney injury or renal failure
-
•
Respiratory: eg, pneumonia, acute respiratory distress syndrome, or pleural effusion
-
•
Hematological: eg, elevated d-dimer, thrombophilia, or thrombocytopenia
-
•
Gastrointestinal: eg, abdominal pain, vomiting, diarrhea, elevated bilirubin, elevated liver enzymes
-
•
Dermatological: eg, rash or mucocutaneous lesions
-
•
Neurological: eg, cerebrovascular accident, aseptic meningitis, encephalopathy, or headache
-
•
No alternative plausible diagnosis
Laboratory evidence of inflammation: one or more of elevated C-reactive protein, erythrocyte sedimentation rate, fibrinogen, procalcitonin, d-dimer, ferritin, lactic acid dehydrogenase, interleukin 6, or neutrophils; or reduced lymphocytes or albumin
Current or recent positive SARS-CoV-2 RT-PCR, antigen, or serology test; or exposure to a suspected or confirmed COVID-19 case within the 4 weeks before onset of symptoms (for this investigation, any NAAT/Ag test in the past or during the MIS-C evaluation was used; only positive serology results from specimens collected before any intravenous immunoglobulin [IVIG] receipt were used; the exposure criterion was not used.)
Given the substantial overlap in clinical and laboratory features between KD and MIS-C, we included children considered to have KD/atypical KD by treating clinicians if they otherwise met MIS-C criteria. We then classified children by whether they had laboratory evidence of past or recent SARS-CoV-2 infection. We defined evidence of SARS-CoV-2 infection as any of the following: reported history of positive nucleic acid amplification or antigen test (NAAT/Ag) in the past, positive NAAT/Ag test during MIS-C illness evaluation, or positive anti-nucleocapsid antibody test during evaluation. Children were classified as having no evidence of SARS-CoV-2 infection if they had no history of a positive NAAT/Ag test, and tested negative by NAAT/Ag and anti-nucleocapsid antibody testing during evaluation. Children who had no evidence of SARS-CoV-2 infection as described above but tested positive for anti-spike antibodies were classified as without evidence of infection because anti-spike antibodies are expected after COVID-19 vaccination; vaccinated children are often not tested for this antibody during MIS-C evaluations. While not necessarily indicating SARS-CoV-2 infection in a vaccinated child, a positive anti-spike antibody test satisfies the SARS-CoV-2 test result criterion of the full CDC MIS-C definition. In addition to using the CDC MIS-C definition, we classified children using the Brighton Collaboration MIS-C case definition, which does not require a positive SARS-CoV-2 test [7]. If described in the medical record, we captured information on exposure to a household member with a positive NAAT/Ag test.
We obtained the number of children aged 5−11 years who received ≥1 COVID-19 vaccine dose through 31 December 2021 from CDC national COVID-19 vaccine data [8].
This activity was determined by the CDC to meet criteria for public health surveillance according to 45 C.F.R.§ 46.102(l)(2); therefore, no institutional review board approval or informed consent was required.
RESULTS
We adjudicated 77 potential cases (Figure 1). None had a previous history of MIS-C. Seven children (9%) had an alternative diagnosis, 1 (1%) did not meet clinical criteria of the 2020 CDC MIS-C case definition, and 69 (90%) met clinical and inflammatory criteria and had no alternative diagnosis. Of the 69, 58 (84%) had evidence of past or recent SARS-CoV-2 infection and therefore met the full CDC MIS-C definition. Four (6%) of the 69 had no evidence of past or recent SARS-CoV-2 infection; only 1 of the 4 was tested for anti-spike antibodies, which were detected, and therefore met full CDC MIS-C definition. For 7 (10%) of the 69, SARS-CoV-2 infection status could not be determined (ie, SARS-CoV-2 testing was incomplete); 4 were tested for anti-spike antibodies, which were detected, and therefore met full CDC MIS-C definition (Figure 1).
Of the 58 children with evidence of SARS-CoV-2 infection, 36 (62%) were male. The most common race/ethnicity categories were white non-Hispanic (25/58, 43%) and black non-Hispanic (13/58, 22%) (Supplementary Table A). Twenty-three (40%) had illness onset November through December 2021 (Supplementary Figure).
Of these 58 children, 33 (57%) had a positive NAAT/Ag test before illness onset, 5 (9%) had positive NAAT/Ag test only during MIS-C illness, and 20 (34%) did not have a positive NAAT/Ag test but were anti-nucleocapsid antibody positive (Table 1 and Supplementary Table B). Of the 33 who had a positive NAAT/Ag test before illness onset, 14 (42%) had a positive test before vaccination, 11 (33%) had received only 1 vaccine dose and subsequently had a positive test, and 8 (24%) had received 2 doses and subsequently had a positive test (for 2, the positive test was ≤ 14 days after dose 2; for 6 it was 23–48 days after dose 2; Supplementary Tables B and C). Among these 33 children with a positive NAAT/Ag test before illness onset, 30 had onset 3–66 days after the positive test, and 1 each had onset 339, 398, and 451 days after the positive test (2 of the last 3 had anti-nucleocapsid antibody testing and both tested positive). Of the 20 children without a positive NAAT/Ag test but who tested anti-nucleocapsid antibody positive, 8 (40%) had known household exposure to a NAAT/Ag-positive case, at 12−39 days before illness onset.
