Abstract
Introduction
Docetaxel plus ramucirumab could be a promising treatment for chemo-naive elderly patients with NSCLC, but high incidence of febrile neutropenia (FN) is a critical concern. We thus adopted a routine primary prophylactic pegylated-granulocyte-colony stimulating factor (PEG-G-CSF) to reduce FN and maximize the efficacy of docetaxel plus ramucirumab in elderly patients.
Methods
This is a single arm phase 2 trial for chemo-naive elderly patients (aged ≥75 y) with advanced NSCLC. Docetaxel (60 mg/m2, d 1) plus ramucirumab (10 mg/kg, d 1) with PEG-G-CSF (3.6 mg, d 2) was administered every 3 weeks until progression. The primary end point was overall response rate (ORR) (expected ORR: 35%).
Results
Between February 2018 and January 2021, 54 patients were enrolled. Median age was 78 (range: 75–86). A total of 21 (38.9%) partial response, 22 (40.7%) stable disease, nine (16.7%) progressive disease, and two (3.7%) not assessable were confirmed, resulting in ORR of 38.9% (90% confidence interval [CI]: 27.7%–51.0%) and disease control rate of 79.6%. Median progression-free survival and overall survival were 5.2 (95% CI: 4.2–6.9) and 12.7 (95% CI: 10.2–18.9) months, respectively. There were one (1.9%) FN, two (3.7%) bleeding grade greater than or equal to 3, and one (1.9%) treatment-related death (pneumonitis). Pneumonitis occurred in five patients (9.3%). Main adverse events grade greater than or equal to 3 were observed: four (7%) thrombocytopenia; three (5.6%) neutropenia; six (11.1%) hyposodium; five (9.3%) infection; five (9.3%) hypertension; four (7.4%) anorexia; and three (5.6%) oral mucositis.
Conclusions
Docetaxel plus ramucirumab with PEG-G-CSF revealed efficacy and safety for chemo-naive elderly patients with NSCLC. Primary prophylactic PEG-G-CSF highly prevented FN.
Keywords: Docetaxel, Ramucirumab, Febrile neutropenia, pegylated-granulocyte-colony stimulating factor, elderly, Non–small cell lung cancer
Introduction
As societies are aging worldwide, elderly patients with lung cancer, especially NSCLC represents an increasing trend. Before immune-oncology era, chemotherapy (chemo) was the standard of care for patients with advanced and metastatic disease. Molecular-targeted therapies are generally recommended even for elderly patients with NSCLC harboring driver oncogene alterations such as EGFR mutations, ALK fusions, ROS1-fusions, and BRAF mutations. In those without such gene alterations, cytotoxic agents are still indicated. Cytotoxic monotherapies such as vinorelbine, gemcitabine, and docetaxel are standard regimens for elderly patients with NSCLC.1, 2, 3 Some studies have revealed effectiveness of carboplatin-based platinum doublets, such as carboplatin plus weekly paclitaxel/nab-paclitaxel or pemetrexed.4, 5, 6 In Japan, docetaxel monotherapy is a standard of care for chemo-naive elderly patients with advanced NSCLC, according to the results of our phase 3 trial comparing docetaxel versus vinorelbine monotherapies (WJTOG9904).4 However, its therapeutic effect is not exemplary, and advancement of cytotoxic regimens has reached a plateau.
Ramucirumab is a fully-human immunoglobulin G1 monoclonal antibody that binds with high affinity to vascular endothelial growth factor (VEGF) receptor-2, preventing VEGF binding and activation.7 In a pivotal phase 3 study (REVEL), docetaxel plus ramucirumab revealed superior overall response rate (ORR) and progression-free survival (PFS) over docetaxel monotherapy in second-line chemo for advanced NSCLC. These differences in ORR and PFS were translated into overall survival (OS) benefit, and docetaxel plus ramucirumab revealed superior OS over docetaxel monotherapy.8 This evidence prompted us to investigate docetaxel plus ramucirumab for chemo-naive elderly patients. However, in a similarly designed Japanese randomized phase 2 trial (JVCG), febrile neutropenia (FN) was observed in 34.2% of docetaxel plus ramucirumab arm.9 This high incidence of FN is a clinical concern when using docetaxel plus ramucirumab for elderly patients. Gandara et al.10 revealed that FN was more frequent in Japanese than in Western populations even when the same regimen was administered. To overcome this problem, we adopt pegylated-granulocyte-colony stimulating factor (PEG-G-CSF) support. The ASCO practice guideline recommends primary prophylactic G-CSF when the risk of FN is approximately 20% or higher.11 PEG-G-CSF revealed reduction of FN incidence administered only once a cycle in many types of cancers.12 In patients with NSCLC, incidence of FN could also be reduced by PEG-G-CSF. On the basis of the above background, we hypothesize that primary prophylactic PEG-G-CSF reduces FN and maximizes the efficacy of docetaxel plus ramucirumab in Japanese elderly patients with NSCLC.
