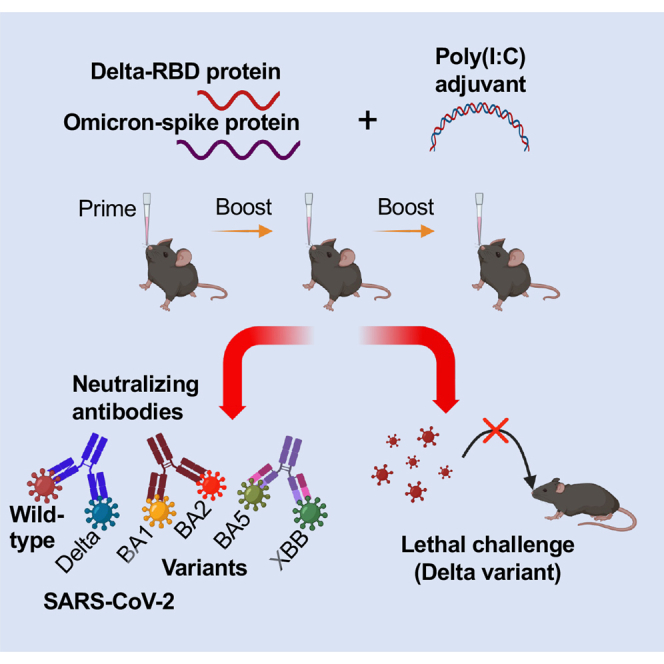
Summary
Mucosal COVID-19 vaccines are needed to block SARS-CoV-2 infection at the mucosal site. Intranasal delivery of a glycosylated Delta variant receptor-binding domain (Delta-RBD) mucosal vaccine elicited potent and balanced systemic antibody titers comparable to those induced by the intramuscular injection of the same vaccine or Omicron-S subunit vaccine, as well as high mucosal IgA antibody responses. It elicited broadly neutralizing antibodies against the original SARS-CoV-2 strain, Delta and Omicron BA1/BA2 variants, completely protecting transgenic mice from lethal challenge with a Delta variant, including complete absence of weight loss. Of note, intramuscular priming with the Omicron-S protein followed by intranasal boosting with the Delta-RBD protein improved the vaccine’s ability to generate broad-spectrum neutralizing antibodies against recent BA5 and XBB Omicron variants. Overall, this vaccine has the potential to prevent the SARS-CoV-2 infection of the respiratory mucosa, while the i.m. priming and i.n. boosting vaccination strategy may offer protection against known and emerging SARS-CoV-2 variants.
Subject areas: Immunology, Virology
Graphical abstract
Highlights
-
•
Glycosylated Delta-RBD mucosal vaccine elicited broadly neutralizing antibody (nAb)
-
•
It induced potent systemic IgG and mucosal IgA antibody responses in immunized mice
-
•
The vaccine completely protected mice against Delta variant without weight loss
-
•
Priming with Omicron-spike further improved nAb titer against Omicron variants
Immunology; Virology
Introduction
The global Coronavirus Disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has led to extensive economic damage and devastating loss of human life. Although SARS-CoV-2 has a much lower mortality rate than SARS-CoV and Middle East respiratory syndrome (MERS)-CoV, which caused the 2002 epidemic and 2012 outbreaks, respectively, it has much broader and long-lasting consequences.1,2,3,4,5 As of September 06, 2023, SARS-CoV-2 has infected at least 770 million people and caused over 6.95 million deaths.6
The SARS-CoV-2 genome encodes four structural proteins, namely the spike (S), envelope, nucleocapsid, and membrane proteins, among which the surface S protein plays a critical role in viral infection.7 The S protein of SARS-CoV-2 is composed of the S1 and S2 subunits. Viral infection is initiated by binding of the receptor-binding domain (RBD) in the S1 subunit to its cellular receptor, angiotensin-converting enzyme 2 (ACE2).8 Thus, the S protein, and specifically its RBD fragment, are important targets for the development of effective COVID-19 vaccines.9,10 Multiple SARS-CoV-2 variants have been characterized to date, including Alpha, Beta, Gamma, Delta, and Omicron (as well as its subvariants).11,12 Although the available COVID-19 vaccines targeting the S protein and/or its RBD exhibit high potency against the earlier SARS-CoV-2 strains and variants, they fail to induce broad-spectrum neutralizing antibodies and therefore high levels of protection against the currently dominant SARS-CoV-2 Omicron variants.13,14,15
On May 05, 2023, the World Health Organization (WHO) declared that COVID-19 was no longer a Public Health Emergency of International Concern.16,17 Additionally, the United States COVID-19 public health emergency came to an end on May 11, 2023, thereby concluding the 3-year COVID-19 pandemic.18 However, the challenge of preventing deaths from the high transmissible and mutation-prone SARS-CoV-2 remains.19 Therefore, the development of effective vaccines to prevent infection with current and future SARS-CoV-2 variants with pandemic potential is still a priority.
The immunoglobulin (Ig)G Fc fragment has been used as a vehicle for the effective intranasal delivery of vaccines to mucosal surfaces, and subsequent induction of mucosal and systemic immune responses against respiratory viral pathogens such as MERS-CoV, respiratory syncytial virus, and influenza virus.20,21,22 Because SARS-CoV-2 is a respiratory viral pathogen, the mucosal or intranasal (i.n.) delivery of COVID-19 vaccines (i.e., via the IgG Fc fragment) is expected to elicit both local and systemic immune responses with superior levels of protection than the intramuscular (i.m.) delivery of the vaccines.23,24
We previously showed that the glycosylation of amino acid residues 519 and 521 within the RBD of the original SARS-CoV-2 strain masked a non-neutralizing epitope containing residue 519, and induced significantly higher systemic neutralizing antibody responses than the non-glycosylated form of the RBD; this led to superior protection against infection with the original SARS-CoV-2 strain.25 In the present study, we designed a mucosal COVID-19 vaccine by fusing a glycosylated Delta variant RBD to the Fc fragment of human IgG, and evaluated its ability to elicit systemic and mucosal immune responses, as well as protect K18-human (h)ACE2 transgenic model mice against lethal SARS-CoV-2 challenge. In addition, we determined how different routes of administration affected the broadly neutralizing activity and protective efficacy of the glycosylated Delta-RBD mucosal vaccine and a trimeric S protein of SARS-CoV-2 Omicron variant (Omicron-S)-based vaccine. Finally, we assessed whether the neutralizing activity and protection of our mucosal vaccine could be further improved by priming with Omicron-S subunit vaccine.
