Abstract
Background
Stakeholders and healthcare professionals have an essential role in the elimination of Hansen's Disease. Of these, pharmacists provide core services that assist the management of these patients with the supply of medicines and clinical actions.
Objectives
To summarize evidence on the role of pharmacist in the management of Hansen's Disease.
Methods
A literature search was performed in MEDLINE, Embase, Scopus, Web of Sciences, LILACS, and Google Scholar for studies published until September 29th, 2022 without language restriction. Studies that reported actions provided by pharmacists in the management of patients with Hansen's Disease were included. The pharmacist interventions identified in the studies were described based on key domains in DEPICT v.2. Two independent reviewers performed study selection and data extraction and any disagreements were resolved by third and fourth authors.
Results
A total of 751 records were identified, of which 8 studies fully met the eligibility criteria. Most of them were conducted in Brazil (n = 5), in an ambulatory setting (n = 8) and the most common study design was descriptive cross-sectional (n = 6). Different roles of pharmacists were identified, such as medication review, therapeutic drug monitoring, patient education, drug information, and dispensing. All studies described pharmacist interventions for patients through one-on-one contact and face-to-face. Pharmacists were responsible for patient counseling (n = 8), suggestions for change in therapy (n = 2), and monitoring results report (n = 2). The studies reported benefits associated with pharmacist interventions, despite the limited descriptions regarding these actions.
Conclusions
Few studies that described the activities of pharmacists in the management of Hansen's Disease were found. As the studies did not offer a satisfactory level of description and quality, further research should be conducted to strengthen this field.
Keywords: Pharmacists, Pharmaceutical services, Hansen's Disease, Leprosy, Review
1. Introduction
Neglected tropical diseases (NTDs) encompass a wide range of 20 conditions, including parasitic, viral, bacterial, fungal, and non-communicable diseases, primarily found in tropical regions, with a particular impact on impoverished communities. They have profound health, social, and economic consequences for over one billion individuals, affecting both their physical and cognitive development.1,2 According to the World Health Organization (WHO), environmental conditions are often linked to the epidemiology of NTDs, making their public health control challenging.1
Among the major NTDs, Hansen's Disease deserves mention. This condition, also known as leprosy, is a chronic infectious disease that is caused by a specific type of bacteria called Mycobacterium leprae, transmitted through droplets from the nose and mouth during frequent contact with untreated cases.3,4 The disease affects several body systems, mainly the skin and peripheral nervous system. This results in various signs and symptoms including numbness, erythema, painless nonpruritic skin lesions, eyebrow hair loss, and tubercles.5,6 If not identified early, the condition can lead to irreversible nerve damage and long-term disability. This includes the loss of sensory and motor function in the nerves supplying the eyes, hands and feet. Consequently, it can also lead to deformities like lagophthalmos, claw hand, claw toes, and footdrop.7,8
Hansen's Disease continues in >120 countries, with an annual report of >200,000 new cases, although the elimination of the disease as a public health problem, defined as a prevalence of <1 per 10,000 population, was achieved in most countries.3 Recently, the WHO released the “Towards zero leprosy: global leprosy (Hansen's Disease) strategy 2021–2030”, defining interruption of transmission as a key goal and including four strategic pillars to achieve it: implement integrated, scale-up prevention alongside integrated active case detection, manage the disease and its complications to prevent disability, and combat stigma and ensure human rights.9 It is worth noting that individuals affected by leprosy also face significant financial challenges. They leave their jobs to receive treatment or lose them due to stigma and discrimination. Furthermore, the costly medications employed for disease management and its associated complications can lead to heightened expenses for the healthcare system.10
In this context, stakeholders and healthcare professionals have an essential role in the elimination of Hansen's Disease. Previous studies reported the role of nurses11,12 and physicians13 in the management of this disease, but few studies describe pharmacists' work experiences. Pharmacists play key roles in preventing and managing NTDs. They serve as communicator, quality drug supplier, collaborator, trainer and supervisor as a health promoter.14,15 Additionally, pharmacists deliver clinical services for patients undergoing multidrug therapy and/or specialty drugs. This entails providing medication counseling, closely monitoring the patient, and ensuring consistent follow-up. For instance, women of reproductive age who are using thalidomide to treat Hansen's Disease require contraceptive counseling, as part of teratogenic risk management programs.14 Therefore, this scoping review aimed to identify and summarize the role of pharmacist in the management of Hansen's Disease. To our knowledge, there is no scoping review or systematic review on this topic.
2. Methods
This review was performed following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement for Scoping Reviews (PRISMA-ScR)16 and the review protocol was registered in the Open Science Framework platform (https://doi.org/10.17605/OSF.IO/7JRAK).