Materials and Methods
Study Design and Objective
This is a prospective multicenter single arm phase 2 study conducted by West Japan Oncology Group. The primary end point was ORR. Secondary end points were PFS, OS, disease control rate, and safety. Initial registration period was set at two years from February 2018. Both follow-up and analysis periods were decided at one year. This study was conducted in compliance with the principles of the Declaration of Helsinki and was registered in the University Hospital Medical Information Network database (UMIN000030598) and the Japan Registry of Clinical Trials (jRCTs051180232).
Eligibility Criteria
Key inclusion criteria included: aged more than or equal to 75 years; written informed consent: histologically or cytologically proven NSCLC; with measurable lesions; neither symptomatic brain metastases nor spinal cord metastases; no symptomatic superior vena cava syndrome; neither poorly controlled pericardial fluid, pleural effusion, nor ascites; no risk factor of pulmonary bleeding on the basis of radiographic findings (cancer infiltration in the main blood vessel or its narrowing, intra-tumor cavitation, or tumor exposure to the central airway involving the segmental branch); Eastern Cooperative Oncology Group performance status (PS) 0/1; adequate laboratory data; neither history of cytotoxic chemotherapies nor ramucirumab (after failure of EGFR/ALK tyrosine kinase inhibitors [TKIs] and immune-checkpoint inhibitors [ICIs] were eligible); adjuvant chemo within 24 weeks; no major surgery within 28 days; no palliative radiation therapies within 14 days; and neither history of chemotherapies nor thoracic radiation therapy for other cancers.
Key exclusion criteria described: active double cancer; systemic infection requiring antibiotics; interstitial lung disease or idiopatic pulmonary fibrosis detected by chest-computed tomography (CT); uncontrolled diabetes melitus; poorly controlled hypertension; clinically relevant heart failure or uncontrolled arrythmia; uncontrolled thromboembolism; human immunodeficiency virus infection; bowel obstruction, infectious colitis or chronic diarrhea; severe psychological disorder; severe liver cirrhosis; continuous prescription of steroids or immunosuppresants; prescription of anticoagulant; prescription of non-steroidal anti-inflammatory drugs or antiplatelets; fever more than or equal to 38°C; massive hemoptysis within 2 months; arterial thrombosis, such as myocardial infarction, unstable angina, cerebrovascular attacks, or transient cerebral ischemic attacks, within 6 months; severe wounds, ulcers, or bone fractures within 28 days; severe bleeding, including gastrointestinal bleeding and vasculitis within 3 months; gastrointestinal perforation or fistulae within 6 months; history of hypersensitivity to drugs, including polysorbate 80; and scheduled major surgery during the trial period.
Treatment Plan
Intravenous docetaxel (60 mg/m2, d 1) plus ramucirumab (10 mg/kg, d 1) with subcutaneous PEG-G-CSF (3.6 mg, d 2) every 3 weeks was administered until progression. Continuous docetaxel or ramucirumab monotherapy were permitted when intolerable toxicities occurred, while clinical benefit was obtained by each drug. Dose reduction of docetaxel from 60 mg/m2 to 50 mg/m2 could be performed in cases of : FN; grade 4 neutropenia more than or equal to 7 days; non-hematological toxicity grade greater than or equal to 3; or the physicians consider dose-reduction necessary from a safety viewpoint. When similar adverse events (AEs) occurred, docetaxel dosage could be further reduced to 40 mg/m2. When these AEs occurred after dose reduction to 40 mg/m2, docetaxel was terminated. PEG-G-CSF was used in each dose of docetaxel and was not be used without docetaxel. Ramucirumab was terminated in cases of : hypersensitivity; venous thromboembolism grade greater than or equal to 2; arterial thromboembolism; grade 4 hypertension; hemorrhage of the central nervous system; posterior reversible encephalopathy syndrome; hemorrhage (excluding bronchopulmonary hemorrhage) grade greater than or equal to 3; bronchopulmonary hemorrhage grade greater than or equal to 2; pneumonitis grade greater than or equal to 2; congestive heart failure grade greater than or equal to 3; tracheoesophageal fistula; gastrointestinal perforation; liver cirrhosis-related hepatic encephalopathy or hepatorenal syndrome; nephrotic syndrome (persistent, 24-h protein level: >3 g); recurrent proteinuria (proteinuria with a 24-h protein level of >2 g was observed 3 times, or there is no decrease in the 24-h protein level to 2 g within 3 wk); or the physicians consider the continuation of drug administration difficult.
Efficacy and Safety Evaluation
CT or magnetic resonance imaging scan of the brain, CT scans of the chest and abdomen, a bone scan or positron emission tomography scan, and an electrocardiogram were required before enrollment. Tumor assessments were carried out at baseline, every 6 weeks until 24 weeks from enrollment, and every 9 weeks thereafter. The tumor response was evaluated in accordance with the Response Evaluation Criteria in Solid Tumors, version 1.1. AEs were evaluated using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0.