Table 1.
SARS-CoV-2 Laboratory Testing and Number of COVID-19 Vaccine Doses Received for the 69 Children Who Met Clinical and Inflammatory Criteria of 2020 CDC MIS-C Definition and Had No Alternative Diagnosis
| SARS-CoV-2 Infection Status | No. | Positive NAAT/Ag Test During MIS-C Illness/No. Tested | Positive Anti-Nucleocapsid Ab Test/No. Tested | No. Who Received Only 1 Vaccine Dose Before Illness Onset | No. Who Received 2 Vaccine Doses Before Illness Onset |
|---|---|---|---|---|---|
| With evidence of past or recent SARS-CoV-2 infection a | 58 | ||||
| History of positive NAAT/Ag test before MIS-C illness. | 33 | 3/26 | 19/20 | 15 | 18 |
| Positive NAAT/Ag test before vaccine dose 1 | 14 | 2/13b | 8/8 | 10 | 4 |
| Positive NAAT/Ag test after vaccine dose 1 or dose 2 | 19 | 1/15c | 11/12d | 5 | 14 |
| Only positive NAAT/Ag test was during MIS-C illnesse | 5 | 5/5 | 2/2 | 1 | 4 |
| No positive NAAT/Ag test; anti-nucleocapsid Ab test positivef | 20 | 0/20 | 20/20 | 6 | 14 |
| Without evidence of past or recent SARS-CoV-2 infection | 4 | ||||
| No positive NAAT/Ag test, anti-nucleocapsid Ab test negative, anti-spike Ab test positivea | 1 | 0/1 | 0/1 | 1 | 0 |
| No positive NAAT/Ag test, anti-nucleocapsid Ab test negative, anti-spike Ab test not performed | 3 | 0/3 | 0/3 | 3 | 0 |
| SARS-CoV-2 infection status could not be determined (incomplete SARS-CoV-2 testing) | 7 | ||||
| No positive NAAT/Ag test, anti-nucleocapsid Ab test not performed, anti-spike Ab test positivea | 4 | 0/4 | NA | 1 | 3 |
| No positive NAAT/Ag test, anti-nucleocapsid Ab test not performed, anti-spike Ab test not performed | 3 | 0/3 | NA | 1 | 2 |
Additional information on timing of previous NAAT/Ag tests and vaccination is in Supplementary Tables B and C.
Abbreviations: Ab, antibody; CDC, Centers for Disease Control and Prevention; MIS-C, multisystem inflammatory syndrome in children, NAAT/Ag, SARS-CoV-2 nucleic acid amplification/antigen test; NA, not applicable.
Met 2020 CDC MIS-C definition because of positive NAAT/Ag or serology test. While not necessarily indicating SARS-CoV-2 infection in a vaccinated child, a positive anti-spike antibody test satisfies the SARS-CoV-2 test result criterion of the CDC MIS-C definition.
These positive tests were 3 and 27 days after child’s previous positive test.
This positive test was 40 days after child’s previous positive test.
Child who tested negative on anti-nucleocapsid antibody test tested positive by NAAT/Ag 21 days earlier.
For one child who had 2 vaccine doses, a household member had NAAT/Ag-positive test approximately 40 days before child’s MIS-C illness onset. For another child who had 2 vaccine doses (illness onset 51 days after first and 3 days after second vaccine dose), a household member had NAAT/Ag-positive test approximately 5 weeks before child’s MIS-C illness onset. This child died.
For 8 of the 20 children, a household member had NAAT/Ag-positive test before child’s MIS-C illness (range approximately 12–39 days before illness onset).
Among these 58 children, 26 (45%) had shock, 15 (26%) had left ventricular ejection fraction (LVEF) < 55%, 5 (9%) had coronary artery dilation/ectasia but none had coronary artery aneurysms (Supplementary Table D). Fifty-one (88%) met Brighton level 1 (definitive case); 3 (5%) had discharge diagnosis of KD/atypical KD. Fifty-seven (98%) were discharged home. One child who had a positive NAAT test at the hospital died with cause of death assessed as MIS-C associated with COVID-19 by state medical examiner. The child’s MIS-C illness onset was approximately 5 weeks after a household member tested NAAT/Ag positive and 3 days after COVID-19 vaccine dose 2.
