Abstract
Motivation
Microbial communities have a profound impact on both human health and various environments. Viruses infecting bacteria, known as bacteriophages or phages, play a key role in modulating bacterial communities within environments. High-quality phage genome sequences are essential for advancing our understanding of phage biology, enabling comparative genomics studies and developing phage-based diagnostic tools. Most available viral identification tools consider individual sequences to determine whether they are of viral origin. As a result of challenges in viral assembly, fragmentation of genomes can occur, and existing tools may recover incomplete genome fragments. Therefore, the identification and characterization of novel phage genomes remain a challenge, leading to the need of improved approaches for phage genome recovery.
Results
We introduce Phables, a new computational method to resolve phage genomes from fragmented viral metagenome assemblies. Phables identifies phage-like components in the assembly graph, models each component as a flow network, and uses graph algorithms and flow decomposition techniques to identify genomic paths. Experimental results of viral metagenomic samples obtained from different environments show that Phables recovers on average over 49% more high-quality phage genomes compared to existing viral identification tools. Furthermore, Phables can resolve variant phage genomes with over 99% average nucleotide identity, a distinction that existing tools are unable to make.
Availability and implementation
Phables is available on GitHub at https://github.com/Vini2/phables.
1 Introduction
Bacteriophages (hereafter ‘phages’) are viruses that infect bacteria, which influence microbial ecology and help modulate microbial communities (Edwards and Rohwer 2005, Rodriguez-Valera et al. 2009). Phages are considered the most abundant biological entity on earth, totalling an estimated 1031 particles (Comeau et al. 2008). Since their discovery by Frederick Twort in 1915 (Twort 1915), phages have been isolated from many diverse environments (Keen 2015). When sequencing technologies were first developed, phage genomes were the first to be sequenced due to their relatively small genome size (Sanger et al. 1977). With the advent of second-generation sequencing technologies, the first metagenomic samples to be sequenced were phages (Breitbart et al. 2002). The availability of advanced sequencing technologies has facilitated the investigation of the effects of phages on the functions of microbial communities, especially in the human body’s niche areas. For example, phages residing in the human gut have a strong influence on human health (Łusiak-Szelachowska et al. 2017) and impact gastrointestinal diseases such as inflammatory bowel disease (IBD) (Norman et al. 2015). To date, our understanding of the diversity of phages is limited, as most have not been cultured due to the inherent difficulty of recovering phages from their natural environments. Although countless millions of phage species are thought to exist, only 26 048 complete phage genomes have been sequenced according to the INfrastructure for a PHAge REference Database (INPHARED) (Cook et al. 2021) (as of the September 2023 update).
Metagenomics has enabled the application of modern sequencing techniques for the culture-independent study of microbial communities (Hugenholtz et al. 1998). Metagenomic sequencing provides a multitude of sequencing reads from the genetic material in environmental samples that are composed of a mixture of prokaryotic, eukaryotic, and viral species. Metagenomic analysis pipelines start by assembling sequencing reads from metagenomic samples into longer contiguous sequences that are used in downstream analyses. Most metagenome assemblers (Peng et al. 2011, Namiki et al. 2012, Li et al. 2015, Nurk et al. 2017) use ‘de Bruijn graphs’ (Pevzner et al. 2001) as the primary data structure where they break sequencing reads into smaller pieces of length k, known as k-mers, and represent -mers as vertices and k-mers as edges. After performing several simplification steps, the final ‘assembly graph’ represents sequences as vertices and connection information between these sequences as edges (Nurk et al. 2017, Mallawaarachchi et al. 2020a). Non-branching paths in the assembly graph (paths where all vertices have an in-degree and out-degree of one, except for the first and last vertices) are referred to as ‘unitigs’ (Kececioglu and Myers 1995). Unitigs are entirely consistent with the read data and belong to the final genome(s). Assemblers condense unitigs into individual vertices and resolve longer optimized paths through the branches into contiguous sequences known as ‘contigs’ (Bankevich et al. 2012). As the contextual and contiguity information of reads is lost in de Bruijn graphs, mutations in metagenomes with high strain diversity appear as ‘bubbles’ in the assembly graph where a vertex has multiple outgoing edges (branches) which eventually converge as incoming edges into another vertex (Pevzner et al. 2001, 2004). Assemblers consider these bubbles as errors and consider one path of the bubble corresponding to the dominant strain (Bankevich et al. 2012) or terminate contigs prematurely (Li et al. 2015). Moreover, most metagenome assemblers are designed and optimized for bacterial genomes and fail to recover viral populations with low coverage and genomic repeats (Roux et al. 2017, Sutton et al. 2019). However, previous studies have shown that contigs connected to each other are more likely to belong to the same genome (Mallawaarachchi et al. 2020a,b, 2021). Hence, the assembly graph retains important connectivity and neighbourhood information within fragmented assemblies. This concept has been successfully applied to develop tools such as GraphMB (Lamurias et al. 2022), MetaCoAG (Mallawaarachchi and Lin 2022a,b), and RepBin (Xue et al. 2022), where the assembly graphs are utilized in conjunction with taxonomy-independent metagenomic binning methods to recover high-quality metagenome-assembled genomes (hereafter MAGs) of bacterial genomes. Moreover, assembly graphs have been used for bacterial strain resolution in metagenomic data (Quince et al. 2021). However, limited studies have been conducted to resolve phage genomes in metagenomic data, particularly in viral-enriched metagenomes.
Computational tools have enabled large-scale studies to recover novel phages entirely from metagenomic sequencing data (Simmonds et al. 2017) and gain insights into interactions with their hosts (Nayfach et al. 2021b, Roach et al. 2022b, Hesse et al. 2022). While exciting progress has been made towards identifying new phages, viral dark matter remains vast. Current methods are either too slow or result in inaccurate or incomplete phage genomes. Generating high-quality phage genomes via de novo metagenome assembly is challenging due to the modular and mosaic nature of phage genomes (Hatfull 2008, Belcaid et al. 2010, Lima-Mendez et al. 2011). Repeat regions can result in fragmented assemblies and chimeric contigs (Casjens and Gilcrease 2009, Merrill et al. 2016). Hence, current state-of-the-art computational tools rely on the combination of either more conservative tools based on sequence- and profile-based screening [e.g. MetaPhinder (Jurtz et al. 2016)] or machine learning approaches based on nucleotide signatures [e.g. Seeker (Auslander et al. 2020), refer to Supplementary Table S1 in Section S1]. Resulting predictions are then evaluated using tools such as CheckV (Nayfach et al. 2021a) and VIBRANT (Kieft et al. 2020) to categorize the predicted phages based on their completeness, contamination levels, and possible lifestyle (virulent or temperate) (McNair et al. 2012). Due to the supervised nature of the underlying approaches, most of these tools cannot characterize novel viruses that are significantly different from known viruses. Moreover, the approach used by these tools can be problematic with fragmented assemblies where contigs do not always represent complete genomes. In an attempt to address this limitation, tools such as MARVEL (Amgarten et al. 2018) and PHAMB (Johansen et al. 2022) were developed to identify viral metagenome-assembled genomes (vMAGs) of phages from metagenomic data. These programs rely on existing taxonomy-independent metagenomic binning tools such as MetaBAT2 (Kang et al. 2019) or VAMB (Nissen et al. 2021) and attempt to predict viral genome bins from this output using machine learning techniques.
