Abstract
Pemphigus vulgaris (PV) is an autoimmune blistering disease affecting the skin and mucosa. It clinically presents as painful erosions, mainly in the oral cavity, and flaccid blisters and erosions on the skin. Steven-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) is a severe mucocutaneous drug hypersensitivity reaction characterized by painful, dusky, erythematous eruptions that often progress to blisters, erosions, and extensive epidermal detachment accompanied by systemic symptoms. Herein, we report the case of a 48-year-old man who presented with extensive skin and mucosal lesions following the ingestion of an unknown medication. The patient’s symptoms clinically mimicked SJS, and accordingly, a preliminary diagnosis of SJS/TEN was made. However, clinical investigation of skin biopsy and direct immunofluorescence assay results indicated PV, and a final diagnosis of PV was established.
Key words: Stevens-Johnson syndrome, pemphigus vulgaris, toxic epidermal necrolysis, paraneoplastic pemphigus
Introduction
Pemphigus vulgaris (PV) is an autoimmune blistering disease characterized by the presence of circulating immunoglobulin (Ig) G autoantibodies against proteins that play an important role in cell-cell adhesion in the skin and mucous membranes, resulting in bullous lesions and erosions. PV is divided into two types depending on the cells involved: the mucosal type, with mainly oral lesions without skin involvement, and the mucocutaneous type, which involves both oral and skin lesions.1 Stevens-Johnson syndrome/ toxic epidermal necrolysis (SJS/TEN) is a rare, life-threatening mucocutaneous adverse drug reaction characterized by fullthickness epidermal necrolysis and exfoliation affecting both the skin and mucous membranes, accompanied by systemic symptoms. 2 That was clinically presented.
Case Report
An otherwise healthy 48-year-old man presented to the emergency department with extensive, painful skin and mucosal lesions that had persisted for almost 2 months. He previously had tonsillitis and had taken an unknown oral antibiotic, following which he developed this progressive skin eruption. Upon examination, diffuse ulcerations and extensive epidermal detachment involving the face and trunk, associated with erosions and denuded areas of the lips, tongue, soft and hard palate, nasal mucosa, and genitalia, were observed (Figure 1). The affected body surface area was 25%. The patient was clinically diagnosed with SJS/TEN secondary to the unknown antibiotic he had taken based on the clinical findings without histopathology. The patient was treated with a single dose of etanercept along with cyclosporine dosed at 3 mg/kg/day for 3-4 weeks without improvement; thus, skin biopsies were performed to re-evaluate the initial diagnosis. Histopathological examination revealed suprabasal blister formation and acantholysis. The blister cavity contained acantholytic cells, eosinophils, and neutrophils. There was a moderate perivascular inflammatory infiltrate composed of lymphocytes, plasma cells, neutrophils, and eosinophils (Figure 2). Direct immunofluorescence showed intercellular deposition of IgG in the epidermis, with negative results for C3, IgM, and IgA. Serological evaluation using an enzyme-linked immunosorbent assay (ELISA) was positive for IgG autoantibodies against desmogleins (Dsg) 1 and 3. Therefore, a diagnosis of PV was established. An extensive malignancy workup, including imaging and blood tests, was performed, and the results were unremarkable. Treatment was initiated with the administration of rituximab infusion at a dosage of 1,000 mg twice (day 1 and day 15). The patient showed significant improvement, with almost complete regression of the lesions (Figure 3).
Discussion and Conclusions
Pemphigus includes a group of rare acquired autoimmune bullous skin diseases. PV is the most common and severe form of pemphigus. PV can occur at any age but is often seen in middleaged and older populations. It is characterized by the presence of IgG autoantibodies directed against keratinocyte surface antigens, primarily Dsg 3 and, to a lesser extent, Dsg 1; these are desmosomal structural proteins that maintain keratinocyte adhesion.1,3 SJS/TEN is a severe cutaneous hypersensitivity reaction with a high mortality rate.2 Clinically, PV patients present with painful erosions, mainly in the oral cavity, and flaccid blisters and erosions on the skin. Patients with SJS/TEN usually present with painful, dusky, erythematous eruptions that often progress to blisters, erosions, and extensive epidermal detachment accompanied by systemic symptoms.4 However, it can be quite difficult to distinguish one from the other because, as in our case, the clinical presentation of PV mimics that of SJS/TEN. Our patient was misdiagnosed and treated for SJS/TEN in the emergency room because the diagnosis relied solely on the clinical presentation. Histopathology can be used to differentiate these two conditions. Histopathology of SJS/TEN shows full-thickness necrosis of the epidermis and a sparse lymphocytic infiltrate, while in PV, intraepidermal blistering with suprabasal epidermal acantholysis is usually seen, giving rise to a tombstone appearance with an eosinophilic infiltrate in the blister.4,5 The gold-standard diagnostic test for PV is direct immunofluorescence microscopy, which shows IgG and C3 deposition between keratinocytes in a net-like pattern in the epidermis. Serology can also detect antigen-specific IgG antibodies against Dsg 1 or Dsg 3 by indirect immunofluorescence or ELISA.
Figure 1.