2.1. Databases and search strategy
A comprehensive search of published literature until September 29th, 2022 was performed in MEDLINE (via PubMed), Scopus, Embase, Web of Science, and LILACS (Latin American and Caribbean Health Sciences Literature) databases to identify relevant studies. Additionally, a grey literature search was conducted in Google Scholar up to the 60 registers, excluding patents and citations, to identify studies that were not indexed in the databases listed above. No language and period time filter was applied for all databases. The search strategy included combinations of keywords related to the pharmacist, pharmaceutical services, and Hansen's Disease. The full strategy search for all databases can be found in Appendix S1.
2.2. Study selection
Studies that reported work experiences provided by pharmacists in the management of patients with Hansen's Disease were included. Studies that did not clarify the role of the pharmacist, described other healthcare professionals or interdisciplinary teams without reporting the involvement of pharmacists, and studies published in non-Roman characters were excluded. Finally, reviews, books or book chapters, editorials, comments, conference proceedings or abstracts, and guidelines were also excluded.
The studies retrieved from the databases were transferred using the Rayyan QCRI platform17 to remove duplicate files, analyze the titles and abstracts of the studies, and examine the full texts of the studies that were previously selected based on their abstracts. Two independent reviewers (E.C.O·D and N.R.S.) independently assessed the titles, abstracts, and full texts of all studies identified. Any discrepancies were resolved by the third and fourth reviewers (M.B.V. and T.M.L).
2.3. Data extraction and synthesis of results
The following data were extracted: author, year of publication, country, Journal Citation Reports (JCR) 2021, citescore, publication type, setting, target population, sample size, activities and-or responsibilities, objectives, main findings, and limitations. The activities and-or responsibilities were categorized into three domains adapted from the International Pharmaceutical Federation (FIP) (2020)18 and Visacri, Figueiredo, and Lima (2021)19: “access and supply of medicines”, “patient care” and “support for healthcare professionals”. Two reviewers (E.C.O·D and N.R.S.) independently completed the data extraction using a preformatted Microsoft Excel spreadsheet that had been standardized through team consensus. Disagreements were resolved by the third and fourth authors (M.B.V. and T.M.L.).
Moreover, the pharmacist interventions reported in the included studies were classified based on key domains in the Descriptive Elements of Pharmacist Intervention Characterization Tool (DEPICT)20 version 2: 1) contact with recipient (how the contact with the recipient occurs); 2) method of communication with recipient; 3) setting of the intervention (where the recipient received the service); 4) action(s) taken by pharmacist (what is done to address the identified problems); and 5) materials that support action(s) (items developed or provided by the pharmacist as part of the service). Two independent reviewers (M.B.V. and T.M.L.) classified data and any conflicts were resolved through consensus.
The findings of this scoping review are presented through a narrative synthesis due to the diverse nature of the included studies. The studies were categorized based on publication characteristics and summarized in tables. The original ideas and concepts of the included studies were acknowledged and respected.
3. Results
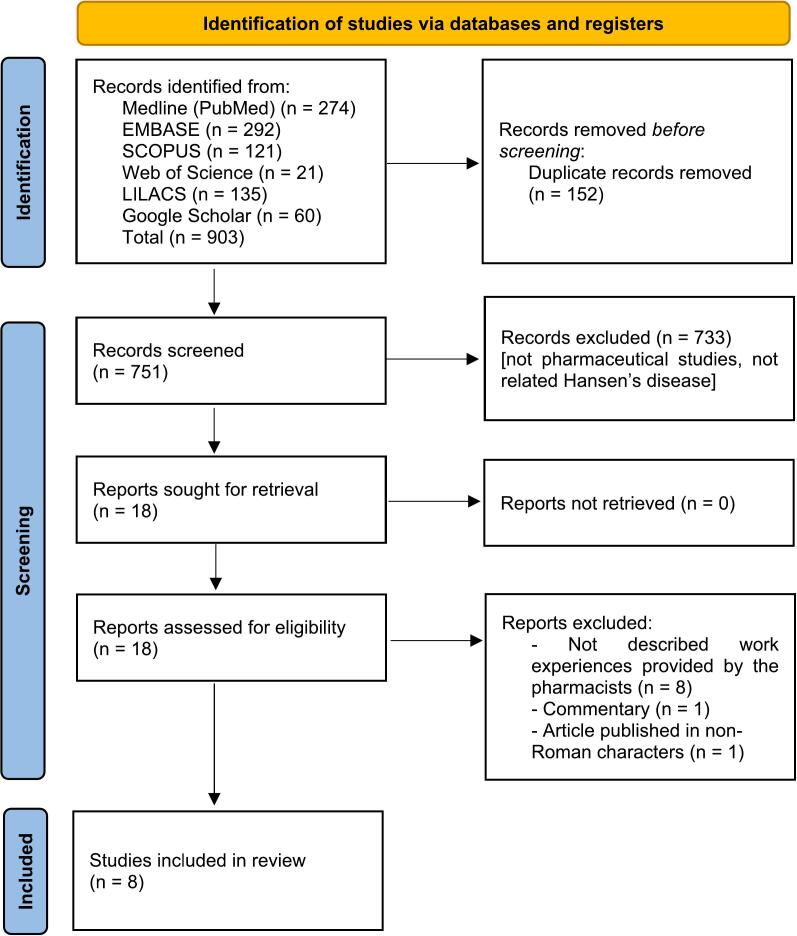
A total of 751 records were identified in the electronic search. After removing duplicates and reviewing the titles and abstracts, 15 articles were selected for full-text reading. No relevant studies were found through the examination of the reference lists of the included studies. Of these, eight studies21, 22, 23, 24, 25, 26, 27, 28 met the eligibility criteria and were included in this review. A flowchart of the literature search is shown in Fig. 1. The references for the excluded studies as well as the reasons for their exclusion, are available in Appendix S2.
