Abstract
An efficient methodology to form 4-alkoxy- and 4-aryloxybenzo[d][1,2,3]triazines via an intramolecular heterocyclization of 1-azido-2-[isocyano(p-tosyl)methyl]benzenes under basic conditions has been developed. DFT calculations have been performed to further understand the mechanism of this heterocyclization, which occurs in good to excellent yields with a broad scope.
Introduction
1,2,3-Benzotriazines are important N-heterocycles that exhibit a wide range of biological activities.1 For instance, this heterocyclic scaffold has been reported in derivatives (Figure 1) that act as antidepressants (binding to 5-HT1A receptors),2 anesthetics,3 antifungal agents,4 and antihypertensives,5 among others. Moreover, the AMPAR-positive allosteric modulator (AMPA-PAM) tulrampator is in clinical trials as a possible treatment for Alzheimer’s disease, dementia, and mild cognitive impairment.6
Figure 1.
Examples of biologically active benzotriazines.
In addition, compounds of this kind have also been described as pesticides,7 dyes,8 recording and imaging materials,1a and synthetic intermediates. Thus, this scaffold is a versatile building block for the synthesis of ortho-arylated,9 alkenylated,10 and hydroxylated11 benzamides, as well as various azaheterocycles12 via metal-catalyzed denitrogenative transannulation reactions.
1,2,3-Benzotriazines are typically prepared via the diazotization of 2-aminobenzamides in the presence of NaNO2 and a strong acid,1 although the harsh acidic conditions needed for this method and the use of sodium nitrite can lead to the release of toxic nitrogen oxides and the potential formation of nitrosamines, the latter of which are especially undesirable in drugs, even as traces at ppb levels.13 Consequently, milder reagents and reaction conditions have been reported recently for this transformation, despite most of them still requiring the use of nitrites or similar N-atom donor reagents.14 As such, the search for different and efficient approaches to these molecules remains of great significance.
p-Tosylmethyl isocyanide (TosMIC) and its derivatives are densely functionalized building blocks with three groups that can engage in a multitude of reactions: the isocyanide moiety, acidic protons in the α-position, and the tosyl group.15 As part of a research program aimed at expanding TosMIC chemistry to the preparation of six-membered heterocycles,16 our group has recently developed a new method for the synthesis of isoquinolines and γ-carbolines via a heterocyclization that takes advantage of the capacity of isocyanides to act both as nucleophiles and electrophiles.17
Herein we report the use of these reagents to synthesize a different azaheterocyclic scaffold—1,2,3-benzotriazine—that occurs thanks to another of their remarkable properties: their acidic α-carbon atom and the tendency of the isocyanide and tosyl groups to act as a leaving group. This new methodology involves an intramolecular heterocyclization of 1-azido-2-[isocyano(p-tosyl)methyl]benzenes under basic conditions, followed by the insertion of a phenol or an alkyl alcohol.
Results and Discussion
In order to test the feasibility of the proposed heterocyclization, 1-azido-2-[isocyano(p-tosyl)methyl]benzene (3a, R = H) was prepared in two steps: treatment of commercially available 2-azidobenzaldehyde (1a) with formamide, chlorotrimethylsilane, and p-toluenesulfonic acid to form N-(α-tosylbenzyl)formamide 2a, and dehydration of this intermediate using phosphorus oxychloride and triethylamine (Scheme 1).18 In a similar manner, we also synthesized the TosMIC derivatives 3b–3e in good overall yields (36–52% in two steps), although an extra initial reaction to prepare the 2-azidobenzaldehydes 1b–1e is necessary in those cases. Compounds 3b, 3c, and 3d contain a halogen in different positions of the aryl moiety, and 3e contains a trifluoromethyl group.
Scheme 1. Synthesis of TosMIC Derivatives 3a–3e.
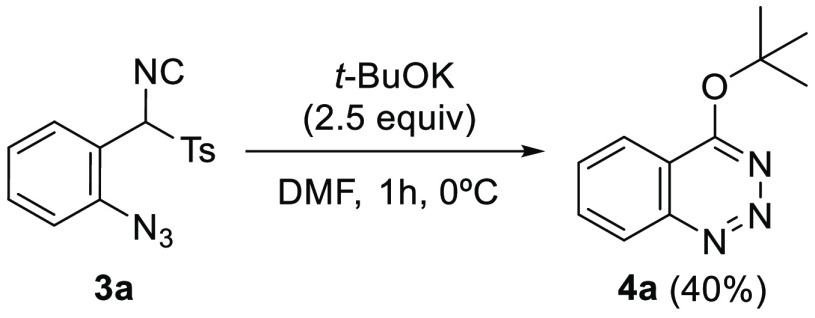
Our first attempt at the proposed heterocyclization involved the treatment of isonitrile 3a with a bulky base, such as t-BuOK in a polar solvent such as DMF (Scheme 2). The idea was to force α-deprotonation of the TosMIC moiety, expecting a subsequent nucleophilic attack of the anion formed at the electrophilic azide19 to carry out the desired cyclization and finally form the benzo[d][1,2,3]triazin-4(3H)-one. Interestingly, despite using a non-nucleophilic base in the process, the main product achieved in the reaction was 4-(tert-butyloxy)benzotriazine (4a) in 40% yield.
Scheme 2. Synthesis of 4-tert-Butyloxybenzotriazine 4a.
Taking this result into account, we speculated that a more nucleophilic alkoxide could improve the result of the cyclization, and for that reason, we tried the same reaction with an allyloxy anion. As we anticipated, the treatment of isonitrile 3a with three equivalents of sodium allyloxide, prepared in situ from allylic alcohol and NaH, in DMF as solvent, afforded 4-(allyloxy)benzotriazine (4b) in a better yield (54%, Table 1, entry 1). The use of other polar solvents led to variable yields, and THF was found to offer the best result of all those employed (entries 2–4). The temperature and concentration of the starting material in the reaction mixture were also studied (entries 5–8), and the best conditions found were 0.04 M at 0 °C (entry 6). Thus, benzotriazine 4b was isolated in an excellent yield (88%, 93% when calculated by 1H NMR using ethylene carbonate as the internal standard) after 1 h under the optimized conditions.
Table 1. Optimization of the Formation of 4b.
| entry | solvent | conc [M] | T (°C) | yield (%)a |
|---|---|---|---|---|
| 1 | DMF | 0.08 | 0 | 54 |
| 2 | MeCN | 0.08 | 0 | 75 |
| 3 | DMSO | 0.08 | rt | 7 |
| 4 | THF | 0.08 | 0 | 78 |
| 5 | THF | 0.08 | –20 | 66 |
| 6 | THF | 0.04 | 0 | 93 |
| 7 | THF | 0.04 | –20 | 63 |
| 8 | THF | 0.02 | 0 | 82 |
Calculated by 1H NMR employing ethylene carbonate as internal standard.
With the optimized conditions in hand, the scope of the heterocyclization was examined (Scheme 3). The procedure proved successful with a variety of sodium alkoxides prepared in situ from an alcohol and NaH. The list includes primary alcohols such as methyl, butyl, allyl, propargyl, benzyl, and pyridin-2-ylmethyl alcohol; secondary alcohols such as isopropyl, s-butyl, 1,1,1,3,3,3-hexafluoropropan-2-yl alcohol, and dl-menthol; and tertiary alcohols such as tert-butyl and 2-methylbut-3-yn-2-yl alcohol. Additionally, TosMIC derivatives 3b–3e were tested, and 4-(allyloxy)benzotriazines 4o–4r were obtained in high yields from sodium allyloxide prepared in situ. In general, no decrease in the reaction yields was detected when bulkier alcohols were employed.
Scheme 3. Substrate Scope for 4-Alkyloxybenzotriazines 4.
After demonstrating the feasibility of synthesizing 4-(alkyloxy)benzotriazines 4 from TosMIC derivatives, we considered the possibility of expanding the scope of these cyclizations to the use of phenols. Thus, isonitrile 3a was treated with 3 equiv of sodium phenoxide, prepared in situ from phenol and NaH, in THF as solvent at 0 °C for 1 h to afford 4-phenoxybenzotriazine (5a) in 79% yield. Although this was a good result, we explored other reaction conditions in an attempt to improve the yield achieved (Table 2).
Table 2. Optimization of the Formation of 5a.
| entry | base | solvent | T (°C) | yield (%)a |
|---|---|---|---|---|
| 1 | NaH | THF | 0 | 79 |
| 2 | NaH | DMF | 0 | 54 |
| 3 | Na2CO3 | THF | 0 | 0b |
| 4 | Na2CO3 | DMF | 0 | 72 |
| 5c | Na2CO3 | DMF | 0 | 74 |
| 6d | Na2CO3 | DMF | 0 | 55 |
| 7c | Na2CO3 | DMF | rt | 66 |
| 8c | Na2CO3 | DMF | –20 | 38 |
| 9c,e | Na2CO3 | DMF | 0 | 50 |
| 10c,f | Na2CO3 | DMF | 0 | 23 |
| 11 | Na2CO3 | MeCN | 0 | 23 |
| 12 | Na2CO3 | NMP | 0 | 42 |
| 13 | Na2CO3 | DMSO | 0 | 59 |
| 14 | K2CO3 | DMF | 0 | 65 |
| 15 | Cs2CO3 | DMF | 0 | 61 |
| 16 | K3PO4 | DMF | 0 | 72 |
| 17 | DBU | DMF | 0 | 52 |
| 18 | DABCO | DMF | 0 | 0b |
| 19 | DBN | DMF | 0 | 48 |
Calculated by 1H NMR employing ethylene carbonate as internal standard.
Starting material recovered.
