Abstract
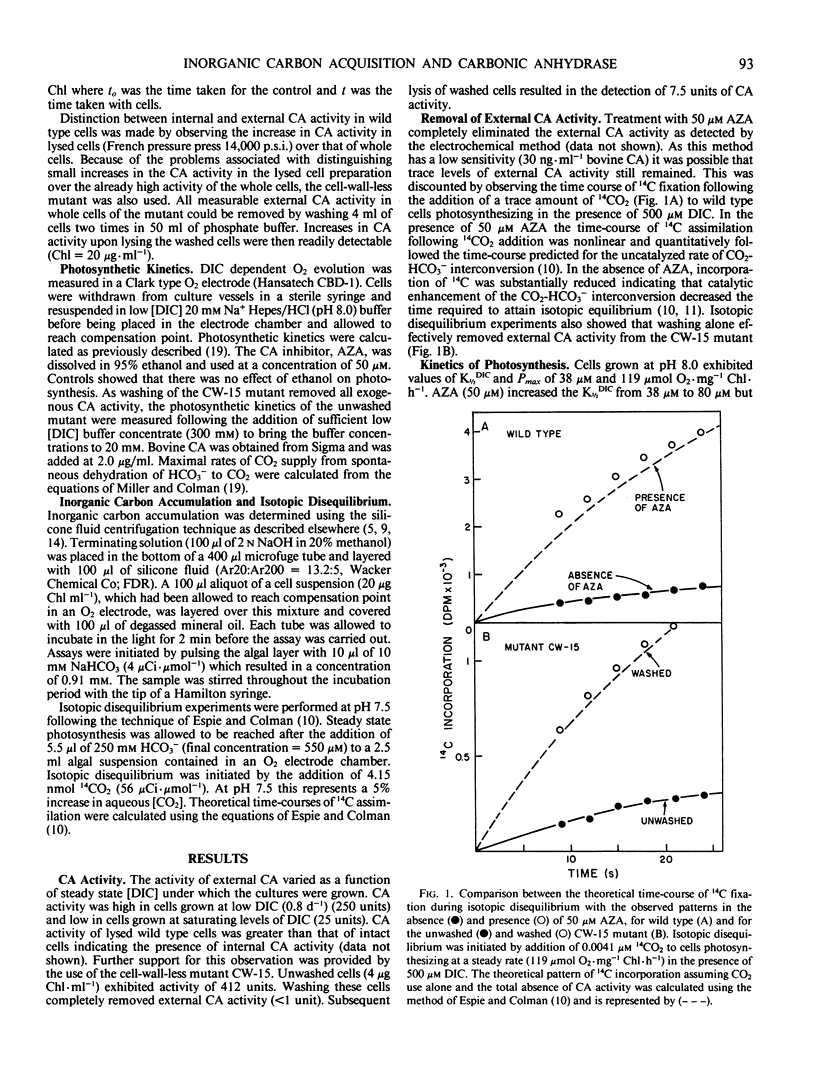
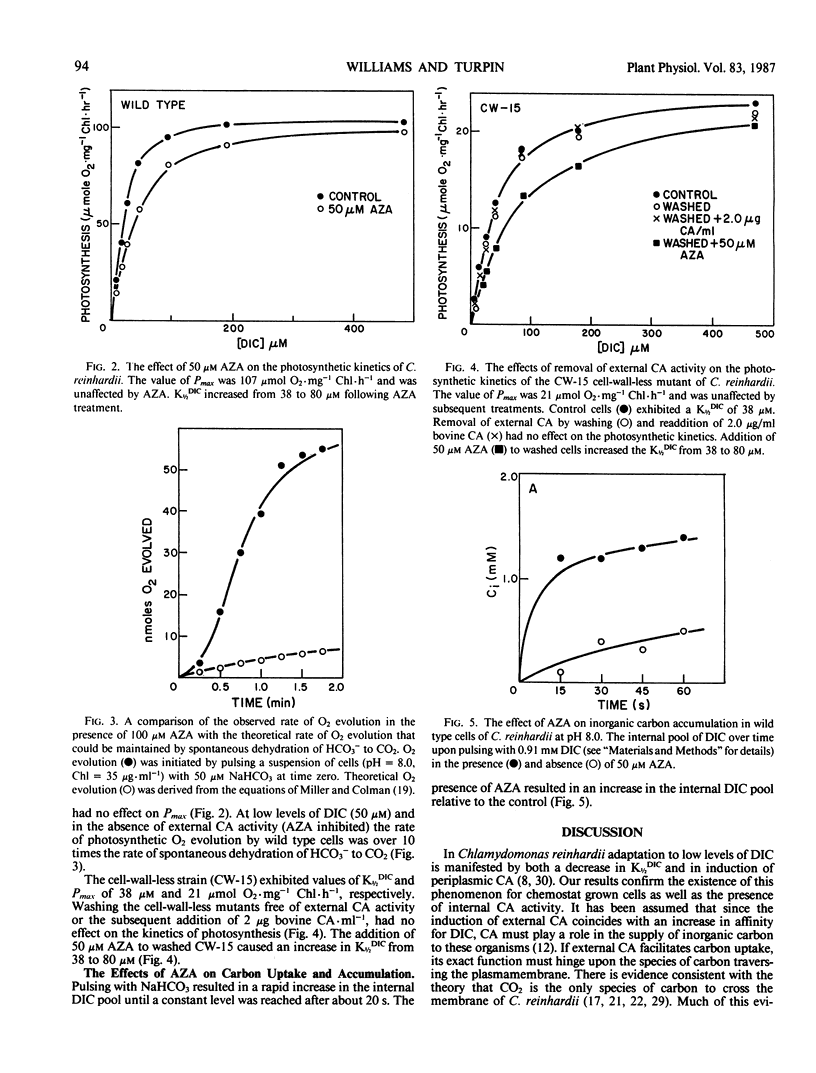
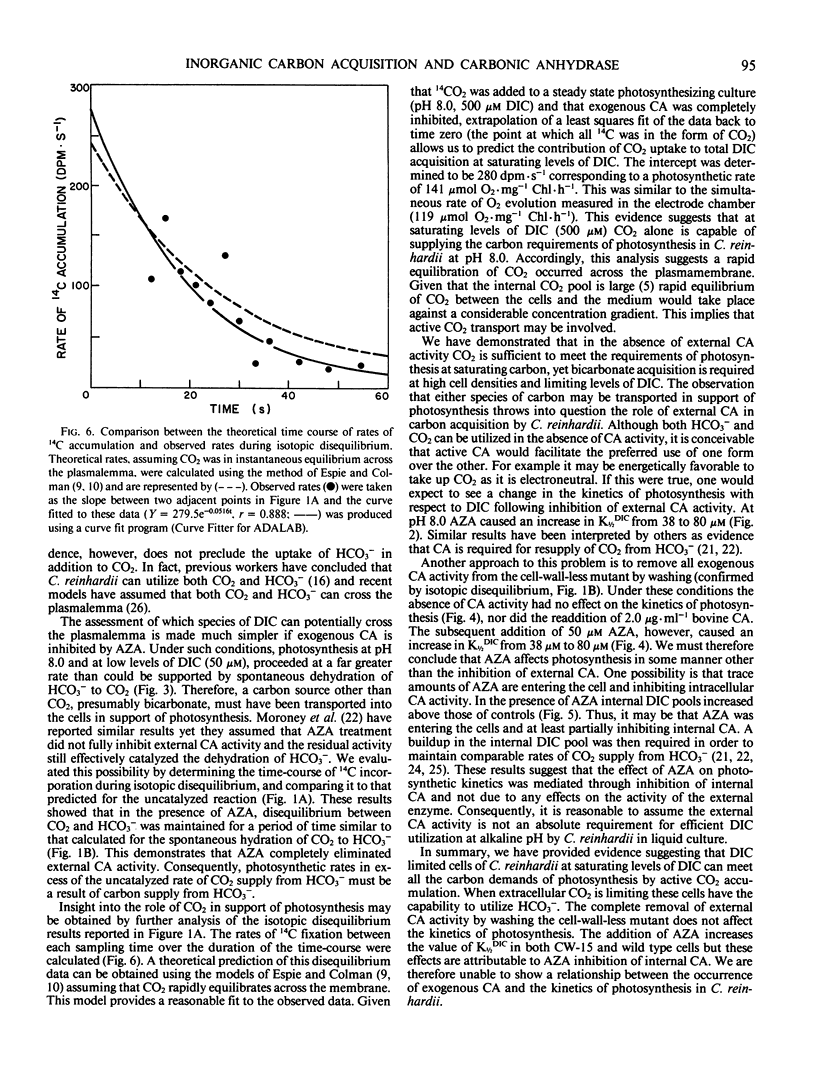
The role of external carbonic anhydrase in inorganic carbon acquisition and photosynthesis by Chlamydomonas reinhardii at alkaline pH (8.0) was studied. Acetazolamide (50 micromolar) completely inhibited external carbonic anhydrase (CA) activity as determined from isotopic disequilibrium experiments. Under these conditions, photosynthetic rates at low dissolved inorganic carbon (DIC) were far greater than could be maintained by CO2 supplied from the spontaneous dehydration of HCO3− thereby showing that C. reinhardii has the ability to utilize exogenous HCO3−. Acetazolamide increased the concentration of DIC required to half-saturate photosynthesis from 38 to 80 micromolar, while it did not affect the maximum photosynthetic rate. External CA activity was also removed from the cell-wall-less mutant (CW-15) by washing. This had no effect on the photosynthetic kinetics of the algae while the addition of acetazolamide to washed cells (CW-15) increased the K½DIC from 38 to 80 micromolar. Acetazolamide also caused a buildup of the inorganic carbon pool upon NaHCO3 addition, indicating that this compound partially inhibited internal CA activity. The effects of acetazolamide on the photosynthetic kinetics of C. reinhardii are likely due to the inhibition of internal rather than a consequence of the inhibition of external CA. Further analysis of the