Abstract
Rationale
In addition to rare genetic variants and the MUC5B locus, common genetic variants contribute to idiopathic pulmonary fibrosis (IPF) risk. The predictive power of common variants outside the MUC5B locus for IPF and interstitial lung abnormalities (ILAs) is unknown.
Objectives
We tested the predictive value of IPF polygenic risk scores (PRSs) with and without the MUC5B region on IPF, ILA, and ILA progression.
Methods
We developed PRSs that included (PRS-M5B) and excluded (PRS-NO-M5B) the MUC5B region (500-kb window around rs35705950-T) using an IPF genome-wide association study. We assessed PRS associations with area under the receiver operating characteristic curve (AUC) metrics for IPF, ILA, and ILA progression.
Measurements and Main Results
We included 14,650 participants (1,970 IPF; 1,068 ILA) from six multi-ancestry population-based and case–control cohorts. In cases excluded from genome-wide association study, the PRS-M5B (odds ratio [OR] per SD of the score, 3.1; P = 7.1 × 10−95) and PRS-NO-M5B (OR per SD, 2.8; P = 2.5 × 10−87) were associated with IPF. Participants in the top PRS-NO-M5B quintile had ∼sevenfold odds for IPF compared with those in the first quintile. A clinical model predicted IPF (AUC, 0.61); rs35705950-T and PRS-NO-M5B demonstrated higher AUCs (0.73 and 0.7, respectively), and adding both genetic predictors to a clinical model yielded the highest performance (AUC, 0.81). The PRS-NO-M5B was associated with ILA (OR, 1.25) and ILA progression (OR, 1.16) in European ancestry participants.
Conclusions
A common genetic variant risk score complements the MUC5B variant to identify individuals at high risk of interstitial lung abnormalities and pulmonary fibrosis.
Keywords: idiopathic pulmonary fibrosis, interstitial lung abnormalities, polygenic risk score, MUC5B
At a Glance Commentary
Scientific Knowledge on the Subject
In addition to rare genetic variants and the MUC5B locus, common genetic variants contribute to idiopathic pulmonary fibrosis (IPF) risk. The predictive power of common variants outside the MUC5B locus for IPF and interstitial lung abnormalities (ILA) is unknown.
What This Study Adds to the Field
Common genetic risk variants outside the MUC5B region, when combined into a polygenic score, predict IPF, ILA, and ILA progression. A polygenic score combined with the MUC5B promoter variant has substantial predictive power and may prove useful in the clinical setting.
Idiopathic pulmonary fibrosis (IPF) is the most common type of interstitial lung disease (ILD), and it often leads to chronic respiratory failure and death (1). Upon diagnosis, IPF has a median survival of only 3–5 years, and patients may already have a significant burden of fibrosis, with impairments in oxygenation and ventilation (2, 3). Therefore, there is an important need to develop risk prediction tools that will help to identify individuals at high risk of developing IPF before the development of symptoms and/or progressive lung fibrosis.
In addition to clinical risk factors that are associated with a higher risk for IPF (i.e., older age, male sex, smoking, etc.), genetic variants confer susceptibility. Rare variants related to telomerase maintenance (4), cell–cell adhesion (5), surfactant protein function (6), and alveolar epithelial integrity have been identified in several studies and are associated with a higher risk of IPF. Common variants in these and other pathways are also associated with IPF susceptibility, with by far the strongest association from the common MUC5B promoter polymorphism (rs35705950-T), as the prevalence of its risk allele (T) is >50% among patients with IPF. Some of these variants have also been associated with interstitial lung abnormalities (ILAs), which are computed tomography (CT)-based radiologic abnormalities in the lung parenchyma that may represent earlier stages of pulmonary fibrosis (7–9). Although ILA might progress to IPF, ILA shares both unique and distinct genetic characteristics with IPF (10, 11). Furthermore, ILA can progress to multiple types of ILD, not just IPF.
Although several studies have examined the associations of individual genetic variants with IPF, the proportion of IPF risk that can be predicted by common genetic variation is less clear. Given that individual genetic variants have small effects, summing the effects of variants into genetic or polygenic risk scores (PRSs) can capture a greater degree of phenotypic variability. PRSs have been used to improve disease prediction in cardiovascular (12–14) and other lung diseases like chronic obstructive pulmonary disease (COPD) (15, 16) and asthma (17–20). However, IPF is unusual among complex pulmonary diseases because of the MUC5B rs35705950-T variant, which is quite common (minor allele frequency 0.11 in Europeans) and has a large effect (OR, ∼5 for IPF [21, 22], OR, 2–3 for ILA [9, 23] in heterozygotes). The implications of such a genetic architecture on polygenic risk prediction methods are not well understood (24).