0.08 M THF solution of the starting material.
0.16 M THF solution of the starting material.
2 equiv of phenol and base added.
1 equiv of phenol and base added.
First, we tested a weaker base, namely Na2CO3, which is able to deprotonate the acidic proton of phenols, and although the formation of 5a was not observed using THF as solvent, a 72% yield was achieved with DMF. Next, different concentrations of the starting material, temperatures, and number of equivalents of sodium phenoxide were examined, but no improvement was found. Finally, an evaluation of the use of other polar solvents, such as MeCN, NMP, and DMSO, and bases, such as K2CO3, CsCO3, K3PO4, DBU, DABCO, and DBN, in the reaction also led to lower yields than the initial attempt.
Once again, the optimized conditions were employed to expand the scope of the cyclization. Isonitriles 3a–3e were treated with 3 equiv of a variety of sodium aryloxides, prepared in situ from substituted phenol derivatives and NaH, in THF as solvent to afford 4-aryloxybenzotriazines 5 in moderate to high yields (Scheme 4). Substituents of phenol derivatives include electron-withdrawing and -donating groups at different positions of the aromatic ring. Heterocycles such as 2-hydroxypyridine and 5-hydroxyindole were also used successfully, as was the natural product α-tocopherol.
Scheme 4. Substrate Scope of 4-Aryloxybenzotriazines 5.
The heterocyclization reaction developed was rationalized by way of a plausible mechanistic hypothesis that involves an initial α-deprotonation of the TosMIC moiety followed by a nucleophilic attack of the anion formed on the electrophilic azide,19 which would lead to a cyclization process and loss of the tosyl group. Thereafter, the presence of a second equivalent of the aryl- or alkyloxide would allow a nucleophilic substitution in which the isonitrile would act as a leaving group, thus forming the final benzotriazine.
To gain insight into this mechanistic hypothesis, density functional calculations were performed (Scheme 5). In the presence of a base (tBuOK), the TosMIC anion I is generated, and N1 undergoes a 6-endo-trig cyclization with the isocyanide, thus resulting in the formation of II via TS (ΔG‡ = +12.3 kcal/mol). Upon losing the tosyl group, the nonaromatic benzotriazine III is generated in an exergonic process. This compound can be attacked by a tert-butoxide group and thermodynamically driven elimination of CN, thus, leading to the formation of 4a.
Scheme 5. Mechanistic Hypothesis and DFT Calculations.
Conclusions
In summary, a novel and efficient synthetic methodology to afford 4-alkoxy- and 4-aryloxybenzo[d][1,2,3]triazines has been developed. This process involves an intramolecular heterocyclization of 1-azido-2-[isocyano(p-tosyl)methyl]benzenes under basic conditions, followed by the insertion of an alkyl alcohol or a phenol, and occurs in good to excellent yields with a broad scope. DFT calculations have been performed to further understand the mechanism of this heterocyclization, which opens up a new way to obtain 1,2,3-benzotriazines without the need for harsh conditions or metal catalysis.
Experimental Section
General Experimental Details
All reactions involving air-sensitive compounds were carried out under an inert atmosphere (Ar). Dry solvents, where necessary, were dried with an MBRAUN MB-SPS-800 apparatus. Starting materials sourced from commercial suppliers were used as received unless otherwise stated. 2-Azidobenzaldehydes were prepared from the corresponding 2-nitro- or 2-fluorobenzaldehydes according to literature procedures.20 Reactions were monitored using analytical TLC plates (Merck; silica gel 60 F254, 0.25 mm), and compounds were visualized with UV radiation. Silica gel grade 60 (70–230 mesh, Merck) was used for column chromatography. All melting points were determined in open capillary tubes on a Stuart Scientific SMP3 melting point apparatus (uncorrected). 1H and 13C NMR spectra were recorded on a Varian Mercury VX-300, Varian Unity 500 MHz, and Bruker Ascend 400 MHz spectrometer at room temperature. Chemical shifts are given in parts per million (δ) downfield from tetramethylsilane, with calibration on the residual protiosolvent used (δH = 7.26 ppm and δC = 77.2 ppm for CDCl3). Coupling constants (J) are in hertz (Hz), and signals are described as follows: s, singlet; d, doublet; t, triplet; q, quadruplet; bs, broad singlet; dd, double doublet; apt, apparent triplet; dq, double quadruplet; ddd, double doublet of doublets, and m, multiplet. High-resolution analysis (HRMS) were performed on an Agilent 6210 time of-flight LC/MS.
General Procedure for the Formation of N-((2-Azidophenyl)(tosyl)methyl)formamides 2a–2e
A 250 mL dry Schlenk flask was charged with 2 M acetonitrile and 2 M toluene, 1 equiv of the appropriate benzaldehyde (1), 2.5 equiv of formamide, and 1.1 equiv of chlorotrimethylsilane under an argon atmosphere. After heating the solution in a sand bath at 50 °C for 4–5 h, 1.5 equiv of p-toluenesulfonic acid prepared by a previous procedure18 was added and heating was continued for an additional 4–5 h. The solution was cooled to room temperature, and 55 mL of diethyl ether were added. The resulting mixture was cooled to 0 °C and held there for 1 h, and the precipitated white solid was collected using a Büchner funnel. The reaction flask was washed with 35 mL of diethyl ether, and this rinse was poured over the filter cake. After washing with diethyl ether a second time, the solid was dried under vacuum for 5–10 h to give the corresponding N-(α-tosyl-2-azido-benzyl)formamide 2 which was used in the next step without further purification.
N-((2-Azidophenyl)(tosyl)methyl)formamide (2a)
Obtained as a white solid (7.10 g, 21.5 mmol, 93%) following the general procedure outlined above using commercially available compound 1a(21) (3.40 g, 23.1 mmol). Rf = 0.3 (Hexane/EtOAc 1:1). Mp 151 °C (decomposition). 1H NMR (400 MHz, DMSO-d6) δ 9.84 (dd, J = 10.5, 1.5 Hz, 1H), 8.04 (d, J = 1.3 Hz, 1H), 7.67 (dd, J = 7.8, 1.5 Hz, 1H), 7.62–7.57 (m, 2H), 7.52 (td, J = 7.7, 1.4 Hz, 1H), 7.45–7.40 (m, 2H), 7.36–7.20 (m, 2H), 6.62 (d, J = 10.5 Hz, 1H), 2.40 (s, 3H). 13C{1H} NMR (101 MHz, DMSO-d6) δ: 160.6, 145.2, 138.7, 133.3, 131.4, 129.9, 129.7, 129.1, 125.2, 121.9, 118.8, 64.6, 21.1. HRMS (ESI-TOF) calcd for C15H15N4O3S [M + H]+: 331.0859. Found: 331.0864.
N-((2-Azido-3-chlorophenyl)(tosyl)methyl)formamide (2b)
Obtained as a white solid (4.2 g, 11.4 mmol, 94%) following the general procedure outlined above using compound 1b(22) (3.93 g, 21.6 mmol). Rf = 0.48 (Hexane/EtOAc 1:1). Mp 160 °C (decomposition). 1H NMR (400 MHz, DMSO-d6) δ 9.92 (dd, J = 10.5, 1.5 Hz, 1H), 8.03 (d, J = 1.3 Hz, 1H), 7.67 (td, J = 8.1, 6.6 Hz, 4H), 7.50–7.41 (m, 3H), 6.81 (d, J = 10.6 Hz, 1H), 2.41 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.6, 145.5, 135.1, 133.0, 132.0, 129.9, 129.0, 128.6, 127.6, 126.6, 125.5, 65.6, 21.2. HRMS (ESI-TOF) calcd for C15H14ClN4O3S [M + H]+: 365.0470. Found: 365.0472.
N-((2-Azido-4-bromophenyl)(tosyl)methyl)formamide (2c)
Obtained as a white solid (5.9 g, 14.4 mmol, 65%) following the general procedure outlined above using compound 1c(22) (5.11 g, 22.6 mmol). Rf = 0.3 (Hexane/EtOAc 1:1). Mp 155 °C (decomposition). 1H NMR (400 MHz, DMSO-d6) δ 9.90 (d, J = 10.4 Hz, 1H), 8.02 (s, 1H), 7.60 (dd, J = 19.7, 9.7 Hz, 4H), 7.48–7.40 (m, 3H), 6.56 (d, J = 10.3 Hz, 1H), 2.41 (s, 3H). 13C{1H} NMR (400 MHz, DMSO-d6) δ: 160.5, 145.3, 140.5, 133.5, 131.5, 129.8, 129.1, 128.2, 124.0, 121.8, 121.2, 64.3, 21.2. HRMS (ESI-TOF) calcd for C15H14BrN4O3S [M + H]+: 408.8907. Found: 408.8902.
N-((2-Azido-6-bromophenyl)(tosyl)methyl)formamide (2d)
Obtained as a white solid (7.76 g, 19.0 mmol, 90%) following the general procedure outlined above using compound 1d(23) (4.8 g, 21.2 mmol). Rf = 0.4 (Hexane/EtOAc 1:1). Mp 144 °C (decomposition). 1H NMR (400 MHz, DMSO-d6) δ 9.48 (dd, J = 10.6, 1.5 Hz, 1H), 8.09 (d, J = 1.3 Hz, 1H), 7.74–7.65 (m, 2H), 7.53 (dd, J = 7.2, 2.0 Hz, 1H), 7.49–7.38 (m, 4H), 7.06 (d, J = 10.5 Hz, 1H), 2.41 (s, 3H). 13C{1H} NMR (101 MHz, DMSO-d6) δ: 161.1, 145.2, 140.7, 134.7, 132.3, 129.8, 129.5, 128.8, 127.6, 121.4, 120.1, 70.3, 21.1. HRMS (ESI-TOF) calcd for C15H14BrN4O3S [M + Na]+: 430.9784. Found: 430.9786.