Statistical Analysis
We decided the threshold ORR to be 20%, and the expected ORR 35%. On the basis of this assumption, the number of patients needed to provide the 80% power for a one-sided 0.05 level of type I error was calculated to be 59. Taking ineligible patients into account, the sample size was set at 65. The 90% and 95% confidence intervals (CIs) for ORR were calculated with the Clopper-Pearson method. PFS was defined as time from enrollment to date of disease progression or death from any cause. OS was defined as time from enrollment to death from any cause. PFS and OS were analyzed using the Kaplan-Meier method, and median values and corresponding 95% CIs were calculated using the Brookmeyer-Crowley method. All statistical analyses were performed with SAS version 9.4 (SAS Institute).
Results
Between February 2018 and January 2021, 54 patients were enrolled. Enrollment period was extended from 2 to 3 years, but this trial was terminated before full enrollment (N = 65), because of slow accrual after approval of front-line immunotherapy. The statistical power of our study reached 75%, on the basis of final enrollment of 54 cases.
Baseline patient characteristics are shown in Table 1. Median age was 78 (range: 75–86), and there were 37 (69%) aged less than 80 and 17 (32%) aged more than or equal to 80. Males accounted for 80%. Three (6%) stage III, 15 (28%) recurrence after surgery, and 36 (67%) stage IV patients were enrolled. Forty (74%) adenocarcinoma, 8 (15%) squamous carcinoma, and 6 (11%) others were included. Six (11%) were EGFR-mutation positive, and 45/3 (83%/6%) negative/unknown. A total of 43 (80%) were chemo-naive, and 5/4/3 (9%/7%/6%) received ICI/EGFR-TKI/adjuvant chemo as prior therapies (one duplication). A total of 17 (30%) prior surgery and 10 (19%) prior radiation therapies were performed.
Table 1.
Baseline Characteristics
| Characteristics | n (%) |
|---|---|
| Age | |
| Median, range | 78, 75–86 |
| <80 | 37 (69) |
| ≥80 | 17 (31) |
| Sex | |
| Male | 43 (80) |
| Female | 11 (20) |
| ECOG performance status | |
| 0 | 20 (37) |
| 1 | 34 (63) |
| Smoking status | |
| Former/current | 31/11 (57/20) |
| Never | 12 (22) |
| Stage | |
| III/recurrence | 3/15 (6/28) |
| IV | 36 (67) |
| Histology | |
| Adeno | 40 (74) |
| Squamous/others | 8/6 (15/11) |
| EGFR mutation | |
| Positive | 6 (11) |
| Negative/unknown | 45/3 (83/6) |
| Prior chemotherapy | |
| Chemo-naive | 43 (80%) |
| ICI/EGFR TKI/adjuvant chemo | 5/4/3 (9%/7%/6%)a |
| Prior surgery | |
| Post-surgery | 16 (30) |
| None | 38 (70) |
| Prior radiation | |
| Irradiated | 10 (19) |
| None | 44 (81) |
Chemo, chemotherapy; ECOG, Eastern Cooperative Oncology Group; ICI, immune-checkpoint inhibitor; TKI, tyrosine kinase Inhibitor.
One duplication.
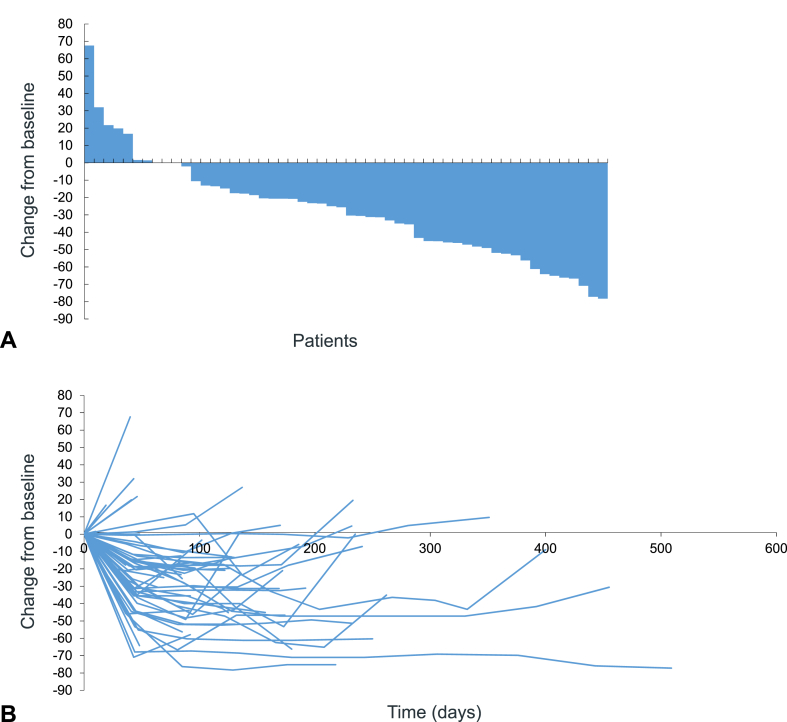
A total of 21 (38.9%) partial response, 22 (40.7%) stable disease, nine (16.7%) progressive disease, and two (3.7%) not assessable were confirmed, resulting in ORR of 38.9% (90% CI: 27.7%–51.0% and 95% CI: 25.9%–53.1%) and disease control rate of 79.6% (Table 2). Figure 1A and B reveal waterfall and spider plots.