Metagenomic binning tools are designed to capture nucleotide and sequence coverage-specific patterns of different taxonomic groups; therefore, sequences from viruses with low and uneven sequence coverage are often inaccurately binned. Many metagenomic binning tools filter out short sequences [e.g. shorter than 1500 bp (Kang et al. 2019)], which further result in the loss of essential regions in phage genomes that are often present as short fragments in the assembly (Casjens and Gilcrease 2009). Moreover, most metagenomic binning tools struggle to distinguish viruses from genetically diverse populations with high strain diversity and quasispecies dynamics. These tools do not resolve the clustered sequences into contiguous genomes and the bins produced often contain a mixture of multiple strains resulting in poor-quality MAGs (Meyer et al. 2022). Existing solutions developed for viral quasispecies assembly only consider one species at a time (Baaijens et al. 2020, Freire et al. 2021, 2023) and cannot be applied to complex metagenomes. Despite the recent progress, it is challenging for currently available tools to recover complete high-quality phage genomes from metagenomic data, and a novel approach is required to address this issue. The use of connectivity information from assembly graphs could overcome these challenges [as shown in previous studies on bacterial metagenomes (Lamurias et al. 2022, Mallawaarachchi and Lin 2022a, 2022b)] to enable the recovery of high-quality phage genomes.
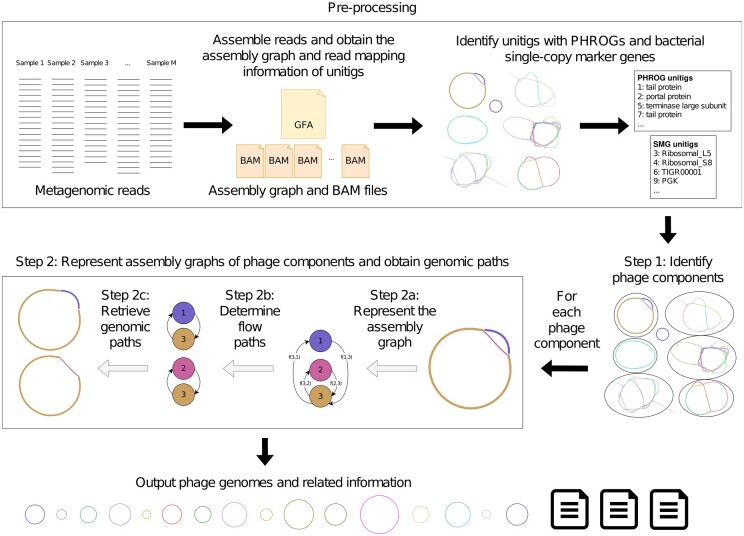
In this article, we introduce Phables, a software tool that can resolve phage genomes from viral metagenome assemblies. First, Phables identifies phage-like components in the assembly graph using conserved genes. Second, using read mapping information, graph algorithms and flow decomposition techniques, Phables identifies the most probable combinations of varying phage genome segments within a component, leading to the recovery of accurate phage genome assemblies (Fig. 1). We evaluated the quality of the resolved genomes using different assessment techniques and demonstrate that Phables produces complete and high-quality phage genomes.
Figure 1.
Phables workflow. Preprocessing: assemble reads, obtain the assembly graph and read mapping information, and identify unitigs with PHROGs and bacterial single-copy marker genes. Step 1: Identify phage components from the initial assembly. Step 2: For each phage component, represent the assembly graph, determine the flow paths and retrieve the genomic paths. Finally, output phage genomes and related information.
2 Materials and methods
Figure 1 presents the overall workflow of Phables. Reads from single or multiple viral metagenomic samples are assembled, and the assembly graph and read mapping information are obtained. The unitig sequences from the assembly graph are extracted and screened for Prokaryotic Virus Remote Homologous Groups (PHROGs) (Terzian et al. 2021) and bacterial single-copy marker genes. Phables identifies sub-graphs known as ‘phage components’ and resolves separate phage genomes from each phage component. Finally, Phables outputs the resolved phage genomes and related information. Each step of Phables is explained in detail in the following sections.
2.1 Preprocessing
The preprocessing step performed by Phables uses an assembly graph and generates the read mapping information and the gene annotations required for Step 1 in the workflow. We recommend Hecatomb (Roach et al. 2022a) to assemble the reads into contigs and obtain the assembly graph. However, Phables can work with any assembly graph in the Graphical Fragment Assembly (GFA) format.
The unitig sequences are extracted from the assembly graph, and the raw sequencing reads are mapped to the unitigs using Minimap2 (Li 2018) and Samtools (Li et al. 2009). Phables uses CoverM (https://github.com/wwood/CoverM) [available from Koverage (https://github.com/beardymcjohnface/Koverage)] to calculate the read coverage of unitigs, using the reads from all samples, and records the mean coverage (the average number of reads that map to each base of the unitig).
Phables identifies unitigs containing PHROGs (Terzian et al. 2021). PHROGs are viral protein families commonly used to annotate prokaryotic viral sequences. MMSeqs2 (Steinegger and Söding 2017) is used to identify PHROGs in unitigs using an identity cutoff of 30% and an e-value of less than (by default).
Next, Phables identifies unitigs containing bacterial single-copy marker genes. Most bacterial genomes have conserved genes known as single-copy marker genes (SMGs) that appear only once in a genome (Dupont et al. 2012, Albertsen et al. 2013). FragGeneScan (Rho et al. 2010) and HMMER (Eddy 2011) are used to identify sequences containing SMGs. SMGs are considered to be present if more than 50% (by default) of the gene length is aligned to the unitig. The list of SMGs is provided in Supplementary Table S2 in Section S2.
2.2 Step 1: Identify phage components
Phables identifies components from the final assembly graph where all of its unitigs do not have any bacterial SMGs (identified from the preprocessing step) and at least one unitig contains one or more genes belonging to a PHROG for at least one of the PHROG categories: ‘head and packaging’, ‘connector’, ‘tail’ and ‘lysis’, which contain known phage structural proteins and are highly conserved in tailed phages (Auslander et al. 2020) (refer to Supplementary Fig. S1 in Section S3 for an analysis of the PHROG hits to all known phage genomes). The presence of selected PHROGs ensures the components are phage-like and represent potential phage genomes. The absence of bacterial SMGs further ensures that the components are not prophages. These identified components are referred to as ‘phage components’. Components that are comprised of a single circular unitig (the two ends of the unitig overlap) or a single linear unitig and that satisfy the above conditions for genes are considered phage components only if the unitig is longer than the predefined threshold that is set to 2000 bp by default, as this is the approximate lower bound of genome length for tailed phages (Luque et al. 2020).
2.3 Step 2: Represent assembly graphs of phage components and obtain genomic paths
2.3.1 Step 2a: Represent the assembly graph
Following the definitions from STRONG (Quince et al. 2021), we define the assembly graph for a phage component where is a collection of vertices corresponding to unitig sequences that make up a phage component and directed edges represent connections between unitigs. Each directed edge is defined by a starting vertex and an ending vertex (the arrow denotes the direction of the overlap), where indicates whether the overlap occurs between the original sequence, indicated by a sign or its reverse complement, indicated by a sign.
The weight of each edge irrespective of the orientation of the edge, termed is set to the minimum of the read coverage values of the two unitigs and . We also define the confidence of each edge irrespective of whether the overlap occurs between the original sequence or its reverse complement, termed as the number of paired-end reads spanning across . Here, the forward read maps to unitig and the reverse read maps to unitig . We also define the confidence of paths termed as the number of paired-end reads spanning across . Here, the forward read maps to unitig and the reverse read maps to unitig . Paired-end information has been used in previous studies for assembling viral quasispecies to untangle assembly graphs (Freire et al. 2023). Moreover, paired-end reads are widely used in manual curation steps to join contigs from metagenome assemblies and extend them to longer sequences (Chen et al. 2020). The more paired-end reads map to the pair of unitigs, the more confident we are about the overlap represented by the edge (refer to Supplementary Fig. S2 in Section S4 for histograms of edge confidence).
2.3.2 Step 2b: Determine flow paths
Phables models the graph of the phage component as a flow decomposition problem and obtains the genomic paths with their corresponding coverage values. We define three cases based on the number and arrangement of unitigs present in the phage component as shown in Fig. 2.
Figure 2.