Although a prior study demonstrated that additional variants beyond those identified as genome-wide significant contribute to genetic risk in patients with IPF (7), the cumulative impact of all common genetic variants—genome-wide significant, and those below the significance threshold—in independent cohorts characterized for both IPF and ILA has not been previously assessed. We hypothesized that an IPF-derived PRS would be associated with a higher risk of IPF and ILA in population-based and case–control cohorts. Because of the known large effect size of rs35705950-T, similar to other genetic risk scores in diseases with a large effect variant (25), we examined whether a PRS excluding the MUC5B region (PRS-NO-M5B) was associated with IPF and ILA and whether the PRS-NO-M5B and rs35705950-T together could explain more phenotypic variability and improve prediction compared with either genetic risk factor alone.
Methods
Study Populations
All studies obtained informed consent and approval from local institutional review boards and ethics committees. Cohort genotyping details can be found in the online supplement.
IPF study populations
Denver
The Denver cohort consisted of two subsets: a sample used for training and a sample used for testing. The Denver testing sample included only samples nonoverlapping with those used in the Allen and colleagues (7) genome-wide association study (GWAS); thus, for IPF, the Denver testing set was used as an external validation cohort. Additional details regarding the Denver testing cohort can be found in the online supplement.
Lung Tissue Research Consortium
The LTRC (Lung Tissue Research Consortium) recruited individuals ⩾21 years of age who were undergoing lung surgery. Details regarding study inclusion criteria have been previously published (26). IPF case identification was based on American Thoracic Society/European Respiratory Society guidelines (27) for IPF diagnosis or a pathologic diagnosis of usual interstitial pneumonitis or honeycomb lung, as previously reported (28).
ILA study populations
Genetic Epidemiology of COPD
The COPDGene (Genetic Epidemiology of COPD) study recruited 10,198 non-Hispanic White and African American participants 45–80 years of age with ⩾10 pack-years of smoking (29). Anthropometric and survey data, spirometry, CT imaging, and blood were collected at baseline and at the 5- and 10-year follow-up visits.
Framingham Heart Study
The FHS (Framingham Heart Study) is a community-based cohort in Framingham, Massachusetts, that was started in 1948 (original cohort) and was extended with the offspring cohort in 1971 (30). The study was then extended to the third-generation (Gen3) cohort in 2002 and is comprised of children from the large offspring cohort families. Spirometry was performed at exams 5–9 of the offspring cohort and exams 1–2 of the Gen3 cohort (31).
Multi-Ethnic Study of Atherosclerosis
MESA (Multi-Ethnic Study of Atherosclerosis) is an ongoing prospective U.S.-based cohort of community-dwelling adults that was originally designed to examine subclinical cardiovascular disease (32). A subset of participants agreed to undergo genetic testing.
Subpopulations and Intermediate Outcome Measures in COPD Study
SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) recruited individuals aged 40–80 years with a smoking history ⩾20 pack-years (30). Recruitment included nonsmokers and smokers without COPD, smokers with mild-to-moderate COPD, and smokers with severe COPD.
Statistical Analyses
Overview of study design
A schematic of the study design is shown in Figure 1. PRSs were computed using GWAS summary statistics from Allen and colleagues (7) and included thousands of SNPs below a threshold of genome-wide significance. These PRSs were evaluated in the above cohorts. We tested the association of a PRS excluding the MUC5B region (500-kb window [i.e., ±250 kb] around rs35705950-T) and rs35705950-T with IPF, ILA, and ILA progression in specific cohorts using multivariable logistic regression models.
Figure 1.
Schematic of study design. AUC = area under the receiver operating characteristic curve; COPDGene = Genetic Epidemiology of COPD; FHS = Framingham Heart Study; GWAS = genome-wide association study; ILA = interstitial lung abnormalities; IPF = idiopathic pulmonary fibrosis; LTRC = Lung Tissue Research Consortium; M5B = mucin 5B (oligomeric/gel-forming) (MUC5B); MESA = Multiethnic Study of Atherosclerosis; PRS = polygenic risk score; PRS-M5B = a PRS including the MUC5B region; PRS-NO-M5B = a PRS excluding the MUC5B region (500-kb window [i.e., ±250 kb]).