Table 2.
Objective Response
| Confirmed Response | n (%) |
|---|---|
| Complete response | 0 |
| Partial response | 21 (38.9) |
| Stable disease | 22 (40.7) |
| Progressive disease | 9 (16.7) |
| Not assessable | 2 (3.7) |
| Response rate: 38.9% (90% CI: 27.7%–51.0%, 95%CI: 25.9%–53.1%) | |
| Disease control rate: 79.6% (95% CI: 66.5%–89.4%) | |
CI, confidence interval.
Figure 1.
Waterfall (A) and spider (B) plots.
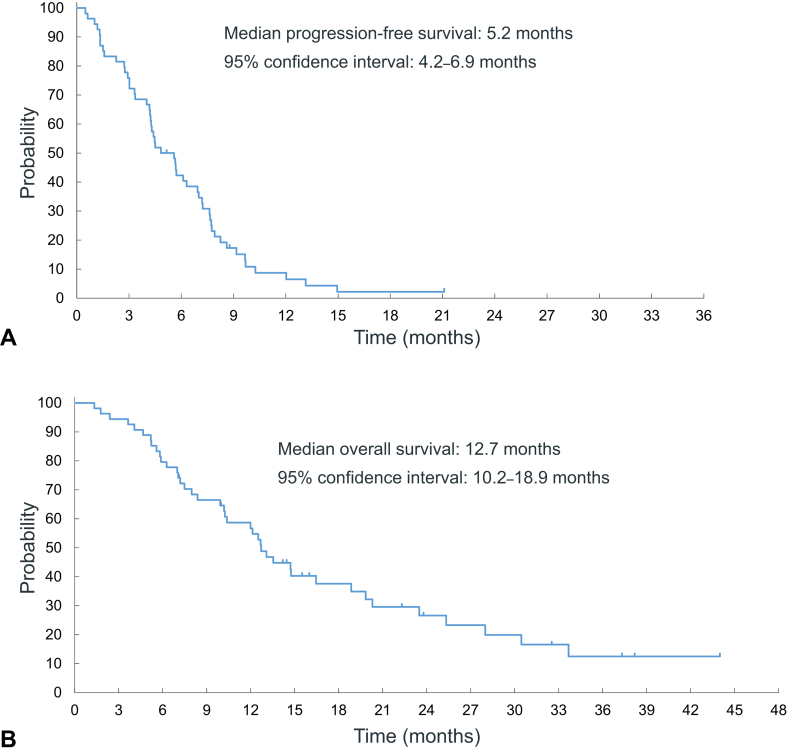
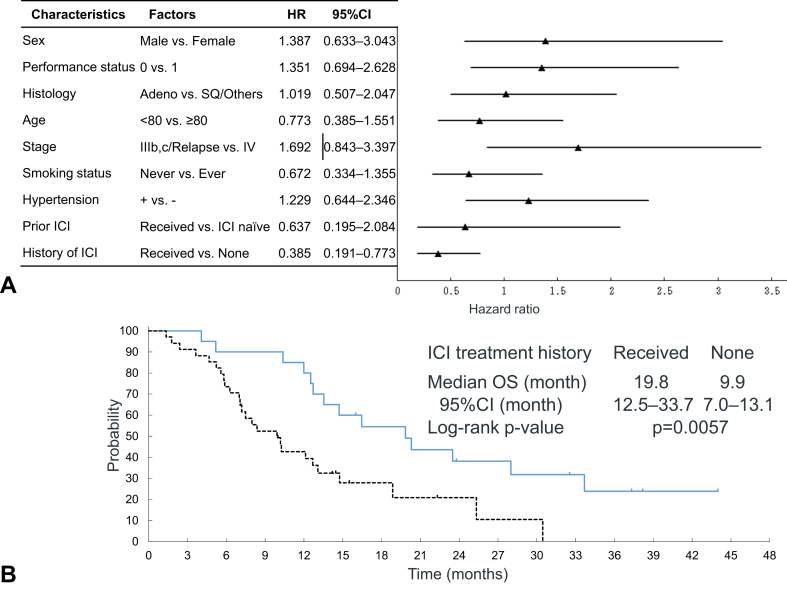
Median PFS and OS were 5.2 (95% CI: 4.2–6.9) and 12.7 (95% CI: 10.2–18.9) months, respectively (Fig. 2A and B). One-year PFS rate was 8.7%. 1-, 2-, and 3-year OS rates were 56.8%, 26.6%, and 12.5%. Subgroup analysis of OS found history of ICI as a prognostic factor for longer survival (Fig. 3A). Figure 3B exhibits Kaplan-Meier plots of OS by ICI treatment history. Patients with ICI treatment had better prognosis than those without (median OS: 19.8 [95% CI: 12.5–33.7] mo versus 9.9 [95% CI: 7.0–13.1] mo, p = 0.0057). One-, 2-, and 3-year OS rates of receiving ICI treatment versus no ICI treatment were 80.0% versus 42.7%, 38.2% versus 21.0%, and 23.9% versus 0%, respectively.
