Abstract
Rationale
Obstructive sleep apnea is characterized by frequent reductions in ventilation, leading to oxygen desaturations and/or arousals.
Objectives
In this study, association of hypoxic burden with incident cardiovascular disease (CVD) was examined and compared with that of “ventilatory burden” and “arousal burden.” Finally, we assessed the extent to which the ventilatory burden, visceral obesity, and lung function explain variations in hypoxic burden.
Methods
Hypoxic, ventilatory, and arousal burdens were measured from baseline polysomnograms in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Osteoporotic Fractures in Men (MrOS) studies. Ventilatory burden was defined as event-specific area under ventilation signal (mean normalized, area under the mean), and arousal burden was defined as the normalized cumulative duration of all arousals. The adjusted hazard ratios for incident CVD and mortality were calculated. Exploratory analyses quantified contributions to hypoxic burden of ventilatory burden, baseline oxygen saturation as measured by pulse oximetry, visceral obesity, and spirometry parameters.
Measurements and Main Results
Hypoxic and ventilatory burdens were significantly associated with incident CVD (adjusted hazard ratio [95% confidence interval] per 1 SD increase in hypoxic burden: MESA, 1.45 [1.14, 1.84]; MrOS, 1.13 [1.02, 1.26]; ventilatory burden: MESA, 1.38 [1.11, 1.72]; MrOS, 1.12 [1.01, 1.25]), whereas arousal burden was not. Similar associations with mortality were also observed. Finally, 78% of variation in hypoxic burden was explained by ventilatory burden, whereas other factors explained only <2% of variation.
Conclusions
Hypoxic and ventilatory burden predicted CVD morbidity and mortality in two population-based studies. Hypoxic burden is minimally affected by measures of adiposity and captures the risk attributable to ventilatory burden of obstructive sleep apnea rather than a tendency to desaturate.
Keywords: obstructive sleep apnea, cardiovascular disease, hypoxic burden, arousals, ventilatory burden
At a Glance Commentary
Scientific Knowledge on the Subject
Obstructive sleep apnea (OSA) is associated with increased risk of cardiovascular morbidity and mortality. OSA is characterized by frequent reductions in ventilation during sleep, leading to oxygen desaturations and/or arousals from sleep. However, the apnea– hypopnea index and other conventional OSA severity metrics do not adequately characterize these downstream effects of airway obstruction, potentially contributing to the null findings in the randomized controlled trials of continuous positive airway pressure therapy.
What This Study Adds to the Field
In this study, physiological burdens of OSA (ventilatory burden, hypoxic burden, and arousal burden) were obtained from two community-based prospective cohort studies comprising more than 4,500 adults. The associations of these metrics with incident cardiovascular disease and all-cause mortality were assessed. Our findings show that both ventilatory and hypoxic burdens predict incident cardiovascular disease and mortality with similar hazard ratios, whereas arousal burden does not. In addition, we examined the extent to which the hypoxic burden captures the risk attributable to OSA in comparison with available measures of visceral obesity. Our findings show that the hypoxic burden is minimally affected by measures of adiposity, spirometry, and baseline oxygen saturation and largely captures the risk attributable to the ventilatory burden of OSA rather than a tendency to desaturate.
Obstructive sleep apnea (OSA) is a common condition associated with an increased risk of cardiovascular disease (CVD) and mortality (1, 2). It has generally been accepted that the conventional metrics of OSA severity, such as the apnea–hypopnea index (AHI), do not adequately characterize heterogeneity in OSA-related physiological stressors and subtypes (3–5). Furthermore, it has been speculated that inadequate characterization of the frequent reductions in ventilation and ensuing hypoxemia and/or arousals by these frequency-based metrics may have contributed to the null findings in the randomized controlled trials (RCTs) of continuous positive airway pressure (CPAP) therapy, potentially because of inclusion of low-risk individuals in these trials (6–11).
OSA is characterized by frequent reductions in ventilation during sleep, leading to oxygen desaturations and/or arousals from sleep. AHI and other conventional OSA severity metrics do not provide information on the depth (or intensity) and duration of ventilatory deficit, oxygen desaturation, and arousals. Recently, there has been growing interest to incorporate these important characteristics of respiratory events to identify individuals with OSA who are at increased risk of CVD outcomes. Particularly, indices that quantify total OSA-related hypoxemia (12–16) have shown promise to improve risk stratification. For example, hypoxic burden, defined as the OSA-related total area under the desaturation curve, was previously shown to predict cardiovascular and all-cause mortality (14) and incident heart failure (15) in observational cohorts, as well as incident CVD in a clinical cohort of patients with OSA (16). In addition, hypoxic burden was associated with increased blood pressure and chronic kidney disease in the MESA (Multi-Ethnic Study of Atherosclerosis) cohort (17, 18). In contrast, there have been limited studies to examine the associations of other key characteristics of OSA, including ventilatory burden and arousal burden, with CVD outcomes. For example, on one hand, a recent study demonstrated that arousal burden, a cumulative measure of the duration of arousals normalized by sleep time, was associated with CVD-related and all-cause mortality (19). On the other hand, the associations of a quantitative measure of total ventilatory burden with these outcomes remain unknown. In the present study, our primary objective was to investigate the associations of physiological burdens of sleep apnea (hypoxic burden, ventilatory burden or ventilatory deficit, and arousal burden) with incident CVD and all-cause mortality in two well-defined population studies across the United States.
In addition, it is plausible that individuals with similar amounts of upper airway obstruction, and therefore similar ventilatory burdens, may exhibit different hypoxic burdens. Therefore, understanding the drivers of hypoxic burden is also critical. Although lack of ventilation may directly reflect OSA-related pathophysiology, measures of baseline saturation, degree of adiposity, and impaired pulmonary function may increase hypoxic burden via indirect pathways (20). For example, elevated visceral obesity could lead to reduced lung volume and thereby smaller oxygen stores, resulting in faster desaturations that tend to increase the hypoxic burden for any given degree of ventilatory burden (20, 21). Therefore, in our secondary set of analyses, we sought to examine the extent to which ventilatory burden explains the variations in hypoxic burden in comparison with other anthropometric/demographic, polysomnographic, and spirometric factors, including age, sex, race, body mass index (BMI), abdominal obesity, wakefulness, oxygen saturation as measured by pulse oximetry (SpO2), and FVC.
Methods
Study Samples
MESA cohort
This study follows the current reporting for observational studies (22). MESA is a community-based, prospective cohort study designed to examine the risk factors associated with the development of subclinical CVD outcomes in middle-aged or older adults without clinically evident CVD at baseline (23). During the MESA Exam 5, 2,237 men and women underwent type 2 polysomnograms (PSGs) between 2010 and 2013. All participants completed a standardized questionnaire to assess their medical, sleep, and lifestyle habits at Exam 5 (24). Institutional review board approval was obtained from each study site, and each participant provided written informed consent.
Sleep study
A total of 2,035 PSGs are available on the National Sleep Research Resource website (www.sleepdata.org) (25). A 15-channel monitor (Compumedics Ltd.) was used to collect sleep study data, including finger pulse oximetry, which was sampled at 1 Hz; electroencephalography; electrooculography; chin electromyography; inductance bands; and a nasal cannula. A centralized sleep reading center (Brigham and Women’s Hospital, Boston, MA) scored the studies using standardized criteria (24). Respiratory events were identified if amplitude reduction on the nasal pressure exceeded 30% for hypopneas and 90% for apneas for at least 10 seconds. AHI calculation included all apneas plus hypopneas associated with ⩾3% desaturation or arousal. In addition, AHI4, defined as all apneas plus hypopneas associated with ⩾4% desaturation, was also calculated. In this study, participants with follow-up data from MESA Exam 5 (through 2018) were included. The exclusion criteria were incomplete data for core exposures and outcomes.
Clinical and anthropometric measures
Baseline characteristics, including demographic information, medical history, and smoking, were obtained using standardized questionnaires. Blood pressure was measured according to current guidelines (24), and American Diabetes Association criteria were used for diabetes (26). Height, weight, and BMI were measured in all subjects. In addition, indices of body fat distribution, such as waist and hip circumference, were measured with a steel measuring tape at the umbilicus and hip at the maximal circumference of the buttocks, respectively. Body surface area was calculated using the previously validated methods (27). Finally, body fat composition was measured in kilograms via full-body bioelectrical impedance analysis using the Valhalla BCS-2 Body Composition Scale and printer (28).
Spirometry
We used spirometry data from the MESA Lung Study (29), performed during MESA Sleep Study (N = 1,433 subjects). Prebronchodilator lung function was measured following the recommendation from the American Thoracic Society/European Respiratory Society guidelines (30). All spirometry data were reviewed in a central reading center using a race-neutral approach (31).
