Abstract
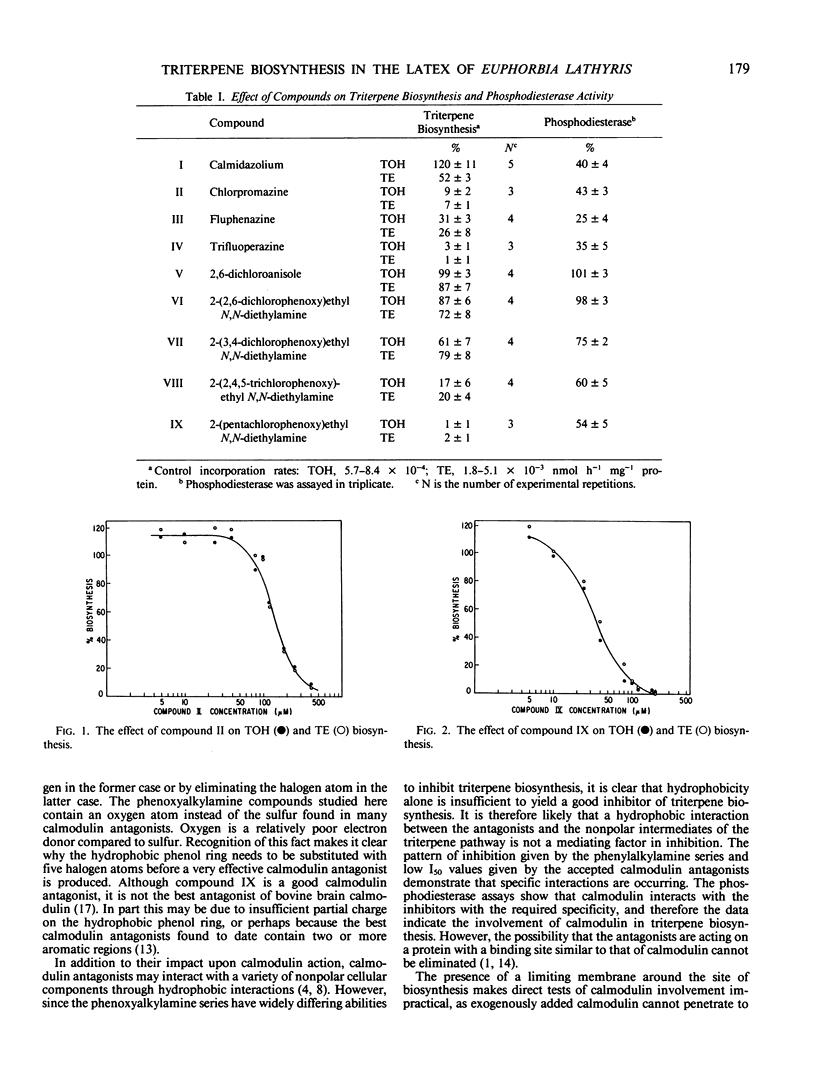
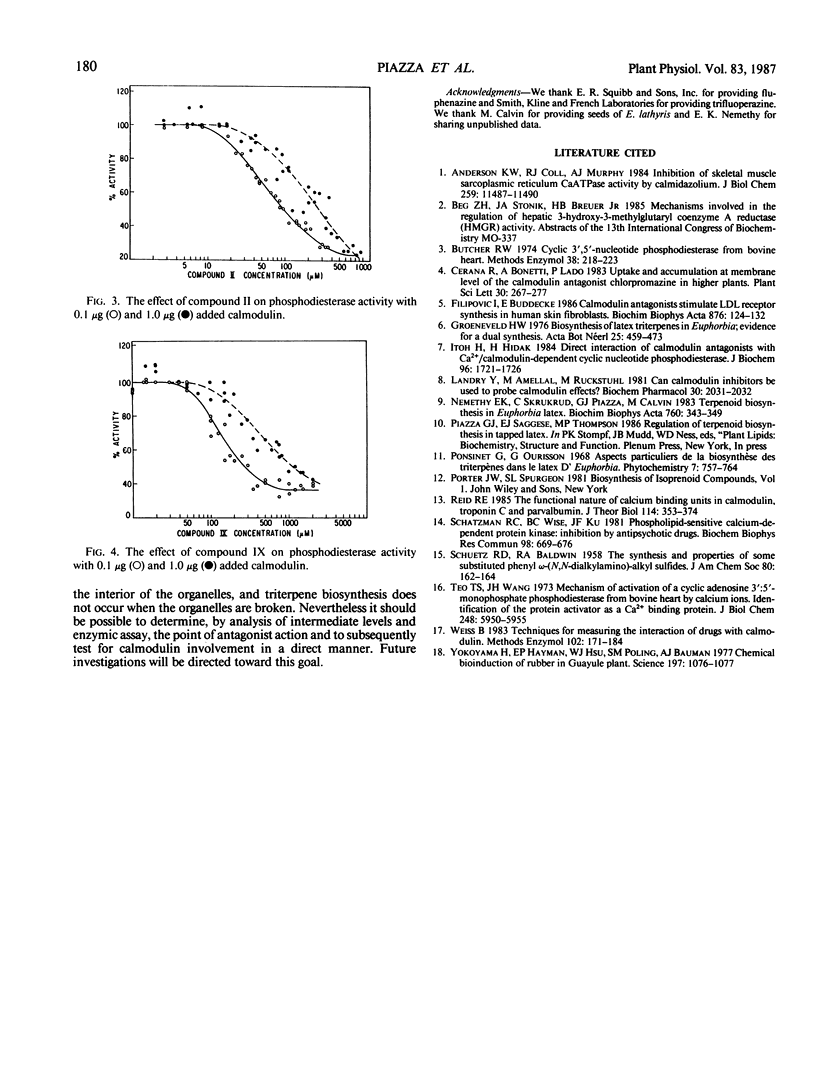
Recognized calmodulin antagonists and chlorinated phenoxyalkylamines were tested as inhibitors of mevalonate incorporation into triterpenols and their fatty acid esters in a centrifuged pellet from the latex of Euphorbia lathyris. The calmodulin antagonists, chlorpromazine (II), fluphenzine, and trifluoperazine were good inhibitors; I50 values for II and trifluoperazine were 150 and 55 micromolar, respectively. Inhibition by the phenoxyalkylamines increased with increasing chlorine substitution, and I50 for 2-(pentachlorophenoxy)ethyl N,N-diethylamine (IX) was 35 micromolar. The calmodulin-stimulated phosphodiesterase catalyzed hydrolysis of cAMP was used as an assay to quantitate the calmodulin antagonism of the tested compounds. Compounds II and IX were calmodulin antagonists over a concentration range similar to their effective range in the biosynthesis of triterpenes. The antagonism of the chlorinated phenoxy compounds increased in parallel to their inhibitory effect upon triterpene biosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. W., Coll R. J., Murphy A. J. Inhibition of skeletal muscle sarcoplasmic reticulum CaATPase activity by calmidazolium. J Biol Chem. 1984 Sep 25;259(18):11487–11490. [PubMed] [Google Scholar]
- Butcher R. W. Cyclic 3',5'-nucleotide phosphodiesterase from bovine heart. Methods Enzymol. 1974;38:218–223. doi: 10.1016/0076-6879(74)38035-4. [DOI] [PubMed] [Google Scholar]
- Filipovic I., Buddecke E. Calmodulin antagonists stimulate LDL receptor synthesis in human skin fibroblasts. Biochim Biophys Acta. 1986 Mar 21;876(1):124–132. doi: 10.1016/0005-2760(86)90325-5. [DOI] [PubMed] [Google Scholar]