Figure 2.
Kaplan-Meier plots of progression-free survival (A) and overall survival (B).
Figure 3.
Subgroup analysis of overall survival (A) and Kaplan-Meier plots of overall survival by history of immunotherapy (B). SQ, squamous; ICI, immune-checkpoint inhibitor; HR, hazard ratio; CI, confidence interval; and OS, overall survival.
The median number of treatment cycles of docetaxel was 5 (range: 1–15), of ramucirumab 5 (range: 1–20). Dose reduction of docetaxel was performed to 50 mg at 22 (40.7%) cases, 40 mg at four cases (7.4%), and terminated at four cases (7.4%). Ramucirumab was skipped in 15 cases (27.8%) and terminated in two cases (3.7%) because of AEs.
A total of 39 patients (72.2%) received post-study treatments. A total of 34 (63.0%) systemic therapy, 15 (27.8%) radiation therapy, and one (1.9%) surgery were carried out. Systemic therapies included: 18 (33.3%) cytotoxic monotherapies; 17 (31.5%) immunotherapies; nine (16.7%) platinum doublets; and four (7.4%) EGFR TKIs. Second, third, fourth, and fifth-line systemic therapies were administered in 34 (63.0%), 11 (20.4%), six (11.1%), and one (1.9%), respectively.
Table 3 shows safety profile, including AEs: incidence greater than or equal to 10% or any grade greater than or equal to 3. There were one (1.9%) FN, two (3.7%) bleeding grade greater than or equal to 3, and one (1.9%) treatment-related death (grade 5 pneumonitis). Pneumonitis occurred in five patients (9.3%) (grade 1/2/3/4/5: two [3.7%]/one [1.9%]/one [1.9%]/zero [0%]/one [1.9%]). Hematological toxicities grade greater than or equal to 3: four (7.4%) thrombocytopenia; and three (5.6%) neutropenia, while non-hematological toxicities grade greater than or equal to 3: six (11.1%) hyposodium; 5 (9.3%) hypertension; four (7.4%) anorexia; and three (5.6%) oral mucositis were observed.
Table 3.
Safety Profile
| Adverse Events | Grade 1 | Grade 2 | Grade ≥3 |
|---|---|---|---|
| Laboratory | |||
| Leukopenia | 1 (1.9) | 12 (22.2) | 1 (1.9) |
| Anemia | 21 (38.9) | 12 (22.2) | 2 (3.7) |
| Thrombocytopenia | 28 (51.9) | 10 (18.5) | 4 (7.4) |
| Neutropenia | 5 (9.3) | 7 (13.0) | 3 (5.6) |
| AST increased | 25 (46.3) | 2 (3.7) | 1 (1.9) |
| ALT increased | 9 (16.7) | 1 (1.9) | 1 (1.9) |
| Albumin decreased | 8 (14.8) | 29 (53.7) | 1 (1.9) |
| Creatinine increased | 8 (14.8) | 0 (0) | 0 (0) |
| Hyper-Sodium | 6 (11.1) | 0 (0) | 0 (0) |
| Hypo-Sodium | 20 (37.0) | 0 (0) | 6 (11.1) |
| Hyper-Potassium | 13 (24.1) | 2 (3.7) | 0 (0) |
| Hypo-Potassium | 15 (27.8) | 0 (0) | 1 (1.9) |
| Hyper-Calcium | 2 (3.7) | 0 (0) | 1 (1.9) |
| Hypo-Calcium | 13 (24.1) | 2 (3.7) | 0 (0) |
| Ramucirumab-related | |||
| Hypertension | 5 (9.3) | 5 (9.3) | 5 (9.3) |
| Proteinuria | 14 (25.9) | 12 (22.2) | 1 (1.9) |
| Thromboembolism | 0 (0) | 1 (1.9) | 0 (0) |
| Pulmonary bleeding | 3 (5.6) | 2 (3.7) | 0 (0) |
| Extra-pulmonary bleeding | 12 (22.2) | 3 (5.6) | 2 (3.7) |
| Others | |||
| Febrile neutropenia | 0 (0) | 0 (0) | 1 (1.9) |
| Interstitial lung disease | 2 (3.7) | 1 (1.9) | 2 (3.7)a |
| Peripheral neuropathy | 5 (9.3) | 2 (3.7) | 0 (0) |
| Nausea | 15 (27.8) | 9 (16.7) | 0 (0) |
| Vomiting | 8 (14.8) | 1 (1.9) | 0 (0) |
| Fever | 17 (31.5) | 3 (5.6) | 0 (0) |
| Heart failure | 0 (0) | 0 (0) | 1 (1.9) |
| Diarrhea | 6 (11.1) | 2 (3.7) | 1 (1.9) |
| Infection | 0 (0) | 7 (13.0) | 5 (9.3) |
| Pleural effusion | 1 (1.9) | 3 (5.6) | 1 (1.9) |
| Malaise | 18 (33.3) | 5 (9.3) | 1 (1.9) |
| Dyspnea | 2 (3.7) | 2 (3.7) | 2 (3.7) |
| Oral mucositis | 6 (11.1) | 9 (16.7) | 3 (3.7) |
| Anorexia | 15 (27.8) | 6 (11.1) | 4 (5.6) |
| Alopecia | 4 (7.4) | 3 (5.6) | 0 (0) |
| Nail change | 6 (11.1) | 2 (3.7) | 2 (3.7) |
| Fatigue | 1 (1.9) | 3 (5.6) | 1 (1.9) |
| Rash | 5 (9.3) | 1 (1.9) | 0 (0) |
| Edema | 7 (13.0) | 7 (13.0) | 1 (1.9) |
| Constipation | 6 (11.1) | 3 (5.6) | 0 (0) |
| Dysgeusia | 3 (5.6) | 5 (9.3) | 0 (0) |
Note: All values are n (%).