Osteoporotic Fractures in Men Study cohort
The MrOS (Osteoporotic Fractures in Men Study) (https://mrosonline.ucsf.edu/; May 9, 2023) was used to examine the external validity of the longitudinal associations in MESA. The parent MrOS is a community-based, prospective cohort study of 5,994 men aged ⩾65 years recruited from six centers across the United States (2000–2002). It was designed to describe the epidemiology of osteoporosis and fractures in older men (32, 33). From 2003 to 2005, 3,135 men from the MrOS cohort participated in the ancillary MrOS Sleep Study and underwent comprehensive sleep evaluations, including full home PSG, as described previously. Ethical approval was obtained from local institutional review boards, and all participants provided informed consent.
Sleep study
In-home sleep studies using one night of unattended PSG (Safiro, Compumedics, Inc.) were performed with recording of central electroencephalography, bilateral electrooculography, chin electromyography, an electrocardiogram, nasal pressure and thermistor (for airflow measurement), chest and thoracic inductance plethysmography, finger pulse oximetry, body position, and leg movements. Similar to the MESA study, apneas were identified if thermistry-based airflow was absent or nearly absent for at least 10 seconds. Hypopneas were identified when there was at least 30% reduction in airflow (by thermistry or nasal pressure) or thoracoabdominal movement for at least 10 seconds. In MrOS, SpO2 signals were captured by fingertip pulse oximeters (Nonin) sampled at 1 Hz.
Of the 3,135 participants who completed the sleep study, the PSGs of 2,896 were available on the National Sleep Research Resource website (25). After excluding 269 individuals with incomplete data, a total of 2,627 participants were included in this analysis.
OSA-related Physiological Burdens
Hypoxic burden
As described previously (14, 15, 34), hypoxic burden, calculated from the pulse oximeter signal, is a single metric that encapsulates the frequency, depth, and duration of respiratory event-related oxygen desaturations. It is defined as the total area under the desaturation curve of SpO2 associated with respiratory events (all events based on ⩾30% reduction in airflow, regardless of desaturation or arousal) per hour of sleep (14, 15, 34). All individual desaturations were aligned with respect to the end of respiratory events and ensemble averaged (defined as a method to capture the characteristics of the recurring signal associated with a respiratory event) to quantify a subject-specific search window that is used to measure the total hypoxic burden (percentage min/h). For the hypoxic burden, the desaturation duration is based on the subject-specific search window (see the online supplement for details on hypoxic burden calculation).
Ventilatory burden
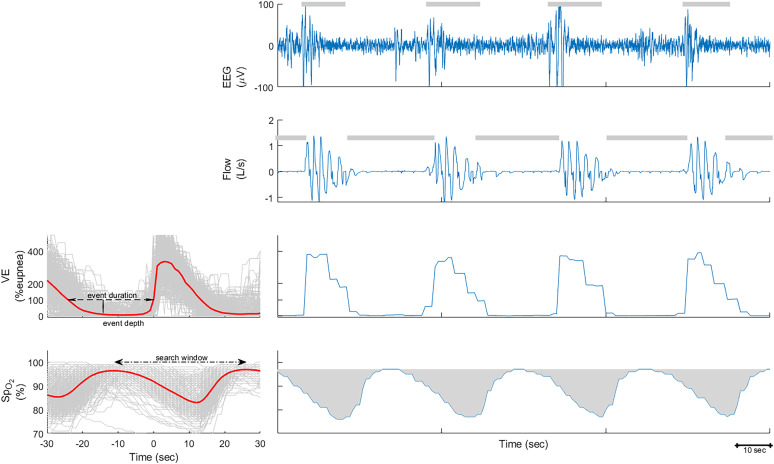
To quantify ventilatory burden, all breaths were automatically identified from the nasal pressure signal using previously validated methods (13, 35–38). The detected breaths were used to measure e, expressed as the percentage of eupneic ventilation (35). As previously described (35, 36), eupneic ventilation was defined as the mean ventilation during a 7-minute period. To quantify ventilatory burden, similar to hypoxic burden quantification, all ventilatory signals were aligned with respect to the end of events and then ensemble averaged (Figure 1). For each participant, the average ventilatory burden per event was defined as the multiplication of the average ventilation during the respiratory event (i.e., event depth [13, 36]) and average duration of respiratory events. The total ventilatory burden (percentage eupnea × min/h) for each participant was defined as the multiplication of respiratory event rate (events/h) and average ventilatory area per event (percentage eupnea × min/event). For the ventilatory burden, event duration was based on manual scoring of respiratory events (see the online supplement for details on ventilatory burden calculation).
Figure 1.
Example of hypoxic burden, ventilatory burden, and arousal burden calculation. Left: The overlaid e and oxygen saturation as measured by pulse oximetry (SpO2) signals associated with all respiratory events for one individual. These signals were synchronized at the termination of respiratory events (time 0) and averaged to calculate the average event depth and duration and the search window to calculate the hypoxic burden. Right: A 3-minute period, including EEG with scored arousals, airflow with scored respiratory events, and ventilation and SpO2 signals.
Arousal burden
Arousal burden was defined as the total duration of all arousals divided by the total sleep time and expressed as the percentage of sleep time as previously described (19).
Outcomes
MESA
Covariates and outcomes were extracted from the clinical examination, questionnaires, and adjudication of reported events. Incident CVD and all-cause mortality during the follow-up period between the MESA Sleep Study and the end of 2018 were extracted from the MESA datasets. As described previously, the MESA events committee adjudicated outcomes on the basis of regular follow-up calls in addition to review of medical records and death certificates (27, 39, 40). In MESA, the primary endpoint was incident hard CVD (MESA definition of hard CVD), defined as a composite of myocardial infarction, resuscitated cardiac arrest, stroke (not transient ischemic attack), and death resulting from coronary heart disease (CHD) or stroke. Secondary outcomes included incident all CVD (defined as incident hard CVD, definite angina, probable angina, other atherosclerotic death, or other CVD death), hard CHD (defined as myocardial infarction, resuscitated cardiac arrest, or CHD death), and all CHD (defined as hard CHD, definite angina, or probable angina). For incident outcomes, those with preexisting CVD or CHD at MESA Exam 5 were excluded.
MrOS
In MrOS, participants were contacted every 4 months after the sleep study. Reported deaths were confirmed with death certificates and medical records. Incident CVD events were based on MrOS sleep visit and adjudication of incident events by the central coordinating center until 2018 (32, 33). Incident CVD, the primary endpoint in MrOS, was defined as a composite of any type of fatal or nonfatal cardiovascular event, including CHD, cerebrovascular disease events, peripheral vascular disease, other CVD events, and any heart failure. For incident analysis, we excluded those with preexisting CVD, defined as self-reported diagnosis of CHD, cerebrovascular disease, peripheral vascular disease, and/or heart surgery. Cardiovascular mortality was based on the underlying cause of death as determined by a study physician adjudicator. Cause of death due to CVD was broadly categorized by International Classification of Diseases, Ninth Revision, codes (codes 396.9–442, 966.71, 785.51), cancer (codes 141.9–208.0), and other causes (reported codes not in previous categories).
Statistical Analysis
Descriptive statistics
Baseline characteristics and PSG parameters are summarized as mean (±SD) or median (interquartile range [IQR]) for numerical variables and as proportions for nominal variables.
Primary analysis: associations of OSA-related physiological burdens with longitudinal outcomes
Primary exposures were the “physiological burdens” described above. To determine the association between the exposures and incident outcomes, four Cox regression models with different levels of adjustment were constructed: Model 1 included age, sex, race/ethnicity, and BMI; Model 2 included covariates in Model 1 plus hypertension, diabetes mellitus, and smoking status; and Model 3 included variables in Model 2 and baseline SpO2 (wakefulness SpO2 in MESA and preevent SpO2 in MrOS). Model 4 included all the covariates in Model 3 plus the desaturation sensitivity, which was defined as the ratio of hypoxic burden/ventilatory burden. We included this variable to adjust for “tendency to desaturation.” In additional sensitivity analyses (MESA only), BMI was replaced with alternative measures of visceral obesity, including waist circumference, hip circumference, waist/hip ratio, waist/height ratio, and total body fat. Similar to our previous analyses, all exposures were logarithmically transformed and standardized (14, 15, 17). Hazard ratios (HRs) and 95% confidence intervals (CIs) were reported. The proportional hazard assumption (a test to measure if the effect is not proportional over time) was tested using the scaled Schoenfeld residuals against the transformed time.