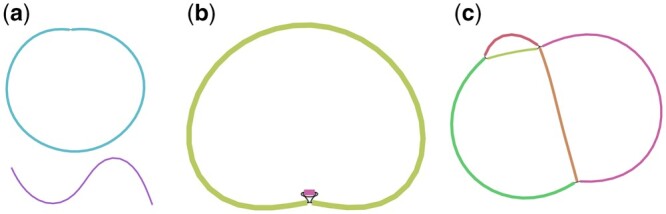
Cases of phage components. (a) Case 1 represents a phage component with one circular or linear unitig. (b) Case 2 represents a phage component with two circular unitigs connected to each other. (c) Case 3 represents a phage component that is more complex with multiple unitigs and multiple paths.
Case 1: When the phage component has only one circular or linear unitig longer than the predefined threshold , Phables considers this unitig as one genome. The genomic path is defined as the unitig sequence itself.
Case 2: The phage components in Case 2 have two circular unitigs connected together where at least one is longer than the predefined threshold . This is an interesting case as the shorter unitig corresponds to the ‘terminal repeats’ of phages. Some phages have double-stranded repeats at their termini which are a few hundred base pairs in length and are exactly the same in every virion chromosome (i.e. they are not permuted) (Casjens and Gilcrease 2009). The terminal repeats are generated by a duplication of the repeat region in concert with packaging (Chung et al. 1990, Zhang and Studier 2004) (refer to Supplementary Fig. S3 in Section S5). This type of end structure could be overlooked when a phage genome sequence is determined by shotgun methods because sequence assembly can merge the two ends to give a circular sequence. Phables successfully resolves these terminal repeats to form complete genomes. To resolve the phage component in Case 2, we consider the shorter unitig as the terminal repeat. Now we combine the original sequence of the terminal repeat to the beginning and end of the longer unitig (refer to Supplementary Fig. S3 in Section S5). The coverage of the path will be set to the coverage of the longer unitig.
Case 3: In Case 3, we have more complex phage components where there are more than two unitigs forming branching paths, and we model them as a minimum flow decomposition (MFD) problem. The MFD problem decomposes a directed acyclic graph (DAG) into a minimum number of source-to-sink () paths that explain the flow values of the edges of the graph (Vatinlen et al. 2008, Dias et al. 2022). The most prominent applications of the MFD problem in bioinformatics include reconstructing RNA transcripts (Tomescu et al. 2013, Shao and Kingsford 2017, Gatter and Stadler 2019) and viral quasispecies assembly (Baaijens et al. 2020). The MFD problem can be solved using integer linear programming (ILP) (Schrijver 1998).
In the viral metagenomes, we have identified structures containing several phage variant genomes, that are similar to viral quasispecies often seen in RNA viruses (Domingo and Perales 2019). Hence, Phables models each of the remaining phage components as an MFD problem and uses the minimum flow decomposition using integer linear programming (MFD-ILP) implementation from Dias et al. (2022). MFD-ILP finds a flow decomposition with a set of flow paths and associated weights such that the number of flow paths is minimized. These flow paths represent possible genomic paths. An example of a phage component with possible paths is shown in Fig. 3.
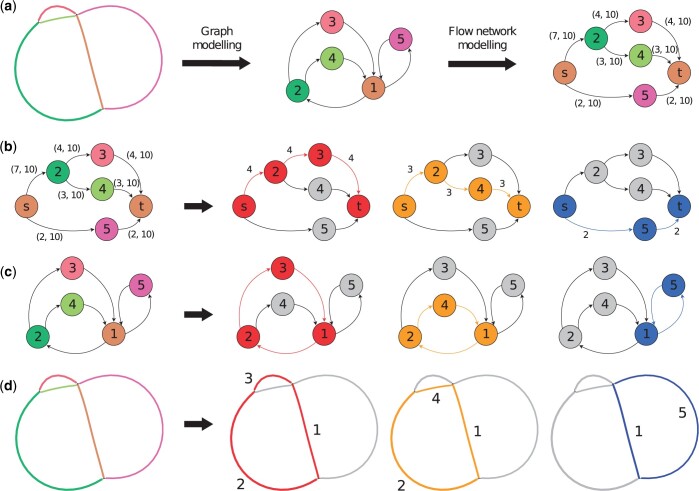
Figure 3.
Modelling of an example Case 3 phage component. Example of a Case 3 phage component (a) being modelled as a graph, a flow network and resolved into paths denoted using (b) flow network visualization with flow values, (c) graph visualization with directed edges, and (d) Bandage (Wick et al. 2015) visualization (with corresponding unitig numbers). Here, three flow paths (, , and ) can be obtained corresponding to three phage genomes. The thick black arrows in B to D denote the resolution into three paths.
First, we convert the assembly graph of the phage component into a DAG. We start by removing ‘dead-ends’ from . We consider a vertex to be a dead-end if it has either no incoming edges or no outgoing edges, which arise due to errors at the start or end of reads that can create protruding chains of deviated edges (Bankevich et al. 2012). Dead-ends are particularly problematic in later steps of Phables as they can affect the continuity of genomic paths. Hence, their removal ensures that all the possible paths in the graph form closed cycles. We eliminate dead-ends by recursively removing vertices with either no incoming edges or no outgoing edges. Note that removing one dead-end can cause another vertex that is linked only to the removed one to become a dead-end, hence the removal process is done recursively.
Since a Case 3 phage component forms a cyclic graph as shown in Fig. 3a, we have to identify a vertex to represent the source/sink (referred to as ) in order to convert the graph to a DAG and model it as a flow network. Starting from every vertex (), we conduct a breadth-first-search and identify an iterator, , where ‘vertices’ is the non-empty list of vertices at the same distance ‘level’ from the ‘source’. The method that generates this iterator is known as and we use the NetworkX implementation (https://networkx.org/documentation/stable/reference/algorithms/). We extract the vertices in the final layer and check if their successors are equal to . If this condition holds for some vertex in , we consider this vertex to be the vertex. If more than one vertex satisfies the condition to be a vertex, then we pick the vertex corresponding to the longest unitig as the vertex. This process is carried out to find a vertex common to the flow paths (refer to Supplementary Algorithm S1 in Section S6). As an example, consider vertex 1 in Fig. 3a. When we do a breadth-first-search starting from vertex 1, the vertices in the last layer in our iterator will be 3 and 4. The successor of both 3 and 4 is vertex 1. Since the successors of the vertices in the last layer are the same as the starting vertex, we consider vertex 1 as the vertex.
The edges of that are weighted according to unitig coverage, may not always satisfy the conservation of flow property because of uneven sequencing depths at different regions of the genomes (Peng et al. 2011). Hence, we use inexact flow networks that allow the edge weights to belong to an interval. Once we have identified a vertex, we separate that vertex into two vertices for the source and sink . We create an inexact flow network from to and model the rest of the vertices and edges in . For example, in Fig. 3b vertex 1 is broken into two vertices and t, and the network flows from to . For every edge , we have associated two positive integer values and , satisfying , where , , is the coverage multiplier parameter (1.2 by default) and is the maximum coverage of a unitig in the phage component. In Fig. 3b, each edge has two values that define the flow interval for the inexact flow network . This modelling ensures that the flow through each edge is bounded by a relaxed interval between the edge weight and the maximum coverage within the component. For example, in Fig. 3b, the edge has a weight of 4 (which is the minimum of the read coverage values of the two unitigs 2 and 3 obtained from Step 2a). Here, and for the component, so we set and .
Next, we define a set of simple paths , where the edges that form each path have paired-end reads spanning across them, i.e. ( by default). Enforcing these paths to contain paired-end reads ensures that genuine connections are identified and reflected in at least one decomposed path. For example, in Fig. 3b, the edge has 10 paired-end reads spanning across the edge. Hence, we add the path to . Moreover, for a path passing through the junction (where the in-degree and out-degree are non-zero), we add the path to , if or if is less than a predefined threshold (100 by default). This allows Phables to specify longer subpaths across complex junctions.
Now we model our inexact flow network as a minimum inexact flow decomposition (MIFD) problem and determine a minimum-sized set of paths and associated weights with each where the following conditions hold.