N-((2-azido-4-(trifluoromethyl)phenyl)(tosyl)methyl)formamide (2e)
Obtained as a white solid (4.3 g, 10.7 mmol, 77%) following the general procedure outlined above using compound 1e(21) (3.0 g, 13.9 mmol). Rf = 0.3 (Hexane/EtOAc 1:1). Mp 168 °C (decomposition). 1H NMR (400 MHz, DMSO-d6) δ 9.96 (dd, J = 10.5, 1.5 Hz, 1H), 8.06–8.00 (m, 1H), 7.89 (d, J = 8.2 Hz, 1H), 7.73 (dd, J = 8.4, 1.8 Hz, 1H), 7.69–7.61 (m, 3H), 7.51–7.41 (m, 3H), 6.69 (d, J = 10.5 Hz, 1H), 2.42 (s, 3H). 13C{1H} NMR (400 MHz, DMSO-d6) δ: 162.9, 160.6, 145.5, 140.1, 133.1, 130.9, 129.8, 129.1, 128.1, 126.0, 125.5, 121.7 (q, J = 3.7 Hz), 116.2 (q, J = 4.0 Hz), 64.3, 21.2. 19F NMR (376 MHz, DMSO-d6) δ −61.41. HRMS (ESI-TOF) calcd for C16H14BrF3N4O3S [M + H]+: 399.0733. Found: 399.0721.
General Procedure for the Formation of 1-Azido-2-(isocyano(tosyl)methyl)benzenes 3a–3e
A 250 mL dry Schlenk flask was charged with 1 equiv of the appropriate N-(α-tosyl-2-azido-benzyl)formamide 2 and 48 mM of THF. Phosphorus oxychloride (2 equiv) was added, and the resulting solution was stirred for 5 min at 25 °C. After the solution was cooled to 0 °C, 6 equiv of triethylamine were added slowly over 30–45 min while keeping the internal reaction temperature below 10 °C. After the triethylamine addition is complete, the reaction was warmed to room temperature and held there for 30–45 min. Ethyl acetate (140 mL) and water (140 mL) were added sequentially to the reaction, the mixture was stirred for 5 min, and, after transfer of the mixture to a separatory funnel, the aqueous layer was removed. The organic layer was washed with water (2 × 140 mL), saturated NaHCO3 solution (140 mL), and brine (70 mL), and the combined organic layers were dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified by flash chromatography using mixtures of hexane and EtOAc as eluents to obtain the corresponding α-tosyl-2-azidobenzyl isocyanide 3.
1-Azido-2-(isocyano(tosyl)methyl)benzene (3a)
Obtained as a yellow solid (571.9 mg, 1.83 mmol, 60%) following the general procedure outlined above using compound 2a (1.0 g, 3.03 mmol). Rf = 0.3 (Hexane/EtOAc 2:1). Mp 106–111 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 8.3 Hz, 2H), 7.51 (ddd, J = 8.0, 7.5, 1.5 Hz, 1H), 7.47 (dd, J = 7.9, 1.5 Hz, 1H), 7.39 (d, J = 8.5 Hz, 2H), 7.23 (td, J = 7.6, 0.9 Hz, 1H), 7.18 (dd, J = 8.1, 0.8 Hz, 1H), 6.06 (s, 1H), 2.49 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ: 165.5, 146.9, 139.4, 132.4, 131.3, 130.6, 130.0, 129.7, 125.4, 118.6, 118.5, 70.4, 22.0. HRMS (ESI-TOF) calcd for C15H13N4O2S [M + H]+: 313.0754. Found: 313.0764.
2-Azido-1-chloro-3-(isocyano(tosyl)methyl)benzene (3b)
Obtained as a yellow solid (356.7 mg, 1.03 mmol, 38%) following the general procedure outlined above using compound 2b (1.0 g, 2.74 mmol). Rf = 0.3 (Hexane/EtOAc 5:1). Mp 129 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 7.80–7.73 (m, 2H), 7.54–7.47 (m, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.36 (d, J = 7.8 Hz, 1H), 7.29–7.17 (m, 1H), 6.26 (d, J = 2.5 Hz, 1H), 2.50 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 166.3, 147.2, 135.9, 133.3, 130.9, 130.6, 130.4, 130.2, 127.8, 126.9, 122.9, 71.3, 22.0. HRMS (ESI-TOF) calcd for C15H12ClN4O2S [M + Na]+: 369.0183. Found: 369.0190.
2-Azido-4-bromo-1-(isocyano(tosyl)methyl)benzene (3c)
Obtained as a yellow solid (350.6 mg, 0.90 mmol, 73%) following the general procedure outlined above using compound 2c (500 mg, 1.22 mmol). Rf = 0.3 (Hexane/EtOAc 3:1). Mp 105 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J = 7.9 Hz, 2H), 7.41 (d, J = 8.0 Hz, 2H), 7.39–7.30 (m, 3H), 5.98 (s, 1H), 2.50 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 166.0, 147.1, 140.7, 131.1, 130.9, 130.6, 130.2, 128.8, 126.4, 121.7, 117.6, 70.0, 22.0. HRMS (ESI-TOF) calcd for C15H12BrN4O2S [M + Na]+: 412.9678. Found: 412.9678.
1-Azido-3-bromo-2-(isocyano(tosyl)methyl)benzene (3d)
Obtained as a yellow solid (450.7 mg, 1.15 mmol, 48%) following the general procedure outlined above using compound 2d (1.0 g, 2.44 mmol). Rf = 0.3 (Hexane/EtOAc 1.5:1). Mp 130 °C (violent decomposition). 1H NMR (400 MHz, CDCl3) δ 7.90–7.81 (m, 2H), 7.52–7.38 (m, 3H), 7.33 (td, J = 8.1, 2.7 Hz, 1H), 7.16 (ddd, J = 8.1, 6.2, 1.1 Hz, 1H), 6.44 (s, 1H), 2.49 (d, J = 2.1 Hz, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 167.2, 146.9, 141.8, 133.3, 132.7, 130.3, 130.2, 129.8, 128.3, 119.4, 117.8, 74.6, 22.0. HRMS (ESI-TOF) calcd for C15H12BrN4O2S [M + Na]+: 412.9678. Found: 412.9676.
2-Azido-1-(isocyano(tosyl)methyl)-4-(trifluoromethyl)benzene (3e)
Obtained as a yellow solid (637,6 mg, 1.68 mmol, 67%) following the general procedure outlined above using compound 2e (1.0 g, 2.51 mmol). Rf = 0.3 (Hexane/EtOAc 3:1). Mp 93 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 7.82–7.75 (m, 2H), 7.63 (d, J = 8.2 Hz, 1H), 7.48 (dd, J = 8.4, 1.7 Hz, 1H), 7.45–7.39 (m, 3H), 6.08 (s, 1H), 2.51 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 166.4, 147.3, 140.4, 134.6 (q, J = 33.4 Hz), 131.0, 130.6, 130.5, 130.3, 122.14, 122.05 (q, J = 3.7 Hz), 122.0, 115.5 (q, J = 3.8 Hz), 69.9, 22.0. 19F NMR (376 MHz, CDCl3) δ −63.2. HRMS (ESI-TOF) calcd for C15H12F3N4O2S [M + H]+: 381.0628. Found: 381.0627.
General Procedure for the Formation of Benzo[d][1,2,3]triazines 4 and 5
NaH (3.0 equiv) was dissolved in THF (0.2 M) and stirred for about 5 min under Ar. The corresponding alcohol or phenol (3.0 equiv) was added at rt. After 2 min, the mixture was cooled to 0 °C and a solution of the corresponding isocyanide (3) (1 equiv) in THF (0.04 M) was added. The reaction mixture was stirred at 0 °C for 1 h. After this time, the reaction mixture was warmed to room temperature and diluted with ethyl acetate and water. The aqueous layer was extracted 2 times with ethyl acetate, and the collected organic phases were then washed one more time with brine. After evaporation of the solvent, the crude material was purified by flash chromatography using mixtures of hexane/EtOAc as eluents to obtain the corresponding triazine 4 or 5.
4-(tert-Butoxy)benzo[d][1,2,3]triazine (4a)
Obtained as a light brown solid (12.9 mg, 0.06 mmol, 40%) following the general procedure outlined above using compound 3a (50 mg, 0.16 mmol). Rf = 0.3 (Hexane/EtOAc 4:1). Mp 137 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ: 8.30 (d, J = 8.3 Hz, 1H), 8.14 (d, J = 8.1 Hz, 1H), 7.98 (t, J = 7.6 Hz, 1H), 7.85 (t, J = 7.6 Hz, 1H), 1.82 (s, 9H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.3, 146.0, 134.3, 132.5, 127.8, 122.4, 112.3, 85.1, 28.4. HRMS (ESI-TOF) calcd for C11H14N3O [M + H]+: 204.1131. Found: 204.1130.
4-(Allyloxy)benzo[d][1,2,3]triazine (4b)
Obtained as a green-brown solid (165.2 mg, 0.88 mmol, 88%) from compound 3a (1.0 mmol, 312.4 mg) following the same general procedure described above. Rf = 0.3 (Hexane/EtOAc 4:1). Mp 110 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.31 (d, J = 8.4 Hz, 1H), 8.16 (d, J = 8.2 Hz, 1H), 7.98 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 7.85 (ddd, J = 8.2, 7.1, 1.1 Hz, 1H), 6.16 (ddt, J = 17.1, 10.4, 5.8 Hz, 1H), 5.48 (dq, J = 17.2, 1.4 Hz, 1H), 5.33 (dq, J = 10.4, 1.2 Hz, 1H), 5.21 (dt, J = 5.8, 1.3 Hz, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.4, 146.0, 134.8, 132.9, 131.8, 128.0, 122.0, 119.6, 111.2, 68.9. HRMS (ESI-TOF) calcd for C10H10N3O [M + H]+: 188.0818. Found: 188.0826.