Secondary analysis: hypoxic burden/arousal burden versus ventilatory burden (MESA)
To assess the extent to which ventilatory burden captures the variability in hypoxic burden (dependent variable) in unadjusted and adjusted analyses, multiple linear regression models were created, and the standardized coefficient for ventilatory burden and a measure of the overall fit of each model were quantified using the MESA dataset. Model 1 quantifies the unadjusted association between hypoxic burden and ventilatory burden. Model 2 also adjusted for age, sex, race/ethnicity, BMI, and body surface area. Model 3 adjusted Model 2 for wakefulness SpO2 and percentage in supine position. Model 4 was similar to Model 2 but replaced BMI for waist circumference, a measure of visceral obesity that may better explain the lung volume–related variations in hypoxic burden. Finally, Model 5 adjusted Model 3 for prebronchodilator raw FVC (31). Additional models in sensitivity analyses were similar to Model 3, although separately replacing BMI for the other measures of visceral obesity described above. The contribution of each variable to the overall variation was estimated using partial R2 using the partial eta-square method for linear models (41). In a sensitivity analysis, to further investigate whether the association between hypoxic burden and ventilatory burden varied by BMI categories, the sample was stratified into two groups (BMI <32 kg/m2 vs. BMI ⩾32 kg/m2), and the linear regression analysis described in Model 1 was repeated in each stratum. Finally, similar models were created to assess the extent to which ventilatory burden captures the variability in arousal burden.
Additional exploratory and sensitivity analyses (MESA)
Event-level association of hypoxic burden and ventilatory burden
Linear mixed-effect analyses examined the within-individual association between ventilatory burden and hypoxic burden after considering “subject” as a random effect (N = 374,399 events). Similar to between-subject analysis, we used Models 1–5 as described above (see the Secondary analysis section).
Associations of hypoxic burden versus total sleep time with oxygen saturation below 90% and CVD (MESA)
Both hypoxic burden and total sleep time with oxygen saturation below 90% (T90) have shown significant associations with incident CVD (16). Exploratory analyses tested the association of these two metrics with the primary CVD outcome, both when modeled separately and when placed into one model together. (Absence of collinearity was tested using variance inflation factor.)
Ventilatory/hypoxic burden and conventional OSA metrics (MESA)
The relationships between ventilatory/hypoxic burden and conventional OSA metrics, including AHI, T90, and arousal index, were examined using regression models, both in the overall sample and in the OSA subgroup (i.e., AHI4, ⩾5 events/hour). Finally, additional sensitivity analyses in MESA tested the association of ventilatory/hypoxic burden with longitudinal outcomes after 1) excluding participants who were receiving CPAP in the baseline sleep study (n = 72) and 2) restricting the sample to OSA (AHI4, ⩾5 events/h). All statistical analyses were performed using the R statistical software package (R Foundation for Statistical Computing; www.r-project.org), and a P value <0.05 was considered statistically significant.
Results
Descriptive Statistics
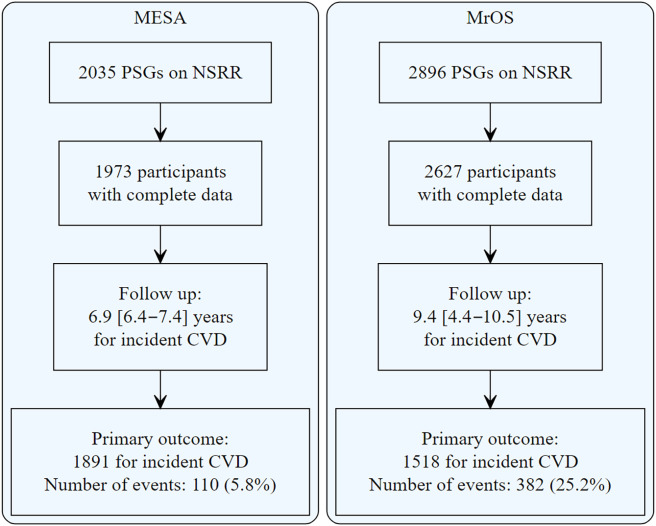
The study flowchart is shown in Figure 2. In MESA, 1,973 middle-aged or older men and women were included, and in MrOS, 2,627 older men were included. The baseline characteristics of participants in the MESA and MrOS studies are shown in Table 1. In MESA, the median [IQR] age was 67 [61–75] years, 59.5% of participants were aged ⩾65 years, and 46.4% were male. The hypoxic burden was 37.5 [19.2–73.0]% min/h, the median ventilatory burden was 318 [174–619]% eupnea × min/h, and the median arousal burden was 7.2 [5.1–10.2]% sleep time. A total of 1,433 participants had data on spirometry. In this subset, the median FVC was 3,001 [2,425–3,733] ml. In MrOS, the median age was 76 [72–80] years. The hypoxic burden was 43.8 [24.0–77.0]% min/h, the median ventilatory burden was 457 [241–839]% eupnea × min/h, and the median arousal burden was 7.5 [5.6–10.0]% sleep time.
Figure 2.
Study sample flowchart. CVD = cardiovascular disease; MESA = Multi-Ethnic Study of Atherosclerosis; MrOS = Osteoporotic Fractures in Men Study; NSRR = National Sleep Research Resource; PSG = polysomnogram.
Table 1.
Baseline Characteristics of Study Sample in Multi-Ethnic Study of Atherosclerosis and Osteoporotic Fractures in Men Study
| Variable | MESA (N = 1,973) | MrOS (N = 2,627) |
|---|---|---|
| n (%) or Median [IQR] | n (%) or Median [IQR] | |
| Demographics | ||
| Age, yr | 67 [61–75] | 76 [72–80] |
| Sex, male, n (%) | 917 (46.4%) | 2,627 (100%) |
| Race/ethnicity | ||
| Non-Hispanic White, n (%) | 720 (36.5%) | 2,383 (90.7%) |
| Chinese, n (%) | 237 (12.0%) | 76 (2.9%) |
| Black, n (%) | 543 (27.5%) | 92 (3.5%) |
| Hispanic/Latino, n (%) | 473 (23.9%) | 48 (1.8%) |
| Other, n (%) | — | 28 (1%) |
| BMI, kg/m2 | 27.9 [24.7–31.8] | 27 [25.0–29.0] |
| Hip circumference, cm | 103.5 [97.2–112.0] | 102 [97.0–107] |
| Body surface area, m2 | 1.8 [1.7–2.0] | — |
| Smoking status | ||
| Never smoker, n (%) | 933 (47.2%) | 1,054 (40.1%) |
| Former smoker, n (%) | 902 (45.7%) | 1,525 (58.0%) |
| Current smoker, n (%) | 138 (7.0%) | 48 (1.8%) |
| Comorbidities | ||
| Hypertension, n (%) | 1,118 (56.6%) | 1,303 (49.8%) |
| Diabetes, n (%) | 218 (11.0%) | 358 (13.3%) |
| COPD, n (%) | 33 (1.7%) | 134 (5.1%) |
| Sleep characteristics | ||
| TST, min | 369 [315–417] | 361 [317–401] |
| Time in REM, % | 18.4 [13.9–22.5] | 19.6 [14.7–23.8] |
| Time in supine position, % | 35.1 [12.9–64.2] | 30.2 [11.2–57.6] |
| AHI, events/h | 17.7 [9.4–32.7] | 13 [6.0–24.0] |
| AHI4, events/h | 8.3 [2.9–18.9] | 8.0 [3.0–12.6] |
| % Sleep time <90% SpO2 | 0.62 [0.05–3.37] | 1.0 [0.0–4.0] |
| Baseline SpO2, %* | 96 [95–97] | 95 [94.6–96.8] |
| Ventilatory burden, % eupnea* min/h | 318 [174–619] | 457 [241–839] |
| Hypoxic burden, % min/h | 37.5 [19.2–73.0] | 43.8 [24.0–77.0] |
| Arousal burden, % | 7.2 [5.1–10.2] | 7.5 [5.6–10.0] |
| ESS score | 5.0 [3.0–8.0] | 6.0 [3.0–8.0] |
Definition of abbreviations: AHI = apnea–hypopnea index based on 3% drop in SpO2 or arousal; AHI4 = apnea–hypopnea index based on 4% drop in SpO2; BMI = body mass index; COPD = chronic obstructive pulmonary disease; ESS = Epworth Sleepiness scale; MESA = Multi-Ethnic Study of Atherosclerosis; MrOS = Osteoporotic Fractures in Men Study; SpO2 = oxygen saturation as measured by pulse oximetry; TST = total sleep time.
Baseline SpO2 was defined as wakefulness SpO2 in MESA and preevent SpO2 in MrOS.
