Abstract
Objective
Patient-reported outcome measures (PROMs) are useful standardized tools to measure current patient health status and well-being. While there are existing constipation-related PROMs, the majority of PROMs were not developed with adequate patient involvement and few examined content validity. Accordingly, the current study aimed to develop a constipation PROM with multiple phases of patient and clinician involvement.
Methods
To generate PROM items, 15 patients with chronic constipation (age range =28–79 years, 10 females) underwent a qualitative interview exploring their experiences with chronic constipation. Following that, eight clinical experts completed the content validity index (CVI) ratings of all the items generated to assess content validity. Based on results of the content validity assessment, relevant items were maintained and 12 participants with chronic constipation were re-interviewed to obtain feedback about comprehensibility, comprehensiveness and relevance.
Results
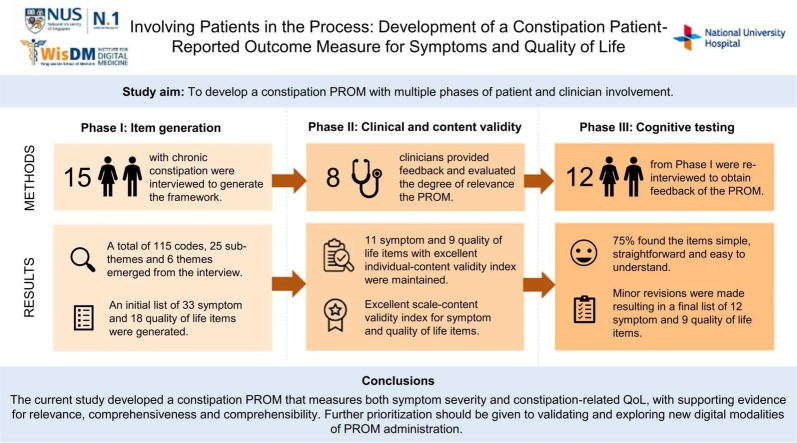
Six themes and 25 sub-themes emerged from the qualitative interview, and an initial list of 33 symptom items and 18 quality of life (QoL) items were generated. Based on the CVIs calculated, 11 symptom items and nine QoL items were maintained with the scale-content validity index indicating excellent content validity. Overall, participants indicated the PROM to be relevant, comprehensive and easy to understand however, minor amendments were made to improve the three qualities of interest.
Conclusion
The current study developed a constipation PROM that measures both symptom severity and constipation-related QoL, with supporting evidence for relevance, comprehensiveness and comprehensibility. Further prioritization should be given to validating and exploring new digital modalities of PROM administration.
Keywords: Patient-reported outcome measures, Constipation, Quality of life, Digital health
Graphical Abstract
Highlights
-
•
Constipation management often relies on self-report and PROMs developed with minimal patient input.
-
•
This study developed a PROM with patient and clinician input to assess constipation symptoms and quality of life.
-
•
A well-validated PROM empowers patients in self-care and monitoring, paving the way for personalized digital health tracking.
1. Introduction
Chronic constipation is a prevalent gastrointestinal condition that affects approximately 10–15% of the global population. [1] In 2017–18, treatment for constipation cost the National Health Service (NHS) £ 162 million and in the United States, direct medical costs for constipation were estimated to be $235 million annually. [2], [3] Further economic burden of constipation can be observed through the decrease in work productivity, with constipation patients reporting significantly higher absenteeism and medical visits compared to controls. [4] Patients with chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C) subtypes experience a variety of symptoms including infrequent bowel movements, excessive straining, hard stools, bloating and abdominal pain. [5], [6] Treatment can range from dietary and lifestyle changes to pharmacological therapy in a stepwise or complementary manner. [7] Given the heterogenous nature of constipation and the combinations of pharmacological and non-pharmacological interventions available, the diagnosis and management of chronic constipation can be complex and challenging. As each treatment should ideally be individualized, continual monitoring of symptoms and refinement of management strategies are essential to successful treatment progress. [7].
An integral part of symptom monitoring includes obtaining an accurate and up-to-date status of health and well-being through patient self-reporting. In this instance, the usage of a relevant, reliable and validated patient-reported outcome measure (PROM) can be useful in monitoring intervention success in terms of clinical symptoms and quality of life (QoL) outcomes in patients. [8] PROMs are standardized and validated questionnaires that measure patients’ perception of their own health status and/or well-being. [9] In conjunction with the usage of PROMs for research purposes, PROMs have been increasingly adopted in clinical practice, particularly high volume services that require close tracking of patients’ progress (e.g. hip and knee replacement, cancer treatment, haemodialysis). [10], [11], [12] The integration of PROMs in clinical practice has been reported to be feasible with varying benefits including improvement of clinician-patient communication, the ability to tailor patient therapy and care, and increased patient empowerment. [13], [14].
To fully capture patients’ perspective, PROM developers should be conscious of involving patients early in the development process and consistently throughout development. [15] Nevertheless, patient involvement in the development and implementation of PROMs are lacking with a review reporting only 9.3% of patients (out of 193 PROMs reviewed) were involved in the development of frameworks or domains of PROMs. [15] Similarly in constipation, our systematic review reported that the majority of PROMs (14 out of 23) scored “inadequate” for development based on the COSMIN (COnsensus-based Standards for the selection of health Measurement INstruments) guidelines due to the absence of patient input in item development or feedback regarding the relevance, comprehensibility and comprehensiveness of the PROM. [16], [17] The lack of patient involvement in the development process has shown to result in PROMs that may not optimally reflect a patient’s perspective due to the inclusion of unimportant items and exclusion of important aspects to the patients. [18] Beyond PROM development, research studies have increasingly included patients’ perspective through patient advocate or patient peer reviewer roles in the development and implementation of new intervention designs and clinical treatments.
