Abstract
Rationale
Supplemental oxygen is widely administered to ICU patients, but appropriate oxygenation targets remain unclear.
Objectives
This study aimed to determine whether a low-oxygenation strategy would lower 28-day mortality compared with a high-oxygenation strategy.
Methods
This randomized multicenter trial included mechanically ventilated ICU patients with an expected ventilation duration of at least 24 hours. Patients were randomized 1:1 to a low-oxygenation (PaO2, 55–80 mm Hg; or oxygen saturation as measured by pulse oximetry, 91–94%) or high-oxygenation (PaO2, 110–150 mm Hg; or oxygen saturation as measured by pulse oximetry, 96–100%) target until ICU discharge or 28 days after randomization, whichever came first. The primary outcome was 28-day mortality. The study was stopped prematurely because of the COVID-19 pandemic when 664 of the planned 1,512 patients were included.
Measurements and Main Results
Between November 2018 and November 2021, a total of 664 patients were included in the trial: 335 in the low-oxygenation group and 329 in the high-oxygenation group. The median achieved PaO2 was 75 mm Hg (interquartile range, 70–84) and 115 mm Hg (interquartile range, 100–129) in the low- and high-oxygenation groups, respectively. At Day 28, 129 (38.5%) and 114 (34.7%) patients had died in the low- and high-oxygenation groups, respectively (risk ratio, 1.11; 95% confidence interval, 0.9–1.4; P = 0.30). At least one serious adverse event was reported in 12 (3.6%) and 17 (5.2%) patients in the low- and high-oxygenation groups, respectively.
Conclusions
Among mechanically ventilated ICU patients with an expected mechanical ventilation duration of at least 24 hours, using a low-oxygenation strategy did not result in a reduction of 28-day mortality compared with a high-oxygenation strategy.
Clinical trial registered with the National Trial Register and the International Clinical Trials Registry Platform (NTR7376).
Keywords: oxygen, intensive care medicine, hyperoxia, hypoxia, mechanical ventilation
At a Glance Commentary
Scientific Knowledge on the Subject
Sufficient arterial oxygenation is crucial for maintaining physiological balance and organ function. Oxygen therapy is widely applied in acutely ill patients; however, excessive use of oxygen carries the risk of atelectasis, pulmonary inflammation, and toxicity. Previous randomized clinical trials yielded inconsistent results regarding the best oxygenation targets in clinical practice, creating uncertainty. Further exploration is required to establish consistent guidelines.
What This Study Adds to the Field
This randomized clinical trial found no difference in 28-day mortality between patients treated with lower versus higher oxygenation targets. The present findings make a valuable contribution to the existing evidence on oxygenation targets in ICU patients. Compared with previous studies, a much larger contrast of 40 mm Hg in arterial oxygen concentrations was achieved. This contrast is crucial for demonstrating potential intervention effects.
Arterial oxygen concentrations are fundamental in maintaining a physiological balance and ensuring proper function of various organ systems. Patients with hypoxia are at risk for cell injury, tissue damage, and organ failure. In this context, oxygen therapy is a lifesaving intervention and is therefore widely and liberally applied in acutely ill patients. The administration of high oxygen concentration has also been associated with beneficial effects because of antibacterial properties and counteraction of vasodilation (1, 2). However, several studies have shown that liberal oxygen therapy with supranormal arterial oxygen concentrations is not without risks (3, 4). Excessive oxygen administration may cause atelectasis, vasoconstriction, inflammation, and toxicity because of an imbalance in reactive oxygen species (5, 6).
Several randomized clinical trials (RCTs) have been conducted to identify the optimal oxygenation targets in mechanically ventilated ICU patients (7–13). One trial showed a lower mortality rate with lower oxygenation targets (9), and six other trials reported no difference in mortality between the higher and lower targets (7, 8, 10–13). Results from individual or aggregated data analyses have been inconclusive so far, which may be influenced by differences in the study population (subgroups), different targets (either oxygen saturation as measured by pulse oximetry [SpO2] or PaO2), lack of power, or insufficient contrast between groups (14, 15). Goals for arterial oxygenation are increasingly implemented, but clinical practice guidelines and clinician behavior do not consistently rely on directive data from robust interventional studies (16).
Our aim was to provide additional data regarding the general adult ICU population using PaO2 targets that are widely used in clinical practice. Accordingly, we conducted a multicenter, binational trial to test whether the use of conservative oxygen therapy results in reduced 28-day mortality compared with liberal oxygen therapy in mechanically ventilated ICU patients. Some of the results of this study were previously reported in the form of an abstract (17, 18).
Methods
Study Design
This investigator-initiated parallel group RCT was conducted in eight ICUs in the Netherlands and one ICU in Italy. Ethical approval was granted for all centers by the Medical Ethical Committee of Leiden, The Hague, and Delft (P18.109). The protocol was prospectively registered in the National Trial Register and the International Clinical Trials Registry Platform under number NTR7376 and published (19).
The study was funded by the Dutch Research Council (project number 401.16.009). An independent data and safety monitoring board (DSMB) periodically reviewed blinded efficacy and safety data, with the option to request unblinded data if required.
Participants
All patients aged 18 years or older with an expected mechanical ventilation time of 24 hours or longer were screened for eligibility. The main exclusion criteria included a decision to withhold life-sustaining treatment, acute respiratory distress syndrome (ARDS) with a PaO2/FiO2 ratio less than 150 mm Hg, acute decompensation of chronic obstructive pulmonary disease (COPD), severe not rapidly reversible low cardiac output shock (cardiac index, ⩽2 L/min/m2), venoarterial extracorporeal membrane oxygenation, underlying diseases with an indication for hyperoxygenation, severe anemia (hemoglobin, <4.0 mmol/L) that is not rapidly reversible, and uncontrollable intracranial hypertension (19). Patients with ARDS who had a PaO2/FiO2 ratio less than 150 mm Hg were excluded from the study because they were likely to require very high FiO2 for prolonged periods if assigned to the high PaO2 target group. Patients with COPD were excluded from the study because they commonly have chronically low PaO2 values. The full list of inclusion and exclusion criteria can be found in Appendixes E1 and E2 in the online supplement.