Primary Analysis: Associations of OSA-related Physiological Burdens with Longitudinal Outcomes
In MESA, during a median [IQR] follow-up of 6.9 [6.4–7.4] years, there were a total of 110 new hard CVD events. For secondary outcomes, there were 75 new CHD hard events, 145 CVD all events, and 91 CHD all events. During this period, a total of 196 all-cause deaths were recorded. The test of proportional hazard assumption for the primary outcome was not significant for individual variables as well as for the overall model (P = 0.84). In all models, higher hypoxic burden was associated with an increased risk of CVD (hard and all), all-cause mortality, and CHD (hard and all). In the fully adjusted model, every 1-SD increase in hypoxic burden was significantly associated with a 45% (95% CI, 14–84%) increased risk of incident hard CVD, 33% (95% CI, 8–63%) increased risk of incident all CVD events, 21% (95% CI, 2–44%) increased risk of all-cause mortality, 47% (95% CI, 11–96%) increased risk of incident hard CHD, and 42% (95% CI, 10–82%) increased risk of all CHD (Table 2). It is worth noting that replacing BMI with other measures of visceral obesity in the fully adjusted model did not affect the association of hypoxic burden with incident CVD and all-cause mortality (see Table E1 in the online supplement). Ventilatory burden was also significantly associated with these outcomes (hard CVD, 35% [95% CI, 9–67%]; all CVD, 32% [95% CI, 9–59%]; all-cause mortality, 24% [95% CI, 5–45%]; hard CHD, 41% [95% CI, 8–84%]; and all CHD, 38% [95% CI, 9–76%]; the numbers represent the increased risk per 1-SD increase in ventilatory burden) (Table 2). In contrast, there were no significant associations between arousal burden and primary or secondary outcomes in MESA (Table 2).
Table 2.
Associations of Hypoxic Burden, Ventilatory Burden and Arousal Burden with Incident Cardiovascular Disease (Hard, Primary Outcome), All-Cause Mortality, Incident Cardiovascular Disease, and Coronary Heart Disease in Multi-Ethnic Study of Atherosclerosis Cohort
| Model 1 (HR [95% CI]) | Model 2 (HR [95% CI]) | Model 3 (HR [95% CI]) | Model 4 (HR [95% CI]) | |
|---|---|---|---|---|
| Hypoxic burden | ||||
| Cardiovascular disease (hard) (n = 1,891) | 1.40 [1.12, 1.76] * | 1.40 [1.11, 1.75] * | 1.43 [1.13, 1.80] * | 1.45 [1.14, 1.84] * |
| Cardiovascular disease (all) (n = 1,848) | 1.29 [1.06, 1.57]† | 1.29 [1.06, 1.57]† | 1.32 [1.08, 1.61] * | 1.33 [1.08, 1.63] * |
| All-cause mortality (n = 1,973) | 1.25 [1.06, 1.47] * | 1.25 [1.06, 1.47] * | 1.24 [1.05, 1.47]† | 1.21 [1.02, 1.44]† |
| Coronary heart disease (hard) (n = 1,925) | 1.46 [1.10, 1.92] * | 1.43 [1.09, 1.87]† | 1.46 [1.11, 1.92] * | 1.47 [1.11, 1.96] * |
| Coronary heart disease (all) (n = 1,880) | 1.39 [1.09, 1.79] * | 1.38 [1.08, 1.76]† | 1.42 [1.10, 1.82] * | 1.42 [1.10, 1.82] * |
| Ventilatory burden | ||||
| Cardiovascular disease (hard) (n = 1,891) | 1.35 [1.08, 1.67] * | 1.34 [1.08, 1.66] * | 1.35 [1.09, 1.67] * | 1.38 [1.11, 1.72] * |
| Cardiovascular disease (all) (n = 1,848) | 1.28 [1.06, 1.54]† | 1.28 [1.06, 1.53]† | 1.28 [1.07, 1.55] * | 1.32 [1.09, 1.59] * |
| All-cause mortality (n = 1,973) | 1.19 [1.02, 1.40]† | 1.20 [1.02, 1.40]† | 1.19 [1.02, 1.40]† | 1.24 [1.05, 1.45]† |
| Coronary heart disease (hard) (n = 1,925) | 1.39 [1.07, 1.81]† | 1.36 [1.05, 1.76]† | 1.37 [ 1.06, 1.77]† | 1.41 [ 1.08, 1.84]† |
| Coronary heart disease (all) (n = 1,880) | 1.38 [1.08, 1.76]† | 1.36 [1.07, 1.73]† | 1.37 [1.08, 1.73] * | 1.38 [1.09, 1.76] * |
| Arousal burden | ||||
| Cardiovascular disease (hard) (n = 1,891) | 1.15 [0.95, 1.39] | 1.14 [0.94, 1.38] | 1.15 [0.95, 1.39] | — |
| Cardiovascular disease (all) (n = 1,848) | 1.11 [0.94, 1.31] | 1.10 [0.93,1.30] | 1.11 [0.94, 1.31] | — |
| All-cause mortality (n = 1,973) | 1.11 [0.96, 1.27] | 1.08 [0.94, 1.25] | 1.08 [0.94, 1.24] | — |
| Coronary heart disease (hard) (n = 1,925) | 1.19 [0.94, 1.50] | 1.18 [0.94,1.48] | 1.19 [0.94, 1.49] | — |
| Coronary heart disease (all) | 1.18 [0.96, 1.47] | 1.17 [0.95, 1.45] | 1.18 [0.96, 1.47] | — |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio per 1-SD increase in hypoxic burden, ventilatory burden, and arousal burden.
Statistical significance is highlighted in bold.
Model 1: age + sex + race + body mass index.
Model 2: Model 1 + hypertension + diabetes + smoking status.
Model 3: Model 2 + wakefulness oxygen saturation as measured by pulse oximetry.
Model 4: Model 3 + desaturation sensitivity (defined as hypoxic burden/ventilatory burden).
P < 0.01.
P < 0.05.
In MrOS, over a median [IQR] follow-up of 9.4 [4.4–10.5] years for incident CVD and 12.0 [7.7–12.6] years for mortality, there were a total of 382 new CVD events. Hypoxic burden was associated with an increased risk of incident CVD (13% [95% CI, 2–26%]) (Table 3), CVD death (22% [95% CI, 10–35%]) (Table 3), and all-cause mortality (6% [95% CI, 0–12%]). Ventilatory burden was also associated with incident CVD (12% [95% CI, 1–25%]) (Table 3), and CVD mortality (21% [95% CI, 9–35%]) but not all-cause mortality (5% [95% CI, −1%, 12%]. In MrOS, arousal burden was associated only with CVD mortality (11% [95% CI, 0–23%]) (Table 3).
Table 3.
Associations of Hypoxic Burden, Ventilatory Burden, and Arousal Burden with Incident Cardiovascular Disease, Cardiovascular Mortality, and All-Cause Mortality in Osteoporotic Fractures in Men Study Cohort
| Model 1 (HR [95% CI]) | Model 2 (HR [95% CI]) | Model 3 (HR [95% CI]) | Model 4 (HR [95% CI]) | |
|---|---|---|---|---|
| Hypoxic burden | ||||
| Incident CVD (n = 1,518) | 1.12 [1.01, 1.25] * | 1.12 [1.01, 1.24] * | 1.12 [1.00, 1.24] * | 1.13 [1.02, 1.26] * |
| Cardiovascular death (n = 2,627) | 1.24 [1.12, 1.38] † | 1.23 [1.11, 1.37] † | 1.24 [1.11, 1.37] † | 1.22 [1.10, 1.35] † |
| All-cause mortality, (n = 2,627) | 1.06 [1.00, 1.13] * | 1.06 [1.00, 1.13] * | 1.06 [1.01, 1.13] * | 1.06 [1.00, 1.12]‡ |
| Ventilatory burden | ||||
| Incident CVD (n = 1,518) | 1.12 [1.01, 1.24] * | 1.12 [1.01, 1.25] * | 1.13 [1.01, 1.25] * | 1.12 [1.01, 1.25] * |
| Cardiovascular death (n = 2,627) | 1.16 [1.05, 1.28] § | 1.16 [1.05, 1.28] § | 1.16 [1.05, 1.28] § | 1.21 [1.09, 1.35] † |
| All-cause mortality (n = 2,627) | 1.03 [0.98, 1.10] | 1.04 [0.98, 1.10] | 1.04 [0.98, 1.10] | 1.05 [0.99, 1.12]‡ |
| Arousal burden | ||||
| Incident CVD (n = 1,483) | 1.07 [0.96, 1.18] | 1.06 [0.96, 1.18] | 1.06 [0.96, 1.18] | — |
| Cardiovascular death (n =2,564) | 1.12 [1.01, 1.24] * | 1.11 [1.00, 1.23] * | 1.11 [1.00, 1.23] * | — |
| All-cause mortality (n = 2,564) | 0.99 [0.94, 1.05] | 0.98 [0.93,1.04] | 0.98 [0.93, 1.04] | — |
Definition of abbreviations: CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio per 1-SD increase in hypoxic burden, ventilatory burden, and arousal burden.
Statistical significance is highlighted in bold.
Model 1: age + race + body mass index.
Model 2: Model 1 + hypertension + diabetes + smoking status + chronic obstructive pulmonary disease.
Model 3: Model 2 + preevent oxygen saturation as measured by pulse oximetry.
Model 4: Model 3 + desaturation sensitivity (defined as hypoxic burden/ventilatory burden).
P < 0.05.
P < 0.001.
P < 0.1.
P < 0.01.