There has been an increasing push for patient involvement in existing and new PROM development by regulatory boards, such as the Food and Drug Administration (FDA). [19] Following the recommendation of the FDA and the COSMIN checklist, the current study aimed to develop a new chronic constipation PROM with the involvement of patients from development of PROM items to obtaining feedback on comprehensibility, comprehensiveness and relevance.
2. Methods
The novel constipation PROM was developed based on the COSMIN guidelines to ensure relevance, comprehensiveness and comprehensibility. [17] The PROM was developed in three phases: i) Phase I (Item generation): qualitative interviews with participants to gather concepts of interest to generate a conceptual framework; ii) Phase II (Clinical and content validity): input from clinicians to assess clinical relevance and content validity; and iii) Phase III (Cognitive testing): input from participants to revise and improve provisional items. The study procedures were approved by the National University of Singapore Institutional Review Board (IRB; IRB number: NUS-IRB-2020–696).
2.1. Recruitment
Participants with chronic constipation were recruited from the National University Hospital (NUH) in Singapore. The study clinician, a fully trained consultant gastroenterologist at NUH identified potential participants and obtained their verbal consent to be contacted by the research team via phone calls or email. Interested participants were contacted and provided with an overview of the study, along with a copy of the participant information sheet. Participants were screened and included based on the criteria listed in Table 1.
Table 1.
Inclusion and exclusion criteria of participants.
| Inclusion/Exclusion | Criteria |
|---|---|
| Inclusion criteria |
|
| Exclusion criteria |
|
IBS-C; irritable bowel syndrome with constipation
2.2. Phase I: Item generation
As the study aimed to incorporate patients’ perspectives of their chronic constipation health outcomes and quality of life into the PROM development process, patients with chronic constipation were interviewed in Phase I to provide researchers with insights to generate relevant PROM items. Fifteen participants with chronic constipation completed Phase I. All participants were fluent in English. Sixty-minute semi-structured interviews were conducted either in-person or via video-conferencing, depending on participant’s preference. Informed consent for the study was obtained prior to commencing the interviews and all participants consented to audio-recording for transcription purposes. An interview guide was developed based on a systematic review [16] and input from the study clinician. The open-ended questions covered patients’ experience with chronic constipation (see Supplementary Material). All interviews were conducted in English between March and July 2021. Participants were reimbursed for their time and all data collected were de-identified and stored in a secure database.
Interviews lasted between 29 and 97 minutes. All interview recordings were transcribed verbatim and inductive thematic analysis was used to identify emerging or recurring themes. Primary coding, during which data from the transcripts were descriptively labelled to generate initial codes, was conducted. Based on the primary codes, secondary codes were generated through categorizing of labelled data. Finally, categories emerging from secondary coding were analyzed and grouped into broader, overarching themes. [20] Initial codes and frameworks were developed independently by two researchers, and a final set of codes and broader themes were generated from discussion and iterations. All themes and sub-themes recurred in both females and males.
Based on the themes and sub-themes from the interview, the PROM items were generated through multiple discussions, including consultation with the study clinician regarding clinical relevance, and referral to interview transcripts for item phrasing.
2.3. Phase II: Clinical and content validity
Following item generation, the provisional questionnaire was submitted for content validity assessment by eight clinical experts who are familiar with the construct of interest (3 gastroenterologists, 2 medical residents, a rheumatologist, a geriatrician and a psychologist). Experts were asked to rate the degree of relevance of each candidate item to the measured domain of each item on a 4-point Likert scale (1 =not relevant, 2 =somewhat relevant, 3 =quite relevant, 4 =highly relevant). Experts were also asked to provide narrative comments for additional feedback.
For both domains, the content validity of individual items was determined using the item-content validity index (I-CVI; ), which represents the proportion of experts who gave a relevance rating of 3 or 4 for each item. [21] Items that met the minimum I-CVI of.78 for excellent content validity were included in the provisional PROM. [22], [23], [24] The scale-content validity index based on average method (S-CVI/Ave; ) was then calculated for each domain, which represents the proportion relevance of the scale assessed by all experts. Items with a minimum S-CVI/Ave of.90 were considered to have excellent content validity. [22], [23], [24].
2.4. Phase III: Cognitive testing
Following input from the clinical experts, cognitive testing of the provisional PROM was conducted to assess comprehensibility, comprehensiveness and relevance. Participants from Phase I were contacted and scheduled for a 30-minute semi-structured interview. Interviews were conducted either in-person or via video-conferencing, depending on participant’s preference. The provisional PROM was completed via pen-and-paper for in-person interviews and screen-sharing function for online interviews. During the interview, participants were asked to complete the provisional PROM and ‘think aloud’ during the process. Following completion of the PROM, participants were asked open-ended questions that covered overall feedback, comprehension, relevance, comprehensiveness and length (see Supplementary Material). Twelve participants with chronic constipation completed Phase III. All interviews were audio-recorded and conducted in English between November 2021 and January 2022. Participants were reimbursed for their time and all data collected were de-identified and stored in a secure database.
Interviews lasted between 20 and 46 minutes. Similar to Phase I, all interview recordings were transcribed verbatim and inductive thematic analysis was used to identify emerging or recurring themes. Initial codes and frameworks were developed independently by two researchers, and final set of codes and broader themes were generated from discussion and iterations. A list of items to address was generated and the provisional PROM was amended based on feedback from participants and input from the study clinician.
3. Results
3.1. Participant characteristics
Participant demographic data for Phase I and III are presented in Table 2.
Table 2.