Randomization and Blinding
Patients were assessed for eligibility by clinicians and, when appropriate, randomized within 2 hours after intubation to either the low-oxygenation (conservative) or the high-oxygenation (liberal) group with secure web-based randomization software developed by Castor EDC/CDMS (20) using computer-generated variable block randomization with a 1:1 ratio and stratification based on study site. Clinicians and outcome assessors were not blinded for the intervention, and data analysts remained blinded. Informed consent was obtained according to national regulations and, if possible, before randomization. Given the emergency setting of this trial, deferred consent from a proxy was permitted. If a patient died before delayed informed consent could be obtained, their data were still included in the analysis. Patients were excluded from the study if informed consent was not obtained within 5 days after randomization.
Trial Procedures
Oxygenation was targeted at maintaining a PaO2 concentration between 55 and 80 mm Hg for patients in the low-oxygenation group and between 110 and 150 mm Hg for patients in the high-oxygenation group. In addition to blood gas measurements, oxygen could also be adjusted on the basis of SpO2. The target SpO2 range was 91–94% for the low-oxygenation group and 96–100% for the high-oxygenation group. Oxygenation targets were pursued until ICU discharge or 28 days after randomization, whichever came first. At least one arterial blood sample per shift was collected while patients were mechanically ventilated (three per 24 h). If PaO2 values fell outside the specified ranges, the FiO2 or positive end-expiratory pressure (PEEP) could be adjusted accordingly at the discretion of the treating physician. To guide this process, the protocol specified a recommended PEEP and FiO2 table (see Table E1). To prevent prolonged exposure to high inspiratory oxygen concentrations used solely to achieve the high oxygenation target, the protocol allowed clinicians to temporarily decrease FiO2 to 0.8 and limit the PEEP to a maximum of 15 cm H2O if the FiO2 was higher than 0.8 or the PEEP was higher than 14 cm H2O for more than 2 hours. In those cases, the achievability of the PaO2 targets was reassessed every 2 hours. When the patient was extubated, oxygenation targets were still pursued. For patients randomized to the low-oxygenation group, supplemental oxygen was generally avoided, unless PaO2 fell below 55 mm Hg. Patients in the high-oxygenation group received a nasal cannula of 5 L of oxygen, unless the PaO2 exceeded 150 mm Hg.
Rescue therapy, such as prone position, recruitment maneuvers, or extracorporeal membrane oxygenation, was applied only for clinical indications and not solely to achieve the study PaO2 targets. The use of a high FiO2 during planned interventions involving upper airways (e.g., bronchoscopy) was permitted but restricted to the shortest possible duration. Further details of the study protocol were previously published (19).
Data Collection
Data from the patient data management system and from the Dutch National Intensive Care Evaluation (NICE) registry database were collected and recorded in an electronic case report form designed with Castor EDC (20, 21). The Acute Physiology and Chronic Health Evaluation IV score (22) was used to assess disease severity upon admission, whereas Sequential Organ Failure Assessment scores (23) were used to evaluate daily disease severity. Acute and chronic diagnoses were registered on the basis of data definitions provided by the NICE registry (21). Further details regarding the data collected in the electronic case report form can be found in the published study protocol (19).
Outcomes
The primary outcome measure was all-cause mortality at Day 28 after randomization. Secondary outcomes included the number of ventilator-free days and alive at Day 28; ICU and hospital lengths of stay (LOSs); ICU, hospital, and 90-day mortality; and ischemic events. Ventilator-free days were defined as the number of days that a patient was alive and free from invasive ventilation, calculated from the time of randomization, provided that the period of unassisted breathing lasted at least 24 consecutive hours (24). Serious adverse events (SAEs) were categorized as follows: PaO2 of <37.5 mm Hg; SpO2 <80% for longer than 10 minutes; cardiac arrest; or intestinal, cerebral, cardiac, or peripheral limb ischemia.
Statistical Analysis
On the basis of an expected mortality of 24% in the control group (25), the original sample size was determined to be 1,512 patients to detect an absolute difference of 6% between the two study groups, with a two-sided α of 0.05 and a power of 80%. After careful consideration and in concordance with the DSMB, we decided to stop the study prematurely after inclusion of 664 patients. The main reason for the early termination of the study was the COVID-19 pandemic, which significantly increased the workload for all participating ICUs and resulted in a substantial decrease in patient enrollment. An estimation was made that continuing at the current pace of enrollment would require an additional 5 years to reach the intended inclusion range. As a result, recruitment was stopped on November 21, 2021.
For the primary endpoint of 28-day mortality, rates were calculated according to a modified intention-to-treat principle, including all patients except those who did not provide signed informed consent or who were excluded after randomization on the basis of exclusion criteria. Differences were assessed using a chi-square test. A two-sided hypothesis test was performed with a significance level of 0.05, and the results were presented as relative risk with two-sided 95% confidence intervals. In addition, a per-protocol analysis was performed that only considered patients in the low-oxygenation group if 50% or more of the PaO2 values in the arterial blood gas analysis were equal to or below 80 mm Hg and only considered patients in the high-oxygenation group if 50% or more of the PaO2 values in the arterial blood gas analysis were equal to or above 110 mm Hg.
For the secondary endpoints, continuous variables with a normal distribution were presented as means and SDs, and variables with a nonnormal distribution were presented as medians and interquartile ranges (IQRs). Differences between groups were assessed using a Mann-Whitney U test. Categorical variables were presented as frequencies and percentages, and a chi-square test was used to analyze differences. Survival curves were calculated using the Kaplan-Meier method and compared using the log-rank test. Statistical significance was defined as a P value <0.05 in a two-sided test. When appropriate, 95% confidence intervals were used to express statistical uncertainty. In addition, an exploratory post hoc subgroup analysis was conducted to assess the heterogeneity of treatment effects. Patients were divided into subgroups on the basis of diagnosis criteria of the NICE Acute Physiology and Chronic Health Evaluation IV admission diagnosis model (21). Solely the largest subgroups were included in the analysis, including patients with sepsis, pneumonia, cardiac arrest, abdominal causes, and stroke. In addition, subgroups predefined as patients with ARDS (PaO2/FiO2, <200 mm Hg) or elevated lactate concentration (>2 mmol/L) at ICU admission were included in the analysis. Statistics for both primary and secondary endpoints were calculated as described above.