Secondary Analysis: Physiological and Other Correlates of Hypoxic Burden (MESA)
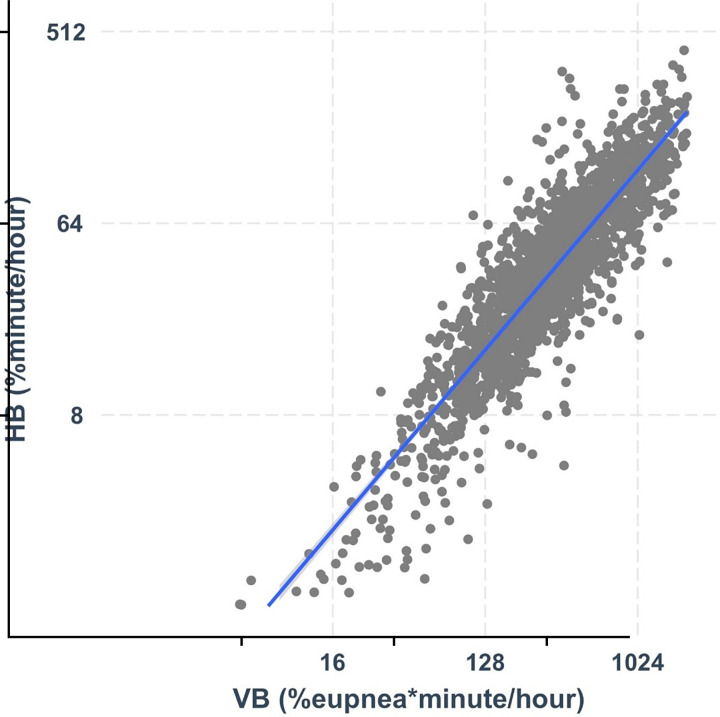
Ventilatory burden was strongly associated with hypoxic burden, such that 78% of the variability in hypoxic burden was explained by ventilatory burden (R2 = 0.78) (Table 4, Figure 3). Adjusting for age, race, sex, BMI, and body surface area improved the model by only 2% (Table 4), whereas BMI and Black race were the additional significant predictors in this model. The addition of wakefulness SpO2 and percentage in supine position improved the model by only 2% (Table 4, Model 3). Further adjustments for FVC or replacing BMI with measures of visceral obesity did not meaningfully change these observations (Table 4, Models 4 and 5). When we examined the contribution of each variable using the partial R2 method, in Model 4, 74% of the variation in hypoxic burden was explained by the ventilatory burden; in contrast, only 6%, 2%, and 2% of the variations were explained by BMI, wakefulness SpO2, and race, respectively (Table 4). Similar to these between-subject findings, linear mixed-effect analyses demonstrated a strong association between hypoxic burden and ventilatory burden within individuals and across respiratory events in all models (Table E2). Finally, the results of the stratified analysis by BMI (BMI <32 kg/m2 vs. BMI ⩾32 kg/m2) revealed a similar association between the ventilatory burden and the hypoxic burden (R2 = 0.79 and 0.77 for BMI <32 kg/m2 and BMI ⩾32 kg/m2, respectively). In contrast, the ventilatory burden explained only 26% of the variability in the arousal burden in all models (Table E3).
Table 4.
Linear Model between Sleep Apnea–Specific Hypoxic Burden across Ventilatory Burden, Age, Sex, Race, Body Mass Index, Supine Position, FVC, and Waist Circumference in Multi-Ethnic Study of Atherosclerosis Cohort
| Model 1 (n = 1,973) |
R 2 | Model 2 (n = 1,973) |
R 2 | Model 3 (n = 1,973) |
R 2 | Model 4 (n = 1,851) |
R 2 | Model 5 (n = 1,433) |
R 2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ventilatory burden (per 1 SD) | 0.88 (0.86, 0.90) * | 0.78 | 0.85 (0.83, 0.87) * | 0.74 | 0.84 (0.82, 0.86) * | 0.73 | 0.85 (0.83, 0.87) * | 0.74 | 0.84 (0.82, 0.87) * | 0.73 |
| Age (per 1 SD) | 0.02 (−0.00, 0.04) | 0 | 0.01 (−0.01, 0.03) | 0 | −0.00 (−0.02, 0.02) | 0 | 0.01 (−0.02, 0.03) | 0 | ||
| Sex (male vs. female) | 0.02 (−0.02, 0.06) | 0 | 0.01 (−0.04, 0.05) | 0 | −0.05 (−0.09, −0.00) † | 0 | 0.06 (−0.01, 0.13) | 0 | ||
| Race | 0.01 | 0.01 | 0 | 0.02 | ||||||
| Black vs. White | −0.11 (−0.16, −0.06) * | −0.10 (−0.15, −0.04) * | −0.08 (−0.13, −0.03) ‡ | −0.17 (−0.24, −0.10) * | ||||||
| Chinese vs. White | −0.02 (−0.09, 0.05) | −0.02 (−0.09, 0.05) | −0.02 (−0.09, 0.05) | −0.09 (−0.17, −0.01) † | ||||||
| Hispanic vs. White | −0.02 (−0.07, 0.04) | −0.00 (−0.05, 0.05) | 0.01 (−0.04, 0.07) | −0.07 (−0.14, −0.01) † | ||||||
| BMI (per 1 SD) | 0.14 (0.12, 0.16) * | 0.07 | 0.13 (0.11, 0.15) * | 0.06 | 0.13 (0.11, 0.16) * | 0.06 | ||||
| Supine position (per 1 SD) | 0.03 (0.01, 0.05) ‡ | 0 | 0.03 (0.01, 0.05) ‡ | 0 | 0.03 (0.01, 0.05) † | 0 | ||||
| Wake SpO2 (per 1 SD) | −0.06 (−0.08, −0.04) * | 0.01 | −0.06 (−0.08, −0.04) * | 0.02 | −0.06 (−0.08, −0.04) * | 0.02 | ||||
| Waist circumference (per 1 SD) | 0.12 (0.09, 0.14) * | 0.05 | ||||||||
| FVC (per 1 SD) | −0.05 (−0.09, −0.01) † | 0 | ||||||||
| Model R2 | 0.78 | 0.80 | 0.80 | 0.80 | 0.80 |
Definition of abbreviations: BMI = body mass index; SpO2 = oxygen saturation as measured by pulse oximetry.
Hypoxic burden was standardized (per 1 SD).
Statistical significance is highlighted in bold.
P < 0.001.
P < 0.05.
P < 0.01.
Figure 3.
Hypoxic burden (y-axis) is strongly associated with ventilatory burden (x-axis). The R2 value for this association was 0.79. HB = hypoxic burden; VB = ventilatory burden.
Additional Exploratory and Sensitivity Analyses (MESA)
In the exploratory analysis, although the association of T90% with the primary outcome was significant in the fully adjusted model (hard CVD: HR, 1.31 [95% CI, 1.04–1.65]), it became nonsignificant after further adjusting for hypoxic burden (HR, 1.09 [95% CI, 0.81–1.48]; variance inflation factor, <1.61). The association of hypoxic burden with the primary outcome was significant, and the corresponding HR remained similar before and after adding T90% to the model (HR, 1.49 [95% CI, 1.15–1.95] vs. 1.40 [95% CI, 1.01–1.96], respectively).
It is worth noting that the correlation of ventilatory/hypoxic burden and conventional metrics of OSA severity were weaker than those of ventilatory burden and hypoxic burden and further decreased in individuals with OSA (Figures E1 and E2). Last, the association of ventilatory burden/hypoxic burden with incident outcomes did not change meaningfully after excluding those receiving CPAP at the baseline sleep study visit (Table E4) or restricting the sample to the OSA subgroup (Table E5).
Discussion
In this study, the main goal was to understand whether hypoxic burden is likely operating as a specific measure of OSA-related stress. We found that the hypoxic burden predicted incident CVD and all-cause mortality in two diverse community-based samples (Table 2). Moreover, OSA-related ventilatory burden also predicted incident CVD, CVD-related mortality, and all-cause mortality but was slightly weaker than the hypoxic burden (Tables 2 and 3). Adjusting for desaturation sensitivity (“propensity to desaturate”) or an alternative measure of adiposity did not alter these findings. Finally, our data revealed that the ventilatory burden (i.e., total OSA-related reduction in airflow) explained about 80% of variability in hypoxic burden; other factors, including obesity measures (e.g., BMI, waist/height ratio, total body fat), baseline SpO2, and FVC, explained <2% of the observed variability in the hypoxic burden. This study provides additional population-based evidence that hypoxic burden is associated not only with mortality and incident heart failure but also with incident CVD and CHD (novel finding) in a large, well-characterized, and diverse community-based cohort of middle-aged or older adults. For the first time, to our knowledge, we have demonstrated that hypoxic burden is negligibly affected by available measures of adiposity, lung function, and baseline oxygen saturation and largely captures the risk attributable to ventilatory burden of OSA rather than tendency to desaturate. These findings have important implications for both clinical practice and the future design of clinical trials in sleep apnea.