4-Methoxybenzo[d][1,2,3]triazine (4c)
Obtained as a light brown solid (9.3 mg, 0.06 mmol, 60%) following the general procedure outlined above using compound 3a (30.0 mg, 0.09 mmol). Rf = 0.3 (Hexane/EtOAc 4:1). Mp 99 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ: 8.38 (d, J = 8.4 Hz, 1H), 8.19 (d, J = 9.4 Hz, 1H), 8.05 (t, J = 7.0 Hz, 1H), 7.91 (t, J = 8.2 Hz, 1H), 4.35 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 161.0, 145.9, 134.8, 132.9, 128.0, 122.0, 111.1, 55.6. HRMS (ESI-TOF) calcd for C8H8N3O [M + H]+: 162.0662. Found: 162.0666.
4-Butoxybenzo[d][1,2,3]triazine (4d)
Obtained as a white solid (29.0 mg, 0.1 mmol, 74%) from 3a (60.0 mg, 0.2 mmol) following the general method. Rf = 0.39 (Hexane/EtOAc 4:1). Mp 133 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ: 8.36 (d, J = 8.6 Hz, 1H), 8.19 (d, J = 8.2 Hz, 1H), 8.03 (t, J = 7.7 Hz, 1H), 7.89 (t, J = 7.6 Hz, 1H), 4.76 (t, J = 6.2 Hz, 2H), 1.94 (quintet, J = 7.2 Hz, 2H), 1.57 (sextet, J = 7.6 Hz, 2H), 1.02 (t, J = 7.7 Hz, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.8, 145.9, 134.7, 132.8, 127.9, 122.0, 111.2, 68.5, 30.9, 19.4, 13.9. HRMS (ESI-TOF) calcd for C11H14N3O [M + H]+: 204.1131. Found: 204.1130.
4-Isopropoxybenzo[d][1,2,3]triazine (4e)
Obtained as a light brown solid (29.8 mg, 0.16 mmol, 82%) from 3a (60.0 mg, 0.19 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 4:1). Mp 129 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ: 8.28 (d, J = 8.4 Hz, 1H), 8.12 (d, J = 8.2 Hz, 1H), 7.95 (t, J = 7.8 Hz, 1H), 7.81 (t, J = 7.6 Hz, 1H), 5.87–5.76 (m, 1H), 1.47 (dd, J = 6.3, 2.2 Hz, 6H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.3, 146.0, 134.6, 132.6, 127.9, 122.1, 111.5, 72.1, 22.0. HRMS (ESI-TOF) calcd for C10H12N3O [M + H]+: 190.0975. Found: 190.0974.
4-(sec-Butoxy)benzo[d][1,2,3]triazine (4f)
Obtained as a yellow oil (15.7 mg, 0.07 mmol, 80%) from 3a (30 mg, 0.1 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 4:1). Mp 128 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ: 8.35 (dt, J = 8.4, 1.0 Hz, 1H), 8.19 (dt, J = 8.1, 1.0 Hz, 1H), 8.02 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 7.88 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H), 5.73 (h, J = 6.2 Hz, 1H), 1.95 (ddd, J = 14.2, 7.6, 6.7 Hz, 1H), 1.82 (dqd, J = 14.8, 7.5, 5.7 Hz, 1H), 1.51 (d, J = 6.2 Hz, 3H), 1.04 (t, J = 7.5 Hz, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.6, 146.0, 134.6, 132.6, 127.9, 122.1, 111.6, 76.6, 29.0, 19.3, 9.8. HRMS (ESI-TOF) calcd for C11H14N3O [M + H]+: 204.1131. Found: 204.1132.
4-((2-Isopropyl-5-methylcyclohexyl)oxy)benzo[d][1,2,3]triazine (4g)
Obtained as a white solid (14.7 mg, 0.05 mmol, 27%) from 3a (60 mg, 0.2 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 5:1). Mp 120 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.28 (dt, J = 8.3, 1.0 Hz, 1H), 8.12 (dt, J = 8.1, 1.0 Hz, 1H), 7.96 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 7.82 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H), 5.55 (td, J = 10.6, 4.4 Hz, 1H), 2.42–2.32 (m, 1H), 1.97 (pd, J = 6.9, 2.6 Hz, 1H), 1.77–1.55 (m, 4H), 1.24–1.00 (m, 2H), 0.99–0.90 (m, 1H), 0.88 (dd, J = 6.8, 3.9 Hz, 6H), 0.74 (d, J = 6.9 Hz, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.7, 146.1, 134.6, 132.6, 128.0, 122.1, 111.6, 78.5, 47.8, 40.2, 34.5, 31.5, 27.0, 24.1, 22.2, 20.8, 17.1. HRMS (ESI-TOF) calcd for C17H24N3O [M + H]+: 286.1914. Found: 286.1915.
4-((1,1,1,3,3,3-Hexafluoropropan-2-yl)oxy)benzo[d][1,2,3]triazine (4h)
Obtained as a white solid (40.5 mg, 0.14 mmol, 71%) from 3a (60.0 mg, 0.2 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 5:1). Mp 136 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ: 8.53 (d, J = 8.5, 1H), 8.30 (dd, J = 8.1, 1.4, 1H), 8.20 (ddd, J = 8.5, 7.1, 1.4 Hz, 1H), 8.06 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H), 7.02 (hept, J = 6.0 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ: 159.0, 146.8, 136.2, 134.3, 130.1, 128.6, 122.1, 121.5, 110.1, 68.7 (q, J = 70.3, 35.0 Hz). 19F-NMR (376 MHz, CDCl3) δ – 72.97. HRMS (ESI-TOF) calcd for C10H6F6N3O [M + H]+: 298.0410. Found: 298.0410.
4-(Prop-2-yn-1-yloxy)benzo[d][1,2,3]triazine (4i)
Obtained a yellow oil (18.3 mg, 0.10 mmol, 62%) following the general procedure outlined above using compound 3a (50 mg, 0.16 mmol). Rf = 0.3 (Hexane/EtOAc 4:1). 1H NMR (400 MHz, CDCl3) δ: 8.41 (d, J = 8.3 Hz, 1H), 8.25 (d, J = 8.1 Hz, 1H), 8.08 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 7.94 (ddd, J = 8.2, 7.2, 1.1 Hz, 1H), 5.40 (d, J = 2.4 Hz, 2H), 2.62 (t, J = 2.4 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ: 159.8, 146.1, 135.1, 133.2, 128.1, 122.0, 110.9, 77.3, 76.3, 55.8. HRMS (ESI-TOF) calcd for C10H8N3O [M + H]+: 186.0662. Found: 186.0663.
4-((2-Methylbut-3-yn-2-yl)oxy)benzo[d][1,2,3]triazine (4j)
Obtained as a brown solid (28.8 mg, 0.14 mmol, 70%) following the general procedure outlined above using compound 3a (60 mg, 0.19 mmol). Rf = 0.3 (Hexane/EtOAc 4:1). Mp 138 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.36 (dd, J = 7.9, 1.0 Hz, 1H), 8.00–7.79 (m, 3H), 2.76 (s, 1H), 2.11 (s, 6H). 13C{1H} NMR (101 MHz, CDCl3) δ: 168.0, 146.4, 134.6, 133.8, 126.8, 125.8, 118.0, 83.0, 75.8, 73.9, 30.1. HRMS (ESI-TOF) calcd for C12H12N3O [M + H]+: 214.0975. Found: 214.0982.
4-(But-3-yn-1-yloxy)benzo[d][1,2,3]triazine (4k)
Obtained a yellow oil (23.3 mg, 0.12 mmol, 69%) following the general procedure outlined above using compound 3a (60 mg, 0.19 mmol). Rf = 0.25 (Hexane/EtOAc 4:1). 1H NMR (400 MHz, CDCl3) δ 8.37 (d, J = 8.4 Hz, 1H), 8.22 (d, J = 8.2 Hz, 1H), 8.05 (t, J = 7.7 Hz, 1H), 7.91 (t, J = 7.6 Hz, 1H), 4.91–4.83 (m, 2H), 2.87 (td, J = 6.6, 2.9 Hz, 2H), 2.05 (t, J = 2.7 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.4, 146.0, 134.9, 133.0, 128.0, 122.0, 111.0, 79.9, 70.4, 66.0, 19.2. HRMS (ESI-TOF) calcd for C11H10N3O [M + H]+: 200.0818. Found: 200.0819.
4-(Pent-3-yn-1-yloxy)benzo[d][1,2,3]triazine (4l)
Obtained as a yellow-brown solid (20.9. mg, 0.01 mmol, 51%) from 3a (60 mg, 0.20 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 4:1). Mp 125 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.38 (dd, J = 8.4, 1.0 Hz, 1H), 8.23 (dd, J = 8.1, 1.3 Hz, 1H), 8.05 (ddt, J = 8.2, 7.2, 1.0 Hz, 1H), 7.91 (tt, J = 8.0, 1.0 Hz, 1H), 4.82 (t, J = 6.8 Hz, 2H), 2.80 (tq, J = 7.0, 2.6 Hz, 2H), 1.79 (t, J = 2.6 Hz, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.6, 146.0, 134.8, 132.9, 128.0, 122.1, 111.1, 77.9, 74.6, 66.8, 19.5, 3.6. HRMS (ESI-TOF) calcd for C12H12N3O [M + H]+: 214.0975. Found: 214.0978.