Genetic predictors
In accordance with PRS reporting guidelines laid out by Choi and colleagues (33), we have detailed the preparation of genetic data and PRS calculations in the online supplement. PRSs were calculated using lassosum (34), a penalized regression method that accounts for linkage disequilibrium. As a reference panel, we used 10,000 randomly sampled participants from the U.K. Biobank (application #20915), as previously performed (35). Additional details regarding PRS calculation can be found in the online supplement.
Outcomes
We examined the association of genetic predictors with three outcomes: IPF, ILA, and ILA progression. IPF cases were identified in the LTRC and Denver cohorts, as detailed above and in the online supplement.
ILA cases were identified in COPDGene, FHS, MESA, and SPIROMICS. ILA was defined in the Fleischner Society guidelines (36) in COPDGene, FHS, and MESA. In SPIROMICS, ILA included ground-glass or reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, and traction bronchiectasis. ILA progression was determined as previously defined (37, 38) and used to compare those with probable or definite progression to individuals with no disease.
Model specifications and performance evaluations
Ancestry groups were assigned based on concordance of self-identified race and principal components analysis. All models were adjusted for principal components of genetic ancestry, the optimal number of which was defined by each cohort. In addition, we adjusted models for age, sex, and pack-years of smoking (when available) or ever-smoker status (when available). We used the R meta package (39) to perform inverse-variance fixed and random effects meta-analyses. Area under the receiver operating characteristic curve (AUC) analyses were performed using the pROC R package (40) to evaluate model performances, and AUCs were compared using DeLong tests (41). We calculated performance characteristics for using genetics for IPF risk prediction (i.e., PRS and rs35705950-T), as described in the online supplement.
Results
Characteristics of Study Participants
We included 14,650 participants in this study (Table 1). COPDGene and SPIROMICS by design are enriched for smokers and have lower FEV1/FVC and FEV1% predicted than other cohorts. FHS and COPDGene are the only cohorts with adjudication of ILA progression. The Denver cohort used out-of-study control subjects, shown in Table E1 in the online supplement.
Table 1.
Characteristics of Study Participants
| Characteristic | IPF Populations |
ILA Populations |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Denver | LTRC | FHS | COPDGene AA | COPDGene NHW | MESA AA | MESA Asian | MESA His | MESA NHW | SPIROMICS | |
| N | 4,146 | 570 | 1,472 | 1,435 | 4,078 | 568 | 329 | 485 | 933 | 634 |
| Age, yr, mean (SD) | 65.26 (9.40) | 63.10 (10.34) | 57.88 (11.74) | 54.30 (7.19) | 61.39 (8.63) | 69 (9) | 68 (9) | 68 (9) | 70 (9) | 65.74 (8.38) |
| Sex, n (%) | 1,486 (35.8) | 268 (47.0) | 718 (48.8) | 582 (40.6) | 1,944 (47.7) | 306 (54.0) | 163 (50.0) | 263 (54.0) | 482 (52.0) | 289 (45.6) |
| Race, n (%) | ||||||||||
| Non-Hispanic White | 4,146 (100.0) | 514 (90.2) | 1,472 (100.0) | 0 (0.0) | 4,078 (100.0) | 0 (0) | 0 (0) | 0 (0) | 933 (100) | 634 (100.0) |
| African American | 0 (0.0) | 4 (0.7) | 0 (0.0) | 1,435 (100.0) | 0 (0.0) | 568 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0.0) |
| Hispanic | 0 (0.0) | 28 (4.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0) | 485 (100) | 0 (0) | 0 (0.0) |