Demographic data of participants in Phase I and III.
| Demographic categories | Participant characteristics |
|---|---|
| Phase I (n = 15) | |
| Sex | Male (n = 5), Female (n = 10) |
| Ethnicity | Chinese (n = 13) |
| Malay (n = 1) | |
| Indian (n = 1) | |
| Age | Range: 28–78 years; Mean = 53 years |
| Education | Secondary school (n = 5) |
| Diploma or equivalent (n = 1) | |
| Bachelor’s or equivalent (n = 1) | |
| Master’s or equivalent (n = 8) | |
| Comorbidities* | None (n = 3) |
| 1–2 (n = 8) | |
| 3 or more (n = 4) | |
| Phase III (n = 12) | |
| Sex | Male (n = 2), Female (n = 10) |
| Ethnicity | Chinese (n = 11) |
| Malay (n = 1) | |
| Age | Range: 28–79 years; Mean = 52 years |
| Education | Secondary school (n = 4) |
| Diploma or equivalent (n = 1) | |
| Bachelor’s or equivalent (n = 1) | |
| Master’s or equivalent (n = 6) | |
| Comorbidities* | None (n = 2) |
| 1–2 (n = 7) | |
| 3 or more (n = 3) |
Comorbidities: allergic rhinitis, cardiovascular disease, diabetes, fibromyalgia, hepatitis B carrier high blood pressure, high cholesterol, kidney disease, migraine, orthopedic issues, gallstones, gastroesophageal reflux disease.
3.2. Phase I: Item generation
A total of 115 codes (35 symptom codes and 80 QoL-related codes), 25 sub-themes and six themes were generated from the interviews. The overarching domains include symptoms and QoL (i.e. psychological impact, relationships, health-related worries, healthcare experiences and interference in life).
3.2.1. Symptoms
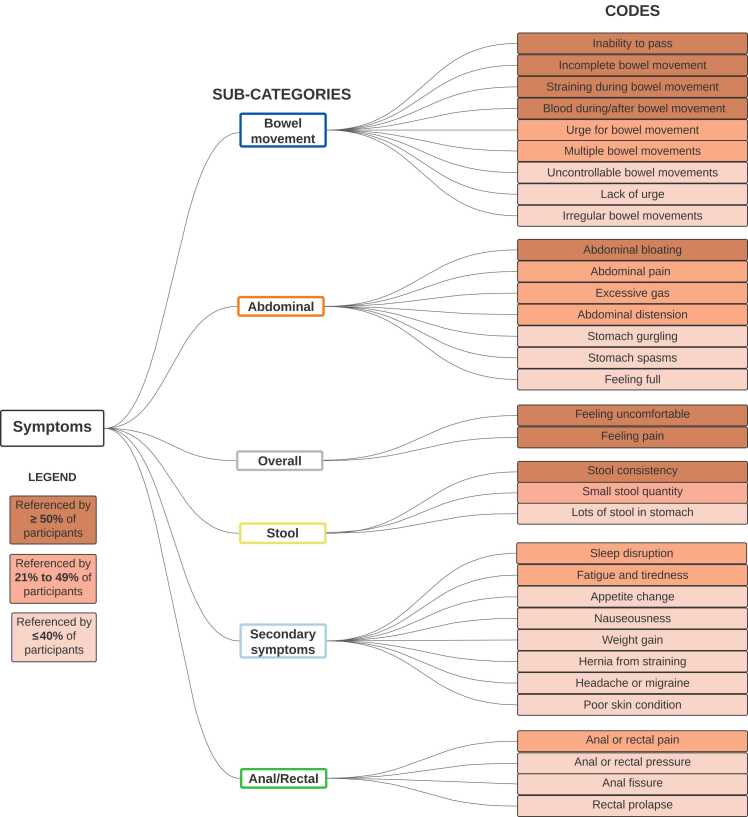
The majority of participants described a general feeling of uncomfortableness and/or pain when asked to describe their experience with chronic constipation. Participant 13 described, “[Constipation] affects me because I can’t feel comfortable. Yeah… when you [have] stools inside, you don’t feel comfortable.” Beyond overall sensation of discomfort and pain, participants also described specific symptoms that can be categorized into the following categories: bowel movement, abdominal, stool, anal/rectal and secondary symptoms. See Fig. 1 for specific symptoms within each category and percentage referenced by participants.
Fig. 1.
Coding tree of constipation-related symptoms and candidate items for the provisional PROM.
3.2.2. Quality of life
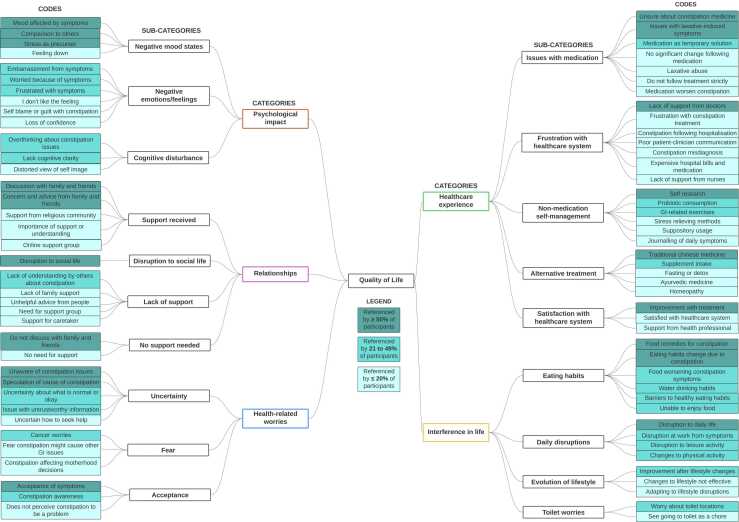
When asked about the impact of chronic constipation on QoL, participants described issues that can be categorized into psychological impact, relationships, health-related worries, healthcare experiences and interference in life. See Fig. 2 for specific QoL-related codes within each category and percentage referenced by participants.
Fig. 2.
Coding tree of constipation-related QoL and candidate times for the provisional PROM.