A path will consist of unitigs with orientation information. The weight will be the coverage of the genome represented by the path .
2.3.3 Step 2c: Retrieve genomic paths
The flow paths obtained from Cases 1 and 2 described in the previous section are directly translated to genomic paths based on the unitig sequences. In Case 3, we get paths from the flow decomposition step (as shown in Fig. 3b). The paths longer than the predefined threshold and have a predefined coverage threshold of (10 by default) or above are retained. For each remaining path, we remove from the path as and are the same vertex and combine the nucleotide sequences of the unitigs corresponding to the vertices and the orientation of edges in the flow path (refer to Fig. 3c and d). Once the genomic paths of phage components are obtained, we record the constituent unitigs, path length (in bp), coverage (i.e. the flow value of the path), and the guanine-cytosine (GC) content of each genomic path.
3 Experimental design
3.1 Simulated phage dataset
We simulated reads from the following four phages with the respective read coverage values and created a simulated phage dataset (referred to as ‘simPhage’) to evaluate Phables.
Enterobacteria phage P22 (AB426868) -
Enterobacteria phage T7 (NC_001604) -
Staphylococcus phage SAP13 TA-2022 (ON911718) -
Staphylococcus phage SAP2 TA-2022 (ON911715) -
The Staphylococcus phage genomes have an average nucleotide identity (ANI) of 96.89%. Paired-end reads were simulated using InSilicoSeq (Gourlé et al. 2019) with the predefined MiSeq error model. We used metaSPAdes (Nurk et al. 2017) from SPAdes version 3.15.5 to assemble the reads into contigs and obtain the assembly graph for the simPhage dataset. Supplementary Tables S3 and S5 in Section S7 summarize the details of the simulations and assemblies.
3.2 Real datasets
We tested Phables on the following real viral metagenomic datasets available from the National Center for Biotechnology Information (NCBI).
Water samples from Nansi Lake and Dongping Lake in Shandong Province, China (NCBI BioProject number PRJNA756429), referred to as ‘Lake Water’
Soil samples from flooded paddy fields from Hunan Province, China (NCBI BioProject number PRJNA866269), referred to as ‘Paddy Soil’
Wastewater virome (NCBI BioProject number PRJNA434744), referred to as ‘Wastewater’
Stool samples from patients with IBD and their healthy household controls (NCBI BioProject number PRJEB7772) (Norman et al. 2015), referred to as ‘IBD’
All the real datasets were processed using Hecatomb version 1.0.1 to obtain a single assembly graph for each dataset (Roach et al. 2022a). Supplementary Tables S3–S5 in Section S7 summarize the information about the datasets and their assemblies.
3.3 Tools benchmarked
We benchmarked Phables with PHAMB (Johansen et al. 2022) (version 1.0.1), a viral identification tool that predicts whether MAGs represent phages and outputs genome sequences. PHAMB takes binning results from a metagenomic binning tool and predicts bins that contain bacteriophage sequences. The MAGs for PHAMB were obtained by running VAMB (version 3.0.8), a binning tool that does not rely on bacterial marker genes, in co-assembly mode on the original contigs with the author-recommended parameter --minfasta 2000 and the --cuda flag. The commands used to run all the tools can be found in Supplementary Section S8.
3.4 Evaluation criteria
3.4.1 Evaluation criteria for binning tools
The resolved genomes from Phables and identified MAGs from PHAMB were evaluated using CheckV version 1.0.1 (Nayfach et al. 2021a) (with reference database version 1.5) which compares bins/genomes against a large database of complete viral genomes. We compare the following metrics from the CheckV results.
CheckV viral quality
Completeness of sequences—number of sequences with 90% completeness
Contamination of sequences—number of sequences with 10% contamination
-
The number and length distribution of sequences with the following warnings
Contig 1.5× longer than expected genome length
High may indicate a large duplication
Since PHAMB predicts all viral bins, we only consider the bins from PHAMB that contain the contigs corresponding to the unitigs recovered by Phables for a fair comparison.
3.4.2 Evaluation criteria for resolved genomes
The number of components resolved by Phables for each case was recorded. The viral quality of the resolved genomes and the unitigs and contigs contained in the corresponding genomic paths were evaluated using CheckV (Nayfach et al. 2021a). Since the reference genomes for the simPhage dataset were available, we evaluated the resolved genomes using metaQUAST (Mikheenko et al. 2016).
4 Results
4.1 Benchmarking results on the simulated phage dataset
We first benchmarked Phables using the simPhage dataset. We evaluated the resolved phage genomes using metaQUAST (Mikheenko et al. 2016). We analysed the genome coverage reported from metaQUAST and the average coverage values reported by Phables. Figure 4 denotes the Bandage (Wick et al. 2015) visualization of the assembly graph from the simPhage dataset and how Phables resolved the complex Case 3 component containing the two Staphylococcus phages.
Figure 4.
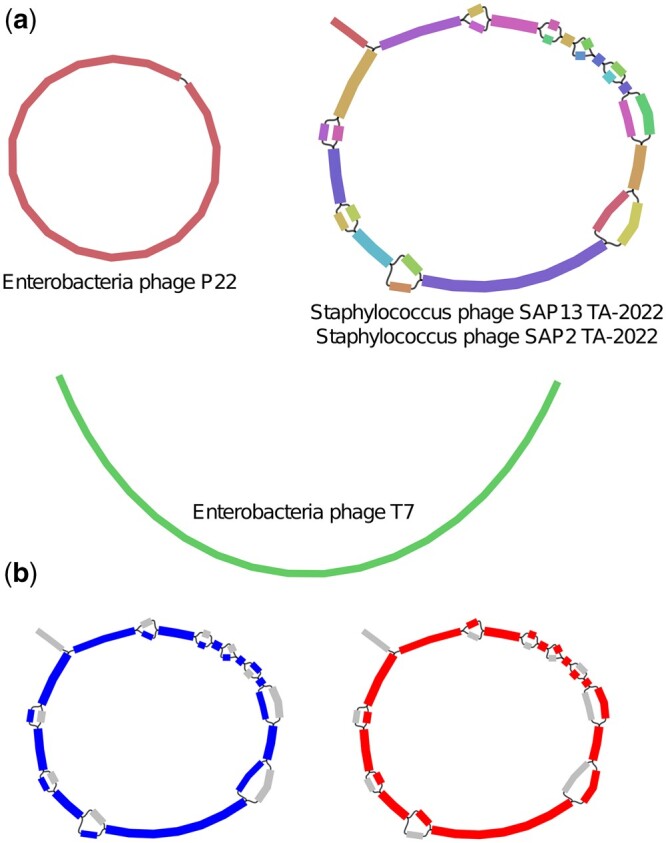
simPhage assembly graph. Visualization of the (a) assembly graph from the simPhage dataset with phage components and (b) resolution of two paths (red and blue) from the Staphylococcus phage component.
Phables recovered the two Staphylococcus phage genomes with over 92% genome coverage (refer to Table 1). The slightly low genome coverage for Staphylococcus phage SAP2 TA-2022 may have been due to the omission of the dead-end which was not properly assembled. Moreover, Phables has recovered the circular genome of Enterobacteria phage P22 and the linear genome of Enterobacteria phage T7 as well. According to Table 1, the coverage values reported from Phables are similar or close to the actual simulated coverage values of the genomes. VAMB failed to run on this dataset as there were fewer contigs than the minimum possible batch size and hence PHAMB could not be run.
Table 1.