| Asian | 0 (0.0) | 16 (2.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0) | 329 (100) | 0 (0) | 0 (0) | 0 (0.0) |
| Other | 0 (0.0) | 8 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0.0) |
| Current smoking status, n (%) | NA | 12 (2.3) | 87 (5.9) | 1,128 (78.6) | 1,560 (38.3) | 62 (11.0) | 10 (3.0) | 27 (6.0) | 65 (7.0) | 185 (29.8) |
| Pack-years of smoking, mean (SD) | NA | 18.33 (25.35) | 17.46 (17.04) | 37.62 (19.89) | 45.80 (24.58) | 11 (19) | 4 (14) | 6 (15) | 14 (25) | 48.56 (26.16) |
| FVC, L, mean (SD) | NA | 2.97 (1.02) | 4.14 (1.07) | 3.29 (0.96) | 3.44 (1.03) | 3.00 (0.84) | 2.86 (0.66) | 3.21 (0.79) | 3.58 (0.87) | 3.51 (1.02) |
| FEV1% predicted, mean (SD) | NA | 81.92 (22.17) | 97.82 (14.91) | 84.79 (25.59) | 74.82 (26.15) | 96.2 (34.0) | 101.0 (19.6) | 95.4 (18.2) | 93.3 (18.8) | 73.78 (25.70) |
| FEV1/FVC, mean (SD) | NA | 0.80 (0.07) | 0.75 (0.07) | 0.72 (0.15) | 0.65 (0.17) | 0.98 (0.12) | 0.99 (0.12) | 1.0 (0.09) | 0.97 (0.11) | 60.10 (15.92) |
| IPF, n (%) | 1,694 (40.9) | 276 (48.4) | NA | NA | NA | NA | NA | NA | NA | NA |
| ILA, n (%) | NA | NA | 170 (11.5) | 107 (7.5) | 332 (8.1) | 66 (12.0) | 35 (11.0) | 56 (12.0) | 120 (13.0) | 182 (29.36) |
| ILA progression (no disease to 4 or 5), n (%) | NA | NA | 118 (8.0) | 40 (2.9) | 179 (4.5) | NA | NA | NA | NA | NA |
| PRS-NO-M5B, mean (SD) | NA | 0.00 (1.00) | 0.03 (1.01) | −0.01 (0.97) | −0.02 (1.00) | −0.04 (0.95) | −0.02 (0.99) | −0.04 (1.03) | 0.01 (1.00) | 0.00 (1.00) |
| rs35705950 T allele, n (%) copies | ||||||||||
| 0 | 2,616 (63.1) | 360 (63.2) | 1,160 (78.8) | 1,352 (96.0) | 3,190 (81.0) | 519 (95.0) | 311 (97.0) | 425 (89.0) | 720 (79.0) | 518 (81.7) |
| 1 | 1,375 (33.2) | 193 (33.9) | 296 (20.1) | 55 (3.9) | 706 (17.9) | 28 (5.0) | 8 (3.0) | 53 (11.0) | 179 (20.0) | 106 (16.7) |
| 2 | 153 (3.7) | 17 (3.0) | 16 (1.1) | 1 (0.1) | 41 (1.0) | 0 | 0 | 1 (0.002) | 11 (1.0) | 10 (1.6) |
Definition of abbreviations: AA = African American; COPD = chronic obstructive pulmonary disease; COPDGene = Genetic Epidemiology of COPD; FHS = Framingham Heart Study; HIS = Hispanic; ILA = interstitial lung abnormalities; IPF = idiopathic pulmonary fibrosis; LTRC = Lung Tissue Research Consortium; MESA = Multiethnic Study of Atherosclerosis; NA = not applicable; NHW = non-Hispanic White; PRS = polygenic risk score; PRS-NO-M5B = PRS excluding the MUC5B region (500-kb window); SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study.
Development of PRSs
We used lassosum (34) to calculate PRSs with (PRS-M5B) and without (PRS-NO-M5B) the MUC5B region. The PRS-M5B lassosum score included 60,762 variants, 60,566 of which were outside of a 500-kb window surrounding rs35705950-T. In the PRS-M5B score, we observed that the effect sizes of rs35705950-T and surrounding variants were particularly large compared with other genomic regions; by contrast, the PRS-NO-M5B had 60,608 variants, and variant effect sizes were more similar across chromosomal regions (Figure E1), suggesting that more variants not in linkage disequilibrium with rs35705950-T were included in this PRS. Further details regarding PRS computations are in the Supplementary Results and Figures E2 and E3. Using linkage disequilibrium score regression, we estimated IPF observed-scale heritability to be ∼28% (SE, 4.9%). Accounting for population prevalence estimates, the PRS-NO-M5B (variance explained, 4–14%) and rs35705950-T (variance explained, 2–11%) together explained ∼8–18% of IPF variability and ∼4% of ILA variability (Table E2).