Because randomization was stratified by site, we conducted an additional analysis that involved including the study site in the analysis of both primary and secondary endpoints. For binary endpoints, we performed a logistic regression analysis, whereas, for continuous endpoints, we conducted a linear regression analysis. In both cases, we included the randomization group and study site as categorical variables.
Interim analyses were not planned beforehand, but after the study started, the DSMB deemed it necessary to conduct interim analyses of mortality. These analyses were planned after the inclusion of 500, 750, and 1,000 patients to ensure the safety of both treatment targets. As per the request of the DSMB, an interim analysis was performed after 500 patients. The interim analysis indicated no significant difference in in-hospital mortality between the two groups. Stopping rules were defined beforehand and can be found in the protocol (19). All statistical analyses were performed using the R language and environment for statistical computing (version 4.0.3; R Foundation for Statistical Computing).
Results
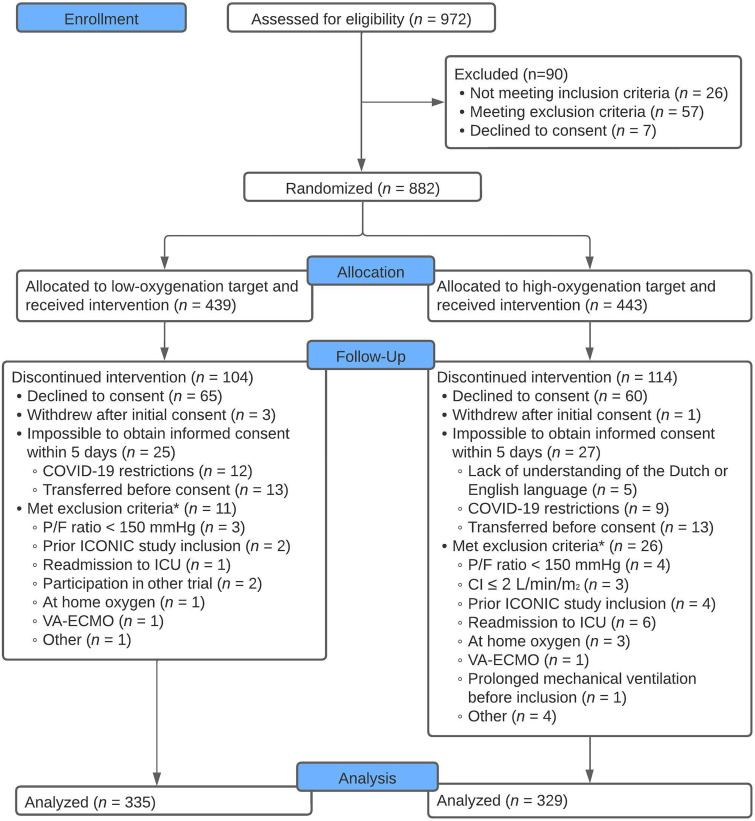
From November 19, 2018, until November 21, 2021, 972 patients were screened for eligibility. In total, 882 patients met the inclusion criteria and were randomized to either the low- or high-oxygenation group. Deferred written informed consent was available for 664 patients (Figure 1). Baseline characteristics were comparable between the groups (Table 1). No patients were lost to follow-up, and endpoint data were available for all patients.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram. The full list of inclusion and exclusion criteria can be found in Appendixes E1 and E2. Data were available for primary and secondary outcomes of all patients. *Patients were withdrawn from the study only if exclusion criteria were present at the time of inclusion. This was checked within 24 hours after randomization. CI = cardiac index; P/F = PaO2/FiO2 ratio; VA-ECMO = venoarterial extracorporeal membrane oxygenation.
Table 1.
Baseline Characteristics of Included Patients
| Low-Oxygenation Targets (55–80 mm Hg) (n = 335) |
High-Oxygenation Target (110–150 mm Hg) (n = 329) |
|
|---|---|---|
| Sex, female, n (%) | 111 (33.1) | 118 (35.9) |
| Age, yr, median [IQR] | 67 [59, 74] | 67 [56, 73] |
| SOFA admission score, median [IQR] | 9 [7, 11] | 9 [7, 11] |
| APACHE IV score on admission, median [IQR] | 87 [66, 107] | 86 [65, 113] |
| Mechanical ventilation in the first 24 h of admission, n (%)* | 289 (87.3) | 296 (92.2) |
| Duration mechanical ventilation before enrollment, min, median [IQR] | 0 [0, 58] | 2 [0, 61] |
| Type of admission, n (%)† | ||
| Medical | 258 (77.2) | 251 (76.3) |
| Emergency surgery | 61 (18.3) | 56 (17) |
| Elective surgery | 15 (4.5) | 22 (6.7) |
| Acute diagnosis, n (%)‡ | ||
| Sepsis | 53 (15.8) | 42 (12.8) |
| Pneumonia§ | 54 (16.1) | 43 (13.1) |
| Cardiac arrest | 89 (26.6) | 96 (29.2) |
| Abdominal | 29 (8.7) | 37 (11.2) |
| Neurologic | 32 (9.6) | 32 (9.7) |
| Trauma | 12 (3.6) | 12 (3.6) |
| Other | 66 (19.7) | 67 (20.4) |
| Chronic diagnosis on admission, n (%)¶ | ||
| Chronic kidney failure | 20 (6) | 22 (6.7) |
| Chronic dialysis | 6 (1.8) | 3 (0.9) |
| COPD (drug dependent) | 39 (11.7) | 37 (11.2) |
| Chronic respiratory insufficiency | 6 (1.8) | 1 (0.3) |
| Cardiovascular insufficiency (NYHA IV) | 2 (0.6) | 9 (2.7) |
| Liver cirrhosis | 14 (4.2) | 14 (4.3) |
| Diabetes | 52 (15.5) | 52 (15.8) |
| Metastasized neoplasm | 8 (2.4) | 5 (1.5) |
| Hematological malignancy | 14 (4.2) | 19 (5.8) |
| Immunological insufficiency | 33 (9.9) | 43 (13.1) |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; COPD = chronic obstructive pulmonary disease; IQR = interquartile range; NYHA = New York Heart Association; SOFA = Sequential Organ Failure Assessment.