- Itoh H., Hidaka H. Direct interaction of calmodulin antagonists with Ca2+/calmodulin-dependent cyclic nucleotide phosphodiesterase. J Biochem. 1984 Dec;96(6):1721–1726. doi: 10.1093/oxfordjournals.jbchem.a135004. [DOI] [PubMed] [Google Scholar]
- Jurs P. C., Hasan M. N., Henry D. R., Stouch T. R., Whalen-Pedersen E. K. Computer-assisted studies of molecular structure and carcinogenic activity. Fundam Appl Toxicol. 1983 Sep-Oct;3(5):343–349. doi: 10.1016/s0272-0590(83)80002-5. [DOI] [PubMed] [Google Scholar]
- Landry Y., Amellal M., Ruckstuhl M. Can calmodulin inhibitors be used to probe calmodulin effects? Biochem Pharmacol. 1981 Jul 15;30(14):2031–2032. doi: 10.1016/0006-2952(81)90217-3. [DOI] [PubMed] [Google Scholar]
- Reid R. E. The functional nature of calcium binding units in calmodulin, troponin C and parvalbumin. J Theor Biol. 1985 Jun 7;114(3):353–374. doi: 10.1016/s0022-5193(85)80171-5. [DOI] [PubMed] [Google Scholar]
- Schatzman R. C., Wise B. C., Kuo J. F. Phospholipid-sensitive calcium-dependent protein kinase: inhibition by antipsychotic drugs. Biochem Biophys Res Commun. 1981 Feb 12;98(3):669–676. doi: 10.1016/0006-291x(81)91166-9. [DOI] [PubMed] [Google Scholar]
- Teo T. S., Wang J. H. Mechanism of activation of a cyclic adenosine 3':5'-monophosphate phosphodiesterase from bovine heart by calcium ions. Identification of the protein activator as a Ca2+ binding protein. J Biol Chem. 1973 Sep 10;248(17):5950–5955. [PubMed] [Google Scholar]
- Weiss B. Techniques for measuring the interaction of drugs with calmodulin. Methods Enzymol. 1983;102:171–184. doi: 10.1016/s0076-6879(83)02018-2. [DOI] [PubMed] [Google Scholar]
- Yokoyama H., Hayman E. P., Hsu W. J., Poling S. M., Bauman A. J. Chemical bioinduction of rubber in guayule plant. Science. 1977 Sep 9;197(4308):1076–1078. doi: 10.1126/science.197.4308.1076. [DOI] [PubMed] [Google Scholar]