Results
Intranasal delivery of glycosylated Delta-receptor-binding domain with or without Omicron-S (i.m.) priming induced potent and balanced systemic antibody responses
To evaluate the ability of the glycosylated Delta-RBD mucosal subunit vaccine to induce systemic immune responses, K18-hACE2 (C57BL/6 (B6) background) mice were i.n. immunized with glycosylated Delta-RBD (Delta-RBD-i.n.) conjugated to the mucosal adjuvant polyinosinic-polycytidylic acid (Poly(I:C)) (Figure 1). Control mice were immunized with 1) Delta-RBD via i.m. (Delta-RBD-i.m.), 2) Omicron-S via i.m. (Omicron-S-i.m.), 3) Omicron-S via i.n. (Omicron-S-i.n.), or 4) Omicron-S via i.m. priming and then Delta-RBD via i.n. boosting (Omicron-S-i.m. + Delta-RBD-i.n.). Phosphate-buffered saline (PBS) was used as the vehicle in all vaccination experiments. Previously optimized Alum and MPL combinations or Poly(I:C) were used as adjuvants for i.m. and i.n. immunizations, respectively.11,21,26 These mice were boosted two times using the same or different immunogens, and their sera were collected 10 days after the last immunization to detect SARS-CoV-2-specific IgG and IgG subtype antibodies (Figure 1A).
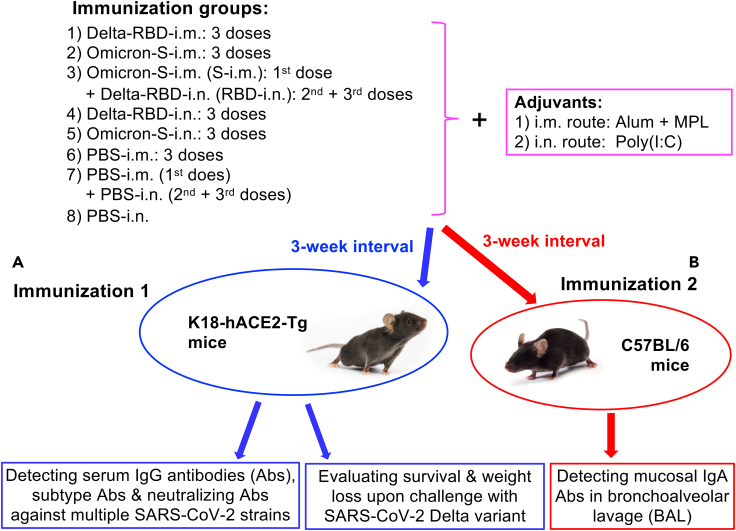
Figure 1.
Mouse immunization procedures and viral challenge schedules
(A) Eight groups of K18-hACE2 (B6) background) mice were immunized with the Delta-RBD protein, Omicron-S protein, or PBS control via intramuscular (i.m.), i.m.-intranasal (i.n.), or i.n. route for three doses at a 3-week interval. Aluminum (Alum) plus monophosphoryl lipid A (MPL) and Poly(I:C) adjuvants were used for i.m. and i.n. routes, respectively. Sera collected from the last immunization were tested for specific IgG antibodies (Abs), subtype Abs, and neutralizing Abs against SARS-CoV-2 original strain and multiple variants. Immunized mice were then challenged with the SARS-CoV-2 Delta variant, and evaluated for protective efficacy via the investigation of survival and weight changes.
(B) Eight groups of B6 mice were immunized as described above, and detected for mucosal IgA Abs from collected bronchoalveolar lavage (BAL) fluid.
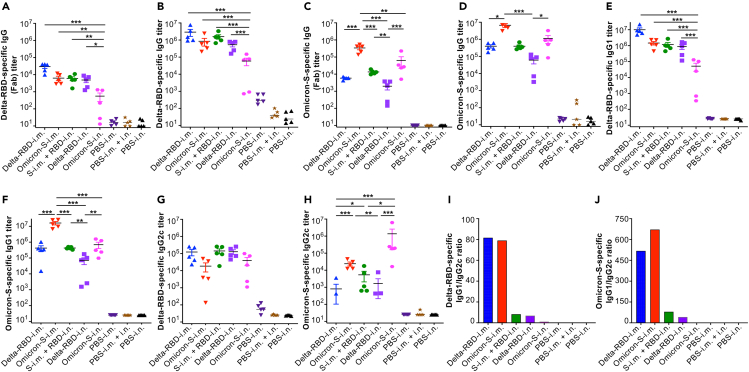
We found that Delta-RBD-i.n. induced potent IgG (Fab or full-length) antibody responses against the Delta-RBD protein, which were comparable to those induced by Delta-RBD-i.m. or Omicron-S-i.m., and Omicron-S-i.m. + Delta-RBD-i.n. priming-boosting, but significantly higher than that elicited by Omicron-S-i.n. alone (Figures 2A and 2B). Immunization with Delta-RBD-i.n. also elicited cross-reactive IgG (Fab or full-length) antibodies against the Omicron-S protein, with the antibody titer being similar to that elicited by Delta-RBD-i.m.; however, the antibody titer following Delta-RBD-i.n. vaccination was significantly lower than that induced by immunization with Omicron-S-i.m. or i.n. (Figures 2C and 2D). Of note, the anti-Omicron-S IgG antibody titer induced by i.n. immunization with Delta-RBD after Omicron-S-i.m. priming was significantly higher than that elicited by vaccination with Delta-RBD-i.n. alone (Figure 2C). Immunization with Delta-RBD-i.n. also elicited a potent IgG1 subtype antibody response, with strong reactivity against Delta-RBD and cross-reactivity against Omicron-RBD; moreover, these antibodies were present at a similar trend to those of the IgG (full-length) antibodies targeting both proteins, but much higher levels than those of the IgG (Fab) antibodies (Figures 2A–2F). In addition, the Delta-RBD-i.n. vaccination induced IgG2c subtype antibodies, which potently recognized Delta-RBD protein and cross-reacted with Omicron-S protein (Figures 2G and 2H). By contrast to the i.m. immunization route, the i.n. delivery of Delta-RBD or Omicron-S induced similar levels of IgG1 and IgG2c antibodies (corresponding to low IgG1/IgG2c ratios) targeting both Delta-RBD and Omicron-S proteins (Figures 2I and 2J). These data indicate that mucosal immunization with the Delta-RBD vaccine alone was sufficient to induce strong and balanced systemic antibody responses.
Figure 2.
Intranasally immunized Delta-RBD protein with or without Omicron-S priming induced systemic IgG and subtype antibodies
Sera were collected from immunized K18-hACE2 mice 10 days after the last immunization, and tested by ELISA for specific IgG (Fab or full-length) and subtype antibodies (Abs).
(A and B) Fab (A) or full-length (B) IgG Abs specific to the Delta-RBD protein.
(C and D) Fab (C) or full-length (D) IgG Abs specific to the Omicron-S protein.