Information on mechanical ventilation in the first 24 hours of admission was missing for four patients in the low-oxygenation group and eight patients in the high-oxygenation group.
Information on type of admission is missing for one patient in the low-oxygenation group.
Acute diagnosis is classified according to the APACHE IV model.
In the low- and high-oxygenation groups, 11 and 8 patients admitted with pneumonia had COVID-19. Information on whether patients were admitted with a COVID-19 infection was available only for patients included in the Netherlands.
More than one chronic diagnosis can be present in the same patient.
Oxygenation
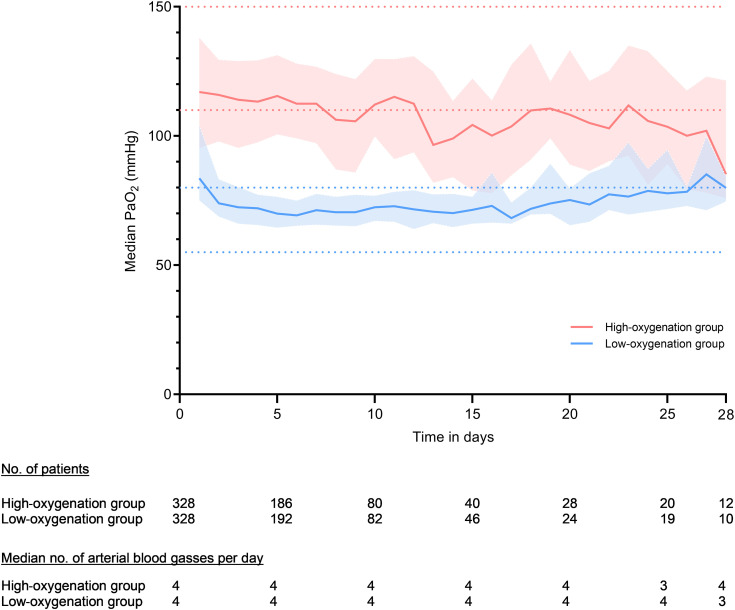
The first PaO2 measured after inclusion in the study was 92.3 mm Hg (IQR, 76.5, 123.2) and 106.5 mm Hg (IQR, 83.3, 147) in the low- and high-oxygenation groups, respectively. More information about the first blood gas analysis can be found in Table E2. During the whole period of mechanical ventilation, the median PaO2 was 75 mm Hg (IQR, 69.8–83.5) in the low-oxygenation group and 115 mm Hg (IQR, 100.3–129.0) in the high-oxygenation group (P < 0.001) (Table 2 and Figure 2). Corresponding median SpO2 values were 95% (IQR, 94–97) and 99% (IQR, 98–100), respectively (P < 0.001) (Table 2 and Figure E1). While spontaneously breathing without mechanical ventilation, the median PaO2 was 75 mm Hg (IQR, 68.3–82.9) in the low-oxygenation group and 85.5 mm Hg (IQR, 73.8–102.8) in the high-oxygenation group. The corresponding median SpO2 values were 95% (IQR, 94–97) and 99% (IQR, 98–100), respectively (P < 0.001) (Table 2 and Figures E2 and E3). Additional data on ventilation are displayed in Table E2 and Figure E4.
Table 2.
Ventilation Data and Outcomes
| Low-Oxygenation Target (55–80 mm Hg) (n = 335) | High-Oxygenation Target (110–150 mm Hg) (n = 329) | P Value | |
|---|---|---|---|
| Ventilation data | |||
| No. of arterial blood gases, mean (SD)* | 30.8 (30.8) | 33.1 (37.6) | 0.38 |
| Duration mechanical ventilation, d, median [IQR] | 3 [1.4, 6.5] | 2.8 [1.4, 6.1] | 0.6 |
| Mechanical ventilation | |||
| PaO2, mm Hg, median [IQR] | 75 [69.8, 83.5] | 115 [100.3, 129] | <0.001 |
| SpO2, %, median [IQR] | 95 [94, 97] | 99 [98, 100] | <0.001 |
| PaCO2, mm Hg, median [IQR] | 39.8 [36, 44.3] | 41.3 [36.8, 45] | 0.054 |
| Off mechanical ventilation | |||
| PaO2, mm Hg, median [IQR] | 75 [68.3, 82.9] | 85.5 [73.8, 102.8] | <0.001 |
| SpO2, %, median [IQR] | 95 [94, 97] | 99 [98, 100] | <0.001 |
| PaCO2, mm Hg, median [IQR] | 37.2 [34.5, 40.6] | 39.8 [36, 43.5] | 0.001 |
| Primary endpoint | |||
| 28-d mortality, n (%) | 129 (38.5) | 114 (34.7) | 0.34 |
| Secondary endpoints | |||
| ICU mortality, n (%) | 109 (32.5) | 94 (28.6) | 0.29 |
| Hospital mortality, n (%) | 127 (37.9) | 111 (33.7) | 0.3 |
| 90-d mortality, n (%) | 144 (43) | 133 (40.4) | 0.56 |
| ICU length of stay, d, median [IQR] | 4.9 [2.3, 10.8] | 4.7 [2.5, 9.9] | 0.89 |
| Hospital length of stay, d, median [IQR] | 14 [5, 26] | 12 [5, 23] | 0.65 |
| Ventilator-free days at Day 28, d, median [IQR] | 18.3 [0, 25.4] | 20.2 [0, 25.7] | 0.36 |
| Serious adverse events, n (%)† | |||
| Serious adverse events | 13 | 22 | — |
| Patients with at least one SAE | 12 (3.6) | 17 (5.2) | — |
| Patients with more than one SAE | 1 (0.3) | 3 (0.9) | — |
| PaO2 <37.5 mm Hg | 0 (0) | 0 (0) | — |
| Ischemia | 10 (3) | 15 (4.6) | — |
| Cerebral | 4 (1.2) | 4 (1.2) | — |
| Cardiac | 0 (0) | 3 (0.9) | — |
| Intestinal | 4 (1.2) | 7 (2.1) | — |
| Extremities | 2 (0.6) | 1 (0.3) | — |
| SpO2 <80% longer than 10 min | 1 (0.3) | 2 (0.6) | — |
| Cardiac arrest | 2 (0.6) | 4 (1.2) | — |
| Other | 0 (0) | 1‡ (0.3) | — |
Definition of abbreviations: IQR = interquartile range; SAE = serious adverse event; SpO2 = oxygen saturation as measured by pulse oximetry.