Of the 4 children who met the clinical and inflammatory criteria of the CDC MIS-C definition and had no evidence of SARS-CoV-2 infection (Figure 1), 3 (75%) were male; 2 were Asian and 2 were another race/ethnicity category. Three had MIS-C illness onset in November and 1 in December 2021 (Supplementary Figure A). Each had symptom onset after vaccine dose 1 (1, 10, 12, and 23 days after vaccination). One (25%) had shock treated with vasoactive medications, 1 (25%) had LVEF <55%, 2 (50%) had coronary aneurysms (Supplementary Tables D and E). Two (50%) met Brighton level 1 (definitive) and 2 (50%) met level 2a (probable). Three (75%) were given discharge diagnosis of KD/atypical KD by treating clinicians; in records available, clinicians mentioned possible MIS-C illness from COVID-19 vaccine in 2. The fourth child was diagnosed with MIS-C. All 4 were discharged home. Through 31 December 2021, 7 269 846 children aged 5−11 years received ≥1 dose of COVID-19 vaccine.
DISCUSSION
We identified 58 children who had MIS-C illness onset within 90 days after COVID-19 vaccination and had evidence of past or recent SARS-CoV-2 infection, and 4 who met the clinical and inflammatory CDC MIS-C criteria and had no evidence of SARS-CoV-2 infection. Vaccine became available for this age group approximately 5−6 weeks before the steep increase in reported COVID-19 cases during which Omicron emerged as the dominant variant (Supplementary Figure A). Given this overlap in timing of vaccine uptake and immense increase in infections, many MIS-C cases from recent SARS-CoV-2 infection were anticipated to occur in this age group and, by coincidence, be temporally associated with COVID-19 vaccination. Demonstrating the widespread high transmission of SARS-CoV-2 to US children in late 2021/early 2022, national anti-nucleocapsid antibody seroprevalence among persons aged 0−17 years was 42% in November 2021, and among children aged 0−11 years increased from 44% in December 2021 to 75% in February 2022 [9].
Whether COVID-19 vaccination contributed to the MIS-C illnesses identified through our passive surveillance cannot be determined. Four children had no known history or laboratory evidence of SARS-CoV-2 infection. Given that 7 269 846 children in our age group had received ≥1 vaccine dose through 31 December 2021, these 4 yield a reporting rate of 0.6 children with MIS-C illness and no evidence of SARS-CoV-2 infection per million vaccinated children. It is possible that these illnesses were KD or a different inflammatory illness or that a recent SARS-CoV-2 infection was not detected by the anti-nucleocapsid antibody test (false negative) [10]. Overall, the clinical features of MIS-C illnesses in our investigation appear similar to cases in younger children from CDC’s MIS-C national surveillance before vaccine availability [11], and to those of the 690 children aged 5−11 years not reported to have received COVID-19 vaccine before their MIS-C onset during November 2021 through March 2022: 31% had shock, 23% had cardiac dysfunction, 10% had coronary artery aneurysms/dilation, and 1% died (CDC MIS-C national surveillance, unpublished data).
Timing of SARS-CoV-2 infection relative to symptom onset of MIS-C can help inform the assessment of whether that infection likely caused the illness while vaccination was probably coincidental. Among the 33 children for whom some information was available on timing of infection, a previous positive NAAT/Ag test was in the distant past for 3 (339, 398, 451 days before MIS-C onset). Two of these 3 children were tested for anti-nucleocapsid antibodies (both positive); whether those antibodies were evidence of only the remote infection, or an additional recent infection with subsequent MIS-C, is unknown.
Limitations of our evaluation included the reliance on passive surveillance systems that likely did not capture all children with MIS-C. We may have included some illnesses that were not MIS-C, including inflammatory disease processes that could have also occurred prepandemic but may have been reported as MIS-C due to overlapping clinical features and the high background anti-nucleocapsid antibody seroprevalence. In addition, we included KD in our analysis because it can be impossible to distinguish between KD and MIS-C, and absence of evidence of SARS-CoV-2 infection would likely influence a clinician to diagnose KD rather than MIS-C (conversely, with evidence of SARS-CoV-2 infection, MIS-C rather than KD). Our surveillance period covered only early vaccine uptake.
There are several case reports of adolescents and adults diagnosed with MIS shortly after COVID-19 vaccination with no laboratory evidence of SARS-CoV-2 infection using currently available assays [12–14]. These reports have the same limitations as our investigation with inability to conclude that COVID-19 vaccination caused or contributed to the illness. Prior results from MIS-C surveillance among US adolescents, and from France, demonstrated that most vaccinated adolescents with MIS-C illness had evidence of past or recent SARS-CoV-2 infection and reporting rates for those without evidence of infection were <1 case/million vaccinated persons [6, 15]. Our findings among children aged 5–11 years are similar and support that, during a period with extensive SARS-CoV-2 circulation, MIS-C illness in children within 90 days after Pfizer-BioNTech COVID-19 vaccination who had no evidence of past or recent SARS-CoV-2 infection was rare (<1 per million vaccinated children).