(E and F) IgG1 (full-length) Abs specific to the Delta-RBD (E) or Omicron-S (F) protein.
(G and H) IgG2c (full-length) Abs specific to the Delta-RBD (G) or Omicron-S (H) protein.
(I and J) IgG/IgG2c ratios calculated based on the Abs specific to the Delta-RBD (I) or Omicron-S (J) protein.
The ELISA plates were coated with the respective protein, and the antibody titer was determined based on the detectable endpoint serum dilution. The data are shown as mean ± standard deviation of the mean (s.e.m.) of five mice in each group. Delta-RBD-i.m. or Delta-RBD-i.n. indicates Delta-RBD protein intramuscular or intranasal immunization. Omicron-S-i.m. or Omicron-S-i.n. indicates Omicron-S protein intramuscular or intranasal immunization. S-i.m. + RBD-i.n. indicates i.m. priming with the Omicron-S protein followed by i.n. boosting with the Delta-RBD protein. Controls, PBS with relevant adjuvants injected via i.m., i.m. + i.n., or i.n. route. ∗ (p < 0.05), ∗∗ (p < 0.01), and ∗∗∗ (p < 0.001) designate significant differences among different groups. The experiments were repeated twice, and similar data were acquired.
Intranasal delivery of glycosylated Delta-receptor-binding domain, particularly Omicron-S (i.m.) priming and glycosylated Delta-receptor-binding domain (i.n.) boost, induced broadly anti-SARS-CoV-2 neutralizing antibodies
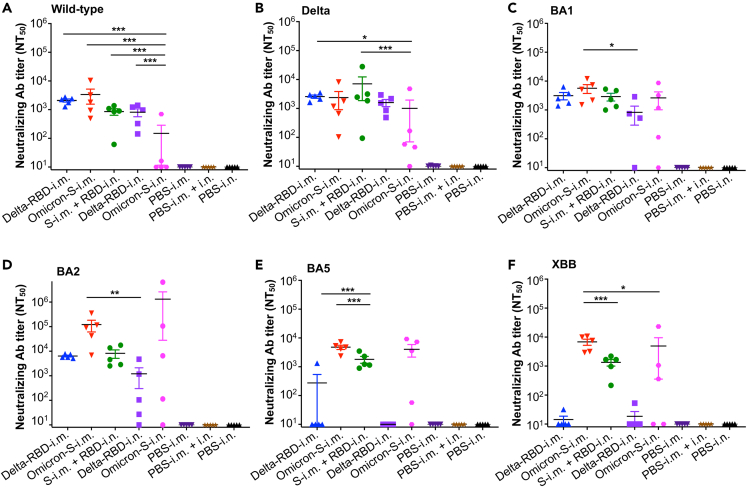
Next, the neutralizing and cross-neutralizing abilities of the glycosylated Delta-RBD mucosal vaccine against the pseudotyped original strain (wild-type) of SARS-CoV-2 and its multiple variants were tested using mouse sera collected from the aforementioned immunization experiments (Figure 1A). Immunization with Delta-RBD-i.n., with or without Omicron-S-i.m. priming, elicited effective neutralizing antibodies against infection with the wild-type and Delta variant pseudoviruses (Figures 3A and 3B). These neutralizing antibody titers were comparable to those induced by i.m. immunization with Delta-RBD or Omicron-S, but were significantly higher, or higher, than those induced by i.n. immunization with Omicron-S. Delta-RBD-i.n. vaccination also induced cross-neutralizing antibodies against the Omicron BA1 and BA2 pseudoviruses (Figures 3C and 3D). Moreover, Delta-RBD-i.n. boosting after Omicron-S-i.m. priming further increased neutralizing antibody titers against Omicron BA2, while also inducing an effective neutralizing antibody response against the Omicron BA5 and XBB pseudoviruses (Figures 3D–3F). Overall, these data indicate that i.n. immunized with Delta-RBD alone induced broadly neutralizing antibodies against the original strain of SARS-CoV-2, as well as the Delta and Omicron (BA1 and BA2) variants, whereas i.m. priming with Omicron-S followed by Delta-RBD-i.n. boosting elicited potent neutralizing antibodies against all SARS-CoV-2 strains tested.
Figure 3.
Intranasally immunized Delta-RBD protein with or without Omicron-S priming induced broadly anti-SARS-CoV-2 neutralizing antibodies
Sera from immunized K18-hACE2 mice 10 days after the last immunization were tested for neutralizing antibodies (Abs) against pseudotyped SARS-CoV-2 wild-type strain and variants.
(A) Neutralizing Abs against pseudotyped original SARS-CoV-2 wild-type strain.
(B) Neutralizing Abs against pseudotyped SARS-CoV-2 Delta variant.
(C) Neutralizing Abs against pseudotyped SARS-CoV-2 Omicron BA1 subvariant.
(D) Neutralizing Abs against pseudotyped SARS-CoV-2 Omicron BA2 subvariant.
(E) Neutralizing Abs against pseudotyped SARS-CoV-2 Omicron BA5 subvariant.
(F) Neutralizing Abs against pseudotyped SARS-CoV-2 Omicron XBB subvariant.
The pseudovirus neutralization assay was performed in 293T cells expressing SARS-CoV-2 receptor, human ACE2 (hACE2/293T), and 50% neutralizing Ab titer was reported as NT50 against respective pseudovirus infection. The data are shown as mean ± s.e.m. of five mice in each group. Delta-RBD-i.m. or Delta-RBD-i.n. indicates Delta-RBD protein intramuscular or intranasal immunization. Omicron-S-i.m. or Omicron-S-i.n. indicates Omicron-S protein intramuscular or intranasal immunization. S-i.m. + RBD-i.n. indicates i.m. priming with the Omicron-S protein followed by i.n. boosting with the Delta-RBD protein. Controls, PBS with relevant adjuvants injected via i.m., i.m. + i.n., or i.n. route. ∗ (p < 0.05), ∗∗ (p < 0.01), and ∗∗∗ (p < 0.001) designate significant differences among different groups. The experiments were repeated twice, and similar data were acquired.