Fig. 1.
Study selection flowchart through literature search.
3.1. Characteristics of the included studies
Studies were conducted in Brazil (n = 5),22,24,26, 27, 28 the United States (n = 2),21,23 and India (n = 1).25 Five studies were published in Portuguese22,24,26, 27, 28 and three in English21,23,25 language and reported between 1986 and 2022. The publication type of the included studies consisted of research articles (n = 7)21, 22, 23, 24, 25, 26, 27 and end-of-graduate course treatise (n = 1).28 Three publications have a JCR21,24,25 and Citescore,21,22,25 with a mean of 1.36 (SD = 1.60 [range 0.222 to 3.204]) and 1.83 (SD = 1.72 [range 0.6 to 3.8]), respectively. All studies had a cross-sectional design and most of them (n = 6)22,24, 25, 26, 27, 28 with a descriptive approach. All studies were performed in the ambulatory setting, and three studies24,26,28 reported the study population for any Hansen's Disease patient, followed by patients in the use of dapsone (n = 3)21,23,25 and thalidomide (n = 2).22,27 The number of patients included in the studies varied, ranging from 7 to 315. The characteristics of the eight studies included in this review are summarized in Table 1.
Table 1.
Characteristics of the studies included in this scoping review.
| Author(s), year | Country | Publication type | JCR 2021 | Citescore | Study design | Setting | Study population |
|---|---|---|---|---|---|---|---|
| Danziger, Piscitelli, 199321 | USA | Research article | 3.204 | 3.8 | Analytical cross-sectional | Clinic | Patients in use of dapsone |
| de Vasconcelos et al., 201722 | Brazil | Research article | NA | 0.6 | Descriptive cross-sectional | Clinic | Patients in use of thalidomide |
| Fischer, West, and Worobec, 198623 | USA | Research article | 0.222 | NA | Analytical cross-sectional | Clinic | Patients in use of dapsone |
| Guerra, Pontes e Randau 202224 | Brazil | Research article | NA | NA | Descriptive cross-sectional | Clinic | Any patients |
| Kannan et al., 200925 | India | Research article | 0.664 | 1.1 | Descriptive cross-sectional | Ambulatory | Patients in use of dapsone |
| Ramos et al., 202226 | Brazil | Research article | NA | NA | Descriptive cross-sectional | Ambulatory | Any patients |
| Sales et al., 202027 | Brazil | Research article | NA | NA | Descriptive cross-sectional | Public community pharmacy | Patients in use of thalidomide |
| Santos, 201328 | Brazil | End-of-graduate course treatise | NA | NA | Descriptive cross-sectional | Primary care | Any patients |
Abbreviations: JCR (Journal Citation Reports), NA (not applicable), USA (United States of America).
3.2. Summary of the results of the included studies
Table 2 summarizes the results of the included studies in detail. Almost all of the studies (n = 7) described pharmacists' activities related to patient care21, 22, 23, 24, 25, 26,28 and four studies described actions related to support for healthcare professionals,21, 22, 23,28 reporting clinical services such as medication review, therapeutic drug monitoring, patient education, and drug information for healthcare professionals. Only one study27 described pharmacists' activity related to access and supply of medicines. All studies described the beneficial actions of the pharmacist interventions.
Table 2.
Summary of the results of the included studies.