During the whole study period.
As reported in the case report form in Castor.
Severe refractory hypotension most likely caused by tamponade.
Figure 2.
Median PaO2 per day during mechanical ventilation. The PaO2 values were calculated on the basis of median PaO2 values per day by study group, whereas median values were taken per patient per day before aggregating the data. Lines represent the achieved median PaO2 per oxygenation group. Faded areas around the lines represent the interquartile ranges. The dotted horizontal lines represent the boundaries of the higher and lower targets. Blood gas data were not available for seven patients in the low-oxygenation group and for one patient in the high-oxygenation group.
Outcomes
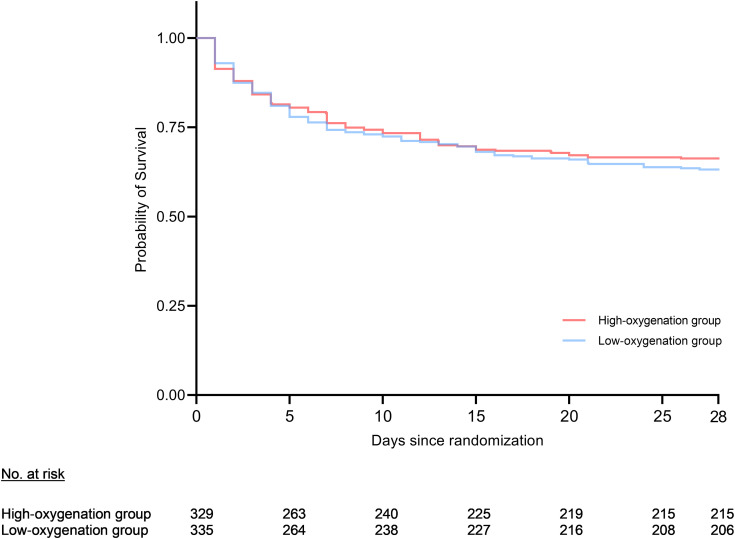
The modified intention-to-treat analysis showed no significant difference in the primary outcome between the two oxygenation groups (P = 0.34). In total, mortality at Day 28 occurred in 129 (38.5%) patients in the low-oxygenation group and 114 (34.7%) patients in the high-oxygenation group (risk ratio, 1.11; 95% confidence interval, 0.9–1.4; P = 0.30). The Kaplan-Meier survival curve (Figure 3) showed no difference in the probability of survival between the two groups (log-rank test; P = 0.4). Similar results regarding 28-day mortality were observed when applying a per-protocol analysis, namely, 82 (35.8%) of 229 patients died in the low-oxygenation group versus 67 (39%) of 171 patients in the high-oxygenation group (Table E3).
Figure 3.
Kaplan-Meier survival curve of survival until Day 28. On Day 28, 129 (38.5%) patients had died within the low-oxygenation group and 114 (34.7%) had died in the high-oxygenation group. Statistical analysis of the Kaplan-Meier curve showed no significant difference (P = 0.4; adjusted for study site, P = 0.4).
No significant differences were observed between the two groups with respect to ICU, hospital, and 90-day mortality (Table 2 and Figure E5). In addition, the analyses of ICU LOS, hospital LOS, and number of ventilator-free days at Day 28 yielded no significant differences (Table 2). The median LOS in the ICU was 4.9 (IQR, 2.3–10.8) days in the low-oxygenation group versus 4.7 (IQR, 2.5–9.9) days in the high-oxygenation group. Adjusted analysis for study site for both primary and secondary endpoints can be found in Table E4.
A total of 13 versus 22 SAEs occurred in the low- and high-oxygenation groups, respectively (Table 2). Ischemic events were the most frequently reported SAE; 10 (3.0%) and 15 (4.6%) occurred in the low- and high-oxygenation groups, respectively. The most common ischemic events were cerebral and intestinal (Table 2).
During the ICU admission, maximal and daily Sequential Organ Failure Assessment scores were comparable in both groups (Figure E6). No differences were found for predefined primary and secondary endpoints within the subgroups. Details of this analysis can be found in Table E5.
Discussion
In this multicenter randomized trial, which included mechanically ventilated adult ICU patients, no significant difference was found in 28-day mortality between patients treated with a low- or a high-oxygenation strategy. In addition, we did not find evidence of a between-group difference in ICU, in-hospital, or 90-day mortality; ventilator-free days; LOS; or ischemic events.
Our findings are in line with recent studies showing similar outcomes of ICU patients, regardless of oxygenation targets (8, 10–12), but they are in contrast with earlier studies suggesting better survival with less oxygen (9) or a benefit for high-oxygenation targets regarding SAEs (7). The first publication reporting higher mortality after adjustment for severity of illness in ICU patients with high PaO2 values originated from an ICU registry in the Netherlands in 2008 (6). Since then, many observational studies were performed in various subsets of ICU patients. A meta-analysis of these studies showed that hyperoxia was associated with higher mortality, but the heterogeneity of studied populations and the observational nature of studies warranted a cautious interpretation of these findings (4). Results from the first RCT on oxygenation in ICU patients were published in 2016 and demonstrated that a conservative protocol for oxygen therapy versus conventional therapy resulted in lower ICU mortality (9). This RCT appeared to confirm the results from earlier observational studies. However, since then, four additional RCTs have been published, all showing no differences in mortality between patients treated with conservative versus liberal oxygen targets (7, 8, 10, 11). In addition, the very recent cluster-randomized PILOT trial (Pragmatic Investigation of Optimal Oxygen Targets Trial), which compared three SpO2 targets (90%, 94%, and 98%), also showed no differences in outcome (12). It should be noted that in every previous trial, other definitions of low- and high-oxygenation targets were used.