4-(Benzyloxy)benzo[d][1,2,3]triazine (4m)
Obtained as a light yellow solid (19.4 mg, 0.08 mmol, 85%) from 3a (30 mg, 0.10 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 3:1). Mp 152 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.39 (dd, J = 8.4, 1.0 Hz, 1H), 8.22 (dt, J = 8.1, 1.0 Hz, 1H), 8.05 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 7.89 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H), 7.66–7.54 (m, 2H), 7.48–7.35 (m, 3H), 5.81 (s, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.6, 146.1, 135.5, 134.8, 133.0, 128.9, 128.8, 128.0, 122.1, 111.2, 70.0. HRMS (ESI-TOF) calcd for C14H12N3O [M + H]+: 238.0975. Found: 238.0973.
4-(Pyridin-2-ylmethoxy)benzo[d][1,2,3]triazine (4n)
Obtained as a light green solid (16.7 mg, 0.07 mmol, 73%) from 3a (30 mg, 0.10 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 3:1). Mp 166 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.59 (ddd, J = 4.9, 1.8, 1.0 Hz, 1H), 8.33 (dt, J = 8.4, 1.0 Hz, 1H), 8.21 (dt, J = 8.2, 1.0 Hz, 1H), 7.99 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 7.85 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H), 7.69 (td, J = 7.7, 1.8 Hz, 1H), 7.53 (dt, J = 7.8, 1.1 Hz, 1H), 7.22 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 5.84 (s, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.5, 155.3, 149.9, 146.1, 137.0, 134.9, 133.1, 128.0, 123.4, 122.8, 122.1, 111.1, 70.4. HRMS (ESI-TOF) calcd for C13H11N4O [M + H]+: 239.0927. Found: 239.0931.
4-(Allyloxy)-8-chlorobenzo[d][1,2,3]triazine (4o)
Obtained as a yellow solid (13.5 mg, 0.07 mmol, 70%) from 3b (30 mg, 0.1 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 4:1). Mp 131 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.15 (dd, J = 8.2, 1.3 Hz, 1H), 8.09 (dd, J = 7.7, 1.3 Hz, 1H), 7.82 (t, J = 7.9 Hz, 1H), 6.21 (ddt, J = 17.3, 10.4, 5.9 Hz, 1H), 5.55 (dq, J = 17.1, 1.5 Hz, 1H), 5.41 (dq, J = 10.5, 1.2 Hz, 1H), 5.28 (dt, J = 5.9, 1.4 Hz, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.1, 142.7, 135.1, 133.7, 132.9, 131.5, 120.8, 120.0, 112.8, 69.5. HRMS (ESI-TOF) calcd for C10H9ClN3O [M + H]+: 222.0429. Found: 222.0430.
4-(Allyloxy)-7-bromobenzo[d][1,2,3]triazine (4p)
Obtained as a light gray solid (29.5 mg, 0.11 mmol, 72%) from 3c (60.0 mg, 0.15 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 5:1). Mp 87–88 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.54 (d, J = 1.9 Hz, 1H), 8.09 (d, J = 8.6 Hz, 1H), 7.99 (dd, J = 8.7, 1.9 Hz, 1H), 6.21 (ddt, J = 17.2, 10.4, 5.9 Hz, 1H), 5.55 (dq, J = 17.2, 1.5 Hz, 1H), 5.41 (dq, J = 10.4, 1.2 Hz, 1H), 5.27 (dt, J = 5.9, 1.3 Hz, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.2, 146.5, 136.6, 131.5, 130.5, 129.4, 123.7, 120.0, 109.8, 69.2. HRMS (ESI-TOF) calcd for C10H9BrN3O [M + H]+: 265.9924. Found: 265.9923.
4-(Allyloxy)-5-bromobenzo[d][1,2,3]triazine (4q)
Obtained as a light yellow solid (12.3 mg, 0.05 mmol, 60%) from 3d (30 mg, 0.08 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 5:1). Mp 126 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 8.1 Hz, 1H), 8.15 (d, J = 7.8 Hz, 1H), 7.83 (t, J = 8.1 Hz, 1H), 6.24 (ddt, J = 16.5, 10.8, 5.9 Hz, 1H), 5.62 (d, J = 17.3 Hz, 1H), 5.40 (d, J = 10.5 Hz, 1H), 5.29 (d, J = 5.6 Hz, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ: 159.0, 147.3, 138.8, 134.8, 131.6, 128.0, 119.6, 116.5, 111.3, 69.8. HRMS (ESI-TOF) calcd for C10H9BrN3O [M + H]+: 265.9924. Found: 265.9928.
4-(Allyloxy)-7-(trifluoromethyl)benzo[d][1,2,3]triazine (4r)
Obtained as a yellow solid (25.8. mg, 0.10 mmol, 77%) from 3e (50 mg, 0.13 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 4:1). Mp 92 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.67 (dt, J = 1.8, 0.9 Hz, 1H), 8.37 (dt, J = 8.5, 0.8 Hz, 1H), 8.09 (dd, J = 8.5, 1.7 Hz, 1H), 6.22 (ddt, J = 17.2, 10.4, 5.9 Hz, 1H), 5.57 (dq, J = 17.2, 1.4 Hz, 1H), 5.43 (dq, J = 10.4, 1.2 Hz, 1H), 5.30 (dt, J = 5.9, 1.3 Hz, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.0, 145.2, 136.6 (q, J = 33.6 Hz), 131.3, 128.7 (q, J = 3.1 Hz), 125.9 (q, J = 4.3 Hz), 123.8, 123.0 (q, J = 274.7 Hz), 120.3, 112.9, 69.5. 19F NMR (376 MHz, CDCl3) δ −63.3. HRMS (ESI-TOF) calcd for C11H9F3N3O [M + H]+: 256.0692. Found: 256.0696.
4-Phenoxybenzo[d][1,2,3]triazine (5a)
Obtained as a brown solid (32.8 mg, 0.15 mmol, 77%) following the general procedure outlined above using compound 3a (60 mg, 0.19 mmol). Rf = 0.3 (Hexane/EtOAc 4:1). Mp 166 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.48–8.37 (m, 2H), 8.13 (ddd, J = 8.4, 7.1, and 1.4 Hz, 1H), 8.01 (ddd, J = 8.2, 7.1, and 1.2 Hz, 1H), 7.55–7.46 (m, 2H), 7.39–7.30 (m, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 161.5, 152.2, 146.6, 135.3, 133.4, 130.0, 128.2, 126.5, 122.1, 121.7, 111.0. HRMS (ESI-TOF) calcd for C13H10N3O [M + H]+: 224.0818. Found: 224.0824.
4-(3-Fluorophenoxy)benzo[d][1,2,3]triazine (5b)
Obtained as a light yellow solid (16.5 mg, 0.07 mmol, 71%) from 3a (30 mg, 0.10 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 4:1). Mp 144 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.47 (d, J = 8.4, 1H), 8.39 (dd, J = 8.1, 1.4, 1H), 8.15 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 8.03 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H), 7.47 (td, J = 8.2, 6.4 Hz, 1H), 7.19–7.02 (m, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 163.3 (d, J = 248.2 Hz), 161.3, 152.8 (d, J = 10.9 Hz), 146.7, 135.5, 133.6, 130.8 (d, J = 9.4 Hz), 128.3, 122.0, 117.6 (d, J = 3.5 Hz), 113.6 (d, J = 21.1 Hz), 110.0 (d, J = 24.7 Hz), 109.9. 19F-NMR (376 MHz, CDCl3) δ – 110.24. HRMS (ESI-TOF) calcd for C13H9FN3O [M + H]+: 242.0724. Found: 242.0726.
4-(3-Chlorophenoxy)benzo[d][1,2,3]triazine (5c)
Obtained as a light brown solid (16.2 mg, 0.06 mmol, 65%) from 3a (30.0 mg, 0.1 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 4:1). Mp 178 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.40 (dt, J = 8.4, 1.0 Hz, 1H), 8.31 (dd, J = 8.1, 1.4, 1H), 8.08 (ddd, J = 8.5, 7.1, 1.4 Hz, 1H), 7.96 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H), 7.37 (t, J = 8.1 Hz, 1H), 7.35–7.22 (m, 2H), 7.19 (ddd, J = 8.2, 2.3, 1.0 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ: 161.3, 152.5, 146.7, 135.5, 135.3, 133.7, 130.7, 128.3, 126.8, 122.5, 121.9, 120.2, 110.8. HRMS (ESI-TOF) calcd for C13H9ClN3O [M + H]+: 258.0429. Found: 258.0431.
4-(4-Chlorophenoxy)benzo[d][1,2,3]triazine (5d)
Obtained as a white solid (15.1 mg, 0.06 mmol, 61%) from 3a (30 mg, 0.10 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 4:1). Mp 155 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.46 (dt, J = 8.4, 1.0 Hz, 1H), 8.39 (dd, J = 8.1, 1.4, 1H), 8.14 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 8.03 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H), 7.51–7.42 (m, 2H), 7.35–7.24 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ: 161.4, 150.6, 146.6, 135.5, 133.6, 131.9, 130.1, 128.3, 123.2, 121.9, 110.9. HRMS (ESI-TOF) calcd for C13H9ClN3O [M + H]+: 258.0429. Found: 258.0430.