Psychological impact. Regarding psychological impact, participants recounted a range of emotions and mood states associated with chronic constipation. Participant 15 shared, “[Having chronic constipation is] very draining, emotionally very draining for me… I mean it has affected me so much that I went through a lull period, whereby it was like depression.” Besides low mood, most participants highlighted instances of embarrassment or worry due to their constipation symptoms. Participant 8 shared, “I vividly remember a meeting I went to, and [my boss] just humiliated me in front of everybody when my stomach made a sound… I just told everybody I [had] something bad for lunch.” Some participants also described cognitive disturbance, including lack of focus and overthinking, which can affect studies, work and sleep.
Relationships. In regards to relationships, the majority of participants stated that they actively shared concerns and discussed with family and/or friends about their constipation. When asked if she discusses her chronic constipation issues with anyone besides her doctor, Participant 5 replied, “Family, yes… [and] some friends, I do share. I’m quite open. I just tell them what I’m suffering from.” Nevertheless, some participants highlighted the lack of support or not needing support outside of the healthcare system for their condition. When asked about their social life, participants often described disruption to their social activities due to restrictive diet, poor mood, low energy or disruptive symptoms such as excessive flatulence and long time spent in the toilet. Participant 14 elaborated, “For me, after a while, all social decisions [have] to calibrate around when I need to move my bowel. If I don’t do it on Saturday, I need to do it on Sunday. Can I do it in the morning such that I am able to go out at night with [my friends]? This kind of mental recalibration, it has become part and parcel of my life, embedded in me like a norm.”
Health-related worries. When describing their issue with chronic constipation, participants often conveyed feelings of uncertainty and fear related to their health. Feelings of uncertainty mostly revolved around their symptoms, cause of constipation and next steps in constipation management. For instance, Participant 10 highlighted her confusion by stating, “I don’t actually know what is IBS. I know what it means – irritable bowel syndrome, but I don’t know… why is it so irritable? Why suddenly it become irritated? For what? What did I do? So, is it because of the poor choices of food when I was young?” In regards to fear, participants often expressed concern that constipation might be a precursor to or a sign of more severe medical issues, particularly stomach or colon cancer. Nevertheless, some participants expressed some positivity through awareness and acceptance of their symptoms. Participant 15 shared, “I think my problem, or rather I would say that things become better when I start[ed] to accept… acceptance and commitment. And trying to recognize rather than trying to fight against it. I think that was big hurdle for me. It was an emotional breakthrough, a mental breakthrough for me.”
Healthcare experience. Part of the experience of dealing with chronic constipation includes interaction with the healthcare system. Participants often expressed difficulties and frustration with their medication usage, as well as the need for sustained and supportive engagement with the healthcare system. In regards to medication, participants described challenges relating to uncertainty about the medication itself (e.g. function, side effects, safety of long-term intake) and laxative-induced side effects. When asked about her current medication intake for constipation management, Participant 13 described, “The Dulcolax [is] a bit too much, then I [had] 4 or 5 [bowel movements] that day so it was actually quite bad. I was constantly at the toilet for 4–5 days… So [the doctor] told me – you should buy 30 tablets of Dulcolax to try. Seriously, one tablet already gone 5 times that one day so, I’m not sure whether I will really take the 30 tablets.” The participants’ frustration further extends to the interactions with the healthcare system due to the lack of specialized care, particularly from general practitioners, and the lack of consistent follow-ups for constipation management. Nevertheless, with adequate support from the healthcare system and perceivable improvement from treatment, participants expressed satisfaction with the received care. Participant 7 described her satisfaction with follow-up phone calls, “So the nurse actually did call me one week after to check on my condition. I told her that I have this stomach pain at midnight after taking the medication [and] the nurse did actually help me reflect [this] to the doctor, then doctor changed the medication for me. So overall, I find that this treatment [is] quite good and actually higher than expectations.”
Interference in life. Participants described the impact of chronic constipation on their eating habits, daily activities and lifestyle. To manage their chronic constipation, the majority of participants altered their diet to incorporate more fibre (through fruits and vegetables) and reduce chilli, dairy and red meat intake. Some participants also described restricting fermentable oligosaccharides, disaccharides, monosaccharides and polyols (i.e. low FODMAP diet). Participant 14 highlighted the restrictive nature of adhering to a low FODMAP diet in Singapore, “So, when I did [the] FODMAP, I mean I was shocked to know that all Asian food… I cannot take at all. If I go take economy rice, I ask the uncle (local way of referring to the stall owner) – is there anything without onion? He looked at his tray and he looked at me and said sorry, no. What am I supposed to do?” Besides diet, participants described disruption to their work and day-to-day activities due to the long period of time spent in the bathroom, uncontrollable bowel movement due to laxatives, uncertainty about toilet locations, and embarrassment from symptoms such as stomach gurgling and flatulence. The sense of frustration due to daily disruptions was summed up by Participant 12 who mentioned, “It’s an inconvenience in my life because you are so used to [having a bowel movement] in the morning and then the rest of the day, you would be free. But now in the morning, you don’t [have a bowel movement] then the whole day you’re struggling. That is one very bad experience.”
Based on the themes and codes identified from the qualitative interview, provisional questionnaire items with 33 symptom items and 18 QoL-related items were developed.
3.3. Phase II: Clinical and content validity
For the domain of constipation symptoms, 11 symptom items rated as relevant (I-CVI =.86–1.0) were included in the provisional PROM for Phase III. For the constipation-related QoL domain, nine out of 18 items were relevant (I-CVI =.88–1.0) and included in the provisional PROM for Phase III. One of the clinical experts provided narrative feedback stressing the need for personalization of the PROM and enquiry regarding treatment history. Based on the I-CVI scores of the included items, the S-CVI/Ave scores were.95 and.92, indicating excellent content validity for the domains of constipation symptoms and constipation-related QoL respectively. See Table 3 for the CVI scores of the included items.
Table 3.