The fact that several large RCTs performed in different countries do not show an effect of oxygen targets on outcomes of ICU patients can be considered as evidence that different oxygenation strategies do not have an impact on mortality. However, it cannot be ruled out that the absence of an effect from these strategies may be caused by a lack of contrast between the studied targets. In previous studies, the contrast between study groups was at times small, from as low as a difference in arterial oxygen concentrations of 7.5 mm Hg to 15 mm Hg (7–10) or 22 mm Hg (11). Such differences may be too small to demonstrate the effects of a certain oxygenation target. The findings of our study add important contributions to the existing literature because the tested intervention resulted in more contrast between achieved oxygen concentrations, as high as 40 mm Hg. However, we still did not observe an effect on mortality. Thus, we do not consider a lack of contrast to be the main explanation for the absence of a benefit. It is worth noting that a larger contrast in oxygenation between intervention groups does not necessarily mean that a PaO2-related mortality difference can be detected. It is also possible that the lowest mortality risk falls between the studied targets. However, considering that previous RCTs (7, 8, 10, 11) examining slightly different target ranges also showed no difference in outcomes, it is less likely that, in all of these studies, the optimal PaO2 target would have fallen between the studied targets.
One would expect that adhering to higher PaO2 targets would result in increased reliance on invasive mechanical ventilation and a higher need for sedative drugs, potentially leading to a prolonged mechanical ventilation time. However, our results demonstrated that mechanical ventilation time was similar for both groups. This finding is consistent with the ICU-ROX [Evaluating the Effects of Two Approaches to Oxygen Therapy in Intensive Care Unit Patients Requiring Life Support (Mechanical Ventilation)] and the PILOT trial, which also reported similar numbers of ventilator-free days (10, 12). No differences were found between the groups when considering LOS; ICU, hospital, and 90-day mortality; ischemic events; and other SAEs. These findings are in line with earlier studies (8, 10–12). Notably, in one of the previous RCTs, a trend toward a higher incidence of intestinal ischemic events in the low-oxygenation group was reported (7). However, in our trial, we did not find any difference in intestinal or other ischemic events for the two study groups.
The latest literature indicates that the general ICU population does not derive benefits from a low- or high-oxygenation strategy. Yet, there are thoughts that specific subgroups of ICU patients, such as those after cardiac arrest, could benefit from specific targets. The ICU-ROX investigators reported improved outcomes in patients with hypoxic-ischemic encephalopathy when treated with a conservative oxygen strategy (10). Similarly, Kilgannon and colleagues found a higher mortality when patients with cardiac arrest were treated with high concentrations of oxygen (26). However, it should be noted that high oxygenation in the latter study was defined as a PaO2 >300 mm Hg, which is twice as high as the upper limit of our high target. This disparity may explain why our results did not show a difference in outcome for patients with cardiac arrest. In addition, two recent RCTs comparing oxygenation strategies (SpO2 of 90–94% and 98–100% or PaO2 of 68–75 mm Hg or 98–105 mm Hg) in patients with cardiac arrest also found no difference in outcomes (27, 28).
The absence of a difference in mortality related to lower or higher oxygenation targets could also be caused by a lack of statistical power. Interestingly, both the present study and previous RCTs have shown nonsignificant trends toward lower mortality in patients treated with higher oxygenation targets (8, 10, 11). The absolute differences in 90-day mortality ranged from 0.5% to 1.2% in the previously published trials and 2.6% in the present study. However, none of these RCTs did have the power to rule out small mortality effects. Interestingly, two very large trials are ongoing at the moment (Intensive Care Unit Randomized Trial Comparing Two Approaches to Oxygen Therapy (UK-ROX) and the Mega randomized registry trial research program comparing conservative versus liberal oxygenation targets in adults receiving unplanned invasive mechanical ventilation in the ICU (Mega-ROX), including 16,500 and 40,000 patients, respectively) (29). The results of these trials will provide important insights into the possible smaller effects on survival, potentially in favor of higher oxygenation targets.
Some relevant limitations of this study must be considered. First, because of the study’s early termination, we were able to include only 664 of the planned 1,512 patients, which resulted in a lack of statistical power to detect clinically important differences. However, with 664 patients, the ICONIC (Conservative versus Conventional Oxygenation Targets in Intensive Care Patients) trial remains one of the larger RCTs in this field. Second, because inclusion in the study was allowed before consent was obtained (deferred consent), a substantial number of patients were withdrawn from the study after initial inclusion and randomization if written informed consent could not be obtained. Excluding patients after inclusion raises concerns about potential selection bias. According to Dutch legislation, we are not allowed to provide data about this population, and we therefore cannot compare characteristics of excluded patients with patients who were included in our study. To minimize the risk of selection bias, the protocol had strict criteria for patient withdrawal, which was permitted only if patients declined consent or if consent was not given within 5 days after inclusion. In addition, patients could be withdrawn within 24 hours after inclusion if exclusion criteria became apparent at the time of inclusion. Patients who died within 5 days before consent could be obtained remained in the study. Third, some patients randomized to the high PaO2 group were unable to reach this target. If, for example, a patient needed 100% oxygen to reach the high-oxygenation goal for prolonged periods, the protocol allowed lowering the FiO2 to 0.8 to decrease the risk of pulmonary toxicity. This may have diminished the contrast in oxygenation between groups. Nevertheless, the difference between median PaO2 values was still 40 mm Hg. Furthermore, this is representative of real-life treatment in the ICU: High-oxygenation targets are not feasible in patients with very severe pulmonary dysfunction. Fourth, because of the nature of the intervention, it was not possible to blind clinicians to the study intervention. However, the chosen endpoints, such as 28-day mortality and ventilator-free days, are objective and are less likely to be influenced by bias. Moreover, data analysts of this study were blinded for the study intervention. Finally, the findings of our study cannot be generalized to patients with severe ARDS or COPD, because these patients were excluded from participation in this study.