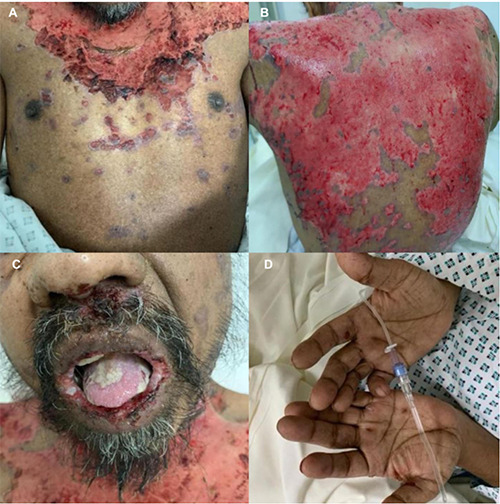
Patient at initial presentation. A,B) Diffuse ulcerations and extensive epidermal detachment involving the trunk; C) face associated with erosions and denuded areas of the lips, tongue, soft and hard palate; D) limbs.
Figure 2.
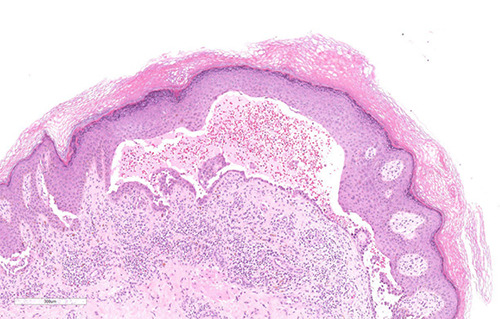
Histological examination of the skin punch biopsy showed suprabasal blister formation and acantholysis. The blister cavity contained acantholytic cells, eosinophils, and neutrophils. There was a moderate perivascular inflammatory infiltrate composed of lymphocytes, plasma cells, neutrophils, and eosinophils (hematoxylin-eosin stain, magnification ×10).
Figure 3.
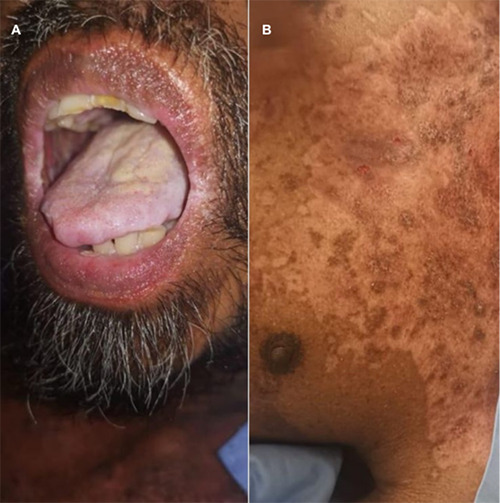
Patient at 2-4 weeks post-treatment. A) Improvement of the lips, and tongue; B) improvement in the chest area.
ELISA reactivity correlates with disease activity; therefore, this test is useful for both the diagnosis and monitoring of disease activity. Thus, it is extremely important to perform these tests to confirm the diagnosis of PV.3,5
There are few reports in the literature on pemphigus mimicking SJS/TEN. Paraneoplastic pemphigus (PNP) is the most common type reported to be confused with SJS/TEN. Hayanga et al. reported a case of PNP that was initially diagnosed as SJS/TEN.4 This patient was admitted to and treated in a burn intensive care unit; the patient did not respond to the cessation of the culprit medications and showed a progression of symptoms, and later, a diagnosis of PNP was established.4 A few similar cases of PNP mimicking SJS/TEN have been reported. The main clinical characteristics in these cases were the presence of diffuse, painful eruptions mainly over the extremities and trunk, along with erosive lesions of the oral, conjunctival, and penile mucosae. Based on the clinical findings, SJS/TEN and PNP were initially suspected. However, after histopathologic and serologic evaluations, the diagnosis of PNP was established.3,6,7
In conclusion, PV can be clinically deceptive and mimic SJS/TEN. Additionally, it is imperative to have a low threshold for skin biopsies, as both diseases can be life-threatening. Early diagnosis and treatment initiation are necessary to achieve a favorable prognosis.
Funding Statement
Funding: no grants from any funding agency in the public, commercial, or not-for-profit sectors were received for this study.
References
- 1.Porro AM, Seque CA, Ferreira MCC, Enokihara MMSS. Pemphigus vulgaris. An Bras Dermatol 2019;94:264-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fakoya AOJ, Omenyi P, Anthony P, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis; extensive review of reports of drug-induced etiologies, and possible therapeutic modalities. Open Access Maced J Med Sci 2018;6:730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLarney RM, Valdes-Rodriguez RH, Isaza-Gonzalez G, et al. Paraneoplastic pemphigus mimicking toxic epidermal necrolysis: an underdiagnosed entity?. JAAD Case Rep 2017;4:67-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayanga AJ, Lee TM, Pannucci CJ, et al. Paraneoplastic pemphigus in a burn intensive care unit: case report and review of the literature. J Burn Care Res 2010;31:826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Zhu X, Li R, et al. Paraneoplastic pemphigus associated with Castleman tumor: a commonly reported subtype of paraneoplastic pemphigus in China. Arch Dermatol 2005;141: 1285-93. [DOI] [PubMed] [Google Scholar]
- 6.Lyon CC, Carmichael AJ. Toxic epidermal necrolysis and paraneoplastic pemphigus. Lancet 1998;352:149. [DOI] [PubMed] [Google Scholar]
- 7.Padhiyar JK, Patel NH, Ninama K, et al. Paraneoplastic pemphigus presenting as toxic epidermal necrolysis: a case report. Nepal J Dermatol Venereol Leprol 2018;16:59-62. [Google Scholar]