Intranasal delivery of glycosylated Delta-receptor-binding domain or Omicron-S protein induced strong mucosal IgA antibody responses
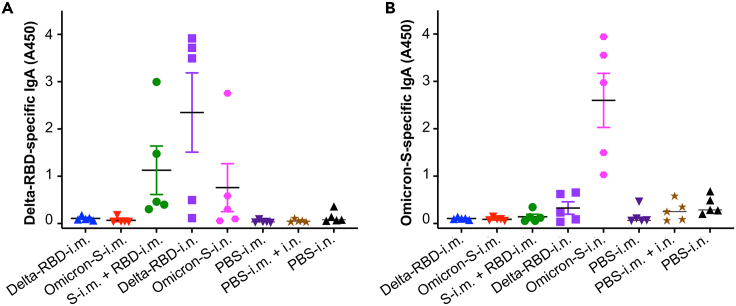
To evaluate the ability of glycosylated Delta-RBD to induce a mucosal immune response, B6 mice were i.n. immunized with this protein or the respective controls. Bronchoalveolar lavage (BAL) fluid was collected from each mouse 1 month after the last immunization (the time point used for the SARS-CoV-2 challenge, as described later in discussion) for specific secretory IgA antibody detection (Figure 1B). The delivery of Delta-RBD-i.n. alone elicited high levels of IgA antibodies specific to Delta-RBD, which showed weaker or background levels of cross-reactivity with Omicron-S (Figures 4A and 4B). Interestingly, the i.n. delivery of Omicron-S alone also induced adequate levels of anti-Omicron-S IgA antibodies (Figure 4B). By contrast, i.m. immunization with Delta-RBD or Omicron-S protein only elicited background levels of IgA antibodies specific to both proteins, which were similar to those induced in the control mice receiving PBS and adjuvant (Figures 4A and 4B). These results reveal that Delta-RBD and Omicron-S via the i.n., but not the i.m., route elicit potent mucosal anti-SARS-CoV-2 antibody responses.
Figure 4.
Intranasally immunized Delta-RBD or Omicron-S protein induced potent mucosal IgA antibody responses
Immunized B6 mice were collected for bronchoalveolar lavage (BAL) fluid one month after the last immunization, and tested by ELISA for SARS-CoV-2-specific IgA antibodies.
(A) IgA antibodies specific to the Delta-RBD protein.
(B) IgA antibodies specific to the Omicron-S protein.
The ELISA plates were coated with the respective protein, and the IgA antibody responses were reported as OD450 values. The data are shown as mean ± s.e.m. of five mice in each group. Delta-RBD-i.m. or Delta-RBD-i.n. indicates Delta-RBD protein intramuscular or intranasal immunization. Omicron-S-i.m. or Omicron-S-i.n. indicates Omicron-S protein intramuscular or intranasal immunization. S-i.m. + RBD-i.n. indicates i.m. priming with the Omicron-S protein followed by i.n. boosting with the Delta-RBD protein. Controls, PBS with relevant adjuvants injected via i.m., i.m. + i.n., or i.n. route. The experiments were repeated twice, and similar data were acquired.
Intranasal delivery of glycosylated Delta-receptor-binding domain completely protected mice from lethal Delta variant challenge without obvious weight loss
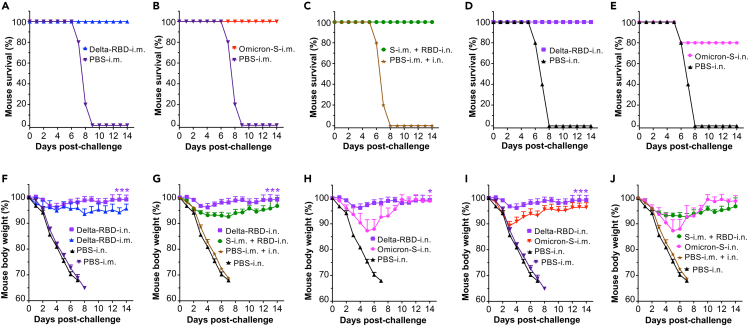
To evaluate the protective efficacy of the glycosylated Delta-RBD mucosal vaccine, immunized K18-hACE2 mice were challenged with the SARS-CoV-2 Delta variant 1 month after their last immunization. Mouse weight changes and overall survival were investigated for 14 days after challenge (Figure 1A). The mice receiving Delta-RBD-i.n., with or without Omicron-S-i.m. priming, and those i.m. immunized with Delta-RBD or Omicron-S all survived the viral challenge (Figures 5A–5D); however, only 80% of the mice i.n. immunized with Omicron-S survived the challenge (Figure 5E). Of note, mice receiving Delta-RBD via the i.m. route experienced weight loss in the first few days post-challenge and maintained the similar weight during the experimental period; by contrast, the mice i.n. immunized with Delta-RBD did not experience obvious weight loss, which showed significantly increased overall weight as compared to the mice receiving Delta-RBD-i.m. or the mice priming with Omicron-S-i.m. and boosting with Delta-RBD-i.n. (Figures 5F and 5G). The mice receiving Omicron-S protein via the i.m. route, and particularly those receiving Omicron-S via the i.n. route, lost significantly more weight than the mice receiving Delta-RBD-i.n. (with or without Omicron-S-i.m. priming), especially on days 2–8 post-challenge (Figures 5H–5J). These data indicate that the i.n. delivery of glycosylated Delta-RBD (with or without Omicron-S-i.m. priming) protected immunized mice from Delta variant infection and prevented SARS-CoV-2-induced weight loss.
Figure 5.
Intranasally immunized Delta-RBD protein with or without Omicron-S priming protected mice from challenge with the SARS-CoV-2 Delta variant
One month after the last immunization of the respective vaccines or PBS control plus adjuvant(s), K18-hACE2 mice were challenged with a SARS-CoV-2 Delta variant (104 PFU/mouse), and mouse survival and weight changes were investigated for 14 days post-challenge.
(A–E) Survival of challenged mice immunized with Delta-RBD-i.m. (A), Omicron-S-i.m. (B), S-i.m. + RBD-i.n. (C), Delta-RBD-i.n. (D), or Omicron-S-i.n. (E).
(F–J) Comparison of weight changes of challenged mice immunized with Delta-RBD-i.n. or Delta-RBD-i.m. (F), Delta-RBD-i.n. or S-i.m. + RBD-i.n. (G), Delta-RBD-i.n. or Omicron-S-i.n. (H), Delta-RBD-i.n. or Omicron-S-i.m. (I), and S-i.m. + RBD-i.n. or Omicron-S-i.n. (J), respectively.
The data (in F–J) are shown as mean +s.e.m. of five mice in each group. Delta-RBD-i.m. or Delta-RBD-i.n. indicates Delta-RBD protein intramuscular or intranasal immunization. Omicron-S-i.m. or Omicron-S-i.n. indicates Omicron-S protein intramuscular or intranasal immunization. S-i.m. + RBD-i.n. indicates i.m. priming with the Omicron-S protein followed by i.n. boosting with the Delta-RBD protein. Controls, PBS with relevant adjuvants injected via i.m., i.m. + i.n., or i.n. route. ∗ (p < 0.05) and ∗∗∗ (p < 0.001) designate significant differences among Delta-RBD-i.n. and other groups.