Evaluation results for the genomes resolved from Phables for the simPhage dataset.
| Genome | Simulated coverage | Phables predicted coverage | Genome coverage (%) |
|---|---|---|---|
| P22 | 100 | 100 | 99.947 |
| T7 | 150 | 150 | 99.599 |
| SAP13 TA-2022 | 200 | 206 | 100.00 |
| SAP2 TA-2022 | 400 | 401 | 92.406 |
4.2 Benchmarking results on the real datasets
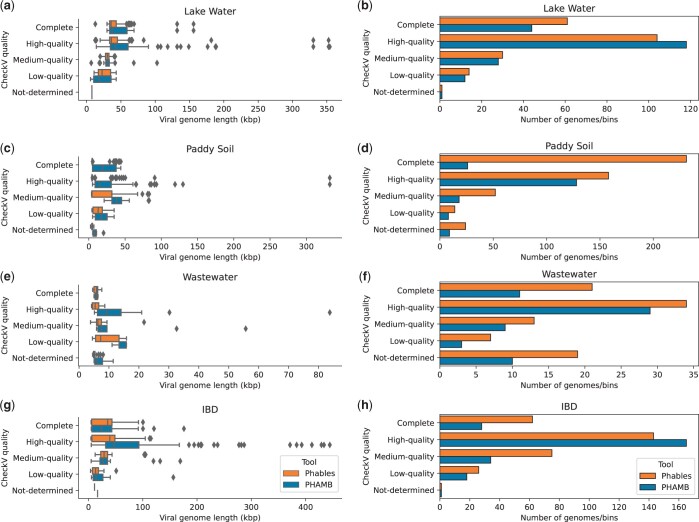
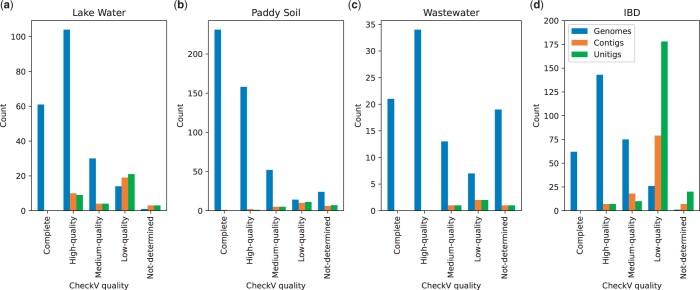
Phables resolves unitigs within phage components to produce multiple complete and high-quality genomes from the viral metagenomes (Fig. 5). The genome quality of Phables results was compared with the vMAG prediction tool PHAMB (Johansen et al. 2022) and evaluated using CheckV (Nayfach et al. 2021a). Figure 5 denotes the comparison of length distributions and bin/genome counts of different CheckV quality categories for Phables and PHAMB results. Unlike Phables, PHAMB has produced genomes with longer sequences as shown in Fig. 5a, c, e, and g, because PHAMB combines all the contigs in a bin to form one long sequence. As denoted in Fig. 5b, d, f, and h, Phables has recovered the greatest number of complete and high-quality genomes combined for all the datasets; 165 in Lake Water, 389 in Paddy Soil, 55 in Wastewater, and 205 in IBD, with 49.54% more genomes recovered than PHAMB on average.
Figure 5.
Comparison of CheckV quality. Genome length distribution (first column of figures) and abundance of genomes (second column of figures) belonging to different CheckV quality categories identified by Phables (denoted in orange) and PHAMB (Johansen et al. 2022) (denoted in blue) for the viral metagenomic datasets Lake Water, Paddy soil, Wastewater, and IBD.
Phables accurately recovers short sequences such as terminal repeats that are challenging for metagenomic binning tools to recover using the assembly graph and produces high-quality genomes. We observed that VAMB incorrectly binned the majority of the short sequences, which reduced the quality of PHAMB results. For example, the repeat sequences in the Case 2 phage components identified by Phables had a mean length of 600 bp in Lake Water, 649 bp in Paddy Soil, 511 bp in Wastewater, and 638 bp in IBD datasets (refer to Supplementary Table S6 in Section S9 for exact lengths of the sequences). All of these short sequences, except for those from the IBD dataset were found in a different bin than the bin of their connected longer sequence in the PHAMB results (8 out of 8 in Lake Water, 2 out of 2 in Paddy Soil, and 1 out of 1 in Wastewater). Phables recovered these short repeat sequences along with their connected longer sequences within a phage component using the connectivity information of the assembly graph.
Phables resulted in a high number of low-quality genomes as determined by CheckV in the Wastewater dataset compared to the other datasets (Fig. 5f). A possible reason for this is that these may be novel phages (as they contain conserved phage markers even though CheckV categorizes them as ‘low-quality’ or ‘not-determined’), and so they are not yet present in the databases that CheckV relies on.
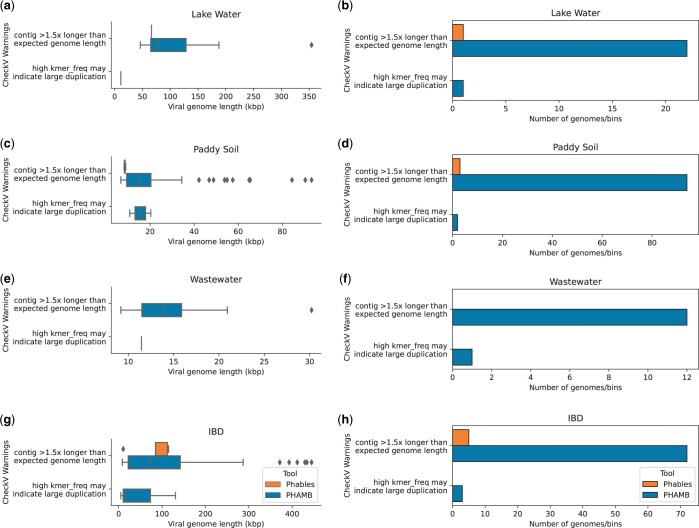
PHAMB does not carry out any resolution steps when combining the contigs of identified MAGs, which results in erroneous genome structures, high levels of contamination and duplications within genomes because of the presence of multiple variant genomes. Such duplications are identified from the warnings reported by CheckV. Hence, we evaluated the number and length distribution of sequences having CheckV warnings and the results are shown in Fig. 6. PHAMB has produced the highest number of genomes with CheckV warnings and produced some very long genomes ( 355–485 kb as shown in Fig. 6a and g), suggesting the combination of two or more variant genomes together in a bin. Only a few genomes produced from Phables (five or less) contain CheckV warnings (refer to Supplementary Table S8 in Section S10 for the exact number of genomes with warnings). These results show that Phables accurately recovers variant genomes including regions like terminal repeats from viral metagenomic samples and produces more high-quality/complete genomes compared to existing state-of-the-art tools.
Figure 6.
Comparison of CheckV warnings. Genome length distribution (first column of figures) and abundance of genomes (second column of figures) having the selected CheckV warnings from Phables (denoted in orange) and PHAMB (Johansen et al. 2022) (denoted in blue) results for the viral metagenomic datasets Lake Water, Paddy soil, Wastewater, and IBD.
4.3 Components resolved and comparison of resolved genomes
The number of phage components resolved by Phables under each case was recorded for all the datasets (refer to Supplementary Table S7 in Section S9 for the exact counts). Most of the resolved components belong to either Case 1 with a single circular unitig or Case 2 with the terminal repeat. When resolving Case 2 components, Phables provides information regarding terminal repeats such as the length of the repeat region, that will be overlooked by other tools. Except for the IBD dataset, Phables was able to resolve all the Case 3 phage components from the rest of the datasets. In a few cases, the Case 3 phage components could not be resolved because Phables was unable to find a vertex for these very complex phage components (refer to Supplementary Fig. S8 in Section S11 for examples of unresolved phage components).
Assemblers attempt to resolve longer paths in the assembly graph by connecting unitigs to form contigs (Bankevich et al. 2012, Kolmogorov et al. 2019). However, they are still unable to resolve complete genomes for complex datasets due to the mosaic nature of phage genomes and produce fragmented assemblies. Phables can be used to resolve these problematic contigs (or unitigs) and obtain high-quality genomes. Figure 7 denotes the comparison of CheckV quality of the genomes resolved in Phables and the unitigs and contigs included in the phage components of Cases 2 and 3. The most complete and high-quality sequences can be found as genomes (61 and 104 for Lake Water, 231 and 158 for Paddy Soil, 21 and 34 for Wastewater, and 62 and 143 for IBD, respectively). In contrast, most medium- and low-quality genomes can be found from contigs and unitigs. Hence, genomes resolved using Phables have higher quality and will be better candidates for downstream analysis than contigs.