Supplementary Material
Financial support.
This work was supported by the Centers for Disease Control and Prevention Clinical Immunization Safety Assessment Project (contract numbers 200-2012-53661 to Cincinnati Children’s Hospital Medical Center and 200-2012-50430-0005 to Vanderbilt University Medical Center).
Footnotes
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Disclaimer.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or the Food and Drug Administration (FDA). Mention of a product or company name is for identification purposes only and does not constitute endorsement by the CDC or the FDA.
Potential conflicts of interest.
S. K.’s institution has received funding from National Institutes of Health (NIH) to conduct clinical trials of Moderna and Janssen COVID-19 vaccines, and funding from Pfizer to conduct clinical trials of Pfizer-BioNTech COVID-19 vaccines. M. A. S. reports funding from NIH, CDC, Pfizer, and Merck; and royalties from UpToDate. K. E. reports grant funding from NIH and CDC; consultant to Bionet, GSK, and IBM; and membership of Data Safety and Monitoring Board for Sanofi, X-4 Pharma, Seqirus, Moderna, Pfizer, Merck, and Roche. C. B. C. reports grants from NIH and Merck; royalties from UpToDate; consulting fees from GlaxoSmithKline, Horizon Pharma, Premier Healthcare, Pfizer, Moderna, Cowen Investments, and Vindico; payment for medicolegal testimony from multiple firms (none related to this investigation); a US patent for staphylococcal antibody (number 10 981 979); participation on a data safety monitoring board or advisory board for Astellas; and is President of the Pediatric Infectious Diseases Society. E. P. S. reports grants from the NIH and Pfizer; consulting fees from Sanofi Pasteur; and serves on the Data Safety Monitoring Board for vaccine trials sponsored by the NIH’s Division of Microbiology and Infectious Diseases. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. COVID data tracker: multisystem inflammatory syndrome in children (MIS-C) Data through November 28, 2022. https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance. Accessed 10 December 2022.
- 3.Zambrano LD, Newhams MM, Olson SM, et al. BNT162b2 mRNA vaccination against COVID-19 is associated with decreased likelihood of multisystem inflammatory syndrome in U.S. children ages 5–18 years. Clin Infect Dis 2022; 76:e90–e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy M, Recher M, Hubert H, et al. Multisystem inflammatory syndrome in children by COVID-19 vaccination status of adolescents in France. JAMA 2022; 327:281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nygaard U, Holm M, Hartling UB, et al. Incidence and clinical phenotype of multisystem inflammatory syndrome in children after infection with the SARS-CoV-2 delta variant by vaccination status: a Danish nationwide prospective cohort study. Lancet Child Adolesc Health 2022; 6:459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yousaf AR, Cortese MM, Taylor AW, et al. Reported cases of multisystem inflammatory syndrome in children aged 12–20 years in the USA who received a COVID-19 vaccine, December, 2020, through August, 2021: a surveillance investigation. Lancet Child Adolesc Health 2022; 6:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2021; 39:3037–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. COVID data tracker: vaccinations in the US https://covid.cdc.gov/covid-data-tracker/#vaccine-delivery-coverage. Accessed 1 May 2022.
- 9.Clarke KE, Jones JM, Deng Y, et al. Seroprevalence of infection-induced SARS-CoV-2 antibodies—United States, September 2021–February 2022. MMWR Morb Mortal Wkly Rep 2022; 71:606–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen LR, Sami S, Vuong N, et al. Lack of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a large cohort of previously infected persons. Clin Infect Dis 2021; 73:e3066–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belay ED, Abrams J, Oster ME, et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr 2021; 175:837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai Q, Nygaard U, Schmidt RC, Zaremba T, Møller AM, Thorvig CM. Multisystem inflammatory syndrome in a male adolescent after his second Pfizer-BioNTech COVID-19 vaccine. Acta Paediatr 2022; 111:125–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yalçinkaya R, Öz FN, Polat M, et al. A case of multisystem inflammatory syndrome in a 12-year-old male after COVID-19 mRNA vaccine. Pediatr Infect Dis J 2022; 41: e87–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varghese M, Alsoub HARS, Koleri J, et al. Multisystem inflammatory syndrome in children (MIS-C) secondary to COVID-19 mRNA vaccination—a case report from Qatar. IDCases 2022; 30:e01606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouldali N, Bagheri H, Salvo F, et al. Hyper inflammatory syndrome following COVID-19 mRNA vaccine in children: a national post-authorization pharmacovigilance study. Lancet Reg Health Eur 2022; 17:100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