Discussion
Currently, several COVID-19 vaccines have been approved for human use and more have been developed preclinically.27,28,29 Although many of the first-generation vaccines induce strong systemic immune responses and protection against the original SARS-CoV-2 strain and early variants, their ability to protect against recent variants is markedly reduced.30,31 SARS-CoV-2 is a mucosal pathogen, which initiates infection in the upper respiratory tract mucosae, before reaching the lower respiratory tract and lungs. Thus, there is a need for mucosal vaccines to block viral transmission at the mucosal site.32,33 Accordingly, next-generation COVID-19 vaccines should be developed to prevent viral infection and transmission via the respiratory route and elicit broad-spectrum neutralizing antibodies targeting multiple SARS-CoV-2 variants.23
Most of the currently developed mucosal COVID-19 vaccines are based on an adenovirus (Ad) vector delivery system.34,35,36 For instance, chimpanzee Ad (ChAd)-vectored trivalent mucosal COVID-19 vaccines expressing SARS-CoV-2 S1, nucleocapsid, and RNA-dependent RNA polymerase proteins induced local and systemic antibodies and protected mice against the original SARS-CoV-2 strain and the Alpha/B.1.1.7 and Beta/B.1.351 variants.34 Moreover, a human Ad5-vectored S-expressing vaccine delivered via i.n. route protected hamsters against severe SARS-CoV-2 infection and transmission.36 Meanwhile, the i.n. injection of a ChAd-vectored SARS-CoV-2-S vaccine elicited systemic and mucosal IgA antibodies, protecting the upper and lower respiratory tracts against SARS-CoV-2 infection.35 Finally, while a systemic S-mRNA vaccine alone elicited weak mucosal immune responses, its combination with a mucosal Ad-S vaccine resulted in the production of potent neutralizing antibodies against the original SARS-CoV-2 strain and the Omicron BA.1.1 subvariant.37
We previously demonstrated that the systemic injection of a glycosylated SARS-CoV-2 wild-type RBD increased neutralizing antibody titers against the original RBD and its multiple variant forms, which offered more protection against SARS-CoV-2 infection than the wild-type RBD without glycosylation.25 Because the Delta variant induces higher levels of severe COVID-19 and death than the wild-type SARS-CoV-2 strain and the other known variants,38,39,40 we decided to focus on it in the present study.
Here, we developed a mucosal COVID-19 subunit vaccine by fusing the glycosylated RBD of the Delta variant to the Fc of human IgG and evaluated its ability to induce mucosal immunity in the presence or absence of an Omicron-S protein. We found that i.n. immunization with the glycosylated Delta-RBD vaccine alone elicited effective IgG and IgG subtype antibody responses. As expected, the antibody titers targeting the Fab of IgG were lower than those targeting the full-length IgG. These antibodies were comparable to those induced by the i.m. immunization with this vaccine, but more balanced systemic antibodies (with lower IgG1/IgG2c ratio). Compared to other vaccination groups, such as Omicron-S-i.m. priming followed by Delta-RBD-i.n. boosting, Delta-RBD-i.n. alone elicited a similar level of Delta-RBD-specific IgG and subtype antibody responses, as well as neutralizing antibodies against the Delta variant, but a more potent Delta-RBD-specific mucosal IgA antibody response in the BAL fluid of model mice, leading to the complete protection of immunized animals against lethal challenge with the Delta variant without obvious weight loss. This phenomenon partially explains the significance of mucosal vaccination in protection against SARS-CoV-2 infection. Moreover, the Delta-RBD-i.n. mucosal vaccine alone also induced effective neutralizing antibodies against the original SARS-CoV-2 strain and early Omicron subvariants (e.g., BA1 and BA2), and its ability to elicit potent and broadly neutralizing antibodies against recent Omicron dominant subvariants (e.g., BA5 and XBB) was significantly increased by priming with Omicron-S.
To conclude, we designed a mucosal COVID-19 vaccine that elicited strong mucosal immune responses and broadly neutralizing antibody responses against multiple SARS-CoV-2 variants in mice, while offering complete protection against SARS-CoV-2-induced weight loss and death. In addition, we developed an effective immunization strategy for improving the potency of broadly neutralizing antibodies, which consisted of i.m. priming with an Omicron-S subunit vaccine followed by i.n. boosting with the Delta-RBD vaccine. Our findings collectively imply that the Delta-RBD-i.n. mucosal vaccine can effectively prevent SARS-CoV-2 transmission at the respiratory mucosa, which can be further improved by Omicron-S priming to protect against the infection of known and emerging SARS-CoV-2 variants with pandemic potential.
Limitations of the study
This study mainly focused on the protective efficacy of the glycosylated Delta-RBD mucosal vaccine against the SARS-CoV-2 Delta variant. Future studies will be performed to evaluate the protection of this vaccine alone or its combination with Omicron-S or other candidate vaccines against current Omicron dominant subvariant or other SARS-CoV-2 variants with pandemic potential.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse IgA antibody-HRP | Invitrogen | Cat# 62-6720 |
| Anti-mouse IgG (Fab) antibody-HRP | Sigma | Cat# A9917; RRID: AB_258476 |
| Anti-mouse-IgG (H+L) antibody-HRP | Invitrogen | Cat# 31430 |
| Anti-Mouse IgG1 antibody-HRP | Invitrogen | Cat# PA1-74421 |
| Anti-Mouse IgG2c antibody-HRP | Invitrogen | Cat# PA1-29288 |
| Experimental models: Cell lines | ||
| hACE2/293T | Laboratory stock | N/A |
| HEK293F | Thermo Fisher Scientific | Cat# K900001 |
| HEK293T | ATCC | Cat# CRL-3216; RRID: CVCL_0063 |
| Chemicals, peptides, and recombinant proteins | ||
| Fat-free milk | Bio-Rad | Cat# 1706404 |
| Polyetherimide (PEI) | Sigma | Cat# 919012 |
| Recombinant DNA | ||
| pLenti-CMV-luciferase | Addgene | Cat# 17477 |
| PS-PAX2 | Addgene | Cat# 12260 |
| Software and algorithms | ||
| Gen5 | BioTek Instruments | N/A |
| GraphPad Prism 9 | Graphpad Software | N/A |
| Bacterial and virus strains | ||
| Live SARS-CoV-2 Delta variant | GISAID | Accession No: EPI_ISL_2331496 |
| Pseudotyped SARS-CoV-2 original strain | GenBank | Accession No: QHR63250.2 |
| Pseudotyped SARS-CoV-2 BA1 subvariant | GISAID | Accession No: EPI_ISL_6795835 |
| Pseudotyped SARS-CoV-2 BA2 subvariant | GISAID | Accession No: EPI_ISL_12030355 |
| Pseudotyped SARS-CoV-2 BA5 subvariant | GISAID | Accession No: EPI_ISL_12043290 |
| Pseudotyped SARS-CoV-2 XBB subvariant | GISAID | Accession No: EPI_ISL_15341139 |
| Other | ||
| Aluminum adjuvant | InvivoGen | Cat# vac-alu-250 |
| Fetal bovine serum (FBS) | R&D Systems | Cat# S11550 |
| Luciferase Assay System | Promega | Cat# E1501 |
| Monophosphoryl lipid A adjuvant | InvivoGen | Cat# vac-mplas |
| Ni-NTA Superflow | Qiagen | Cat# 1018401 |
| nProtein A Sepharose 4 Fast Flow | GE Healthcare | Cat# 17-5280-02 |
| Gibco™ Penicillin and Streptomycin | Thermo Fisher Scientific | Cat# 15140122 |
| Poly(I:C) adjuvant | InvivoGen | Cat# tlrl-picw |
Resource availability
Lead contact
Requests for additional information, resources, or reagents should be directed to the lead contact (ldu3@gsu.edu).