Hypoxic Burden and Incident CVD/Mortality
There has been a growing interest in better identifying high-risk individuals with OSA, mainly because of lack of RCT-level evidence regarding the efficacy of OSA treatment in preventing adverse cardiovascular events (6, 11, 42–45). This issue was recently highlighted in the Agency for Healthcare Research and Quality report (46). One potential reason for the null findings in CPAP RCTs is the use of AHI, a frequency-based metric, to select and enroll participants in these trials. For any given AHI, there are substantial variations in the degree of ventilatory deficit, hypoxic burden, and arousal characteristics (7, 45, 47). The inability to capture these interindividual OSA-related variations makes the AHI potentially a less informative metric of OSA severity. For example, studies from our group and others (12, 14, 19, 48, 49) have demonstrated that hypoxic burden, independent of AHI and other metrics of OSA, was cross-sectionally associated with increased blood pressure (17) and chronic kidney disease (18) and was longitudinally associated with CVD mortality and incident heart failure (in men) (15) in community cohorts and with incident CVD in a clinical cohort of individuals with OSA (16). The findings of the present study expand these observations by identifying hypoxic burden as a predictor of incident CVD and all-cause mortality in two community cohorts of individuals with different degrees of OSA severity.
Ventilatory Burden/Arousal Burden and Incident CVD/Mortality
There are limited data on the association of ventilatory burden and incident outcomes in population studies. For example, Rapoport and colleagues reported the use of breath-to-breath flow amplitude to quantify the severity of OSA beyond the AHI (50). To quantify the total ventilatory burden, one could measure the total area under the ventilatory curve from a eupneic (normal) value. As a reasonable surrogate potentially less affected by the breath-to-breath artifacts/noise/variations in airflow, one could measure the mean reduction in ventilation (across all events) and the mean duration and the frequency of all events. The multiplication of these three dimensions results in an estimated total ventilatory decrement area related to apneas/hypopneas. The research from our group has demonstrated that the mean event depth as estimated this way was strongly associated with pharyngeal collapsibility (i.e., critical closing pressure) (36) and predicted response to oral appliance therapy (13). In this study, we have shown that a relatively simple measure of total ventilatory burden was significantly associated with incident CVD, CHD, and all-cause mortality in two cohorts. The observed associations of ventilatory burden with outcomes were slightly weaker than those of hypoxic burden. Nonetheless, the strong association between ventilatory burden and hypoxic burden (Table 4, Figure 3) suggests that the link between OSA and CVD (i.e., the association of ventilatory burden and CVD) is through OSA-related hypoxemia and that risk is better captured by hypoxic burden (Tables 2–4 and Figure 3) and not a tendency to desaturation. In contrast, in MESA, the arousal burden (19) was not associated with any of these outcomes, and the HRs were substantially lower than those for hypoxic burden or ventilatory burden. These findings were similar in the MrOS cohort (Table 3). These results are consistent with previous findings showing less conclusive associations with incident CVD in general and all-cause mortality in men (19). In the present study, we were not able to conduct sex-specific longitudinal analyses because of limited statistical power for subgroups.
Hypoxic Burden versus Ventilatory Burden
Theoretically, non–OSA-related factors, such as baseline SpO2, lung volume, and metabolic rate, affect the rate of desaturation and thus the total hypoxic burden. Although more studies are needed to determine the factors contributing to increased hypoxic burden in OSA, we attempted to quantify the degree of associations between the hypoxic burden and the ventilatory burden (OSA-related factor) and compare it with that of baseline wakefulness SpO2, abdominal obesity, and lung function (by spirometry). A substantial amount of the variance (∼80%) in hypoxic burden was explained by the OSA-related ventilatory burden. Other potentially non–OSA-related factors contributed to only an additional 1% of variability in hypoxic burden (Table 4). It is possible that in certain clinical settings (e.g., obesity hypoventilation), several of these factors will contribute more to hypoxic burden than what is observed in a general community cohort (51). However, the associations of ventilatory burden and hypoxic burden by categories of BMI (BMI <32 kg/m2 vs. BMI ⩾32 kg/m2) remained similar. Nonetheless, future experimental physiological studies in clinical samples are needed to confirm these observations. Furthermore, in addition to between-subject observations (Table 4), a unique relationship between respiratory event-level ventilatory burden and hypoxic burden was observed (Table E2). Indeed, a larger event-related reduction in ventilation was significantly associated with a larger desaturation area per event, contributing to the observed between-subject association of total ventilatory burden and hypoxic burden.
Hypoxic Burden versus Ventilatory Burden versus Arousal Burden as an OSA Severity Metric beyond AHI
Future prospective studies are needed to identify the best physiological metric(s) of OSA severity. That said, available data from large and well-characterized community and clinical cohorts point to the hypoxic burden as a measure of OSA severity that responds to CPAP (hypoxic burden is zero if there are no apneas or hypopneas), is easy to calculate, is highly correlated with ventilatory burden, and consistently and more precisely predicts longitudinal outcomes. In the present study, we further addressed the association of other aspects of OSA, including ventilatory burden and arousal burden, with CVD outcomes. Of these three measures of physiological burden, hypoxic burden was the strongest and most consistent predictor of incident CVD and mortality, and arousal burden was the weakest. The data from this study suggest that OSA-related ventilatory deficit is linked with CVD outcomes via hypoxic burden and that the nocturnal hypoxia is likely the culprit leading to adverse cardiovascular outcomes. A potential explanation for the weaker association of ventilatory burden with outcomes may be related to the measurement noise related to an accurate quantification of the airflow. Indeed, past studies have discussed challenges with airflow measurements in clinical and home-based settings, mainly because of less standardized and variable ways of measuring airflow (e.g., nasal cannula, thermistor, inductance bands), high prevalence of mouth breathing and less accurate quantification (52, 53) of airflow when it is measured via nasal devices, and the need to calibrate these devices. In contrast, SpO2 measurement is more standardized, is easier, and requires less monitoring than airflow quantification. Finally, although arousals have long been postulated to reflect sympathetic nervous system–related activity and OSA-related sleep disturbances (54), recent data suggest that there may be adaptation over time that influences arousal number, and arousal identification is limited by challenges in consistently identifying discreet changes in EEG activity over background, leading to only modest interscorer agreement (55, 56).
Hypoxic Burden versus T90 and CVD
Similar to hypoxic burden, T90 has been shown to predict increased risk of incident CVD (57, 58). Consistent with our previous findings (14), exploratory analyses in this study showed that after adjusting for hypoxic burden, T90 was not a significant predictor of incident CVD, whereas hypoxic burden was significantly associated with CVD after adjusting for T90. Furthermore, in contrast to hypoxic burden, T90 may be impacted by non–OSA-related conditions such as lung or heart disease (51, 59, 60). Also, T90 may not be an accurate measure of “intermittent” hypoxemia, because it depends on the baseline value of oxygen saturation (51). Although CPAP may decrease a component of T90 that is due to upper airway obstruction, it cannot correct the sustained hypoxemia attributed to an underlying pulmonary and/or cardiac condition (61). As shown in Figures E1 and E2, there are individuals with large T90 but low hypoxic burden. On one hand, these are the participants with low baseline oxygen saturation (of other causes unrelated to OSA). On the other hand, there are many with low T90% but high hypoxic burden. We believe that these individuals will likely benefit from OSA treatment.
Strength and Limitations
This study has several strengths, including the following: 1) good representation of different sex, racial, and ethnic groups in the MESA study; 2) automated and validated methods used to generate the desired metrics; 3) external validation of the associations of the hypoxic and ventilatory burdens with CVD morbidity and mortality in the MrOS cohort; and 4) multiple covariate adjustments and consistency across different outcomes that suggest likely generalizability of the results concerning hypoxic burden. However, the study also had several limitations, including the underrepresentation of younger individuals and individuals with high degrees of comorbidities. In addition, we largely tested the associations with CVD outcomes; future studies are needed to expand these findings to neurocognitive and metabolic outcomes. Although the sample size was comparable to sizes in other OSA-related population studies, larger studies with longer follow-up are needed to confirm our findings. Moreover, well-instrumented physiological studies are needed to identify the determinants of hypoxic burden. Another limitation of this study is the lack of data on CPAP use after the baseline sleep study; however, on the basis of data from the Sleep Heart Health Study, it is expected that the number of individuals who seek treatment during follow-up will be small (26). Furthermore, excluding individuals who were receiving CPAP at the baseline sleep study (n = 72) did not affect our findings (Table E4). Finally, future RCTs in younger individuals are needed to better gauge which aspects of sleep apnea can be used to better identify high-risk patients who would benefit the most from therapies such as CPAP.