Both the present study and previous RCTs showed no differences between the intervention groups. This is in contrast with popular beliefs and common practices, because over the last several years, there appeared to be a strong opinion among healthcare professionals that low-oxygen targets are better than high-oxygen targets (30, 31). Although it is still possible that marked hyperoxia with PaO2 much higher than that studied in the RCTs may increase mortality, it is unlikely that new RCTs comparing conservative oxygenation with marked hyperoxia will ever be conducted in ICU patients.
In conclusion, among adult mechanically ventilated ICU patients with an expected mechanical ventilation duration of at least 24 hours, using a low-oxygenation strategy did not result in a reduction in 28-day mortality compared with a high-oxygenation strategy. It is noteworthy that the trend toward lower mortality in patients treated with higher oxygen targets, as also found in previous studies, precludes definite conclusions regarding what the best oxygen targets are and urges for additional studies.
Acknowledgments
Acknowledgment
The authors express their gratitude for the contributions made by all the authors. We especially honor the memory of Paolo Pelosi for his important contributions to this research. The authors thank J. Wigbers, F. Termorshuizen, C. Klop, L. Dawson, E.Y. Schriel-van den Berg, E. de Vreede, J. Qualm, M. Koopmans, T. Krol, M. Rinket, J.W. Vermeijden, A. Beishuizen, J. van Holten, A.M. Tsonas, M. Botta, T. Winters, J. Horn, F. Paulus, D. Battaglini, L. Ball, and I. Brunetti from the ICONIC study group for their assistance in data collection and data verification.
Footnotes
ICONIC investigators: Jeanette Wigbers, Fabian Termorshuizen, Cintha Klop, Lilian Dawson, Yvonne Schriel-van den Berg, Els de Vreede, Jolanda Qualm, Matty Koopmans, Tim Krol, Martin Rinket, Wytze Vermeijden, Albertus Beishuizen, Jantine van Holten, Anissa Tsonas, Michela Botta, Tineke Winters, Janneke Horn, Denise Battaglini, Lorenzo Ball, and Iole Brunetti.
Supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) (project number 401.16.009). The NWO was not involved in the study design, the collection of the data, the analysis or interpretation of the data, the writing of the report, or the decision to submit the paper for publication.
Author Contributions: E.d.J., H.J.F.H., P.P., M.J.S., D.J.v.W., C.C.A.G., and L.I.v.d.W. contributed to the design of the study. E.d.J., H.J.F.H., P.P., M.J.S., D.J.v.W., C.C.A.G., L.I.v.d.W., A.S.N., M.R.d.P., E.C.B., H.G.R.-d.J., A.C.R., B.G.L., P.L.J.v.d.H., M.J.S., F.P., A.D.C., M.L., F.J.S., N.F.d.K., and F.B.-R. contributed to the data collection. L.I.v.d.W., C.C.A.G., H.J.F.H., E.d.J., and S.L.C. did data analysis and data interpretation. E.d.J. supervised the study. L.I.v.d.W. wrote the first draft with input from H.J.F.H. and E.d.J. All authors contributed to the writing and review of the main manuscript, had full access to all the data in the study, and had final responsibility for the decision to submit the manuscript for publication.
Study materials, including the protocol, are available online. Individual participant data will not be made available. Deidentified data can be made available by the corresponding author on reasonable request.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202303-0560OC on August 8, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
for the ICONIC investigators:
Jeanette Wigbers, Fabian Termorshuizen, Cintha Klop, Lilian Dawson, Yvonne Schriel-van den Berg, Els de Vreede, Jolanda Qualm, Matty Koopmans, Tim Krol, Martin Rinket, Wytze Vermeijden, Albertus Beishuizen, Jantine van Holten, Anissa Tsonas, Michela Botta, Tineke Winters, Janneke Horn, Denise Battaglini, Lorenzo Ball, and Iole Brunetti
References
- 1. Hafner S, Beloncle F, Koch A, Radermacher P, Asfar P. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann Intensive Care . 2015;5:42. doi: 10.1186/s13613-015-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic. The effect of inspired oxygen on infection. Arch Surg . 1984;119:199–204. doi: 10.1001/archsurg.1984.01390140057010. [DOI] [PubMed] [Google Scholar]
- 3. Helmerhorst HJ, Arts DL, Schultz MJ, van der Voort PH, Abu-Hanna A, de Jonge E, et al. Metrics of arterial hyperoxia and associated outcomes in critical care. Crit Care Med . 2017;45:187–195. doi: 10.1097/CCM.0000000000002084. [DOI] [PubMed] [Google Scholar]
- 4. Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, de Jonge E. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med . 2015;43:1508–1519. doi: 10.1097/CCM.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 5. Asfar P, Singer M, Radermacher P. Understanding the benefits and harms of oxygen therapy. Intensive Care Med . 2015;41:1118–1121. doi: 10.1007/s00134-015-3670-z. [DOI] [PubMed] [Google Scholar]
- 6. de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PH, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care . 2008;12:R156. doi: 10.1186/cc7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, et al. LOCO2 Investigators and REVA Research Network Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med . 2020;382:999–1008. doi: 10.1056/NEJMoa1916431. [DOI] [PubMed] [Google Scholar]
- 8. Gelissen H, de Grooth H-J, Smulders Y, Wils E-J, de Ruijter W, Vink R, et al. Effect of low-normal vs high-normal oxygenation targets on organ dysfunction in critically ill patients: a randomized clinical trial. JAMA . 2021;326:940–948. doi: 10.1001/jama.2021.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA . 2016;316:1583–1589. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- 10. Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, Eastwood G, et al. ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group; ICU-ROX Investigators the Australian and New Zealand Intensive Care Society Clinical Trials Group Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med . 2020;382:989–998. doi: 10.1056/NEJMoa1903297. [DOI] [PubMed] [Google Scholar]