Content validity assessment scores of the provisional PROM.
| Item | I-CVI | S-CVI/Ave |
|---|---|---|
| Constipation symptom | .95 | |
| Unable to have a bowel movement | 1.0 | |
| Hard and dry stool | 1.0 | |
| Abdominal bloating | .86 | |
| Feeling incomplete after bowel movement | 1.0 | |
| Straining during bowel movement | 1.0 | |
| Urge to have a bowel movement | 1.0 | |
| Abdominal pain | 1.0 | |
| Excessive gas | .86 | |
| Little amount of stool | .86 | |
| Pressure around anus | .86 | |
| Lack of urge for bowel movement | 1.0 | |
| Constipation-related QoL | .92 | |
| My constipation problems disrupt my daily activities. | 1.0 | |
| Medication is not helping my constipation symptoms. | .88 | |
| I am unsure about how to manage my constipation problems. | .88 | |
| I have to restrict my diet due to my constipation. | .88 | |
| I feel down because of my constipation problems. | 1.0 | |
| I get emotionally bothered by my constipation problems. | .88 | |
| I avoid social activities due to my constipation problems. | .88 | |
| I am coping well with my constipation. | 1.0 | |
| I am worried that my constipation will lead to other health problems. | .88 |
I-CVI, item-content validity index; PROM, patient-reported outcome measure; QoL, quality of life; S-CVI/Ave, scale-content validity index based on average method
Following the content validity assessment, 11 symptoms items and nine QoL-related items were retained in the provisional PROM for further assessment.
3.4. Phase III: Cognitive testing
3.4.1. Comprehensibility, comprehensiveness and relevance
All participants understood the provisional PROM as intended and were able to complete the PROM without assistance. In terms of comprehensibility, most participants found the items and response scales simple, straightforward and easy to understand (75.0%). There was broad consensus across participants that the PROM had a good length (91.7%) and majority found the PROM’s questions comprehensive (58.3%). Many participants also agreed that the items were relevant to their condition (58.3%).
Patient feedback and revisiting the PROM resulted in several minor revisions to improve its comprehensibility and comprehensiveness. See Table 4 for an inventory of revisions following Phase III.
Table 4.
Inventory of revisions to the provisional PROM based on feedback from the cognitive testing session.
| Categories | Revision |
|---|---|
| Comprehensibility | |
| Phrasing of symptoms items |
|
| Phrasing of QoL items |
|
| Phrasing of item scales |
|
| Comprehensiveness | |
| Inclusion of new symptoms items |
|
| Inclusion of new outcome measure for symptoms |
|
PROM, patient-reported outcome measure; QoL, quality of life
The PROM created through Phase I-III consists of 12 symptom items and nine QoL-related items.
4. Discussion
A novel PROM for constipation symptoms and constipation-related QoL with a total of 21 items (12 symptom items and nine QoL-related items) was developed. The development involved input and feedback from patients with CIC and IBS-C, and a diverse group of clinical experts, which resulted in a PROM that was relevant to clinicians and patients, comprehensive and easy to understand.
When speaking to participants in Phase I, the impact of chronic constipation on QoL was evident with the high number of 80 QoL-related codes generated across all the interviews, stressing the importance of including the QoL component in our PROM. Similar to the Patient Assessment of Constipation Quality of Life (PAC-QOL) [25] and Constipation-Related Quality of Life (CRQOL) [26] questionnaires, the participants relayed concerns regarding relationships, psychological impact (e.g. negative mood state, health-related worries and cognitive impairment) and disruptions to daily life. While there were positive experiences highlighted within each theme (e.g. acceptance of symptoms, support from family and friends, improvement after lifestyle changes), the majority of experiences shared were negatively inclined. This is in line with studies that observed activity impairment, and impacted social and psychological functioning in patients with chronic constipation. [27], [28], [29] Beyond these aspects of QoL, participants from the current study highlighted new concepts absent in existing constipation-related QoL PROMs - the importance of healthcare experience, including experience with treatment or medication and interaction with healthcare system. Participants’ frustrations with their healthcare experience often stemmed from uncertainty from recommended medication, laxative-induced side effects, lack of specialized care from general practitioners and lengthy follow-up intervals. Patient satisfaction with treatment (i.e. global satisfaction, effectiveness, side-effects and convenience) and the received care can impact QoL thus, it is an important factor to consider when measuring constipation-related QoL. [30], [31], [32].
After generating the potential PROM items, the current study examined content validity with clinical experts and patients. As the study consisted of participants with co-morbidities that have symptoms overlapping with chronic constipation (e.g., gallstones, gastroesophageal reflux disease), content validity with clinical experts was necessary to ensure that the final list of items were clinically relevant to measuring health outcomes specific to chronic constipation. Overall, participants reported the PROM to be comprehensible, of good length, relevant to their condition and comprehensive. There was consensus on participants’ understanding of symptom items, which is important as differences in perception of symptoms across cultures could affect diagnosis of constipation. [33] The most notable amendment in the light of the participant feedback was an addition of the column to allow patients to indicate their most bothersome symptom(s). Receiving feedback from both the clinical experts and patients strengthened the potential impact of the PROM in being relevant to patient care. Although clinicians often represent their patients’ voice during the revision of new healthcare tools, a significant divergence has been noted in chronic constipation between the clinically measured symptom severity and symptoms that patients find bothersome. [34], [35] For instance, in a survey with 311 primary care physicians, altered stool consistency, abdominal discomfort and infrequent bowel movements were ranked as the three most severe symptoms perceived by the physicians however, abdominal discomfort, abdominal pain, straining and bloating were reported to be the most bothersome symptoms for patients. [35] As symptom bothersome measures the perceived importance of a symptom when it negatively impacts a patient, recognizing bothersome symptoms can aid clinicians better understand patients’ priorities and improve treatment satisfaction. [36], [37].