Conclusions
In this large, well-characterized, and diverse study, hypoxic burden consistently predicted incident CVD and all-cause mortality. Although ventilatory burden predicted these outcomes, hypoxic burden was the strongest predictor of these, and exploratory analyses were consistent with it as a measure of ventilatory burden rather than other factors, such as different measures of visceral fat, baseline SpO2, or lung function. Future larger and longer studies are needed to identify high-risk individuals with OSA who benefit from treatment.
Acknowledgments
Acknowledgment
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org.
Footnotes
Supported by the National Institutes of Health and the American Academy of Sleep Medicine, the Chest Foundation, and the ResMed Foundation (G.L.). S.S. received grant support from the American Heart Association and the National Institutes of Health. D.V. received grant support from the American Heart Association and the American Academy of Sleep Medicine. L.G. received grant support from the American Heart Association. S.R. received partial funding from National Institutes of Health grant R35135818. A.A. received funding from the National Institutes of Health (R01HL153874, R01 HL158765, R21 HL161766), the American Heart Association (19CDA34660137), and the American Academy of Sleep Medicine (188-SR-17, SR-2217). The National Sleep Research Resource was supported by the National Heart, Lung, and Blood Institute (R24 HL114473, 75N92019R002). Data from the Multi-Ethnic Study of Atherosclerosis (MESA) was obtained through support by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. Funding support for the MESA Sleep Study was provided by National Institutes of Health grant HL56984 and National Institute on Aging grant R01 AG070867. This publication was developed under Science to Achieve Results research assistance agreements RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage) awarded by the Environmental Protection Agency (EPA). This publication has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors, and the EPA does not endorse any products or commercial services mentioned in this publication. The Osteoporotic Fractures in Men Study (MrOS) is supported by National Institutes of Health funding. The following institutes provided support: the National Institute on Aging, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Center for Advancing Translational Sciences, and the NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, R01 AG066671, and UL1 TR002369. The National Heart, Lung, and Blood Institute provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839 (https://mrosonline.ucsf.edu). The National Heart, Lung, and Blood Institute provided funding for the ancillary MrOS Sleep Study, “Outcomes of Sleep Disorders in Older Men,” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. The National Sleep Research Resource was supported by the National Heart, Lung, and Blood Institute (R24 HL114473, 75N92019R002).
Author Contributions: G.L. contributed to the study design, data extraction and analysis, and drafting of the manuscript. D.V., W.-H.H, N.E., L.G., H.C.Y., T.-Y.W., L.M., L.T.-M., D.P.W., A.W., and S.S. contributed to the analysis and interpretation of the data and critical revision of the manuscript. R.G.B., T.S., K.L.S., and S.R. oversaw the collection of study data. A.A. contributed to the study design and analysis, interpretation of the data, and critical revision of the manuscript. All authors contributed to interpretation of the data and critical revision of the manuscript and approved the manuscript in its final form.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202209-1808OC on July 7, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med . 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Veasey SC, Rosen IM. Obstructive sleep apnea in adults. N Engl J Med . 2019;380:1442–1449. doi: 10.1056/NEJMcp1816152. [DOI] [PubMed] [Google Scholar]
- 3. Kulkas A, Duce B, Leppänen T, Hukins C, Töyräs J. Severity of desaturation events differs between hypopnea and obstructive apnea events and is modulated by their duration in obstructive sleep apnea. Sleep Breath . 2017;21:829–835. doi: 10.1007/s11325-017-1513-6. [DOI] [PubMed] [Google Scholar]
- 4. Leppänen T, Kulkas A, Mervaala E, Töyräs J. Increase in body mass index decreases duration of apneas and hypopneas in obstructive sleep apnea. Respir Care . 2019;64:77–84. doi: 10.4187/respcare.06297. [DOI] [PubMed] [Google Scholar]
- 5. Borker PV, Reid M, Sofer T, Butler MP, Azarbarzin A, Wang H, et al. Non-REM apnea and hypopnea duration varies across population groups and physiologic traits. Am J Respir Crit Care Med . 2021;203:1173–1182. doi: 10.1164/rccm.202005-1808OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez-Garcia MA, Campos-Rodriguez F, Barbé F, Gozal D, Agustí A. Precision medicine in obstructive sleep apnoea. Lancet Respir Med . 2019;7:456–464. doi: 10.1016/S2213-2600(19)30044-X. [DOI] [PubMed] [Google Scholar]
- 7. Pevernagie DA, Gnidovec-Strazisar B, Grote L, Heinzer R, McNicholas WT, Penzel T, et al. On the rise and fall of the apnea-hypopnea index: a historical review and critical appraisal. J Sleep Res . 2020;29:e13066. doi: 10.1111/jsr.13066. [DOI] [PubMed] [Google Scholar]
- 8. Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med . 2016;194:613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 9. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med . 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 10. Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, Abad J, Duran-Cantolla J, Cabriada V, et al. Spanish Sleep Network Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med . 2020;8:359–367. doi: 10.1016/S2213-2600(19)30271-1. [DOI] [PubMed] [Google Scholar]
- 11. Labarca G, Dreyse J, Drake L, Jorquera J, Barbe F. Efficacy of continuous positive airway pressure (CPAP) in the prevention of cardiovascular events in patients with obstructive sleep apnea: systematic review and meta-analysis. Sleep Med Rev . 2020;52:101312. doi: 10.1016/j.smrv.2020.101312. [DOI] [PubMed] [Google Scholar]
- 12. Kulkas A, Tiihonen P, Julkunen P, Mervaala E, Töyräs J. Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea-hypopnea index. Med Biol Eng Comput . 2013;51:697–708. doi: 10.1007/s11517-013-1039-4. [DOI] [PubMed] [Google Scholar]
- 13. Vena D, Azarbarzin A, Marques M, Op de Beeck S, Vanderveken OM, Edwards BA, et al. Predicting sleep apnea responses to oral appliance therapy using polysomnographic airflow. Sleep (Basel) . 2020;43:zsaa004. doi: 10.1093/sleep/zsaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J . 2019;40:1149–1157. doi: 10.1093/eurheartj/ehy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Azarbarzin A, Sands SA, Taranto-Montemurro L, Vena D, Sofer T, Kim SW, et al. The sleep apnea-specific hypoxic burden predicts incident heart failure. Chest . 2020;158:739–750. doi: 10.1016/j.chest.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trzepizur W, Blanchard M, Ganem T, Balusson F, Feuilloy M, Girault JM, et al. Sleep apnea specific hypoxic burden, symptom subtypes and risk of cardiovascular events and all-cause mortality. Am J Respir Crit Care Med . 2022;205:108–117. doi: 10.1164/rccm.202105-1274OC. [DOI] [PubMed] [Google Scholar]
- 17. Kim JS, Azarbarzin A, Wang R, Djonlagic IE, Punjabi NM, Zee PC, et al. Association of novel measures of sleep disturbances with blood pressure: the Multi-Ethnic Study of Atherosclerosis. Thorax . 2020;75:57–63. doi: 10.1136/thoraxjnl-2019-213533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jackson CL, Umesi C, Gaston SA, Azarbarzin A, Lunyera J, McGrath JA, et al. Multiple, objectively measured sleep dimensions including hypoxic burden and chronic kidney disease: findings from the Multi-Ethnic Study of Atherosclerosis. Thorax . 2021;76:704–713. doi: 10.1136/thoraxjnl-2020-214713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shahrbabaki SS, Linz D, Hartmann S, Redline S, Baumert M. Sleep arousal burden is associated with long-term all-cause and cardiovascular mortality in 8001 community-dwelling older men and women. Eur Heart J . 2021;42:2088–2099. doi: 10.1093/eurheartj/ehab151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jun JC. Dying with OSA, or from it: a cautionary note about novel hypoxia metrics. Am J Respir Crit Care Med . 2022;206:1563–1564. doi: 10.1164/rccm.202206-1052LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brock JM, Billeter A, Müller-Stich BP, Herth F. Obesity and the lung: what we know today. Respiration . 2020;99:856–866. doi: 10.1159/000509735. [DOI] [PubMed] [Google Scholar]
- 22. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet . 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 23. Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep (Basel) . 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bakris G, Ali W, Parati G. ACC/AHA versus ESC/ESH on hypertension guidelines: JACC guideline comparison. J Am Coll Cardiol . 2019;73:3018–3026. doi: 10.1016/j.jacc.2019.03.507. [DOI] [PubMed] [Google Scholar]