Association of Polygenic Risk Scores with IPF, ILA, and ILA Progression
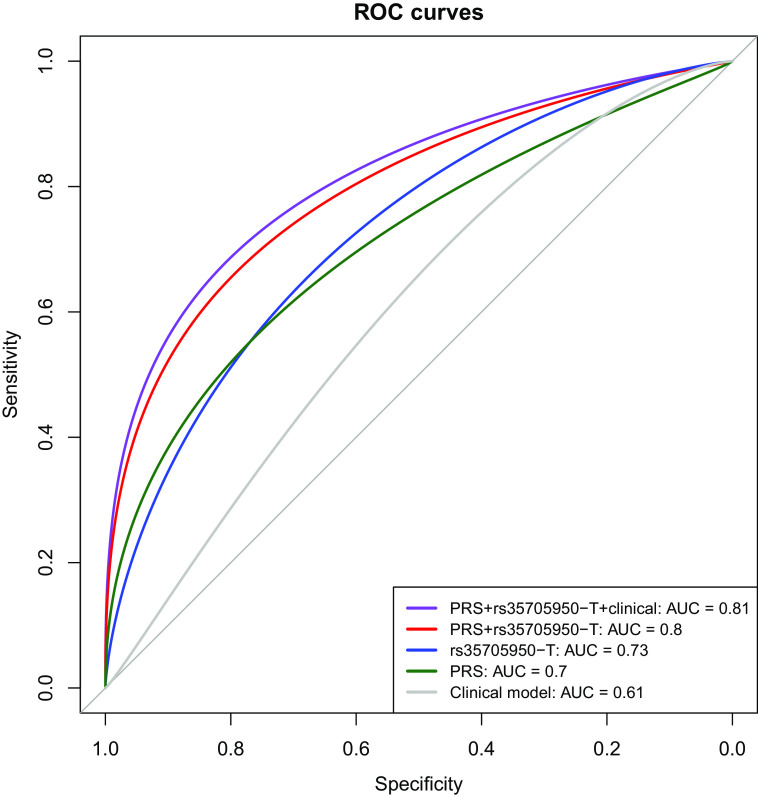
Figure 2 shows the density and quintile plots for multivariable PRS-NO-M5B associations with IPF in the Denver cohort. Being in the top quintile of the PRS-NO-M5B was associated with a log-odds of 2 (odds ratio [OR], ∼7) for IPF compared with being in the first quintile. The PRS-M5B had higher AUC values for IPF and ILA than the PRS-NO-M5B, but models with both the PRS-NO-M5B and rs35705950-T demonstrated higher AUC values than other tested models, both in LTRC and in the Denver testing cohort (Table 2; additional results, Table E3). We observed similar results when using an alternative PRS method and changing the window sizes around rs35705950-T (Supplementary Results, Tables E4 and E5). Hereafter, the PRS-NO-M5B will be referred to as “the PRS.” In Figure 3, we show receiver operating characteristic curves demonstrating an increase in the AUCs for IPF comparing models with rs35705950-T alone, PRS alone, PRS + rs35705950-T, and then starting with a clinical model (age, sex, pack-years of smoking) and adding rs35705950-T, the PRS, and both genetic predictors (Denver testing cohort: P [AUC (PRS + rs35705950-T + clinical factors) vs. AUC (rs35705950-T + clinical factors)] = 2.5 × 10−14). The performance characteristics of using genetics (i.e., PRS and rs35705950-T) are shown in Table E6. The receiver operating characteristic curve for LTRC (which may contain overlapping samples with the Allen and colleagues [7] GWAS) and meta-analyzed AUC values are shown in the online supplement (Figure E4 and Table E7).
Figure 2.
(A) Distribution (density) of the polygenic risk score excluding the MUC5B region (PRS) in the Denver testing cohort. (B) Quintile plot showing adjusted ORs and 95% CIs of the PRS association with IPF for each quintile compared with the first quintile as the reference group (adjusted for clinical data). The colors correspond to quintiles of the PRS in both parts of the figure. Multivariable models were adjusted for age, sex, smoking status, and principal components of genetic ancestry. CI = confidence interval; IPF = idiopathic pulmonary fibrosis; OR = odds ratio.
Table 2.