During Phase III, several participants also raised the need for a PROM that is highly tailored to their individual condition. This includes both the PROM items collected and the recall period. Given the variability in symptom fluctuations (e.g. more symptom changes during initial treatment period vs more stable symptoms following multiple months of successful treatment), some participants expressed that a two-week recall period is too long thus, unable to accurately capture the acute symptom fluctuations during that time, while some participants indicated two weeks to be too short as minimal changes would occur between the subsequent PROM collection timepoints. This was similarly raised by patients with other chronic conditions who stated that the standardized PROMs were not able to fully capture the complex or dynamic nature of their symptoms. [38], [39] For instance, patients undergoing substance use disorder treatment who completed the Treatment Outcomes Profile (TOP) and Clinical Outcome Routine Evaluation – Outcome Measure (CORE-OM) shared that not all contents covered by these PROMs were meaningful to their condition. [39] While the current study involved patients throughout the development of the PROM, the heterogenous nature of constipation and the multitude of treatment options may not be suited for a one-size-fits-all PROM. One potential avenue is to leverage digital health to enable personalization. Using the current provisional PROM as a foundation, an electronic PROM (ePRO) may offer patients the ability to record and track additional symptoms and QoL items that matter to them. In a precedent study, a mobile app-based, personalized ePRO for rheumatology patients was shown to be a feasible and reliable option for remote monitoring of symptoms and treatment efficacy, with the majority of patients preferring the ePRO over paper-based forms. [40] Accordingly, beyond validation of the current PROM, next steps should include developing an ePRO that can be personalized, and is also relevant to both clinicians and patients. Collectively, the datasets realized from ePROs or PROMs may help with the development of even more downstream solutions to help improve treatment outcomes for patients, from enhanced methods of patient stratification, to dynamically targeted interventions and digital biomarkers, among others.
While the current study actively engaged patients and clinical experts for ideas and feedback throughout the development phases, the engagement with the clinical experts, besides the study clinician, was limited to questionnaires and online communication. Qualitative interviewing of clinical experts may provide further insights into clinical relevance and comprehensiveness of the PROM and future investigations should be conducted to further strengthen content validity. Nevertheless, the CVI is a widely used method to quantify content validity and an appropriate indicator when examining multi-item scales. [23] Furthermore, patients were recruited through the study clinician, thus experiences with constipation treatment and levels of treatment satisfaction were similar. Nonetheless, some patients shared that their constipation treatment journey involved previous negative experiences with other healthcare professionals. Future studies should expand recruitment to different levels of healthcare to engage patients who have interacted with other generalists and specialists regarding their constipation issues. This would allow a more in-depth exploration of the impact of treatment satisfaction on patients’ QoL.
There were also some limitations to the socio-demographic factors of the study population. More females were recruited in Phase I and III compared to males. The recruitment outcome of the study reflects the reported prevalence difference of chronic constipation between males and females, where females were 2.2 times more likely to have chronic constipation compared to males. [41] Nevertheless, it should be noted that all themes and sub-themes recurred in both sexes, and all symptom and quality of life items generated in Phase I were included and considered in Phase II. Besides that, the majority of participants in Phases I and III were Chinese. However, it should be noted that the recruitment outcome of the study reflected the study’s attempt to capture the ethnic distribution of Singapore (i.e., 74.3% Chinese, 13.5% Malays, 9.0% Indians and 3.2% others). [42] Finally, about half of the participants in Phase I and III had an education level of master’s or equivalent. An individual's education attainment has been shown to impact health behaviour due to better understanding of health information. [43] Accordingly, participants in the current study may be more aware and articulate about the health outcomes they are interested in. Nevertheless, cognitive testing was conducted with four participants with an education level of secondary school of equivalent to ensure comprehension of the items generated.
Overall, the current study developed a constipation PROM that measures both symptom severity and constipation-related QoL. Based on input and feedback from patients and clinical experts, the created PROM demonstrates evidence for clinical relevance, comprehensiveness and comprehensibility. Next steps in the continual development of the PROM involves a validation study to assess the psychometric properties of each subscale and the overall scale. While the next step involves validating the PROM within the Singapore population, further cross-cultural validation will be required to adapt the current PROM into a different population. For instance, translation adaptions and reassessment of the psychometric properties of the translation need to be conducted to further adapt the PROM into other languages. Given the potential of ePRO for personalization and capturing outcome measures in real-time between clinical follow-ups, further prioritization should also be given to exploring new digital modalities for the administration and personalization of the traditional PROM.
Funding/Support
This work was supported by funding from the Institute for Digital Medicine (WisDM) Translational Research Programme [grant number R-719–000–037–733] at the Yong Loo Lin School of Medicine, National University of Singapore.
Conflict of interest disclosures
AB and DH are co-inventors or previously filed pending patents on artificial intelligence-based therapy development. DH is a shareholder of KYAN Therapeutics, which has licensed intellectual property pertaining to AI-based drug development. VVL, NYL and KTHS have no conflict of interest.
Role of the funder/sponsor
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CRediT authorship contribution statement
VVL: conceptualisation, methodology, investigation, formal analysis, project administration, supervision, writing – original draft, writing – review and editing; NYL: methodology, investigation, data curation, formal analysis, project administration, visualisation, writing – original draft, writing – review and editing; AB: conceptualisation, methodology, supervision, writing – review and editing; KTHS: conceptualisation, methodology, formal analysis, supervision, writing – review and editing; DH: conceptualisation, funding acquisition, supervision, writing – review and editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Dean Ho reports a relationship with KYAN Therapeutics that includes: equity or stocks. Dean Ho and Agata Blasiak has patent Integrated Decision Tree/Artificial Intelligence (AI)-enabled Platform to Dynamically, Continuously, and Sustainably Optimise Agriculture and Food Production Yield pending to N/A. Dean Ho and Agata Blasiak has patent #Saliva Collection Apparatus and Method (International Patent Application No. PCT/SG2021/050485) pending to N/A. Dean Ho and Agata Blasiak has patent #Cognitive Training Platform (European Patent Application No. 20791871.5; US Patent Application No. 17/603,115; Singapore Patent Application No. 11202111204Y) pending to N/A. Agata Blasiak has patent #Invention: Implantable Guide Element and Methods of Fabrication and Use Thereof (US Patent Application No. 17/784,278) pending to Amitabha LAHIRI (NUS).