Figure 7.
Comparison of Phables genomes, contigs and unitigs. Counts of resolved genomes of Phables, unitigs and contigs included in the phage components of Cases 2 and 3 with different CheckV qualities in the viral metagenomic datasets Lake Water, Paddy soil, Wastewater, and IBD.
We compared the similarity between the genomes recovered within each Case 3 phage component for the IBD dataset using pyani (Pritchard et al. 2016), pyGenomeViz (https://moshi4.github.io/pyGenomeViz/), and MUMmer (Marçais et al. 2018) (refer to Supplementary Section S12 for the detailed results). The ANI analysis revealed that the genomes resolved had over 95% ANI with some genomes having over 99% ANI and over 85% alignment coverage. Moreover, as shown in Supplementary Fig. S10, the mosaic genome structure can be clearly seen where some unitigs are shared between genomes and some genomes have unique unitigs. Depending on the size and location within a specific genome, these unitigs potentially correspond to functional modules. Hence, Phables can resolve highly similar variant genomes with mosaic genome structures that the assemblers and binning tools are unable to distinguish.
4.4 Phage components from other assembly methods
We extended our testing of Phables with co-assemblies obtained from other metagenome assemblers including metaSPAdes (Nurk et al. 2017) and MEGAHIT (Li et al. 2015) to show that the components with bubbles observed in the assembly graph are not an artefact of the assembly approach used in Hecatomb. Co-assembly is conducted by combining reads from multiple metagenomes and assembling them together, which increases the sequencing depth and provides sufficient coverage for low-abundance genomes to be recovered (Delgado and Andersson 2022). However, this becomes a computationally intensive approach as the number of samples increases, and hence we have limited the results to just the Lake Water dataset. The results are provided in Supplementary Section S13 and show that the phage component structures are still present in the assemblies and were correctly resolved by Phables, producing more high-quality genomes than PHAMB.
4.5 Implementation and resource usage
The source code of Phables was implemented using Python 3.10.12 and is available as a pipeline (including all the preprocessing steps) developed using Snaketool (Roach et al. 2022c). The commands used to run all the software can be found in Supplementary Section S8. The running times of Phables core methods and running times including the preprocessing steps were recorded for all the datasets and can be found in Supplementary Tables S10 and S11 in Section S14. The core methods of Phables can be run in under 2 min with less than 4 gigabytes of memory for all the datasets.
Phables uses a modified version of the MFD-ILP implementation from Dias et al. (2022) which supports inexact flow decomposition with subpath constraints. Gurobi version 10.0.2 (https://www.gurobi.com/) was used as the ILP solver. To reduce the complexity of the ILP solver, the maximum number of unitigs in a phage component to be solved was limited to 200.
5 Discussion
The majority of the existing viral identification tools rely on precomputed databases and models, only identify whether assembled sequences are of viral origin, and cannot produce complete and high-quality phage genomes. Viral binning tools have been able to overcome these shortcomings up to a certain extent by producing vMAGs, but they are fragmented and do not represent continuous genomes. Generally, the assembly process produces many short contigs where some represent regions which while important are challenging to resolve in phages, such as terminal repeat regions. These short contigs are discarded or binned incorrectly by binning tools, producing incomplete MAGs. Moreover, the mosaic genome structures of phage populations are a widely-documented phenomenon (Hatfull 2008, Belcaid et al. 2010, Lima-Mendez et al. 2011), and cannot be resolved by existing assemblers and binning tools. The resulting MAGs may contain multiple variant genomes assembled together and hence have high contamination.
Here, we introduce Phables, a new tool to resolve complete and high-quality phage genomes from viral metagenome assemblies using assembly graphs and flow decomposition techniques. We studied the assembly graphs constructed from different assembly approaches and different assembly software and consistently observed phage-like components with variation (‘phage components’). Phables models the assembly graphs of these components as a minimum flow decomposition problem using read coverage and paired-end mapping information and recovers the genomic paths of different variant genomes. Experimental results confirmed that Phables recovers complete and high-quality phage genomes with mosaic genome structures, including important regions such as terminal repeats. However, Phables can identify certain plasmids as phages [e.g. ‘phage-plasmids’ (Ravin et al. 1999, Pfeifer et al. 2021, 2022)] because they can encode proteins homologous to phage sequences (refer to Supplementary Section S15). Hence, if users run mixed-microbial communities through Phables, further downstream analysis is required to ensure that the predicted genomes do not include plasmids.
Decomposing assembly graphs has become a popular method to untangle genomes and recover variant genomes from assemblies and while we have successfully used it to obtain mostly circular phage genomes, further work needs to be conducted to handle metagenomes of mixed-microbial communities and recover the range of phage genomes. In the future, we intend to add support for long-read assemblies from dedicated metagenome assemblers that will enable Phables to enforce longer subpaths that will span across more sequences during the flow decomposition modelling. We also intend to extend the capabilities of Phables to recover linear phage genomes from complex components and explore the avenues for recovering high-quality eukaryotic viral genomes from metagenomes.
Supplementary Material
Acknowledgements
The authors thank Prof Christopher Quince for the insights and suggestions regarding the development of methods. This research was undertaken with the resources and services from Flinders University’s High-Performance Computing platform (https://doi.org/10.25957/FLINDERS.HPC.DEEPTHOUGHT) and the National Computational Infrastructure (NCI), Australia.
Contributor Information
Vijini Mallawaarachchi, Flinders Accelerator for Microbiome Exploration, College of Science and Engineering, Flinders University, Adelaide, South Australia 5042, Australia.
Michael J Roach, Flinders Accelerator for Microbiome Exploration, College of Science and Engineering, Flinders University, Adelaide, South Australia 5042, Australia.
Przemyslaw Decewicz, Flinders Accelerator for Microbiome Exploration, College of Science and Engineering, Flinders University, Adelaide, South Australia 5042, Australia; Department of Environmental Microbiology and Biotechnology, Institute of Microbiology, Faculty of Biology, University of Warsaw, Warsaw 02-096, Poland.
Bhavya Papudeshi, Flinders Accelerator for Microbiome Exploration, College of Science and Engineering, Flinders University, Adelaide, South Australia 5042, Australia.
Sarah K Giles, Flinders Accelerator for Microbiome Exploration, College of Science and Engineering, Flinders University, Adelaide, South Australia 5042, Australia.
Susanna R Grigson, Flinders Accelerator for Microbiome Exploration, College of Science and Engineering, Flinders University, Adelaide, South Australia 5042, Australia.
George Bouras, Adelaide Medical School, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, South Australia 5005, Australia; The Department of Surgery—Otolaryngology Head and Neck Surgery, Central Adelaide Local Health Network, Adelaide, South Australia 5000, Australia.
Ryan D Hesse, Flinders Accelerator for Microbiome Exploration, College of Science and Engineering, Flinders University, Adelaide, South Australia 5042, Australia.
Laura K Inglis, Flinders Accelerator for Microbiome Exploration, College of Science and Engineering, Flinders University, Adelaide, South Australia 5042, Australia.
Abbey L K Hutton, Flinders Accelerator for Microbiome Exploration, College of Science and Engineering, Flinders University, Adelaide, South Australia 5042, Australia.
Elizabeth A Dinsdale, Flinders Accelerator for Microbiome Exploration, College of Science and Engineering, Flinders University, Adelaide, South Australia 5042, Australia.
Robert A Edwards, Flinders Accelerator for Microbiome Exploration, College of Science and Engineering, Flinders University, Adelaide, South Australia 5042, Australia.