Multivariable Logistic Models
| IPF (LTRC) |
IPF (Denver) |
ILA (COPDGene NHW) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | AUC | OR (95% CI) | P Value | AUC | OR (95% CI) | P Value | AUC | |
| One genetic predictor per model | |||||||||
| Clinical model* | NA | NA | 0.71 | NA | NA | 0.61 | NA | NA | 0.69 |
| Age | 1.1 (1.02–1.13) | 1.1 × 10−2 | 1.02 (1.01–1.03) | 2.5 × 10−7 | 1.08 (1.06–1.09) | 2.6 × 10−24 | |||
| Sex, male | 3.9 (2.02–7.54) | 4.9 × 10−5 | 1.58 (1.37–1.81) | 2.8 × 10−10 | 0.915 (0.725–1.16) | 4.6 × 10−1 | |||
| Smoking status | NA | NA | 0.63 (0.54–0.73) | 3.0 × 10−9 | NA | NA | |||
| Pack-years of smoking | 1 (0.983–1.02) | 9.6 × 10−1 | NA | NA | 1.01 (1–1.01) | 3.1 × 10−3 | |||
| PRS alone | 3.65 (2.83–4.7) | 1.7 × 10−23 | 0.79 | 2.63 (2.39–2.88) | 9.0 × 10−90 | 0.70 | 1.30 (1.16–1.46) | 6.1 × 10−6 | 0.57 |
| rs35705950-T alone | 3.43 (2.42–4.87) | 5.5 × 10−12 | 0.68 | 4.93 (4.33–5.61) | 3.6 × 10−127 | 0.73 | 1.79 (1.43–2.25) | 5.3 × 10−7 | 0.58 |
| PRS + clinical model | 3.55 (2.7–4.66) | 7.5 × 10−20 | 0.81 | 2.79 (2.52–3.09) | 2.5 × 10−87 | 0.73 | 1.32 (1.18–1.48) | 2.5 × 10−6 | 0.7 |
| rs35705950-T + clinical model | 3.55 (2.43–5.19) | 6.4 × 10−11 | 0.74 | 5.08 (4.43–5.83) | 1.8 × 10−118 | 0.76 | 1.88 (1.48–2.38) | 1.6 × 10−7 | 0.71 |
| Both genetic predictors in a single model with clinical variables | |||||||||
| PRS | 3.49 (2.63–4.64) | 4.9 × 10−18 | 0.84 | 2.74 (2.46–3.05) | 2.4 × 10−75 | 0.81 | 1.31 (1.16–1.47) | 7.4 × 10−6 | 0.72 |
| rs35705950-T | 3.49 (2.26–5.41) | 2.0 × 10−8 | 5.04 (4.34–5.84) | 1.1 × 10−101 | 1.84 (1.45–2.33) | 4.6 × 10−7 | |||
Definition of abbreviations: AUC = area under the receiver operating characteristic curve; CI = confidence interval; COPD = chronic obstructive pulmonary disease; COPDGene = Genetic Epidemiology of COPD; ILA = interstitial lung abnormalities; IPF = idiopathic pulmonary fibrosis; LTRC = Lung Tissue Research Consortium; NA = not applicable; NHW = non-Hispanic white; OR = odds ratio; PRS = polygenic risk score; SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study.
Multivariable logistic models were constructed to test the association of the PRS and the MUC5B rs35705950-T promoter variant with IPF in LTRC and Denver testing cohorts and ILAs in COPDGene NHW. The clinical model included age, sex, and pack-years of smoking or smoking status (as available in each cohort). Models with genetic predictors were adjusted for principal components of genetic ancestry.
Clinical variables are from testing cohorts and are likely overfit.
Figure 3.
Receiver operating characteristic (ROC) curves for idiopathic pulmonary fibrosis prediction in the Denver testing cohort based on a clinical model (age, sex, smoking status) and genetic factors (rs35705950-T, polygenic risk score excluding the MUC5B region [500-kb window (i.e., ±250 kb)] [PRS-NO-M5B]). PRS-NO-M5B is abbreviated to “PRS.” AUCs are shown in the lower right. DeLong P values were used to compare models: P (rs35705950-T + clinical vs. clinical alone) = 0.5; P (PRS + clinical vs. rs35705950-T + clinical factors) = 0.003; P (PRS + rs35705950-T + clinical vs. PRS + clinical factors) = 0.0016. AUC = area under the receiver operating characteristic curve.
The multivariable decision curve analysis comparing the net benefit of each variable across a range of thresholds is shown in Figure E5 and Table E8. Genetics is associated with the greatest net benefit over 20–80% threshold probabilities. In an ascertained population with a 20% decision threshold, obtaining a CT scan on the basis of genetics is equivalent to a strategy that identified 23 IPF cases out of 100 at-risk individuals without conducting any unnecessary CT scans.