4-(4-Iodophenoxy)benzo[d][1,2,3]triazine (5e)
Obtained as a brown solid (27.5 mg, 0.79 mmol, 82%) from 3a (30.0 mg, 0.10 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 4:1). Mp 158 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.46 (d, J = 8.4, 1H), 8.38 (ddd, J = 8.1, 1.5, 1H), 8.14 (dd, J = 8.5, 7.1, 1.4 Hz, 1H), 8.02 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H), 7.85–7.77 (m, 2H), 7.16–7.08 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ: 161.3, 152.0, 146.7, 139.1, 135.5, 133.6, 128.3, 124.0, 122.0, 110.9, 90.6. HRMS (ESI-TOF) calcd for C13H9IN3O [M + H]+: 349.9785. Found: 349.9787.
4-(4-Methoxyphenoxy)benzo[d][1,2,3]triazine (5f)
Obtained as a brown solid (15.2 mg, 0.06 mmol, 62%) from 3a (30 mg, 0.1 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 3:1). Mp 171 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.35 (dd, J = 15.8, 8.2 Hz, 2H), 8.05 (t, J = 7.7 Hz, 1H), 7.93 (t, J = 7.6 Hz, 1H), 7.18 (d, J = 8.1 Hz, 2H), 6.93 (d, J = 8.6 Hz, 2H), 3.78 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 161.8, 157.8, 146.5, 145.6, 135.2, 133.3, 128.1, 122.5, 122.1, 115.0, 111.1, 55.8. HRMS (ESI-TOF) calcd for C14H12N3O2 [M + H]+: 254.0924. Found: 254.0925.
4-(3-Ethynylphenoxy)benzo[d][1,2,3]triazine (5g)
Obtained as a light brown solid (14.4 mg, 0.06 mmol, 61%) from 3a (30 mg, 0.1 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 3:1). Mp 148 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.39 (d, J = 8.4 Hz, 1H), 8.35–8.29 (m, 1H), 8.07 (t, J = 7.8 Hz, 1H), 8.00–7.91 (m, 1H), 7.40 (t, J = 2.4 Hz, 3H), 7.28 (dt, J = 6.7, 2.2 Hz, 1H), 3.09–3.03 (m, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ: 161.4, 151.9, 146.7, 135.5, 133.6, 130.3, 130.0, 128.3, 125.4, 124.1, 122.6, 122.0, 110.9, 82.6, 78.6. HRMS (ESI-TOF) calcd for C15H10N3O [M + H]+: 248.0818. Found: 248.0820.
4-(o-Tolyloxy)benzo[d][1,2,3]triazine (5h)
Obtained as a brown solid (14.5 mg, 0.06 mmol, 64%) from 3a (30 mg, 0.10 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 4:1). Mp 149 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.38 (t, J = 8.6 Hz, 2H), 8.07 (t, J = 7.8 Hz, 1H), 7.95 (t, J = 7.7 Hz, 1H), 7.30–7.22 (m, 2H), 7.20 (d, J = 7.1 Hz, 1H), 7.15 (d, J = 8.2 Hz, 1H), 2.13 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 161.1, 150.8, 146.6, 135.3, 133.4, 131.8, 130.3, 128.2, 127.5, 126.7, 122.0, 121.9, 110.8, 16.4. HRMS (ESI-TOF) calcd for C14H12N3O [M + H]+: 238.0975. Found: 238.0976.
4-(p-Tolyloxy)benzo[d][1,2,3]triazine (5i)
Obtained as a brown solid (17.1 mg, 0.07 mmol, 75%) from 3a (30 mg, 0.10 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 4:1). Mp 150 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.42 (dd, J = 14.4, 8.3 Hz, 2H), 8.12 (t, J = 7.8 Hz, 1H), 8.00 (t, J = 7.6 Hz, 1H), 7.29 (d, J = 7.4 Hz, 2H), 7.21 (dd, J = 8.7, 2.3 Hz, 2H), 2.41 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 161.7, 149.9, 146.5, 136.2, 135.2, 133.3, 130.5, 128.1, 122.1, 121.3, 111.1, 21.1. HRMS (ESI-TOF) calcd for C14H12N3O [M + H]+: 238.0975. Found: 238.0977.
4-(Pyridin-3-yloxy)benzo[d][1,2,3]triazine (5j)
Obtained as a white solid (15.9 mg, 0.07 mmol, 74%) from 3a (30 mg, 0.10 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 1:4). Mp 165 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.69 (d, J = 2.7 Hz, 1H), 8.62 (dd, J = 4.8, 1.4 Hz, 1H), 8.50 (dt, J = 8.5, 0.9 Hz, 1H), 8.43 (ddd, J = 8.0, 1.3, 0.7 Hz, 1H), 8.18 (ddd, J = 8.4, 7.1, 1.4 Hz, 1H), 8.06 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H), 7.80 (ddd, J = 8.4, 2.8, 1.4 Hz, 1H), 7.49 (ddd, J = 8.4, 4.7, 0.7 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ: 161.3, 149.0, 147.5, 146.8, 143.5, 135.7, 133.8, 129.9, 128.4, 124.5, 121.9, 110.8. HRMS (ESI-TOF) calcd for C12H9N4O [M + H]+: 225.0771. Found: 225.0769.
4-((1H-Indol-5-yl)oxy)benzo[d][1,2,3]triazine (5k)
Obtained as a light yellow solid (15.5 mg, 0.06 mmol, 62%) from 3a (30 mg, 0.1 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 3:1). Mp 230 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.50–8.41 (m, 2H), 8.36 (s, 1H), 8.12 (ddd, J = 8.5, 7.3, 1.4 Hz, 1H), 8.01 (ddd, J = 8.2, 7.1, 1.1 Hz, 1H), 7.56 (d, J = 2.3 Hz, 1H), 7.46 (d, J = 8.7 Hz, 1H), 7.31–7.24 (m, 1H), 7.13 (dd, J = 8.7, 2.3 Hz, 1H), 6.59 (td, J = 2.4, 1.1 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ: 162.2, 146.5, 146.0, 135.1, 134.1, 133.2, 128.6, 128.1, 125.9, 122.3, 116.0, 112.8, 112.1, 111.3, 103.4. HRMS (ESI-TOF) calcd for C15H11N4O [M + H]+: 263.0927. Found: 263.0923.
4-(((R)-2,5,7,8-Tetramethyl-2-((4R,8R)-4,8,12-trimethyltridecyl)chroman-6-yl)oxy)benzo[d][1,2,3]triazine (5l)
Obtained as a light yellow solid (37.5 mg, 0.07 mmol, 70%) from 3a (30 mg, 0.1 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 5:1). Mp 155 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.45 (t, J = 6.9 Hz, 2H), 8.12 (t, J = 7.8 Hz, 1H), 8.01 (t, J = 7.6 Hz, 1H), 2.64 (t, J = 6.7 Hz, 2H), 2.14 (s, 3H), 2.01 (s, 3H), 1.97 (s, 3H), 1.90–1.76 (m, 2H), 1.70–1.33 (m, 8H), 1.33–1.19 (m, 11H), 1.18–1.04 (m, 6H), 0.87 (m, 12H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.9, 150.0, 146.5, 142.0, 135.1, 133.3, 128.2, 126.8, 125.0, 123.7, 122.1, 117.9, 110.7, 75.3, 39.5, 37.63, 37.60, 37.56, 37.53, 37.4, 32.94, 32.92, 28.1, 25.0, 24.9, 24.6, 22.9, 22.8, 21.2, 20.8, 19.9, 19.8, 13.3, 12.4, 12.0. HRMS (ESI-TOF) calcd for C36H53N3O2 [M + H]+: 560.4211. Found: 560.4212.
4-(Allyloxy)-8-chlorobenzo[d][1,2,3]triazine (5m)
Obtained as a light brown solid (13 mg, 0.05 mmol, 60%) from 3b (30 mg, 0.09 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 5:1). Mp 146 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.27 (dd, J = 8.1, 1.3 Hz, 1H), 8.11 (dd, J = 7.7, 1.2 Hz, 1H), 7.86 (t, J = 8.0 Hz, 1H), 7.50–7.39 (m, 2H), 7.33–7.21 (m, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 161.3, 152.1, 143.3, 135.5, 134.0, 133.4, 130.1, 126.7, 121.6, 120.9, 112.7. HRMS (ESI-TOF) calcd for C13H9ClN3O [M + H]+: 258.0259. Found: 258.0250.
7-Bromo-4-phenoxybenzo[d][1,2,3]triazine (5n)
Obtained as a brown solid (10.2 mg, 0.03 mmol, 44%) from 3c (30 mg, 0.08 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 5:1). Mp 158 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.62 (d, J = 1.8 Hz, 1H), 8.28 (d, J = 8.6 Hz, 1H), 8.10 (dd, J = 8.7, 1.9 Hz, 1H), 7.56–7.46 (m, 2H), 7.40–7.31 (m, 2H), 7.35–7.28 (m, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ: 161.4, 152.0, 147.1, 137.1, 130.6, 130.1, 130.0, 126.7, 123.8, 121.6, 109.7. HRMS (ESI-TOF) calcd for C13H9BrN3O [M + H]+: 301.9924. Found: 301.9922.
5-Bromo-4-phenoxybenzo[d][1,2,3]triazine (5o)
Obtained as a light yellow solid (19.7 mg, 0.06 mmol, 85%) from 3d (30 mg, 0.08 mmol) following the general method. Rf = 0.3 (Hexane/EtOAc 4:1). Mp 131 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.32 (dd, J = 8.3, 1.1 Hz, 1H), 8.15 (dd, J = 7.7, 1.1 Hz, 1H), 7.82 (t, J = 8.0 Hz, 1H), 7.47–7.37 (m, 2H), 7.31–7.21 (m, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ: 160.0, 151.8, 147.9, 139.2, 135.3, 130.0, 128.2, 126.5, 121.2. HRMS (ESI-TOF) calcd for C13H9BrN3O [M + H]+: 301.9924. Found: 301.9926.