| Author(s), year | Activities | Sample size | Objectives | Main findings | Limitations |
|---|---|---|---|---|---|
| Danziger, Piscitelli, 199321 | Patient care (patient education and therapeutic drug monitoring) | 88 | To analyze the effectiveness of a treatment adherence program of dapsone | Treatment adherence was 70% (1987) and 94.4% (1989). In a 6-period year (1985–90), the treatment adherence of dapsone was 81.6%, increasing over time (p < 0.05) | NR |
| de Vasconcelos et al., 201722 | Patient care (medication review and patient education) | 11 | To identify DRP in patients undergoing the use of thalidomide. | The most common category of DRPs was the “non-quantitative safety problems” (34.8%). A total of 16 pharmacist interventions were performed related to patient counseling (n = 13) and suggestions for change in therapy for physicians (n = 3) | NR |
| Fischer, West, and Worobec, 198623 | Patient care (patient education and therapeutic drug monitoring) | 42 | To analyze the effectiveness of a treatment adherence program of dapsone | Treatment adherence of dapsone increased over time (p < 0.05): baseline (46.7%), 6-month (73.3%), and 18-month (80%). Moreover, a positive significant difference (p < 0.05) on therapeutic response was observed in adherence patients compared to nonadherence patients | NR |
| Guerra, Pontes, and Randau 202224 | Patient care (patient education) | 41 | To identify possible causes related to ADR and treatment nonadherence | Limb numbness, leprosy nodules, body aches/fatigue, light patches, and fever were the most common ADRs reported by interviewed patients, with a moderate level of involvement. Most reported positive aspects of clinical services provided by pharmacists, such as patient counseling about drug administration. The bureaucracy in drug dispensing was cited as a negative aspect. | NR |
| Kannan et al., 200925 | Patient care (patient education) | 54 | To identify ADR related to dapsone | Peripheral neuropathy was the most ADR found in patients (35%), followed by gastrointestinal effects, such as abdominal pain and anorexia (25%). Other ADRs such as headache, insomnia, and dizziness were reported by 20% of patients, and hypersensitivity reactions by 10% of patients. | NR |
| Ramos et al., 202226 | Patient care (medication review and patient education) | 65 | To identify DRP in patients with Hansen's disease | Polypharmacy was common in most patients (n = 39, 60%). A total of 57 DRPs were identified: Drug-nutrient interactions (n = 49; 75.3%), drug-drug interactions (n = 30; 46.1%), dose omission (n = 26; 40.0%), need for laboratory monitoring (n = 25; 38.4%) and need for self-monitoring (n = 16; 24.6%). A total of 143 medications were associated with the identified DRPs, especially dapsone (n = 32; 22.5%), prednisone (n = 21; 14.7%), clofazimine (n = 20; 14.0%), thalidomide (n = 19; 13.3%), and amitriptyline (n = 6; 4.2%). | Incomplete data in medical records and reduced hours for data collection |
| Sales et al., 202027 | Access and supply of medicines (drug dispensing) | 315 | To describe the drug dispensing of thalidomide for Hansen's disease treatment | The year with the highest number of patients undergoing treatment was 2016 (n = 75). The age range of patients was 36 to 55 years, with more males (89.3%). Drowsiness, skin lesions, pain, and swelling were the most ADRs found in patients. | NR |
| Santos, 201328 | Patient care (medication review and patient education) | 7 | To identify DRP in patients with Hansen's disease | Five patients presented ADRs and were classified as a category of DRPs of the “non-quantitative safety problems” | Small sample size |
Abbreviations: ADR (adverse drug reaction), DRP (drug-related problem), NR (not reported).
Regarding the aim of the studies, five22,24, 25, 26,28 identified the drug-related problems in the treatment of patients with Hansen's Disease (two studies22,27 was conducted only for patients in the use of thalidomide), two studies21,23 assessed the compliance monitoring program for dapsone in an outpatient clinic and one study27 described the thalidomide dispensing for Hansen's Disease treatment. Finally, only two studies26,28 reported some limitations such as small sample size and incomplete data in medical records.
3.3. Characteristics of pharmacist interventions based on DEPICT 2
Pharmacist interventions were performed for patients (n = 8)21,28 and-or healthcare professionals (n = 4),21, 22, 23,28 by one-on-one contact and face-to-face communication methods for patients and-or healthcare professionals (n = 8 each),21,28 followed by two studies22,28 with written communication method for healthcare professionals and one study27 using the telephone as a communication method for patients. The places where the recipient received the pharmaceutical service were the primary care setting (n = 8),21, 22, 23, 24, 25, 26, 27, 28 the healthcare office (n = 2),22,28 and the recipient's home (n = 1).27 Pharmacists were responsible for patient counseling (n = 8),21, 22, 23, 24, 25, 26, 27, 28 suggestions for change in therapy (n = 2),22,28 and monitoring results report (n = 2).21,23 Three studies cited the use of materials that support actions adopted by pharmacists such as leaflets,25 auxiliary labels for patients,22 and a list of potential drug-drug interactions for healthcare professionals.28 The description of the pharmacist interventions according to DEPICT version 2 is shown in Table 3.
Table 3.