Having demonstrated that the PRS developed using lassosum was associated with improved predictive performance for ILA in COPDGene (Table 2), we then sought to examine whether this result replicated in additional cohorts. In meta-analyses, the PRS was associated with ILA (OR, 1.25 [95% confidence interval (CI), 1.16–1.36]) in European, but not in non-European, ancestry cohorts (Figure 4A). We also found that the PRS was associated with ILA progression (see Methods) (OR, 1.16 [95% CI, 1.03–1.31]) (Figure 4B). Stratified and interaction analysis results are detailed in the Supplementary Results, Figure E6, and Tables E9 and E10. As previously reported, the rs35705950-T variant was associated with IPF, ILA, and ILA progression (Figure E7).
Figure 4.
Forest plots showing inverse-variance meta-analysis results of multivariable associations of the polygenic risk score (PRS) excluding the MUC5B region (PRS) with (A) interstitial lung abnormalities (ILA) and (B) ILA progression in European and non-European ancestry (when available) cohorts. AA = African American; CI = confidence interval; COPD = chronic obstructive pulmonary disease; COPDGene = Genetic Epidemiology of COPD; FHS = Framingham Heart Study; HIS = Hispanic; MESA = Multiethnic Study of Atherosclerosis; NHW = non-Hispanic white; OR = odds ratio; SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study.
Discussion
In this study of nearly 15,000 participants from six general population and case–control U.S. cohorts, we demonstrated that a PRS excluding the MUC5B region had an effect approaching the MUC5B variant rs35705950-T and improved prediction of IPF in an external validation cohort when added to rs35705950-T. We also observed that the PRS was associated with ILA and ILA progression. These findings lend insight into how the unusual genetic architecture of IPF affects genetic risk prediction of IPF and early stages of pulmonary fibrosis and suggests that common genetic variants outside the MUC5B region in addition to the MUC5B promoter variant can have substantial effects on risk prediction for these traits.
We sought to understand whether separate modeling of genetic risk variants outside of the MUC5B region could add predictive value to the rs35705950-T variant. In Alzheimer’s disease, a similar approach demonstrated that separate modeling of APOE4 and variants outside of this region using a PRS improved disease prediction across a range of PRS calculation methods (25). Among respiratory diseases, IPF genetic architecture is unusual in that the rs35705950-T MUC5B promoter variant is common and of exceptionally large effect size. We found that our prediction results were improved by calculating a PRS excluding the MUC5B region and including it in the same model with the rs35705950-T variant.
Our results suggest that common genetic variants in aggregate and independent of the MUC5B variant contribute substantially to IPF risk. We estimated the observed-scale heritability of IPF, derived from a GWAS of common variants, to be around 28%. In prior work, a polygenic risk score derived from variants with P values below genome-wide significance levels explained 2% of IPF phenotypic variability (7). In the current work, we excluded only the MUC5B region and used an alternative and more powerful polygenic risk score approach to construct the PRS. Although the Nagelkerke R2 values suggested that the PRS explains nearly all the observed IPF heritability, when we account for the low IPF prevalence, we found that the PRS explains ∼4–14% of IPF phenotypic variability (on the liability scale). Despite these modest estimates, the PRS demonstrably improved IPF prediction when added to rs35705950-T and clinical factors. The phenomenon in which a PRS that explains a modest amount of phenotypic variability can be associated with high AUC estimates has been previously described; for rare diseases, such as IPF, variance explained/liability estimates may be low, whereas those in the higher liability groups are more likely to be diseased, and, thus, higher AUCs will be observed (39). Taken together, these findings imply that 1) a substantial portion of IPF genetic risk is due to common variants; 2) genetic risk variants for IPF include tens of thousands of common small effect size variants outside of the MUC5B region below a threshold of genome-wide significance; and 3) the PRS may have potential to be used to risk stratify patients and determine whether routine screening tests are indicated to detect disease before the onset of extensive lung fibrosis.
It has been speculated that several environmental and intrinsic factors together contribute to a higher risk of developing IPF. Our findings suggest that genetic risk summarized by PRS has a significant role in the development of IPF and might have future clinical utility, as it appeared to improve prediction models including clinical risk factors alone. This study demonstrates that other variants (despite having small effect sizes) collectively may still have predictive capabilities in addition to and independent of the MUC5B (rs35705950-T) promoter variant. The identification of additional genetic variants related to IPF risk is an important research gap that may improve the predictive utility of polygenic risk prediction (24).