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
One grade 3, another grade 5.
Discussion
To the best of our knowledge, our study is the first to evaluate the effectiveness of primary prophylactic PEG-G-CSF support in docetaxel plus ramucirumab treatment for chemo-naive elderly patients with advanced NSCLC. FN incidence in our study was only 1.9% (1 of 54 patients), compared with 34% on docetaxel plus ramucirumab arm in a Japanese randomized phase 2 study.8 FN is still an oncologic emergency situation and occasionally induces a fatal AE, particularly in elderly patients. Routine primary prophylactic PEG-G-CSF support could highly reduce the incidence of FN, providing a safer treatment for elderly NSCLC patients.
Our study exhibited ORR of 38.9%. Although a final target enrollment was not completed, ORR exceeded our expected ORR of 35% with a statistical power of 75%, which was near the initial set power of 80%. Our previously conducted WJTOG9904 study revealed that ORRs of docetaxel and vinorelbine were 22.7% and 9.9%, respectively.3 Results of carboplatin-based combination therapies reported ORR of 27% to 37%.4, 5, 6 Median PFS of our study was 5.2 months. The above studies exhibited median PFS of 3 to 8 months.3, 4, 5, 6 Data suggests a potency of docetaxel plus ramucirumab over cytotoxic monotherapies, which could be comparable to carboplatin-based combination regimens in chemo-naive elderly patients with NSCLC.3
Subset analysis revealed a better prognosis in those with ICI treatment history than those without. Some data found efficacy of ICI or chemo-ICI combination therapy for elderly patients.13, 14, 15 Their and our data suggest the priority of ICI treatment even for elderly patients. More effective and safer ICIs or their combination regimens should be preferentially developed for chemo-naive elderly patients with NSCLC, rather than first-line docetaxel plus ramucirumab.
Safety profile except FN suggested a general tolerability of docetaxel plus ramucirumab under PEG-G-CSF support for elderly patients with NSCLC. Frequency of hematological AEs grade greater than or equal to 3 were quite low, especially incidence of neutropenia grade greater than or equal to 3 at 5.6% with primary prophylactic PEG-G-CSF. Severe non-hematological toxicities were also seldom observed. Ramucirumab is a VEGF inhibitor, and a few bleeding events were observed. Careful monitoring of bleeding events is necessary particularly for elderly patients. There were no grade 4 non-hematological toxicities, but one (1.9%) treatment-related death (pneumonitis) was confirmed. Pneumonitis occurred in five patients (9.3%), including one (1.9%) grade 5. Although detailed feedback of the pneumonitic patients’ lungs was unavailable, 78% of enrolled patients were ever smoker, implying a possible smoking-related interstitial lung disease. Careful patient selection is desirable to prevent pneumonitis in docetaxel plus ramucirumab with PEG-G-CSF for elderly patients with NSCLC.
Our study has several limitations. First, our study was terminated before full accrual, and the number of final enrollment (n = 54) did not reach our initial targeted sample size (N = 65). However, the ORR (38.9%) exceeded our expected ORR (35%), suggesting the effectiveness of our studied treatment. Second, our study is a single arm phase 2 study without a reference arm. Synergistic effect of ramucirumab was unable to be evaluated in combination with docetaxel. Nevertheless, historical data of docetaxel monotherapy revealed relatively lower ORR and shorter PFS.3 Our study suggests the potency of additional ramucirumab to docetaxel under PEG-G-CSF support for chemo-naive elderly patients with NSCLC. Third, immunotherapy is already the main stream treatment even for elderly NSCLC patients. Our study was planned before approval of first-line ICI monotherapies or chemo-ICI combination therapies. In current clinical practice, such ICI containing regimens are often used in first-line treatment for “fit” elderly patients. Actually, patient accrual of our study became difficult after approval of first-line ICIs. Our adopted docetaxel plus ramucirumab is a standard of care for the second-line setting. After ICI exposure, docetaxel plus ramucirumab exerted a notable efficacy.16, 17, 18 Therefore, sequential first-line ICIs followed by docetaxel plus ramucirumab might be favorable over exploring first-line docetaxel plus ramucirumab for elderly patients.