4-Phenoxy-7-(trifluoromethyl)benzo[d][1,2,3]triazine (5p)
Obtained as a white solid (23.7. mg, 0.08 mmol, 62%) from 3e (50 mg, 0.13 mmol) following the general method. Rf = 0.4 (Hexane/EtOAc 4:1). Mp 198 °C (decomposition). 1H NMR (400 MHz, CDCl3) δ 8.75 (s, 1H), 8.57 (d, J = 8.8 Hz, 1H), 8.20 (d, J = 8.6 Hz, 1H), 7.53 (t, J = 7.9 Hz, 2H), 7.39 (d, J = 7.3 Hz, 1H), 7.34 (d, J = 8.1 Hz, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ: 161.2, 151.9, 145.8, 137.0 (q, J = 33.8 Hz), 130.2, 129.2 (q, J = 3.0 Hz), 126.9, 126.0 (q, J = 4.3 Hz), 124.3, 123.9, 121.5, 112.8. 19F NMR (376 MHz, CDCl3) δ −63.3. HRMS (ESI-TOF) calcd for C14H9F3N3O [M + H]+: 292.0692. Found: 292.0696.
Acknowledgments
We gratefully acknowledge Dr. A. Salgado for his assistance in the interpretation of the NMR spectra; and MICINN (PID2020-115128RB-I00), Instituto de Salud Carlos III (FEDERfunds, RICORS2040/Kidney Disease, RD21/0005/0005) and Comunidad de Madrid [(INNOREN-CM/P2022/BMD-7221) and Research Talent Attraction Program (2018-T1/IND-10054 and 2022-5A/IND-24227 to E. M.)] for financial support. F. M.-Z. thanks the Universidad de Alcalá for a predoctoral contract.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c01675.
1H, 13C, and 19F NMR spectral data, mass spectrometry data of new compounds and computational data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Ali Munawar M.; Khalid Z.; Adnan Ahmad H.; Khan M.-u.-A.; Gul S. 1,2,3-benzotriazin-4(3H)-ones: synthesis, reactions and applications. Heterocycles 2017, 94, 3–54. 10.3987/REV-16-846. [DOI] [Google Scholar]; b Ziarani G. M.; Mostofi M.; Lashgari N. Chemistry and Biological Activity of [1,2,3]-Benzotriazine Derivatives. Curr. Org. Chem. 2019, 22, 2717–2751. 10.2174/1385272822666181109123711. [DOI] [Google Scholar]
- Fiorino F.; Severino B.; De Angelis F.; Perissutti E.; Frecentese F.; Massarelli P.; Bruni G.; Collavoli E.; Santagada V.; Caliendo G. Synthesis and In-vitro Pharmacological Evaluation of New 5-HT1AReceptor Ligands Containing a Benzotriazinone Nucleus. Arch. Pharm. Chem. Life Sci. 2008, 341, 20–27. 10.1002/ardp.200700151. [DOI] [PubMed] [Google Scholar]
- Caliendo G.; Fiorino F.; Grieco P.; Perissutti E.; Santagada V.; Meli R.; Raso G. M.; Zanesco A.; De Nucci G. Preparation and local anaesthetic activity of benzotriazinone and benzoyltriazole derivatives. Eur. J. Med. Chem. 1999, 34, 1043–1051. 10.1016/S0223-5234(99)00126-9. [DOI] [Google Scholar]
- Ding Z.; Ni T.; Xie F.; Hao Y.; Yu S.; Chai X.; Jin Y.; Wang T.; Jiang Y.; Zhang D. Design, synthesis, and structure-activity relationship studies of novel triazole agents with strong antifungal activity against Aspergillus fumigatus. Bioorg. Med. Chem. Lett. 2020, 30, 126951. 10.1016/j.bmcl.2020.126951. [DOI] [PubMed] [Google Scholar]
- Takai H.; Obase H.; Nakamizo N.; Teranishi M.; Kubo K.; Shuto K.; Hashimoto T. Synthesis and Pharmacological Evaluation of Piperidine Derivatives with Various Heterocyclic Rings at the 4-Position. Chem. Pharm. Bull. 1985, 33, 1104–1115. 10.1248/cpb.33.1104. [DOI] [PubMed] [Google Scholar]
- a Mendez-David I.; Guilloux J.; Papp M.; Tritschler L.; Mocaer E.; Gardier A. M.; Bretin S.; David D. J. S 47445 Produces Antidepressant- and Anxiolytic-Like Effects through Neurogenesis Dependent and Independent Mechanisms. Front Pharmacol. 2017, 8, 462. 10.3389/fphar.2017.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Giralt A.; Gomez-Climent M. A.; Alcala R.; Bretin S.; Bertrand D.; Delgado-García J. M.; Perez-Navarro E.; Alberch J.; Gruart A. The AMPA receptor positive allosteric modulator S 47445 rescues in vivo CA3-CA1 long-term potentiation and structural synaptic changes in old mice. Neuropharmacology 2017, 123, 395–409. 10.1016/j.neuropharm.2017.06.009. [DOI] [PubMed] [Google Scholar]
- a Srinivas K.; Srinivas U.; Bhanuprakash K.; Harakishore K.; Murthy U. S.; Rao V. J. Synthesis and antibacterial activity of various substituted s-triazines. Eur. J. Med. Chem. 2006, 41, 1240–1246. 10.1016/j.ejmech.2006.05.013. [DOI] [PubMed] [Google Scholar]; b Al-Adiwish W. M.; Tahir M. I.; Siti-Noor-Adnalizawati A.; Hashim S. F.; Ibrahim N.; Yaacob W. A. Synthesis, antibacterial activity and cytotoxicity of new fused pyrazolo[1,5-a]pyrimidine and pyrazolo[5,1-c][1,2,4]triazine derivatives from new 5-aminopyrazoles. Eur. J. Med. Chem. 2013, 64, 464–476. 10.1016/j.ejmech.2013.04.029. [DOI] [PubMed] [Google Scholar]; c Zhang R.; Guo W.; Wang G.; Chen X.; Li Z.; Xu X. Synthesis and nematicidal activities of 1,2,3-benzotriazin-4-one derivatives containing benzo[d][1,2,3]thiadiazole against Meloidogyne incognita. Bioorg. Med. Chem. Lett. 2020, 30, 127369. 10.1016/j.bmcl.2020.127369. [DOI] [PubMed] [Google Scholar]
- a Wawrzyniak A. W.; Malinowska-Grabos Z.. Pol. PL 133992, 1986.; b Takeuchi Y.; Higuchi S.. Jpn. Kokai Tokkyo Koho JP 129046 A 20020509, 2002.
- a Thorat V. H.; Upadhyay N. S.; Cheng C.-H. Nickel-Catalyzed Denitrogenative ortho-Arylation of Benzotriazinones with Organic Boronic Acids: an Efficient Route to Losartan and Irbesartan Drug Molecules. Adv. Synth. Catal. 2018, 360, 4784–4789. 10.1002/adsc.201800923. [DOI] [Google Scholar]; b Lin T.; Wang Y.-E.; Cui N.; Li M.; Wang R.; Bai J.; Fan Y.; Xiong D.; Xue F.; Walsh P. J.; Mao J. Nickel-Catalyzed Cross-Electrophile Coupling of 1,2,3-Benzotriazin-4(3H)-ones with Aryl Bromides. J. Org. Chem. 2022, 87, 16567–16577. 10.1021/acs.joc.2c02246. [DOI] [PubMed] [Google Scholar]
- Balakrishnan M. H.; Sathriyan K.; Mannathan S. Nickel-Catalyzed Denitrogenative Cross-Coupling Reaction of 1,2,3- Benzotriazin-4(3H)-ones with Organoboronic Acids: An Easy Access to Ortho-Arylated and Alkenylated Benzamides. Org. Lett. 2018, 20, 3815–3818. 10.1021/acs.orglett.8b01401. [DOI] [PubMed] [Google Scholar]
- Madasamy K.; Balakrishnan M. H.; Korivi R.; Mannathan S. Trifluoroacetic Acid-Mediated Denitrogenative ortho-Hydroxylation of 1,2,3-Benzotriazin-4(3H)-ones: A Metal-Free Approach. J. Org. Chem. 2022, 87, 8752–8756. 10.1021/acs.joc.2c00354. [DOI] [PubMed] [Google Scholar]
- a Wang H.; Yu S. Synthesis of Isoquinolones Using Visible-Light-Promoted Denitrogenative Alkyne Insertion of 1,2,3-Benzotriazinones. Org. Lett. 2015, 17, 4272–4275. 10.1021/acs.orglett.5b01960. [DOI] [PubMed] [Google Scholar]; b Fang Z. J.; Zheng S. C.; Guo Z.; Guo J. Y.; Tan B.; Liu X. Y. Asymmetric Synthesis of Axially Chiral Isoquinolones: Nickel-Catalyzed Denitrogenative Transannulation. Angew. Chem., Int. Ed. 2015, 54, 9528–9532. 10.1002/anie.201503207. [DOI] [PubMed] [Google Scholar]; c Wang N.; Zheng S. C.; Zhang L. L.; Guo Z.; Liu X. Y. Nickel(0)-Catalyzed Denitrogenative Transannulation of Benzotriazinones with Alkynes: Mechanistic Insights of Chemical Reactivity and Regio- and Enantioselectivity from Density Functional Theory and Experiment. ACS Catal. 2016, 6, 3496–3505. 10.1021/acscatal.6b00572. [DOI] [Google Scholar]; d Thorat V. H.; Upadhyay N. S.; Murakami M.; Cheng C. H. Nickel-Catalyzed Denitrogenative Annulation of 1,2,3- Benzotriazin-4-(3H)-ones with Benzynes for Construction of Phenanthridinone Scaffolds. Adv. Synth. Catal. 2018, 360, 284–289. 10.1002/adsc.201701143. [DOI] [Google Scholar]; e Balakrishnan M. H.; Mannathan S. Palladium/Copper-Catalyzed Denitrogenative Alkylidenation and ortho-Alkynylation Reaction of 1,2,3-Benzotriazin-4(3H)-ones. Org. Lett. 2020, 22, 542–546. 10.1021/acs.orglett.9b04297. [DOI] [PubMed] [Google Scholar]