Author contributions
V.M. designed the methods, developed the software, performed all analyses and wrote the paper. M.J.R. preprocessed the datasets. M.J.R. and P.D. assisted with developing the pipeline, optimizing the steps and writing the paper. M.J.R., B.P., S.R.G., P.D., G.B., and L.K.I. tested the software and assisted with the data analysis. S.K.G., R.D.H., and A.L.K.H. curated data. All the authors reviewed the manuscript and provided detailed feedback. P.D., E.A.D., and R.A.E. conceived the project and wrote the paper with input from all authors.
Supplementary data
Supplementary data are available at Bioinformatics online.
Conflict of interest
None declared.
Funding
This work was supported by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases [RC2DK116713], the Australian Research Council [DP220102915], and the Polish National Agency for Academic Exchange (NAWA) Bekker Programme [BPN/BEK/2021/1/00416 to P.D.].
Data and code availability
All the real datasets containing raw sequencing data used for this work are publicly available from their respective studies. The Lake Water dataset was downloaded from NCBI with BioProject number PRJNA756429, the Paddy Soil dataset from BioProject number PRJNA756429, the Wastewater dataset from BioProject number PRJNA434744, and the whole genome sequencing runs of the IBD data from BioProject number PRJEB7772. The sequencing reads for the simPhage dataset, all the assembled data and results from all the tools are available on Zenodo at https://zenodo.org/record/8137197.
The code of Phables is freely available on GitHub under the MIT license and can be found at https://github.com/Vini2/Phables. All analyses in this study were performed using Phables v.1.1.0 with default parameters.
References
- Albertsen M, Hugenholtz P, Skarshewski A et al. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat Biotechnol 2013;31:533–8. [DOI] [PubMed] [Google Scholar]
- Amgarten D, Braga LPP, da Silva AM et al. MARVEL, a tool for prediction of bacteriophage sequences in metagenomic bins. Front Genet 2018;9:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auslander N, Gussow AB, Benler S et al. Seeker: alignment-free identification of bacteriophage genomes by deep learning. Nucleic Acids Res 2020;48:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baaijens JA, Stougie L, Schönhuth A. Strain-aware assembly of genomes from mixed samples using flow variation graphs. In: Schwartz R (ed.), Research in Computational Molecular Biology. Cham: Springer International Publishing, 2020, 221–2. ISBN 978-3-030-45257-5. [Google Scholar]
- Bankevich A, Nurk S, Antipov D et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012;19:455–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcaid M, Bergeron A, Poisson G. Mosaic graphs and comparative genomics in phage communities. J Comput Biol 2010;17:1315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart M, Salamon P, Andresen B et al. Genomic analysis of uncultured marine viral communities. Proc Natl Acad Sci U S A 2002;99:14250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens SR, Gilcrease EB. Determining DNA packaging strategy by analysis of the termini of the chromosomes in tailed-bacteriophage virions. Methods Mol Biol 2009;502:91–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-X, Anantharaman K, Shaiber A et al. Accurate and complete genomes from metagenomes. Genome Res 2020;30:315–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y-B, Nardone C, Hinkle DC. Bacteriophage T7 DNA packaging: III. A “hairpin” end formed on T7 concatemers may be an intermediate in the processing reaction. J Mol Biol 1990;216:939–48. [DOI] [PubMed] [Google Scholar]
- Comeau AM, Hatfull GF, Krisch HM et al. Exploring the prokaryotic virosphere. Res Microbiol 2008;159:306–13. Exploring the prokaryotic virosphere. [DOI] [PubMed] [Google Scholar]
- Cook R, Brown N, Redgwell T et al. INfrastructure for a PHAge REference Database: identification of large-scale biases in the current collection of cultured phage genomes. Phage (New Rochelle) 2021;2:214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado LF, Andersson AF. Evaluating metagenomic assembly approaches for biome-specific gene catalogues. Microbiome 2022;10:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias FH, Williams L, Mumey B et al. Efficient minimum flow decomposition via integer linear programming. J Comput Biol 2022;29:1252–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo E, Perales C. Viral quasispecies. PLoS Genet 2019;15:e1008271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont CL, Rusch DB, Yooseph S et al. Genomic insights to SAR86, an abundant and uncultivated marine bacterial lineage. ISME J 2012;6:1186–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy SR. Accelerated profile hmm searches. PLoS Comput Biol 2011;7:e1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RA, Rohwer F. Viral metagenomics. Nat Rev Microbiol 2005;3:504–10. [DOI] [PubMed] [Google Scholar]
- Freire B, Ladra S, Paramá JR et al. Inference of viral quasispecies with a paired de Bruijn graph. Bioinformatics 2021;37:473–81. [DOI] [PubMed] [Google Scholar]
- Freire B, Ladra S, Paramá JR et al. ViQUF: de novo viral quasispecies reconstruction using unitig-based flow networks. IEEE/ACM Trans Comput Biol Bioinform 2023;20:1550–62. [DOI] [PubMed] [Google Scholar]
- Gatter T, Stadler PF. Ryūtō: network-flow based transcriptome reconstruction. BMC Bioinformatics 2019;20:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlé H, Karlsson-Lindsjö O, Hayer J et al. Simulating illumina metagenomic data with InSilicoSeq. Bioinformatics 2019;35:521–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfull GF. Bacteriophage genomics. Curr Opin Microbiol 2008;11:447–53. Antimicrobials/Genomics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse RD, Roach M, Kerr EN et al. Phage diving: an exploration of the carcharhinid shark epidermal virome. Viruses 2022;14:1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz P, Goebel BM, Pace NR. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol 1998;180:4765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J, Plichta DR, Nissen JN et al. Genome binning of viral entities from bulk metagenomics data. Nat Commun 2022;13:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtz VI, Villarroel J, Lund O et al. MetaPhinder—identifying bacteriophage sequences in metagenomic data sets. PLoS One 2016;11:e0163111. 09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DD, Li F, Kirton E et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 2019;7:e7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kececioglu JD, Myers EW. Combinatorial algorithms for DNA sequence assembly. Algorithmica 1995;13:7–51. [Google Scholar]
- Keen EC. A century of phage research: bacteriophages and the shaping of modern biology. Bioessays 2015;37:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieft K, Zhou Z, Anantharaman K. VIBRANT: automated recovery, annotation and curation of microbial viruses, and evaluation of viral community function from genomic sequences. Microbiome 2020;8:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmogorov M, Yuan J, Lin Y et al. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol 2019;37:540–6. [DOI] [PubMed] [Google Scholar]
- Lamurias A, Sereika M, Albertsen M et al. Metagenomic binning with assembly graph embeddings. Bioinformatics 2022;38:4481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu C-M, Luo R et al. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015;31:1674–6. [DOI] [PubMed] [Google Scholar]
- Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 2018;34:3094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, et al. ; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009;25:2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Mendez G, Toussaint A, Leplae R. A modular view of the bacteriophage genomic space: identification of host and lifestyle marker modules. Res Microbiol 2011;162:737–46. [DOI] [PubMed] [Google Scholar]
- Luque A, Benler S, Lee DY et al. The missing tailed phages: prediction of small capsid candidates. Microorganisms 2020;8:1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łusiak-Szelachowska M, Weber-Dąbrowska B, Jończyk-Matysiak E et al. Bacteriophages in the gastrointestinal tract and their implications. Gut Pathog 2017;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallawaarachchi V, Lin Y. MetaCoAG: binning metagenomic contigs via composition, coverage and assembly graphs. In: Pe’er I (ed.), Research in Computational Molecular Biology. Cham: Springer International Publishing, 2022a, 70–85. ISBN 978-3-031-04749-7. [DOI] [PubMed] [Google Scholar]