Acknowledgement
We would like to thank Yoann Sebastien Sapanel and David Jun Yuan Xi for their assistance with transcriptions in this research.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.csbj.2023.09.034.
Contributor Information
V Vien Lee, Email: vvien.lee@nus.edu.sg.
Agata Blasiak, Email: agata.blasiak@nus.edu.sg.
Kewin Tien Ho Siah, Email: kewin_siah@nuhs.edu.sg.
Dean Ho, Email: biedh@nus.edu.sg.
Appendix A. Supplementary material
Supplementary material.
.
References
- 1.Aziz I., Whitehead W.E., Palsson O.S., Törnblom H., Simrén M. An approach to the diagnosis and management of Rome IV functional disorders of chronic constipation. Expert Rev Gastroenterol Hepatol. 2020;14(1):39–46. doi: 10.1080/17474124.2020.1708718. [DOI] [PubMed] [Google Scholar]
- 2.The Lancet Gastroenterology H. The cost of constipation. Lancet Gastroenterol Hepatol. 2019;4(11):811. doi: 10.1016/s2468-1253(19)30297-3. [DOI] [PubMed] [Google Scholar]
- 3.Martin B.C., Barghout V., Cerulli A. Direct medical costs of constipation in the United States. Manag Care Interface. 2006;19(12):43–49. [PubMed] [Google Scholar]
- 4.Sun S.X., Dibonaventura M., Purayidathil F.W., Wagner J.S., Dabbous O., Mody R. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sci. 2011;56(9):2688–2695. doi: 10.1007/s10620-011-1639-5. [DOI] [PubMed] [Google Scholar]
- 5.Lacy B.E., Mearin F., Chang L., et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–1407. doi: 10.1053/j.gastro.2016.02.031. e5. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri M., Ford A.C., Mawe G.M., et al. Chronic constipation. Nat Rev Dis Prim. 2017;3 doi: 10.1038/nrdp.2017.95. [DOI] [PubMed] [Google Scholar]
- 7.Kassebaum-Ladewski A., Poppers D.M., Brenner D.M. Effective communication strategies and tools for improving treatment outcomes in patients with chronic idiopathic constipation and irritable bowel syndrome with constipation. Am J Gastroenterol. 2022;117(4s) doi: 10.14309/ajg.0000000000001686. S14-s20. [DOI] [PubMed] [Google Scholar]
- 8.Almario C.V., Spiegel B.M.R. Employing irritable bowel syndrome patient-reported outcomes in the clinical trenches. Clin Gastroenterol Hepatol. 2018;16(4):462–466. doi: 10.1016/j.cgh.2017.12.026. e2. [DOI] [PubMed] [Google Scholar]
- 9.Dawson J., Doll H., Fitzpatrick R., Jenkinson C., Carr A.J. The routine use of patient reported outcome measures in healthcare settings. Bmj. 2010;340:c186. doi: 10.1136/bmj.c186. [DOI] [PubMed] [Google Scholar]
- 10.Schoenmakers D.A.L., Schotanus M.G.M., Boonen B., Kort N.P. Consistency in patient-reported outcome measures after total knee arthroplasty using patient-specific instrumentation: a 5-year follow-up of 200 consecutive cases. Knee Surg Sports Trauma Arthrosc. 2018;26(6):1800–1804. doi: 10.1007/s00167-017-4800-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duman-Lubberding S., van Uden-Kraan C.F., Jansen F., et al. Durable usage of patient-reported outcome measures in clinical practice to monitor health-related quality of life in head and neck cancer patients. Support Care Cancer. 2017;25(12):3775–3783. doi: 10.1007/s00520-017-3808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thestrup Hansen S., Kjerholt M., Friis Christensen S., Brodersen J., Hølge-Hazelton B. User experiences on implementation of patient reported outcome measures (PROMs)in a Haematological outpatient clinic. J Patient Rep Outcomes. 2020;4(1) doi: 10.1186/s41687-020-00256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobrozsi S., Panepinto J. Patient-reported outcomes in clinical practice. Hematol 2014, Am Soc Hematol Educ Program Book. 2015;(1):501–506. doi: 10.1182/asheducation-2015.1.501. [DOI] [PubMed] [Google Scholar]
- 14.Øvretveit J., Zubkoff L., Nelson E.C., Frampton S., Knudsen J.L., Zimlichman E. Using patient-reported outcome measurement to improve patient care. Int J Qual Health Care. 2017;29(6):874–879. doi: 10.1093/intqhc/mzx108. [DOI] [PubMed] [Google Scholar]
- 15.Wiering B., de Boer D., Delnoij D. Patient involvement in the development of patient-reported outcome measures: a scoping review. Health Expect. 2017;20(1):11–23. doi: 10.1111/hex.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee V.V., Lau N.Y., Xi D.J.Y., et al. A systematic review of the development and psychometric properties of constipation-related patient-reported outcome measures: opportunities for digital health. J Neurogastroenterol Motil. 2022 doi: 10.5056/jnm22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prinsen C.A.C., Mokkink L.B., Bouter L.M., et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1147–1157. doi: 10.1007/s11136-018-1798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiering B., de Boer D., Delnoij D. Asking what matters: the relevance and use of patient-reported outcome measures that were developed without patient involvement. Health Expect. 2017;20(6):1330–1341. doi: 10.1111/hex.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Food and Drug Administration. Patient-reported outcome measures: Use in medical product development to support labeling claims. 2009: 1–39.