Materials availability
-
•
Special constructs and materials related to this study are available from the lead contact with a signed Material Transfer Agreement.
Experimental model and study participant details
Cell lines
HEK293F, 293T, and 293T expressing human ACE2 receptor (hACE2/293T) cells were cultured at 37°C in the presence of 5% CO2. The 293F cells were cultured in ESF serum-free medium (CSF) (Expression Systems). The 293T and hACE2/293T cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) (R&D Systems) and 1% Gibco™ Penicillin-Streptomycin (Thermo Fisher Scientific).
Viruses
The following SARS-CoV-2 pseudoviruses were used, and they were generated from relevant recombinant plasmids. Specifically, a recombinant wild-type (WT)-S-DNA plasmid encoding full-length S protein of the original SARS-CoV-2 strain (GenBank: QHR63250.2) was constructed by inserting the S gene sequence into pcDNA3.1/V5-His-TOPO vector (Thermo Fisher Scientific). The S-DNA plasmids of Delta variant and Omicron subvariants, including BA1 (GISAID: EPI_ISL_6795835), BA2 (GISAID: EPI_ISL_12030355), BA5 (GISAID: EPI_ISL_12043290), and XBB (GISAID: EPI_ISL_15341139), were constructed to contain the respective mutations at the RBD using the multi-site-directed mutagenesis kit. Live SARS-CoV-2 Delta variant (hCoV-19/USA/MD-HP05647/2021; GISAID: EPI_ISL_2331496) was used in this study.
Mice
Male and female C57BL/6 (B6) mice and female K18-hACE2 transgenic mice (6-8-week-old) were used for the immunization studies. They were randomly assigned to different vaccination groups. The animal protocols were approved by the Institutional Animal Care and Use Committees (IACUC) of Georgia State University and University of Iowa. All mouse experiments were performed according to our approved protocols and the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health.
Method details
Preparation of recombinant protein vaccines
The glycosylated Delta-RBD DNA construct containing a C-terminal Fc tag of human IgG1 was prepared by mutation of residues L452R and T478K of the original RBD sequence (with glycosylation of residues 519 and 521 of the S protein) of SARS-CoV-2 strain (GenBank: QHR63250.2) using a multi-site-directed mutagenesis kit (Agilent Technologies), and insertion into a mammalian cell expression vector.11,41 The Omicron-S DNA construct contained the codon-optimized S sequence of B.1.1.529 variant (GISAID: EPI_ISL_6795835) of SARS-CoV-2 and HexaPro sequences, as well as a C-terminal foldon motif and a His6 tag, which was then inserted into the above vector.11 The amino acid residues were mutated to include substitutions at S371F, T376A, D405N, R408S, L452R, and F486V. The sequence-confirmed recombinant DNA plasmids were transfected into HEK293F cells, and related proteins were purified from culture supernatants using nProtein A Sepharose 4 Fast Flow (for Delta-RBD) (GE Healthcare) and Ni-NTA Superflow (for Omicron-S) (Qiagen), respectively.
Enzyme-linked immunoassay
Enzyme-linked immunoassay (ELISA) was used to test specific IgG and IgA antibodies induced in immunized mouse sera and lung BAL fluid, respectively.21,42 Specifically, ELISA plates were coated with the Delta-RBD or Omicron-S proteins (1 μg/ml) at 4°C overnight, and blocked with the PBST-blocking buffer (2% non-fat milk in PBS containing 0.05% Tween-20) at 37°C for 2 h. The plates were washed with PBST buffer for five times, and then sequentially incubated with mouse sera at serial dilutions or lung BAL fluid collection, and horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Fab-specific, 1:60,000, Sigma; or whole antibody, 1:10,000, Invitrogen), anti-mouse-IgG1 (1:8,000, Invitrogen), anti-mouse-IgG2c (1:10,000, Invitrogen), or anti-mouse IgA (1:3,000, Invitrogen) antibody at 37°C for 1 h. After further washes, the plates were incubated with TMB (3,3’,5,5’-Tetramethylbenzidine) substrate (Sigma), followed by stop of the reaction by H2SO4 (1 N). Absorbance at 450 nm (A450 value) was measured using Cytation 7 Microplate Multi-Mode Reader (BioTek Instruments).
SARS-CoV-2 pseudovirus generation and neutralization assay
These experiments were performed as described below.43,44 The sequence-confirmed recombinant plasmids listed in the virus section were used for generation of pseudoviruses. Each of the above plasmids was respectively co-transfected with PS-PAX2 and pLenti-CMV-luciferase plasmids into 293T cells (ATCC) using the Polyetherimide (PEI) transfection method, followed by collection of pseudovirus-containing supernatants 72 h post-transfection. For pseudovirus neutralization assay, each pseudovirus was respectively incubated with serially diluted mouse sera at 37°C for 1 h, followed by addition of the virus-serum mixture to 293T cells expressing hACE2 (hACE2/293T) (Lab stock) pre-seeded in 96-well plates. After addition of fresh medium 24 h later, the cells were continually cultured for up to 72 h, and lysed using cell lysis buffer, followed by incubation with luciferase substrate (Promega). Relative luciferase activity was then measured using Cytation 7 Microplate Multi-Mode Reader. 50% neutralizing antibody titer (NT50) of each serum sample was calculated accordingly.45
Immunization procedures and sample collection
Two separate immunizations were performed to evaluate immune responses, neutralizing antibodies, and protective efficacy induced by the vaccines (Figure 1).25 First, K18-hACE2 mice (B6 background) were immunized with the following proteins (10 μg protein/mouse) or controls: intramuscularly (i.m.) with 1) Delta-RBD or 2) Omicron-S for three doses at 3 weeks, 3) i.m. with Omicron-S for the 1st dose and intranasally (i.n.) with Delta-RBD for two boosts at 3 weeks, and i.n. with 4) Delta-RBD or 5) Omicron-S for three doses at 3 weeks. PBS injected via i.m., i.m. + i.n., and i.n. routes, respectively, was included as controls. Aluminum (500 μg/mouse) and monophosphoryl lipid A (MPL, 10 μg/mouse) adjuvants (InvivoGen) were used for proteins via the i.m. route (100 μl/mouse), and Poly(I:C) (10 μg/mouse) adjuvant (InvivoGen) was used for proteins via the i.n. route (20 μl/mouse). The use of the above adjuvants for the i.m. and i.n. routes were based on our previously optimized protocols.11,21 Sera were collected 10 days after the last immunization for detection of specific IgG, IgG1, IgG2c, and neutralizing antibodies as described above. Immunized mice were challenged with the SARS-CoV-2 Delta variant four weeks after the last immunization for evaluation of protective efficacy. Second, B6 mice were immunized with each protein or control as described above. Four weeks after the last immunization, BAL fluid was collected from mouse lungs for evaluation of specific IgA antibody response as described above.