- Bharate S. S. Critical Analysis of Drug Product Recalls due to Nitrosamine Impurities. J. Med. Chem. 2021, 64, 2923–2936. 10.1021/acs.jmedchem.0c02120. [DOI] [PubMed] [Google Scholar]
- a Chandrasekhar A.; Sankararaman S. Selective Synthesis of 3-Arylbenzo-1,2,3-triazin-4(3H)-ones and 1-Aryl-(1H)-benzo-1,2,3-triazoles from 1,3-Diaryltriazenes through Pd(0) Catalyzed Annulation Reactions. J. Org. Chem. 2017, 82, 11487–11493. 10.1021/acs.joc.7b02023. [DOI] [PubMed] [Google Scholar]; b Chirila P. G.; Skibinski L.; Miller K.; Hamilton A.; Whiteoak C. J. Towards a Sequential One-Pot Preparation of 1,2,3-Benzotriazin-4(3H)-ones Employing a Key Cp*Co(III)-catalyzed C-H Amidation Step. Adv. Synth. Catal. 2018, 360, 2324–2332. 10.1002/adsc.201800133. [DOI] [Google Scholar]; c Barak D. S.; Mukhopadhyay S.; Dahatonde D. J.; Batra S. NaNO2/I2 as an alternative reagent for the synthesis of 1,2,3-benzotriazin-4(3H)-ones from 2-aminobenzamides. Tetrahedron Lett. 2019, 60, 248–251. 10.1016/j.tetlet.2018.12.025. [DOI] [Google Scholar]; d Ren J.; Yan X.; Cui X.; Pi C.; Wu Y.; Cui X. Iodine-catalysed N-centered [1,2]-rearrangement of 3-aminoindazoles with anilines: efficient access to 1,2,3-benzotriazines. Green Chem. 2020, 22, 265–269. 10.1039/C9GC03567B. [DOI] [Google Scholar]; e Lai Z.; Wang C.; Li J.; Cui S. Redox Cyclization of Amides and Sulfonamides with Nitrous Oxide for Direct Synthesis of Heterocycles. Org. Lett. 2020, 22, 2017–2021. 10.1021/acs.orglett.0c00397. [DOI] [PubMed] [Google Scholar]; f McGrory R.; Faggyas R. J.; Sutherland A. One-pot synthesis of N-substituted benzannulated triazoles via stable arene diazonium salts. Org. Biomol. Chem. 2021, 19, 6127–6140. 10.1039/D1OB00968K. [DOI] [PubMed] [Google Scholar]; g Liu Y.; Qi X.; Zhang W.; Yin P.; Cai Z.; Zhang Q. Construction of Bicyclic 1,2,3-Triazine N-Oxides from Aminocyanides. Org. Lett. 2021, 23, 734–738. 10.1021/acs.orglett.0c03952. [DOI] [PubMed] [Google Scholar]
- a van Leusen D.; van Leusen A. M. Synthetic Uses of Tosylmethyl Isocyanide (TosMIC). Org. React. 2001, 57, 417–666. 10.1002/0471264180.or057.03. [DOI] [Google Scholar]; b Lygin A. V.; de Meijere A. Isocyanides in the Synthesis of Nitrogen Heterocycles. Angew. Chem., Int. Ed. 2010, 49, 9094–9124. 10.1002/anie.201000723. [DOI] [PubMed] [Google Scholar]; c Kaur T.; Wadhwa P.; Sharma A. Arylsulfonylmethyl Isocyanides: a Novel Paradigm in Organic Synthesis. RSC Adv. 2015, 5, 52769–52787. 10.1039/C5RA07876H. [DOI] [Google Scholar]; d Mathiyazhagan A. D.; Anilkumar G. Recent advances and applications of p-toluenesulfonylmethyl isocyanide (TosMIC). Org. Biomol. Chem. 2019, 17, 6735–6747. 10.1039/C9OB00847K. [DOI] [PubMed] [Google Scholar]; e Kumar K. TosMIC: A Powerful Synthon for Cyclization and Sulfonylation. ChemistrySelect 2020, 5, 10298–10328. 10.1002/slct.202001344. [DOI] [Google Scholar]; f Efimov I. V.; Kulikova L. N.; Zhilyaev D. I.; Voskressensky L. G. Recent Advances in the Chemistry of Isocyanides with Activated Methylene Group. Eur. J. Org. Chem. 2020, 2020, 7284–7303. 10.1002/ejoc.202000890. [DOI] [Google Scholar]
- a Mendiola J.; Minguez J. M.; Alvarez-Builla J.; Vaquero J. J. Reaction of 2-Bromomethylazoles and TosMIC: A Domino Process to Azolopyrimidines. Synthesis of Core Tricycle of the Variolins Alkaloids. Org. Lett. 2000, 2, 3253–3256. 10.1021/ol0062087. [DOI] [PubMed] [Google Scholar]; b Baeza A.; Mendiola J.; Burgos C.; Alvarez-Builla J.; Vaquero J. J. Heterocyclizations with Tosylmethyl Isocyanide Derivatives. A New Approach to Substituted Azolopyrimidines. J. Org. Chem. 2005, 70, 4879–4882. 10.1021/jo050029r. [DOI] [PubMed] [Google Scholar]; c Coppola A.; Sánchez-Alonso P.; Sucunza D.; Burgos C.; Alajarín R.; Alvarez- Builla J.; Mosquera M. E. G.; Vaquero J. J. Remote Aryl Cyanation via Isocyanide-Cyanide Rearrangement on Tosylmethyl Isocyanide Derivatives. Org. Lett. 2013, 15, 3388–3391. 10.1021/ol401433x. [DOI] [PubMed] [Google Scholar]
- a Coppola A.; Sucunza D.; Burgos C.; Vaquero J. J. Isoquinoline Synthesis by Heterocyclization of Tosylmethyl Isocyanide Derivatives: Total Synthesis of Mansouramycin B. Org. Lett. 2015, 17, 78–81. 10.1021/ol5032624. [DOI] [PubMed] [Google Scholar]; b Gutiérrez S.; Coppola A.; Sucunza D.; Burgos C.; Vaquero J. J. Synthesis of 1-Substituted Isoquinolines by Heterocyclization of TosMIC Derivatives: Total Synthesis of Cassiarin A. Org. Lett. 2016, 18, 3378–3381. 10.1021/acs.orglett.6b01521. [DOI] [PubMed] [Google Scholar]; c Gutiérrez S.; Sucunza D.; Vaquero J. J. γ-Carboline Synthesis by Heterocyclization of TosMIC Derivatives. J. Org. Chem. 2018, 83, 6623–6632. 10.1021/acs.joc.8b00906. [DOI] [PubMed] [Google Scholar]
- Sisko J.; Mellinger M.; Sheldrake P. W.; Baine N. H. α-Tosylbenzyl Isocyanide. Org. Synth. 2003, 77, 198–205. 10.1002/0471264180.os077.20. [DOI] [Google Scholar]
- Necardo C.; Alfano A. I.; Del Grosso E.; Pelliccia S.; Galli U.; Novellino E.; Meneghetti F.; Giustiniano M.; Tron G. C. Aryl Azides as Forgotten Electrophiles in the Van Leusen Reaction: A Multicomponent Transformation Affording 4-Tosyl-1-arylimidazoles. J. Org. Chem. 2019, 84, 16299–16307. 10.1021/acs.joc.9b02546. [DOI] [PubMed] [Google Scholar]
- a Stokes B. J.; Liu S.; Driver T. G. J. Am. Chem. Soc. 2011, 133, 4702–4705. 10.1021/ja111060q. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dilger A. K.; Pabbisetty K. B.; Corte J. R.; De Lucca I.; Fang T.; Yang W.; Pinto D. J. P.; Wang Y.; Zhu Y.; Mathur A.; Li J.; Hou X.; Smith D.; Sun D.; Zhang H.; Krishnananthan S.; Wu D.-R.; Myers J. E. Jr; Sheriff S.; Rossi K. A.; Chacko S.; Zheng J. J.; Galella M. A.; Ziemba T.; Dierks E. A.; Bozarth J. M.; Wu Y.; Crain E.; Wong P. C.; Luettgen J. M.; Wexler R. R.; Ewing W. R. J. Med. Chem. 2022, 65, 1770–1785. 10.1021/acs.jmedchem.1c00613. [DOI] [PubMed] [Google Scholar]
- Stokes B. J.; Vogel C. V.; Urnezis L. K.; Pan M.; Driver T. G. Org. Lett. 2010, 12, 2884–2887. 10.1021/ol101040p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan A.; Gorantla K. R.; Mallik B. S.; Sharada D. S. Org. Biomol. Chem. 2018, 16, 5113–5118. 10.1039/C8OB00931G. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Dhawan G.; Muthengi A.; Liu S.; Wang W.; Legris M.; Zhang W. Green Chem. 2017, 19, 3851–3855. 10.1039/C7GC01380A. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.