Description of the pharmacist interventions according to the DEPICT version 2.
| Author(s), year | Recipient | Contact with recipient | Methods of communication | Setting of the intervention | Action(s) taken by pharmacist | Materials that support action(s) |
|---|---|---|---|---|---|---|
| Danziger, Piscitelli, 199321 | Patient and HCP | One-on-one (patient) and group (HCP) | Face-to-face (patient and HCP) | Ambulatory (patient and HCP) | Patient counseling (patient) and monitoring results report (HCP) | NR |
| de Vasconcelos et al., 201722 | Patient and HCP | One-on-one (patient and HCP) | Face-to-face (patient) and written (HCP) | Ambulatory (patient) and HCP office (HCP) | Patient counseling (patient) and change or suggestion for change in therapy (HCP) | Auxiliary labels (patient) and NR (HCP) |
| Fischer, West, and Worobec, 198623 | Patient and HCP | One-on-one (patient) and group (HCP) | Face-to-face (patient and HCP) | Ambulatory (patient and HCP) | Patient counseling (patient) and monitoring results report (HCP) | NR |
| Guerra, Pontes, and Randau 202224 | Patient | One-on-one | Face-to-face | Ambulatory | Patient counseling | NR |
| Kannan et al., 200925 | Patient | One-on-one | Face-to-face | Ambulatory | Patient counseling | Leaflets |
| Ramos et al., 202226 | Patient | One-on-one | Face-to-face | Ambulatory | Patient counseling | NR |
| Sales et al., 202026 | Patient | One-on-one | Face-to-face and telephone | Primary care setting and recipient's home | Patient counseling | NR |
| Santos, 201328 | Patient and HCP | One-on-one (patient and HCP) | Face-to-face (patient) and written (HCP) | Ambulatory (patient) and HCP office (HCP) | Patient counseling (patient) and change or suggestion for change in therapy (HCP) | NR (patient) and list of potential drug-drug interactions (HCP) |
Abbreviations: HCP (healthcare professional), NR (not reported).
4. Discussion
To our knowledge, this is the first scoping review aimed to map and summarize scientific evidence on the role of pharmacists in the management of Hansen's Disease. Surprisingly, this scoping review identified only eight relevant studies, four in the Brazilian context, on the work experiences provided by pharmacists in the management of Hansen's Disease, although it is an age-old NTD that still occurs in several countries. Overall, the included studies reported beneficial activities of the pharmacist, such as drug dispensing, patient education, drug information, therapeutic drug monitoring, and medication review. The benefits encompassed improvements in medication adherence, the prevention or resolution of drug-related problems, and the detection of adverse drug reactions. Pharmacist interventions were delivered for patients and healthcare professionals through one-on-one and face-to-face methods, and the action taken by pharmacists was mainly patient counseling within primary care settings. Moreover, the lack of detailed descriptions of these actions and the lack of sufficient scientific rigor of publications was noted. These findings showed that it is crucial for researchers to actively participate in design and quality reports in this field.
In a previous review on the role of nurses in Hansen's Disease, all included studies were from Brazil.11 Similarly, in this scoping review most studies were conducted in the Brazilian context since 2013. This fact may be related to some reasons. According to the Pan American Health Organization, 30,957 new cases were recorded in the Region of the Americas in 2018; of these, 94% of all cases were concentrated in Brazil.29 In addition, the recent epidemiological report published by the Brazilian Ministry of Health showed that 119,698 new cases were recorded between 2017 and 2021 of which 55.7% were male,30 constituting a significant public health problem. From the 1980s onwards, Brazil had institutional initiatives that modified the strategy of care for people affected by Hansen's Disease. In 2010, a guideline on surveillance, care, and control of the disease for the National Health System was published.31 Moreover, the supply of medicines for Hansen's Disease is financed by Federal government funding and dispensed by public community pharmacies, where the pharmacist performs an important role in the health system.32 The two studies conducted in the United States in the 1980s and 1990s coincided with the National Hansen's Disease Program introduced in the same decade.33 Due to the low report of new cases over the years (only 159 in 2020),33 pharmacists and researchers could have lost interest in the field.
On the other hand, countries such as India and Indonesia reported >10,000 new cases in 2019,3 and only one study in the field was identified in this review. One possible reason for this may be attributed to the absence of a description of the role of pharmacists in the management of Hansen's Disease. For example, two studies conducted in the Indian context were excluded for this reason, although the studies were related to drug information and evaluation,34,35 one of the characteristics of the pharmacist's actions. It is important to highlight that the health systems and supporting structures are likely to differ from other settings. This necessitates caution when attempting to apply and extrapolate the findings.
Most studies included in this review were published in journals without an impact factor index, which may be a consequence of the lack of scientific rigor of the publications; thus, their findings need to be analyzed with caution. In addition, regarding the study design and population study, most of them were descriptive cross-sectional with a small sample size. This study design is recognized for its limitations in investigating causal inference or measuring associations between variables of interest as well as the inadequate sample size that may indicate a sampling bias.36 Other study designs, such as experimental studies, can be performed to better understand the effectiveness of the pharmacist's actions.