isotopic disequilibrium experiments at saturating concentration of DIC provided evidence consistent with active CO2 transport by C. reinhardii. The observation that C. reinhardii has the ability to take up both CO2 and bicarbonate throws into question the role of external CA in the accumulation of DIC in this alga.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Berry J. A., Togasaki R. K., Grossman A. R. Identification of Extracellular Carbonic Anhydrase of Chlamydomonas reinhardtii. Plant Physiol. 1984 Oct;76(2):472–477. doi: 10.1104/pp.76.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Grossman A. R. Biosynthesis of carbonic anhydrase in Chlamydomonas reinhardtii during adaptation to low CO(2). Proc Natl Acad Sci U S A. 1984 Oct;81(19):6049–6053. doi: 10.1073/pnas.81.19.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Colman B. Inorganic Carbon Uptake during Photosynthesis : I. A Theoretical Analysis Using the Isotopic Disequilibrium Technique. Plant Physiol. 1986 Apr;80(4):863–869. doi: 10.1104/pp.80.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Colman B. Photosynthesis and inorganic carbon transport in isolated asparagus mesophyll cells. Plant Physiol. 1982 Sep;70(3):649–654. doi: 10.1104/pp.70.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Owttrim G. W., Colman B. Inorganic Carbon Uptake during Photosynthesis : II. Uptake by Isolated Asparagus Mesophyll Cells during Isotopic Disequilibrium. Plant Physiol. 1986 Apr;80(4):870–876. doi: 10.1104/pp.80.