- 11. Schjørring OL, Klitgaard TL, Perner A, Wetterslev J, Lange T, Siegemund M, et al. HOT-ICU Investigators Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med . 2021;384:1301–1311. doi: 10.1056/NEJMoa2032510. [DOI] [PubMed] [Google Scholar]
- 12. Semler MW, Casey JD, Lloyd BD, Hastings PG, Hays MA, Stollings JL, et al. PILOT Investigators and the Pragmatic Critical Care Research Group Oxygen-saturation targets for critically ill adults receiving mechanical ventilation. N Engl J Med . 2022;387:1759–1769. doi: 10.1056/NEJMoa2208415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Panwar R, Hardie M, Bellomo R, Barrot L, Eastwood GM, Young PJ, et al. CLOSE Study Investigators ANZICS Clinical Trials Group. Conservative versus liberal oxygenation targets for mechanically ventilated patients: a pilot multicenter randomized controlled trial. Am J Respir Crit Care Med . 2016;193:43–51. doi: 10.1164/rccm.201505-1019OC. [DOI] [PubMed] [Google Scholar]
- 14. Barbateskovic M, Schjørring OL, Krauss SR, Meyhoff CS, Jakobsen JC, Rasmussen BS, et al. Higher vs lower oxygenation strategies in acutely ill adults: a systematic review with meta-analysis and trial sequential analysis. Chest . 2021;159:154–173. doi: 10.1016/j.chest.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 15. van der Wal LI, Grim CCA, van Westerloo DJ, Schultz MJ, de Jonge E, Helmerhorst HJF. Higher versus lower oxygenation strategies in the general intensive care unit population: a systematic review, meta-analysis and meta-regression of randomized controlled trials. J Crit Care . 2022;72:154151. doi: 10.1016/j.jcrc.2022.154151. [DOI] [PubMed] [Google Scholar]
- 16. Siemieniuk RAC, Chu DK, Kim LH, Güell-Rous MR, Alhazzani W, Soccal PM, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ . 2018;363:k4169. doi: 10.1136/bmj.k4169. [DOI] [PubMed] [Google Scholar]
- 17. Van Der Wal LI, Grim CCA, Helmerhorst HJF, Van Westerloo DJ, Pelosi P, Schultz MJ, et al. Conservative versus liberal oxygenation targets in intensive care unit patients: a multicentre randomised clinical trial. Neth J Crit Care . 2023;31:45–46. doi: 10.1164/rccm.202303-0560OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Der Wal LI, Grim CCA, Helmerhorst HJF, Van Westerloo DJ, Pelosi P, Schultz MJ, et al. 2023. https://www.smartonweb.org/poster/posterhome.php?year=2023
- 19. Grim CCA, van der Wal LI, Helmerhorst HJF, van Westerloo DJ, Pelosi P, Schultz MJ, et al. ICONIC Investigators and PROVE Network ICONIC study—conservative versus conventional oxygenation targets in intensive care patients: study protocol for a randomized clinical trial. Trials . 2022;23:136. doi: 10.1186/s13063-022-06065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castor. 2019. http://www.castoredc.com/
- 21.Arts D, de Keizer N, Scheffer GJ, de Jonge E. Quality of data collected for severity of illness scores in the Dutch National Intensive Care Evaluation (NICE) registry. Intensive Care Med. 2002;28:656–659. doi: 10.1007/s00134-002-1272-z. [DOI] [PubMed] [Google Scholar]
- 22. Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med . 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 23. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med . 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 24. Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med . 2019;200:828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van De Klundert N, Holman R, Dongelmans DA, De Keizer NF. Data resource profile: the Dutch National Intensive Care Evaluation (NICE) registry of admissions to adult intensive care units. Int J Epidemiol . 2015;44:1850–1850h. doi: 10.1093/ije/dyv291. [DOI] [PubMed] [Google Scholar]
- 26. Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, et al. Emergency Medicine Shock Research Network (EMShockNet) Investigators Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA . 2010;303:2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 27. Schmidt H, Kjaergaard J, Hassager C, Mølstrøm S, Grand J, Borregaard B, et al. Oxygen targets in comatose survivors of cardiac arrest. N Engl J Med . 2022;387:1467–1476. doi: 10.1056/NEJMoa2208686. [DOI] [PubMed] [Google Scholar]
- 28. Bernard SA, Bray JE, Smith K, Stephenson M, Finn J, Grantham H, et al. EXACT Investigators Effect of lower vs higher oxygen saturation targets on survival to hospital discharge among patients resuscitated after out-of-hospital cardiac arrest: the EXACT randomized clinical trial. JAMA . 2022;328:1818–1826. doi: 10.1001/jama.2022.17701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young PJ, Arabi YM, Bagshaw SM, Bellomo R, Fujii T, Haniffa R, et al. Protocol and statistical analysis plan for the mega randomised registry trial research program comparing conservative versus liberal oxygenation targets in adults receiving unplanned invasive mechanical ventilation in the ICU (Mega-ROX) Crit Care Resusc. 2022;24:137–149. doi: 10.51893/2022.2.OA4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grim CC, Helmerhorst HJ, Schultz MJ, Winters T, van der Voort PH, van Westerloo DJ, et al. Changes in attitudes and actual practice of oxygen therapy in ICUs after implementation of a conservative oxygenation guideline. Respir Care . 2020;65:1502–1510. doi: 10.4187/respcare.07527. [DOI] [PubMed] [Google Scholar]
- 31. Schjørring OL, Toft-Petersen AP, Kusk KH, Mouncey P, Sørensen EE, Berezowicz P, et al. Intensive care doctors’ preferences for arterial oxygen tension levels in mechanically ventilated patients. Acta Anaesthesiol Scand . 2018;62:1443–1451. doi: 10.1111/aas.13171. [DOI] [PubMed] [Google Scholar]