Over the past few decades, the role of the clinical pharmacist has undergone significant changes across various pharmaceutical practice settings. This evolution has been driven by a shift towards patient-centered care, aiming to address drug-related issues and enhance the overall quality of life for patients.37 In this review, almost all studies reported clinical services, reinforcing the role of clinical pharmacists in this scenario. However, only one study described actions on access and supply of medicines.27 It is known that the key challenge to mitigating Hansen's Disease in endemic countries is access to health facilities and medicines,38 of which the pharmacist's involvement is crucial,39 once early treatment of these patients can lead to a higher success rate and a decrease in the severity of the disease.40 It is also worth mentioning that drug dispensing is a private service of the pharmacist, and cannot be carried out by other health professionals, such as nurses and physicians. Moreover, most clinical pharmacy services are closely linked with the use of medicines, which means that “without medicines, there is no clinical activity.” Therefore, it is important to promote research focused on investigating the difficulties and challenges associated with accessing these medicines.
This review highlighted several clinical activities provided by pharmacists, including patient counseling, drug information, therapeutic drug monitoring, and medication review, with a particular emphasis on promoting medication adherence and managing adverse drug reactions. Meanwhile, physicians are focused on diagnosing and managing Hansen's Disease and its complications10 and nurses are actively involved in epidemiological surveillance, patient education on self-care and patient counseling on the treatment.41,42 Thus, working in a collaborative and interdisciplinary way, physicians, nurses, pharmacists, and other health professionals should provide high quality care for leprosy patients.
DEPICT was developed to improve the characterization of clinical pharmacy services, ensuring consistent reporting and enhancing the reproducibility of interventions in practice.20 All studies reported the key domains of pharmacist interventions, but the descriptions were not in detail. Similar findings were identified in other scoping reviews,19,43 proving that poor description across studies of clinical pharmacy activities is still present. Regarding the key domains of pharmacist interventions reported by the included studies, all of them used face-to-face contact as the level of pharmacist-recipient interaction. It is important to closely monitor these patients to minimize the risk of disability, disfigurement, and social stigma. However, it is not always possible for the physical presence of healthcare professionals in remote areas, and technology, such as telemedicine and tele-education, may be utilized strategies to maintain access to expert healthcare for these patients. A review showed the role of tele-leprology in strengthening routine and referral services and developing sustainable training strategies, although the literature is limited.44 This approach, including telepharmacy, was highlighted during the COVID-19 pandemic, since these tools may be applied in a social distancing context.7
Indeed, pharmacists can play a collaborative role in the management of Hansen's Disease. Based on recent publications,7,45 we highlight possible pharmacist assignments in this field: i) ensure access to comprehensive, well-organized referral facilities for the patients, ii) provide administrative service to ensure access and supply of medicines, iii) management of disease reactions, neuritis, and disabilities, iv) monitoring, support and training on self-care, such as face, hands, and feet care, personal skin hygiene, and medication adherence, v) use of evidence-based guidelines or protocols to guide the conduct, such as to guide the therapeutic drug monitoring of dapsone and rifampicin, vi) patient counseling and drug information, vii) integrate a multidisciplinary team to ensure treatment effectiveness and patient safety, viii) notify adverse drug events with the competent agencies, and ix) combat social stigma and ensure human rights.
This review showed gaps that can be used to improve future studies. Thus, future studies must explore larger and more representative samples, along with robust study designs (i.e., quasi-experimental and clinical trials) to evaluate the effectiveness of the pharmacist's actions.
There are limitations to consider in this review. We may have missed some relevant studies because they were not indexed in the databases searched, published on the websites of institutions or scientific societies, or published in non-Roman characters. In addition, this review did not assess the methodological quality of the included studies, as is inherent to the nature of scoping reviews. Finally, more studies must explore larger and more representative samples, along with robust study designs to evaluate the real effectiveness of the pharmacist's actions.
5. Conclusion
Few studies that reported the activities of pharmacists in the management of Hansen's Disease were identified. All studies were related to clinical services and described patient counseling as the main pharmacist interventions performed, however, the level of detail provided in the descriptions and quality of publication was not deemed satisfactory. We strongly encourage further research with more detailed descriptions to be conducted to enhance the field as well as evaluate the impact of pharmacist activities in patient care with Hansen's Disease.
Funding
No funding was received for this review.
Contributorship including name of guarantor
E.C.O.D. and N.R.S. identified the reports in the databases and collected data from the studies included. M.B.V. and T.M.L. designed the study, drafted and revised the manuscript. All authors approved the final manuscript.
Patient consent
No patient consent was required.