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo W. P., Williams T. G., Birch D. G., Turpin D. H. Photosynthetic Adaptation by Synechococcus leopoliensis in Response to Exogenous Dissolved Inorganic Carbon. Plant Physiol. 1986 Apr;80(4):1038–1040. doi: 10.1104/pp.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Evidence for HCO(3) Transport by the Blue-Green Alga (Cyanobacterium) Coccochloris peniocystis. Plant Physiol. 1980 Feb;65(2):397–402. doi: 10.1104/pp.65.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Turpin D. H., Canvin D. T. Growth and Photosynthesis of the Cyanobacterium Synechococcus leopoliensis in HCO(3)-Limited Chemostats. Plant Physiol. 1984 Aug;75(4):1064–1070. doi: 10.1104/pp.75.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Husic H. D., Tolbert N. E. Effect of Carbonic Anhydrase Inhibitors on Inorganic Carbon Accumulation by Chlamydomonas reinhardtii. Plant Physiol. 1985 Sep;79(1):177–183. doi: 10.1104/pp.79.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Tolbert N. E. Inorganic Carbon Uptake by Chlamydomonas reinhardtii. Plant Physiol. 1985 Feb;77(2):253–258. doi: 10.1104/pp.77.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Spreitzer R. J., Ogren W. L. Carbonic Anhydrase-Deficient Mutant of Chlamydomonas reinhardii Requires Elevated Carbon Dioxide Concentration for Photoautotrophic Growth. Plant Physiol. 1983 Oct;73(2):268–272. doi: 10.1104/pp.73.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]