Challenge of mice with SARS-CoV-2 Delta variant
The challenge experiments were performed as described below.11,25 Four weeks after the last immunization, K18-hACE2 mice were i.n. challenged with a Delta variant of SARS-CoV-2 (104 plaque-forming unit (PFU)/mouse, 50 μl/mouse), and investigated for body weight changes and survival for 14 days post-infection. Mice that were moribund or lost 30% weight were humanely euthanized by cervical dislocation under anesthesia.
Quantification and statistical analysis
Statistical significance among different groups was determined using statistical software (GraphPad Prism 9). Statistical differences of IgG and IgG subtype antibody responses, as well as neutralizing antibody titers, among different groups were calculated using Tukey’s multiple comparison test. Statistical differences of weight changes among different groups after viral challenge were compared using unpaired two-tailed student t test. ∗, ∗∗, and ∗∗∗ indicate P < 0.05, P < 0.01, and P < 0.001, respectively.
Acknowledgments
This study was supported by NIH grants (R01AI157975, R01AI139092, and R01AI137472).
Author contributions
X.G. and A.K.V. carried out the experiments for animal immunization, sample collection, antibody detection, and viral challenge, as well as analyzed the data, and prepared the figures. G.W. and J.S. prepared protein vaccines and pseudoviruses, and performed neutralization assays. S.P. supervised the study and revised the article. L.D. conceived, designed, and supervised the study, wrote, and revised the article.
Declaration of interests
The authors declare no known financial interests. The authors have filed a patent application related to the studies with L.D., G.W. and J.S. as inventors.
Published: September 27, 2023
Contributor Information
Stanley Perlman, Email: stanley-perlman@uiowa.edu.
Lanying Du, Email: ldu3@gsu.edu.
Data and code availability
-
•
All data relevant to the study are included in this paper.
-
•
No code was used in this study.
-
•
Any additional information required for reanalyzing the data reported in this paper is available from the lead contact upon request.
References
- 1.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang N., Shang J., Li C., Zhou K., Du L. An overview of Middle East respiratory syndrome coronavirus vaccines in preclinical studies. Expert Rev. Vaccines. 2020;19:817–829. doi: 10.1080/14760584.2020.1813574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.Xu E., Xie Y., Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat. Med. 2022;28:2406–2415. doi: 10.1038/s41591-022-02001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis H.E., McCorkell L., Vogel J.M., Topol E.J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023;21:133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization WHO Coronavirus (COVID-19) Dashboard. 2023. https://covid19.who.int/
- 7.Wang N., Shang J., Jiang S., Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front. Microbiol. 2020;11:298. doi: 10.3389/fmicb.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan X., Yang Y., Du L. Advances in SARS-CoV-2 receptor-binding domain-based COVID-19 vaccines. Expert Rev. Vaccines. 2023;22:422–439. doi: 10.1080/14760584.2023.2211153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du L., Yang Y., Zhang X., Li F. Recent advances in nanotechnology-based COVID-19 vaccines and therapeutic antibodies. Nanoscale. 2022;14:1054–1074. doi: 10.1039/d1nr03831a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi J., Wang G., Zheng J., Verma A.K., Guan X., Malisheni M.M., Geng Q., Li F., Perlman S., Du L. Effective vaccination strategy using SARS-CoV-2 spike cocktail against Omicron and other variants of concern. NPJ Vaccines. 2022;7:169. doi: 10.1038/s41541-022-00580-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Tracking SARS-CoV-2 Variants. 2023. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- 13.Garcia-Valtanen P., Hope C.M., Masavuli M.G., Yeow A.E.L., Balachandran H., Mekonnen Z.A., Al-Delfi Z., Abayasingam A., Agapiou D., Stella A.O., et al. SARS-CoV-2 Omicron variant escapes neutralizing antibodies and T cell responses more efficiently than other variants in mild COVID-19 convalescents. Cell Rep. Med. 2022;3:100651. doi: 10.1016/j.xcrm.2022.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edara V.V., Manning K.E., Ellis M., Lai L., Moore K.M., Foster S.L., Floyd K., Davis-Gardner M.E., Mantus G., Nyhoff L.E., et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep. Med. 2022;3:100529. doi: 10.1016/j.xcrm.2022.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lasrado N., Collier A.Y., Miller J., Hachmann N.P., Liu J., Sciacca M., Wu C., Anand T., Bondzie E.A., Fisher J.L., et al. Waning immunity against XBB.1.5 following bivalent mRNA boosters. bioRxiv. 2023 doi: 10.1101/2023.01.22.525079. Preprint at. [DOI] [Google Scholar]
- 16.World Health Organization Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. 2023. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic
- 17.Lenharo M. WHO declares end to COVID-19's emergency phase. Nature. 2023 doi: 10.1038/d41586-023-01559-z. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention End of the Federal COVID-19 Public Health Emergency (PHE) Declaration. 2023. https://www.cdc.gov/coronavirus/2019-ncov/your-health/end-of-phe.html
- 19.Kupferschmidt K., Wadman M. It’s still killing and it’s still changing. Ending COVID-19 states of emergency sparks debate. 2023. https://www.science.org/content/article/who-ends-pandemic-emergency-covid-19-deaths-fall