Declaration of Competing Interest
The authors report no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rcsop.2023.100342.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
References
- 1.World Health Organization (WHO) Neglected Tropical Diseases (NTDs) 2023. https://www.who.int/health-topics/neglected-tropical-diseases (accessed 29 September 2023)
- 2.Centers for Diseases Control and Prevention (CDC) Neglected Tropical Disease. 2023. https://www.cdc.gov/globalhealth/ntd/ (accessed 29 September 2023)
- 3.World Health Organization (WHO) Leprosy. 2023. https://www.who.int/news-room/fact-sheets/detail/leprosy (accessed 29 September 2023)
- 4.Centers for Diseases Control and Prevention (CDC) Hansen's Disease (Leprosy) 2023. https://www.cdc.gov/leprosy/ (accessed 29 September 2023)
- 5.Aftab H., Nielsen S.D., Bygbjerg I.C. Leprosy in Denmark 1980-2010: a review of 15 cases. BMC Res Notes. 2016;9:10. doi: 10.1186/s13104-015-1768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X., Zha S., Shui T.J. Presenting symptoms of leprosy at diagnosis: clinical evidence from a cross-sectional, population-based study. PLoS Negl Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0009913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization (WHO) World Health Organization; Geneva: 2020. Leprosy/Hansen Disease: Management of Reactions and Prevention of Disabilities. Technical Guidance. [Google Scholar]
- 8.World Health Organization (WHO) World Health Organization; Geneva: 2018. Guidelines for the Diagnosis, Treatment and Prevention of Leprosy. [Google Scholar]
- 9.World Health Organization (WHO) World Health Organization; Geneva: 2021. Towards Zero Leprosy. Global Leprosy (Hansen’s Disease) Strategy 2021–2030. [Google Scholar]
- 10.Xiong M., Li M., Zheng D., et al. Evaluation of the economic burden of leprosy among migrant and resident patients in Guangdong Province, China. BMC Infect Dis. 2017;17:760. doi: 10.1186/s12879-017-2869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreira A.S., Rocha L.G., Santos V.Y.S., et al. Atuação dos enfermeiros nas ações de controle da hanseníase na atenção primária à saúde: revisão integrativa. Divers J. 2021;6:3949. doi: 10.48017/dj.v6i4.1501. [DOI] [Google Scholar]
- 12.Susanto T., Dewi E.I., Rahmawati I. The experiences of people affected by leprosy who participated in self-care groups in the community: a qualitative study in Indonesia. Lepr Rev. 2017;88:543. doi: 10.47276/lr.88.4.543. [DOI] [Google Scholar]
- 13.White C., Franco-Paredes C. Leprosy in the 21st century. Clin Microbiol Rev. 2015;28:80. doi: 10.1128/CMR.00079-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allayla T.H., Nouri A.I., Hassali M.A. Pharmacist role in global health: a review of literature. Malays J Pharm Sci. 2018;16:45–54. doi: 10.21315/mjps2018.16.1.4. [DOI] [Google Scholar]
- 15.Maity T., Pahari N., Bera K., et al. A literature review on current tropical diseases and the role of pharmacist in public health with special reference to tropical diseases. BioMedRx. 2013;1:4–10. [Google Scholar]
- 16.Tricco A.C., Lillie E., Zarin W., et al. PRISMA extension for scoping reviews (PRISMAScR): checklist and explanation. Ann Intern Med. 2018;169:467. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 17.Ouzzani M., Hammady H., Fedorowicz Z., et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Pharmaceutical Federation (FIP) FIP; The Hague: 2020. Patient Safety. Pharmacists’ Role in Medication without Harm. [Google Scholar]
- 19.Visacri M.B., Figueiredo I.V., Lima T.M. Role of pharmacist during the COVID-19 pandemic: a scoping review. Res Social Adm Pharm. 2021;17:1799. doi: 10.1016/j.sapharm.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotta I., Salgado T.M., Felix D.C., et al. Ensuring consistent reporting of clinical pharmacy services to enhance reproducibility in practice: an improved version of DEPICT. J Eval Clin Pract. 2015;21:584. doi: 10.1111/jep.12339. [DOI] [PubMed] [Google Scholar]
- 21.Danziger L.H., Hill C., Slajchert A.A., et al. Effectiveness of a dapsone compliance program in leprosy. Int J Dermatol. 1993;3:206. doi: 10.1111/j.1365-4362.1993.tb02797.x. [DOI] [PubMed] [Google Scholar]
- 22.de Vasconcelos R.L.H., Santos W.R.P., Sousa A.M.L., et al. Seguimento farmacoterapêutico de pacientes em tratamento com talidomida em um centro especializado em hanseníase. Sci Med. 2017;27 doi: 10.15448/1980-6108.2017.4.27342. ID27342. [DOI] [Google Scholar]
- 23.Fischer J.H., West D.P., Worobec S.M. Evaluation of a continual compliance monitoring program for dapsone in an outpatient Hansen’s disease clinic. Int J Lepr Other Mycobact Dis. 1986;54:517. [PubMed] [Google Scholar]