- 25. Zhang GQ, Cui L, Mueller R, Tao S, Kim M, Rueschman M, et al. The National Sleep Research Resource: towards a sleep data commons. J Am Med Inform Assoc . 2018;25:1351–1358. doi: 10.1093/jamia/ocy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care . 2019;42:S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 27. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol . 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 28. Ogilvie RP, Redline S, Bertoni AG, Chen X, Ouyang P, Szklo M, et al. Actigraphy measured sleep indices and adiposity: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep (Basel) . 2016;39:1701–1708. doi: 10.5665/sleep.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barr RG, Ahmed FS, Carr JJ, Hoffman EA, Jiang R, Kawut SM, et al. Subclinical atherosclerosis, airflow obstruction and emphysema: the MESA Lung Study. Eur Respir J . 2012;39:846–854. doi: 10.1183/09031936.00165410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J . 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 31. Elmaleh-Sachs A, Balte P, Oelsner EC, Allen NB, Baugh A, Bertoni AG, et al. Race/ethnicity, spirometry reference equations, and prediction of incident clinical events: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Am J Respir Crit Care Med . 2022;205:700–710. doi: 10.1164/rccm.202107-1612OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, et al. Osteoporotic Fractures in Men Study Group Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc . 2011;59:2217–2225. doi: 10.1111/j.1532-5415.2011.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, et al. Design and baseline characteristics of the Osteoporotic Fractures in Men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials . 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 34. Azarbarzin A, Sands SA, White DP, Redline S, Wellman A. The hypoxic burden: a novel sleep apnoea severity metric and a predictor of cardiovascular mortality—reply to ‘The hypoxic burden: also known as the desaturation severity parameter’. Eur Heart J . 2019;40:2994–2995. doi: 10.1093/eurheartj/ehz273. [DOI] [PubMed] [Google Scholar]
- 35. Sands SA, Edwards BA, Terrill PI, Taranto-Montemurro L, Azarbarzin A, Marques M, et al. Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am J Respir Crit Care Med . 2018;197:1187–1197. doi: 10.1164/rccm.201707-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vena D, Taranto-Montemurro L, Azarbarzin A, Op de Beeck S, Marques M, Vanderveken OM, et al. Clinical polysomnographic methods for estimating pharyngeal collapsibility in obstructive sleep apnea. Sleep (Basel) . 2022;45:zsac050. doi: 10.1093/sleep/zsac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sands SA, Terrill PI, Edwards BA, Taranto Montemurro L, Azarbarzin A, Marques M, et al. Quantifying the arousal threshold using polysomnography in obstructive sleep apnea. Sleep (Basel) . 2018;41:zsx183. doi: 10.1093/sleep/zsx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mann DL, Terrill PI, Azarbarzin A, Mariani S, Franciosini A, Camassa A, et al. Quantifying the magnitude of pharyngeal obstruction during sleep using airflow shape. Eur Respir J . 2019;54:1802262. doi: 10.1183/13993003.02262-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the Multi-Ethnic Study of Atherosclerosis (MESA) Eur Heart J . 2018;39:2401–2408. doi: 10.1093/eurheartj/ehy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peng AW, Dardari ZA, Blumenthal RS, Dzaye O, Obisesan OH, Iftekhar Uddin SM, et al. Very high coronary artery calcium (≥1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation . 2021;143:1571–1583. doi: 10.1161/CIRCULATIONAHA.120.050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muller KE, Lavange LM, Ramey SL, Ramey CT. Power calculations for general linear multivariate models including repeated measures applications. J Am Stat Assoc . 1992;87:1209–1226. doi: 10.1080/01621459.1992.10476281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Labarca G, Reyes T, Jorquera J, Dreyse J, Drake L. CPAP in patients with obstructive sleep apnea and type 2 diabetes mellitus: systematic review and meta-analysis. Clin Respir J . 2018;12:2361–2368. doi: 10.1111/crj.12915. [DOI] [PubMed] [Google Scholar]
- 43. Labarca G, Saavedra D, Dreyse J, Jorquera J, Barbe F. Efficacy of CPAP for improvements in sleepiness, cognition, mood, and quality of life in elderly patients with OSA: systematic review and meta-analysis of randomized controlled trials. Chest . 2020;158:751–764. doi: 10.1016/j.chest.2020.03.049. [DOI] [PubMed] [Google Scholar]
- 44. Mazzotti DR, Lim DC, Sutherland K, Bittencourt L, Mindel JW, Magalang U, et al. Opportunities for utilizing polysomnography signals to characterize obstructive sleep apnea subtypes and severity. Physiol Meas . 2018;39:09TR01. doi: 10.1088/1361-6579/aad5fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Malhotra A, Ayappa I, Ayas N, Collop N, Kirsch D, Mcardle N, et al. Metrics of sleep apnea severity: beyond the apnea-hypopnea index. Sleep (Basel) . 2021;44:zsab030. doi: 10.1093/sleep/zsab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agency for Healthcare Research and Quality. 2022. https://www.ahrq.gov/topics/sleep-apnea.html [DOI] [PubMed]
- 47. Punjabi NM. COUNTERPOINT: is the apnea-hypopnea index the best way to quantify the severity of sleep-disordered breathing? No. Chest . 2016;149:16–19. doi: 10.1378/chest.14-2261. [DOI] [PubMed] [Google Scholar]
- 48. Azarbarzin A, Sands SA, Younes M, Taranto-Montemurro L, Sofer T, Vena D, et al. The sleep apnea-specific pulse-rate response predicts cardiovascular morbidity and mortality. Am J Respir Crit Care Med . 2021;203:1546–1555. doi: 10.1164/rccm.202010-3900OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Azarbarzin A, Ostrowski M, Hanly P, Younes M. Relationship between arousal intensity and heart rate response to arousal. Sleep (Basel) . 2014;37:645–653. doi: 10.5665/sleep.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rapoport D, Parekh A, Tolbert T, Ayappa I. Beyond the AHI - using breath-by-breath flow amplitude to quantitate severity of OSA. Sleep Med . 2019;64:S313. [Google Scholar]
- 51. Lacedonia D, Carpagnano GE, Aliani M, Sabato R, Foschino Barbaro MP, Spanevello A, et al. Daytime PaO2 in OSAS, COPD and the combination of the two (overlap syndrome) Respir Med . 2013;107:310–316. doi: 10.1016/j.rmed.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 52. Labarca G, Sands SA, Cohn V, Demko G, Vena D, Messineo L, et al. Mouth closing to improve the efficacy of mandibular advancement devices in sleep apnea. Ann Am Thorac Soc . 2022;19:1185–1192. doi: 10.1513/AnnalsATS.202109-1050OC. [DOI] [PubMed] [Google Scholar]
- 53. Marques M, Genta PR, Azarbarzin A, Taranto-Montemurro L, Messineo L, Hess LB, et al. Structure and severity of pharyngeal obstruction determine oral appliance efficacy in sleep apnoea. J Physiol . 2019;597:5399–5410. doi: 10.1113/JP278164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bartels W, Buck D, Glos M, Fietze I, Penzel T. Definition and importance of autonomic arousal in patients with sleep disordered breathing. Sleep Med Clin . 2016;11:435–444. doi: 10.1016/j.jsmc.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 55. Magalang UJ, Chen NH, Cistulli PA, Fedson AC, Gíslason T, Hillman D, et al. SAGIC Investigators Agreement in the scoring of respiratory events and sleep among international sleep centers. Sleep (Basel) . 2013;36:591–596. doi: 10.5665/sleep.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Azarbarzin A, Sands SA, Han S, Sofer T, Labarca G, Stone KL, et al. Relevance of cortical arousals for risk stratification in sleep apnea: a three cohort analysis. J Clin Sleep Med . 2023 doi: 10.5664/jcsm.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Baumert M, Immanuel SA, Stone KL, Litwack Harrison S, Redline S, Mariani S, et al. Composition of nocturnal hypoxaemic burden and its prognostic value for cardiovascular mortality in older community-dwelling men. Eur Heart J . 2020;41:533–541. doi: 10.1093/eurheartj/ehy838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Labarca G, Gower J, Lamperti L, Dreyse J, Jorquera J. Chronic intermittent hypoxia in obstructive sleep apnea: a narrative review from pathophysiological pathways to a precision clinical approach. Sleep Breath . 2020;24:751–760. doi: 10.1007/s11325-019-01967-4. [DOI] [PubMed] [Google Scholar]
- 59. Oldenburg O, Wellmann B, Buchholz A, Bitter T, Fox H, Thiem U, et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J . 2016;37:1695–1703. doi: 10.1093/eurheartj/ehv624. [DOI] [PubMed] [Google Scholar]
- 60. Cabezas E, Pérez-Warnisher MT, Troncoso MF, Gómez T, Melchor R, Pinillos EJ, et al. González-Mangado Sleep disordered breathing is highly prevalent in patients with lung cancer: results of the Sleep Apnea in Lung Cancer Study. Respiration . 2019;97:119–124. doi: 10.1159/000492273. [DOI] [PubMed] [Google Scholar]
- 61. Suzuki K, Miyamoto K, Wakamiya A, Ueda N, Nakajima K, Kamakura T, et al. Impact of nocturnal hypoxemia on the recurrence of atrial tachyarrhythmia after catheter ablation of atrial fibrillation. Heart Vessels . 2022;37:794–801. doi: 10.1007/s00380-021-01969-x. [DOI] [PubMed] [Google Scholar]