- 20.van Erp E.A., Luytjes W., Ferwerda G., van Kasteren P.B. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front. Immunol. 2019;10:548. doi: 10.3389/fimmu.2019.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma C., Li Y., Wang L., Zhao G., Tao X., Tseng C.T.K., Zhou Y., Du L., Jiang S. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: Implication for designing novel mucosal MERS vaccines. Vaccine. 2014;32:2100–2108. doi: 10.1016/j.vaccine.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang R., Xu T., Li Z., Li L., Li C., Li X., Wang Z., Wang S., Wang X., Zhang H. Vaccination with recombinant Lactococcus lactis expressing HA1-IgY Fc fusion protein provides protective mucosal immunity against H9N2 avian influenza virus in chickens. Virol. J. 2023;20:76. doi: 10.1186/s12985-023-02044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knisely J.M., Buyon L.E., Mandt R., Farkas R., Balasingam S., Bok K., Buchholz U.J., D'Souza M.P., Gordon J.L., King D.F.L., et al. Mucosal vaccines for SARS-CoV-2: scientific gaps and opportunities-workshop report. NPJ Vaccines. 2023;8:53. doi: 10.1038/s41541-023-00654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell M.W., Mestecky J. Mucosal immunity: The missing link in comprehending SARS-CoV-2 infection and transmission. Front. Immunol. 2022;13:957107. doi: 10.3389/fimmu.2022.957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi J., Zheng J., Tai W., Verma A.K., Zhang X., Geng Q., Wang G., Guan X., Malisheni M.M., Odle A.E., et al. A glycosylated RBD protein induces enhanced neutralizing antibodies against Omicron and other variants with improved protection against SARS-CoV-2 infecnion. J. Virol. 2022;96:e0011822. doi: 10.1128/jvi.00118-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X., Tai W., Zhang X., Zhou Y., Du L., Shen C. Effects of adjuvants on the immunogenicity and efficacy of a Zika virus envelope domain III subunit vaccine. Vaccines (Basel) 2019;7:161. doi: 10.3390/vaccines7040161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb Y.N. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81:495–501. doi: 10.1007/s40265-021-01480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milane L., Amiji M. Clinical approval of nanotechnology-based SARS-CoV-2 mRNA vaccines: impact on translational nanomedicine. Drug Deliv. Transl. Res. 2021;11:1309–1315. doi: 10.1007/s13346-021-00911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.FDA authorizes Johnson & Johnson COVID-19 vaccine. (2021). Med. Lett. Drugs Ther. 63, 41-42 [PubMed]
- 30.Tuekprakhon A., Nutalai R., Dijokaite-Guraliuc A., Zhou D., Ginn H.M., Selvaraj M., Liu C., Mentzer A.J., Supasa P., Duyvesteyn H.M.E., et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185:2422–2433.e13. doi: 10.1016/j.cell.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carreño J.M., Alshammary H., Tcheou J., Singh G., Raskin A.J., Kawabata H., Sominsky L.A., Clark J.J., Adelsberg D.C., Bielak D.A., et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602:682–688. doi: 10.1038/s41586-022-04399-5. [DOI] [PubMed] [Google Scholar]
- 32.Russell M.W., Moldoveanu Z., Ogra P.L., Mestecky J. Mucosal immunity in COVID-19: A neglected but critical aspect of SARS-CoV-2 infection. Front. Immunol. 2020;11:611337. doi: 10.3389/fimmu.2020.611337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilapitiya D., Wheatley A.K., Tan H.X. Mucosal vaccines for SARS-CoV-2: triumph of hope over experience. EBioMedicine. 2023;92:104585. doi: 10.1016/j.ebiom.2023.104585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Afkhami S., D'Agostino M.R., Zhang A., Stacey H.D., Marzok A., Kang A., Singh R., Bavananthasivam J., Ye G., Luo X., et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell. 2022;185:896–915.e19. doi: 10.1016/j.cell.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan A.O., Kafai N.M., Dmitriev I.P., Fox J.M., Smith B.K., Harvey I.B., Chen R.E., Winkler E.S., Wessel A.W., Case J.B., et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183:169–184.e13. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langel S.N., Johnson S., Martinez C.I., Tedjakusuma S.N., Peinovich N., Dora E.G., Kuehl P.J., Irshad H., Barrett E.G., Werts A.D., Tucker S.N. Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model. Sci. Transl. Med. 2022;14:eabn6868. doi: 10.1126/scitranslmed.abn6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang J., Zeng C., Cox T.M., Li C., Son Y.M., Cheon I.S., Wu Y., Behl S., Taylor J.J., Chakaraborty R., et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci. Immunol. 2022;7:eadd4853. doi: 10.1126/sciimmunol.add4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butt A.A., Dargham S.R., Chemaitelly H., Al Khal A., Tang P., Hasan M.R., Coyle P.V., Thomas A.G., Borham A.M., Concepcion E.G., et al. Severity of illness in persons infected with the SARS-CoV-2 Delta variant vs Beta variant in Qatar. JAMA Intern. Med. 2022;182:197–205. doi: 10.1001/jamainternmed.2021.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor C.A., Patel K., Pham H., Whitaker M., Anglin O., Kambhampati A.K., Milucky J., Chai S.J., Kirley P.D., Alden N.B., et al. Severity of disease among adults hospitalized with laboratory-confirmed COVID-19 before and during the period of SARS-CoV-2 B.1.617.2 (Delta) predominance - COVID-NET, 14 States, January-August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1513–1519. doi: 10.15585/mmwr.mm7043e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wrenn J.O., Pakala S.B., Vestal G., Shilts M.H., Brown H.M., Bowen S.M., Strickland B.A., Williams T., Mallal S.A., Jones I.D., et al. COVID-19 severity from Omicron and Delta SARS-CoV-2 variants. Influenza Other. Respir. Viruses. 2022;16:832–836. doi: 10.1111/irv.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geng Q., Tai W., Baxter V.K., Shi J., Wan Y., Zhang X., Montgomery S.A., Taft-Benz S.A., Anderson E.J., Knight A.C., et al. Novel virus-like nanoparticle vaccine effectively protects animal model from SARS-CoV-2 infection. PLoS Pathog. 2021;17:e1009897. doi: 10.1371/journal.ppat.1009897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang G., Shi J., Verma A.K., Guan X., Perlman S., Du L. mRNA vaccines elicit potent neutralization against multiple SARS-CoV-2 omicron subvariants and other variants of concern. iScience. 2022;25:105690. doi: 10.1016/j.isci.2022.105690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi J., Zheng J., Zhang X., Tai W., Odle A.E., Perlman S., Du L. RBD-mRNA vaccine induces broadly neutralizing antibodies against Omicron and multiple other variants and protects mice from SARS-CoV-2 challenge. Transl. Res. 2022;248:11–21. doi: 10.1016/j.trsl.2022.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S., Zhou Y., Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020;17:613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tai W., Zhang X., Drelich A., Shi J., Hsu J.C., Luchsinger L., Hillyer C.D., Tseng C.T.K., Jiang S., Du L. A novel receptor-binding domain (RBD)-based mRNA vaccine against SARS-CoV-2. Cell Res. 2020;30:932–935. doi: 10.1038/s41422-020-0387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
All data relevant to the study are included in this paper.
-
•
No code was used in this study.
-
•
Any additional information required for reanalyzing the data reported in this paper is available from the lead contact upon request.