In conclusion, our study revealed the efficacy and safety of docetaxel plus ramucirumab with primary prophylactic PEG-G-CSF support for chemo-naive elderly patients with advanced NSCLC. Primary prophylactic PEG-G-CSF highly reduced incidence of FN (1.9%: 1 of 54 patients), relative to historical control data. Our study provided evidence on clinical utility of primary prophylactic PEG-G-CSF for chemo-naive elderly patients. Although there are only few regimens with FN incidence greater than or equal to 20% for NSCLC, primary prophylactic PEG-G-CSF could be useful for frail patients such as elderly and or or poor PS.
CRediT Authorship Contribution Statement
Motoko Tachihara: Investigation, Data curation, Writing-original draft/review and editing.
Akito Hata: Supervision, Project administration, Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing-original draft/review and editing, Visualization, Funding acquisition.
Takaaki Tokito: Investigation, Data curation, Writing-original draft/review and editing.
Satoshi Hara: Investigation, Data curation, Writing-original draft/review and editing.
Hideaki Okada: Investigation, Data curation, Writing-original draft/review and editing.
Hiroshi Tanaka: Investigation, Data curation, Writing-original draft/review and editing.
Yuki Sato: Investigation, Data curation, Writing-original draft/review and editing.
Eriko Tabata: Investigation, Data curation, Writing-original draft/review and editing.
Hiroshi Watanabe: Investigation, Data curation, Writing-original draft/review and editing.
Yusuke Takayama: Investigation, Data curation, Writing-original draft/review and editing.
Ryo Toyozawa: Investigation, Data curation, Writing-original draft/review and editing.
Keiichi Ota: Investigation, Data curation, Writing-original draft/review and editing, Visualization.
Kazushige Wakuda: Investigation, Data curation, Writing-original draft/review and editing.
Atsushi Nakamura: Investigation, Data curation, Writing-original draft/review and editing.
Mototsugu Shimokawa: Investigation, Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing-original draft/review and editing, Visualization.
Nobuyuki Yamamoto: Supervision, Project administration, Investigation, Resources, Writing-original draft/review and editing, Funding acquisition.
Kazuhiko Nakagawa: Supervision, Project administration, Investigation, Resources, Writing-original draft/review and editing, Funding acquisition.
Acknowledgments
This study was supported by Eli Lilly, Japan. The authors thank the patients, their families, and all the investigators who participated in the present study. We appreciate Mr. David Martin for English writing support. Data management and monitoring for the study was conducted by the WJOG under a funding contract with Eli Lilly Japan, Co, Ltd. (Kobe, Japan).
Footnotes
Disclosure: Dr. Tachihara reports as honoraria, Eli Lilly, Chugai, Pfizer, AstraZeneca, Merck Sharp & Dohme, Takeda, Nippon Kayaku, Ono, Bristol-Myers Squibb, Boehringer Ingelheim, Taiho; grants, AstraZeneca; other financial or non-financial interests, Teijin. Dr. Hata reports as honoraria, Eli Lilly, Chugai, Pfizer, AstraZeneca, Boehringer Ingelheim, Taiho; grants, Merck Sharp & Dohme, Eli Lilly, Boehringer Ingelheim, AstraZeneca. Dr. Tokito reports as honoraria, Chugai, AstraZeneca, Merck Sharp & Dohme, Novartis, Bristol-Myers Squibb. Dr. Miura reports as honoraria, AstraZeneca, Ono, Bristol-Myers Squibb, Taiho, Chugai, Merck Sharp & Dohme, Boehringer Ingelheim, Eli Lilly, Novartis, Pfizer, Kyowa Hakko Kirin, Daiichi Sankyo, AbbVie, Nippon Kayaku, AMGEN, Merck, Takeda. Dr. Sato reports as honoraria, AstraZeneca, Merck Sharp & Dohme, Novartis, Chugai, Ono, Pfizer, Taiho, Nippon Kayaku, Bristol-Myers Squibb, Eli Lilly, Takeda, Kyowa Kirin. Dr. Toyozawa reports as honoraria, AstraZeneca, Chugai, Eli Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Novartis, Ono, Pfizer, Taiho, Takeda. Dr. Wakuda reports as honoraria, Chugai, Taiho, Boehringer Ingelheim, Eli Lilly, Ono, Merck Sharp & Dohme, AstraZeneca, Daiichi Sankyo, Janssen, Takeda. Dr. Nakamura reports as honoraria, Merck Sharp & Dohme, Thermo Fisher, AstraZeneca, Eli Lilly, Chugai, Taiho, Nippon Kayaku, Novartis. Dr. Yamamoto reports as honoraria. Merck Sharp & Dohme, AstraZeneca, Ono, Tsumura, Takeda, Chugai, Eli Lilly, Boehringer Ingelheim, Merck, Pfizer; grants, AstraZeneca, Chugai, Eli Lilly, Boehringer Ingelheim, Merck Sharp & Dohme, Prime Research Institute for Medical RWD, Mebix, IQVIA, Toppan printing; other financial interests, Taiho, Chugai, Daiichi Sankyo. Dr. Nakagawa reports as honoraria. Merck Sharp & Dohme, Ono, Chugai, Eli Lilly, Merck, Pfizer, Kyorin; grants, Merck Sharp & Dohme, Daiichi Sankyo, Taiho, Chugai, AstraZeneca, SymBio, Novartis, IQVIA, Covance, AbbVie, Medical Research Support, Boehringer Ingelheim, Syneos Health, Pfizer, Ezai, Eisai, Takeda, Sanofi, EPS, Sysmex, Ono, Eli, Lilly, Bristol-Myers Squibb, A2 Healthcare, Parexel International, Japan Clinical Research Operations, GlaxoSmithKline; royalties, Daiichi Sankyo; other financial interests, Ono, Chugai, Daiichi Sankyo, Takeda. The remaining authors declare no conflict of interest.