- 20.Kolb S.M. Grounded theory and the constant comparative method: valid research strategies for educators. J Emerg Trends Educ Res Policy Stud. 2012;3(1):83–86. [Google Scholar]
- 21.Yusoff M.S.B. ABC of content validation and content validity index calculation. Resource. 2019;11(2):49–54. [Google Scholar]
- 22.Polit D.F., Beck C.T. The content validity index: are you sure you know what's being reported? Critique and recommendations. Res Nurs Health. 2006;29(5):489–497. doi: 10.1002/nur.20147. [DOI] [PubMed] [Google Scholar]
- 23.Polit D.F., Beck C.T., Owen S.V. Is the CVI an acceptable indicator of content validity? Appraisal and recommendations. Res Nurs Health. 2007;30(4):459–467. doi: 10.1002/nur.20199. [DOI] [PubMed] [Google Scholar]
- 24.Lynn M.R. Determination and quantification of content validity. Nurs Res. 1986;35(6):382–385. [PubMed] [Google Scholar]
- 25.Marquis P., De La Loge C., Dubois D., McDermott A., Chassany O. Development and validation of the patient assessment of constipation quality of life questionnaire. Scand J Gastroenterol. 2005;40(5):540–551. doi: 10.1080/00365520510012208. [DOI] [PubMed] [Google Scholar]
- 26.Wang J.Y., Hart S.L., Lee J., Berian J.R., McCrea G.L., Varma M.G. A valid and reliable measure of constipation-related quality of life. Dis Colon Rectum. 2009;52(8):1434–1442. doi: 10.1007/DCR.0b013e3181a51196. [DOI] [PubMed] [Google Scholar]
- 27.Dennison C., Prasad M., Lloyd A., Bhattacharyya S.K., Dhawan R., Coyne K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics. 2005;23(5):461–476. doi: 10.2165/00019053-200523050-00006. [DOI] [PubMed] [Google Scholar]
- 28.Koloski N.A., Jones M., Wai R., Gill R.S., Byles J., Talley N.J. Impact of persistent constipation on health-related quality of life and mortality in older community-dwelling women. Am J Gastroenterol. 2013;108(7):1152–1158. doi: 10.1038/ajg.2013.137. [DOI] [PubMed] [Google Scholar]
- 29.Neri L., Basilisco G., Corazziari E., et al. Constipation severity is associated with productivity losses and healthcare utilization in patients with chronic constipation. U Eur Gastroenterol J. 2014;2(2):138–147. doi: 10.1177/2050640614528175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siqueira H.F.F., Teixeira J.L.A., Lessa Filho R.D.S., et al. Patient satisfaction and quality of life in breast reconstruction: assessment of outcomes of immediate, delayed, and nonreconstruction. BMC Res Notes. 2020;13(1) doi: 10.1186/s13104-020-05058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jneid S., Jabbour H., Hajj A., et al. Quality of life and its association with treatment satisfaction, adherence to medication, and trust in physician among patients with hypertension: a cross-sectional designed study. J Cardiovasc Pharmacol Ther. 2018;23(6):532–542. doi: 10.1177/1074248418784292. [DOI] [PubMed] [Google Scholar]
- 32.Al-Jabi S.W., Zyoud S.H., Sweileh W.M., et al. Relationship of treatment satisfaction to health-related quality of life: findings from a cross-sectional survey among hypertensive patients in Palestine. Health Expect. 2015;18(6):3336–3348. doi: 10.1111/hex.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin A. The changing prevalence of functional constipation: why words matter. Lancet Gastroenterol Hepatol. 2021;6(8):600–602. doi: 10.1016/s2468-1253(21)00140-0. [DOI] [PubMed] [Google Scholar]
- 34.Li B., Mah K., Swami N., et al. Symptom assessment in patients with advanced cancer: are the most severe symptoms the most bothersome? J Palliat Med. 2019;22(10):1252–1259. doi: 10.1089/jpm.2018.0622. [DOI] [PubMed] [Google Scholar]
- 35.Schiller L.R., Dennis E., Toth G. Primary care physicians consider constipation as a severe and bothersome medical condition that negatively impacts patients' lives. ACG. 2004;99:S234–S235. [Google Scholar]
- 36.Hong F., Blonquist T.M., Halpenny B., Berry D.L. Patient-reported symptom distress, and most bothersome issues, before and during cancer treatment. Patient Relat Outcome Meas. 2016;7:127–135. doi: 10.2147/prom.S95593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michel M.C., Oelke M., Goepel M., Beck E., Burkart M. Relationships among symptoms, bother, and treatment satisfaction in overactive bladder patients. Neurourol Urodyn. 2007;26(2):190–195. doi: 10.1002/nau.20367. [DOI] [PubMed] [Google Scholar]
- 38.Greenhalgh J., Gooding K., Gibbons E., et al. How do patient reported outcome measures (PROMs) support clinician-patient communication and patient care? A realist synthesis. J Patient Rep Outcomes. 2018;2 doi: 10.1186/s41687-018-0061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alves P.C., Sales C.M., Ashworth M. "It is not just about the alcohol": service users' views about individualised and standardised clinical assessment in a therapeutic community for alcohol dependence. Subst Abus Treat Prev Policy. 2016;11(1) doi: 10.1186/s13011-016-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richter J.G., Nannen C., Chehab G., et al. Mobile app-based documentation of patient-reported outcomes - 3-months results from a proof-of-concept study on modern rheumatology patient management. Arthritis Res Ther. 2021;23(1) doi: 10.1186/s13075-021-02500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suares N.C., Ford A.C. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(9):1582–1591. doi: 10.1038/ajg.2011.164. [DOI] [PubMed] [Google Scholar]
- 42.Singapore Department of Statistics. Census of population 2020 statistical release 1. 2020: 1–36.
- 43.Friis K., Lasgaard M., Rowlands G., Osborne R.H., Maindal H.T. Health literacy mediates the relationship between educational attainment and health behavior: a danish population-based study. J Health Commun. 2016;21(sup2):54–60. doi: 10.1080/10810730.2016.1201175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.