- 24.Guerra S.K.S., Pontes M.R.L., Randau K.P. Cuidado clínico farmacêutico e estratégia para o uso racional e adesão ao tratamento em pacientes com hanseníase numa Policlínica do Recife. Ciênc Méd Biol. 2022;21:60. doi: 10.9771/cmbio.v21i1.44575. [DOI] [Google Scholar]
- 25.Kannan G., Vasantha J., Rani N.V., et al. Drug usage evaluation of dapsone. Indian J Pharm Sci. 2009;71:456. doi: 10.4103/0250-474X.57301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos M.F., Galete J., Polisel C.G. Cuidado farmacêutico ambulatorial na Hanseníase. Braz J Dev. 2022;8:7213. doi: 10.34117/bjdv8n1-488. [DOI] [Google Scholar]
- 27.Sales A.A., Lima A.N., Damasceno I.A.M., et al. Estudo da dispensação da talidomida no tratamento da hanseníase pela Assistência Farmacêutica do Município de Araguaína-TO. Res Soc Dev. 2020;9 doi: 10.33448/rsd-v9i8.6020. [DOI] [Google Scholar]
- 28.Santos D.V.A. FAMAM; Governador Mangabeira, BA: 2013. Acompanhamento farmacoterapêutico de pacientes cadastrados no programa nacional de controle a hanseniase (pnch) no município de Santo Antônio de Jesus-Bahia. [Google Scholar]
- 29.Pan American Health Organization (PAHO) Leprosy. 2023. https://www.paho.org/en/topics/leprosy (accessed 13 June 2023)
- 30.Ministério da Saúde . Secretaria de Vigilância em Saúde, Ministério da Saúde; Brasília: 2023. Boletim Epidemiológico - Hanseníase 2023. [Google Scholar]
- 31.Ministério da Saúde . Ministério da Saúde; Brasília: 2010. Portaria N° 3.125, de 7 de outubro de 2010 - Aprova as Diretrizes para Vigilância, Atenção e Controle da Hanseníase. [Google Scholar]
- 32.Melo A.C., Trindade G.M., Freitas A.R., et al. Community pharmacies and pharmacists in Brazil: a missed opportunity. Pharm Pract. 2021;19:2467. doi: 10.18549/PharmPract.2021.2.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Health Resources & Services Administration (HRSA) National Hansen's Disease (Leprosy) Program Caring and Curing Since 1894. 2023. https://www.hrsa.gov/hansens-disease (accessed 29 September 2023)
- 34.Pugazhenthan T., Ravichandran, Aravindan U., et al. Evaluation of drug use pattern in Central Leprosy Teaching and Research Institute as a tool to promote rational prescribing. Indian J Lepr. 2017;89:99. [Google Scholar]
- 35.Pugazhenthan T., Venkatesan S., Tamilselvan T., et al. Information on drugs used in management of lepra reactions in commonly used drug information sources in India. Indian J Lepr. 2018;90:129. [Google Scholar]
- 36.Wang X., Cheng Z. Cross-sectional studies: strengths, weaknesses, and recommendations. Chest. 2020;158:S65. doi: 10.1016/j.chest.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Chagas M.O., de Mendonça Lima T., Rebustini F., et al. Instruments to assess the role of the clinical pharmacist: a systematic review. Syst Rev. 2022;11:175. doi: 10.1186/s13643-022-02031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rinaldi A. The global campaign to eliminate leprosy. PLoS Med. 2005;2 doi: 10.1371/journal.pmed.0020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson S. The state of the world's pharmacy: a portrait of the pharmacy profession. J Interprof Care. 2002;16:391. doi: 10.1080/1356182021000008337. [DOI] [PubMed] [Google Scholar]
- 40.Bennett B.H., Parker D.L., Robson M. Leprosy: steps along the journey of eradication. Public Health Rep. 2008;123:198. doi: 10.1177/003335490812300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freitas B.H.B.M., Silva F.B., Jesus J.M.F., et al. Leprosy educational practices with adolescents: an integrative literature review. Rev Bras Enferm. 2019;72:1397–1404. doi: 10.1590/0034-7167-2017-0458. [DOI] [PubMed] [Google Scholar]
- 42.Nascimento G.R.C., Barrêto A.J.R., Brandão G.C.G., et al. Ações do enfermeiro no controle da hanseníase. Rev Eletr Enf. 2011;13(4):743–750. [Google Scholar]
- 43.Franco J., de Souza R.N., Lima T.M., et al. Role of clinical pharmacist in the palliative care of adults and elderly patients with cancer: a scoping review. J Oncol Pharm Pract. 2022;28:664. doi: 10.1177/10781552211073470. [DOI] [PubMed] [Google Scholar]
- 44.Nelson C.A., Kovarik C.L., Morssink C.B. Tele-leprology: a literature review of applications of telemedicine and tele-education to leprosy. Lepr Rev. 2014;85:250–261. [PubMed] [Google Scholar]
- 45.Conselho Federal de Farmácia (CFF) CFF; Brasília: 2023. Resolução n° 747, de 25 de maio de 2023 - Regulamenta as atribuições do farmacêutico em doenças tropicais e negligenciadas, e dá outras providências. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2