Cite this article as: Tachihara M, Hata A, Tokito T, et al. Docetaxel plus ramucirumab with primary prophylactic pegylated granulocyte-colony stimulating factor support for elderly patients with advanced NSCLC: a multicenter prospective single arm phase 2 trial: DRAGON study (WJOG9416L). JTO Clin Res Rep. 2023;4:100569.
References
- 1.Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. The elderly lung cancer vinorelbine Italian study group. J Natl Cancer Inst. 1999;91:66–72. doi: 10.1093/jnci/91.1.66. [DOI] [PubMed] [Google Scholar]
- 2.Gridelli C., Perrone F., Gallo C., et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst. 2003;95:362–372. doi: 10.1093/jnci/95.5.362. [DOI] [PubMed] [Google Scholar]
- 3.Kudoh S., Takeda K., Nakagawa K., et al. Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904) J Clin Oncol. 2006;24:3657–3663. doi: 10.1200/JCO.2006.06.1044. [DOI] [PubMed] [Google Scholar]
- 4.Quoix E., Zalcman G., Oster J.P., et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378:1079–1088. doi: 10.1016/S0140-6736(11)60780-0. [DOI] [PubMed] [Google Scholar]
- 5.Socinski M.A., Langer C.J., Okamoto I., et al. Safety and efficacy of weekly nab®-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:314–321. doi: 10.1093/annonc/mds461. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto I., Nokihara H., Nomura S., et al. Comparison of carboplatin plus pemetrexed followed by maintenance pemetrexed with docetaxel monotherapy in elderly patients with advanced nonsquamous non-small cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2020;6 doi: 10.1001/jamaoncol.2019.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spratlin J.L., Cohen R.B., Eadens M., et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780–787. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garon E.B., Ciuleanu T.E., Arrieta O., et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 9.Yoh K., Hosomi Y., Kasahara K., et al. A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer. 2016;99:186–193. doi: 10.1016/j.lungcan.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Gandara D.R., Kawaguchi T., Crowley J., et al. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol. 2009;27:3540–3546. doi: 10.1200/JCO.2008.20.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith T.J., Khatcheressian J., Lyman G.H., et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 12.Kosaka Y., Rai Y., Masuda N., et al. Phase III placebo-controlled, double-blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy. Support Care Cancer. 2015;23:1137–1143. doi: 10.1007/s00520-014-2597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nosaki K., Saka H., Hosomi Y., et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer. 2019;135:188–195. doi: 10.1016/j.lungcan.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 14.West H., McCleod M., Hussein M., et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto K., Yamada T., Yokoi T., et al. Clinical impact of pembrolizumab combined with chemotherapy in elderly patients with advanced non-small-cell lung cancer. Lung Cancer. 2021;161:26–33. doi: 10.1016/j.lungcan.2021.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Kato R., Hayashi H., Chiba Y., et al. Propensity score-weighted analysis of chemotherapy after PD-1 inhibitors versus chemotherapy alone in patients with non-small cell lung cancer (WJOG10217L) J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida M., Morimoto K., Yamada T., et al. Impact of docetaxel plus ramucirumab in a second-line setting after chemoimmunotherapy in patients with non-small-cell lung cancer: a retrospective study. Thorac Cancer. 2022;13:173–181. doi: 10.1111/1759-7714.14236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiono A., Kaira K., Mouri A., et al. Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients. Thorac Cancer. 2019;4:775–781. doi: 10.1111/1759-7714.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]