- Mallawaarachchi V, Lin Y. Accurate binning of metagenomic contigs using composition, coverage, and assembly graphs. J Comput Biol 2022b;29:1357–76. PMID: 36367700. [DOI] [PubMed] [Google Scholar]
- Mallawaarachchi V, Wickramarachchi A, Lin Y. GraphBin: refined binning of metagenomic contigs using assembly graphs. Bioinformatics 2020a;36:3307–13. [DOI] [PubMed] [Google Scholar]
- Mallawaarachchi VG, Wickramarachchi AS, Lin Y. GraphBin2: Refined and Overlapped Binning of Metagenomic Contigs Using Assembly Graphs. In: Kingsford C and Pisanti N (eds), 20th International Workshop on Algorithms in Bioinformatics (WABI 2020), volume 172 of Leibniz International Proceedings in Informatics (LIPIcs), Dagstuhl, Germany: Schloss Dagstuhl–Leibniz-Zentrum für Informatik, 2020b, 8:1–8:21, ISBN 978-3-95977-161-0. [Google Scholar]
- Mallawaarachchi VG, Wickramarachchi AS, Lin Y. Improving metagenomic binning results with overlapped bins using assembly graphs. Algorithms Mol Biol 2021;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marçais G, Delcher AL, Phillippy AM et al. MUMmer4: a fast and versatile genome alignment system. PLoS Comput Biol 2018;14:e1005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair K, Bailey BA, Edwards RA. PHACTS, a computational approach to classifying the lifestyle of phages. Bioinformatics 2012;28:614–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill BD, Ward AT, Grose JH et al. Software-based analysis of bacteriophage genomes, physical ends, and packaging strategies. BMC Genomics 2016;17:679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F, Fritz A, Deng Z-L et al. Critical assessment of metagenome interpretation: the second round of challenges. Nat Methods 2022;19:429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheenko A, Saveliev V, Gurevich A. MetaQUAST: evaluation of metagenome assemblies. Bioinformatics 2016;32:1088–90. [DOI] [PubMed] [Google Scholar]
- Namiki T, Hachiya T, Tanaka H et al. MetaVelvet: an extension of velvet assembler to de novo metagenome assembly from short sequence reads. Nucleic Acids Res 2012;40:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayfach S, Camargo AP, Schulz F et al. CheckV assesses the quality and completeness of metagenome-assembled viral genomes. Nat Biotechnol 2021a;39:578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayfach S, Páez-Espino D, Call L et al. Metagenomic compendium of 189,680 DNA viruses from the human gut microbiome. Nat Microbiol 2021b;6:960–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen JN, Johansen J, Allesøe RL et al. Improved metagenome binning and assembly using deep variational autoencoders. Nat Biotechnol 2021;39:555–60. [DOI] [PubMed] [Google Scholar]
- Norman JM, Handley SA, Baldridge MT et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 2015;160:447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurk S, Meleshko D, Korobeynikov A et al. Metaspades: a new versatile metagenomic assembler. Genome Res 2017;27:824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Leung HCM, Yiu SM et al. Meta-IDBA: a de novo assembler for metagenomic data. Bioinformatics 2011;27:i94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevzner PA, Tang H, Tesler G. De novo repeat classification and fragment assembly. Genome Res 2004;14:1786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevzner PA, Tang H, Waterman MS. An Eulerian path approach to DNA fragment assembly. Proc Natl Acad Sci USA 2001;98:9748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer E, Bonnin RA, Rocha EPC. Phage-plasmids spread antibiotic resistance genes through infection and lysogenic conversion. mBio 2022;13:e01851–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer E, Moura de Sousa JA, Touchon M et al. Bacteria have numerous distinctive groups of phage–plasmids with conserved phage and variable plasmid gene repertoires. Nucleic Acids Res 2021;49:2655–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard L, Glover RH, Humphris S et al. Genomics and taxonomy in diagnostics for food security: soft-rotting enterobacterial plant pathogens. Anal Methods 2016;8:12–24. [Google Scholar]
- Quince C, Nurk S, Raguideau S et al. STRONG: metagenomics strain resolution on assembly graphs. Genome Biol 2021;22:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravin NV, Svarchevsky AN, Dehò G. The anti-immunity system of phage-plasmid N15: identification of the antirepressor gene and its control by a small processed RNA. Mol Microbiol 1999;34:980–94. [DOI] [PubMed] [Google Scholar]
- Rho M, Tang H, Ye Y. FragGeneScan: predicting genes in short and error-prone reads. Nucleic Acids Res 2010;38:e191. 08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach MJ, Beecroft SJ, Mihindukulasuriya KA et al. Hecatomb: an end-to-end research platform for viral metagenomics. bioRxiv, 10.1101/2022.05.15.492003, 2022a, preprint: not peer reviewed. [DOI]
- Roach MJ, McNair K, Michalczyk M et al. Philympics 2021: prophage predictions perplex programs. F1000Res 2022b;10:758. [Google Scholar]
- Roach MJ, Pierce-Ward NT, Suchecki R et al. Ten simple rules and a template for creating workflows-as-applications. PLoS Comput Biol 2022c;18:e1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Valera F, Martin-Cuadrado A-B, Rodriguez-Brito B et al. Explaining microbial population genomics through phage predation. Nat Rev Microbiol 2009;7:828–36. [DOI] [PubMed] [Google Scholar]
- Roux S, Emerson JB, Eloe-Fadrosh EA et al. Benchmarking viromics: an in silico evaluation of metagenome-enabled estimates of viral community composition and diversity. PeerJ 2017;5:e3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F, Air GM, Barrell BG et al. Nucleotide sequence of bacteriophage ϕX174 DNA. Nature 1977;265:687–95. [DOI] [PubMed] [Google Scholar]
- Schrijver A. Theory of Linear and Integer Programming. Chichester: John Wiley & Sons; 1998. [Google Scholar]
- Shao M, Kingsford C. Accurate assembly of transcripts through phase-preserving graph decomposition. Nat Biotechnol 2017;35:1167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P, Adams MJ, Benkő M et al. Virus taxonomy in the age of metagenomics. Nat Rev Microbiol 2017;15:161–8. [DOI] [PubMed] [Google Scholar]
- Steinegger M, Söding J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat Biotechnol 2017;35:1026–8. [DOI] [PubMed] [Google Scholar]
- Sutton TDS, Clooney AG, Ryan FJ et al. Choice of assembly software has a critical impact on virome characterisation. Microbiome 2019;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzian P, Olo Ndela E, Galiez C et al. PHROG: families of prokaryotic virus proteins clustered using remote homology. NAR Genom Bioinform 2021;3:lqab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomescu AI, Kuosmanen A, Rizzi R et al. A novel min-cost flow method for estimating transcript expression with RNA-Seq. BMC Bioinformatics 2013;14 Suppl 5:S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twort F. An investigation on the nature of ultra-microscopic viruses. Lancet 1915;186:1241–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatinlen B, Chauvet F, Chrétienne P et al. Simple bounds and greedy algorithms for decomposing a flow into a minimal set of paths. Eur J Oper Res 2008;185:1390–401. [Google Scholar]
- Wick RR, Schultz MB, Zobel J et al. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics 2015;31:3350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H, Mallawaarachchi V, Zhang Y et al. RepBin: constraint-based graph representation learning for metagenomic binning. AAAI 2022;36:4637–45. [Google Scholar]
- Zhang X, Studier F. Multiple roles of T7 RNA polymerase and T7 lysozyme during bacteriophage T7 infection. J Mol Biol 2004;340:707–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the real datasets containing raw sequencing data used for this work are publicly available from their respective studies. The Lake Water dataset was downloaded from NCBI with BioProject number PRJNA756429, the Paddy Soil dataset from BioProject number PRJNA756429, the Wastewater dataset from BioProject number PRJNA434744, and the whole genome sequencing runs of the IBD data from BioProject number PRJEB7772. The sequencing reads for the simPhage dataset, all the assembled data and results from all the tools are available on Zenodo at https://zenodo.org/record/8137197.
The code of Phables is freely available on GitHub under the MIT license and can be found at https://github.com/Vini2/Phables. All analyses in this study were performed using Phables v.1.1.0 with default parameters.