Although our PRS model contains many genetic variants, the dramatic reduction in the cost of genetic assays means that the practical implementation of obtaining an individual’s PRS involves a simple blood test and a standard commercial array. This genetic information costs <$100, can be used to calculate PRSs for other diseases, only needs to be obtained once in a lifetime, and can be obtained at a young age (in contrast to clinical factors, which change over time). We demonstrate that common genetic variants can provide excellent discrimination (AUC, 0.8) for predicting IPF. Conversely, clinical factors only provided modest discriminative capacity (AUC, 0.61). Taken together, our results suggest that genetics (i.e., the PRS and rs35705950-T) can be used, with high accuracy, to risk stratify those in whom IPF is suspected.
To understand how the PRS could be used for risk stratification, we calculated test performance characteristics in several scenarios. Considering the scenario in which patients are screened from a cohort enriched for lung disease, we performed a decision curve analysis. The “treatment” would be a CT scan to confirm the presence of fibrosis. Genetics demonstrated a clear net benefit over a wide range of treatment thresholds, whereas clinical factors added little to a “CT scan all” approach. The combined genetic markers exhibited high negative predictive values in both an ascertained and general population sample. A negative test based on genetics would indicate a low likelihood of IPF. Taken together, our results suggest that genetics, available through a simple blood test, may have utility in deciding which patients ought to undergo additional testing for pulmonary fibrosis.
As we have previously demonstrated genetic overlap between ILA and IPF (10), we tested our IPF-derived PRS in ILA. It is possible that ILA prediction developed from an ILA GWAS would provide improved prediction of ILA. However, developing a PRS with good predictive capabilities requires a large GWAS and a trait with high heritability and nonoverlapping samples for testing. The largest ILA GWAS included 1,699 ILA cases (10); because all our cohorts were used in this GWAS, there also would not be a suitable population in which to test an ILA PRS.
We found that the PRS was associated with ILA, although with smaller effect sizes than observed on IPF, suggesting the involvement of both overlapping and divergent pathogenic mechanisms between ILA and IPF, as previously suggested by genetic (10) and transcriptomic analyses (11). The weaker association of the PRS with ILA compared with the association with IPF further supports the idea that IPF and ILA share common genetic origins. Although the rs35705950-T variant has been associated with ILA progression, we observed that the PRS was associated with ILA progression and thus may be helpful in early prediction of pulmonary fibrosis development. Some ILAs can progress to IPF and other ILD types, and research to determine the clinical trajectories of ILA remains ongoing. Therefore, the PRS may capture only a subset of ILA progressors who develop IPF or ILDs with similar pathogenic mechanisms.
There is a growing appreciation that identifying individuals before the onset of fibrosis is necessary to prevent fulminant disease (42, 43). However, several questions remain unanswered. First, the role of genetic scores in higher-risk populations, such as first-degree adult relatives of patients with clinically diagnosed pulmonary fibrosis (44), or in those harboring rare genetic variants, is unclear. Second, as ILA itself is associated with increased mortality, disentangling shared and distinct mechanisms for ILA versus IPF may provide an opportunity to offer personalized therapeutic interventions for those individuals at higher risk to develop pulmonary fibrosis. Despite sharing clinical features with and sometimes progressing to ILD, ILA is a distinct phenotype with unique genetic variants and gene expression patterns (10, 11). The utility of genetic prediction for identifying and managing ILA is yet to be determined. Finally, risk stratification likely involves an integrated assessment of clinical features, imaging, genetic risk, and other biomarkers (45); in addition, future studies will be needed to determine if early intervention improves outcomes among those at risk to develop clinically apparent pulmonary fibrosis.
Although our study includes multiple populations and adds significantly to our understanding of how the genetic architecture of IPF influences genetic risk prediction, there are several limitations. Participants in the Denver and LTRC studies were included in the Allen and colleagues (7) GWAS, which raises a concern that overfitting could have affected our scores. To address these issues, we tested the PRS in a set of Denver cases that were not included in the prior GWAS. In addition, although the majority of cohorts defined ILA using Fleischner Society guidelines (36), SPIROMICS included a broader range of radiologic abnormalities (see Methods); despite this limitation, we still observed a significant association of the PRS with ILA in SPIROMICS. Although the PRS was associated with ILA progression, we were unable to evaluate associations with FVC decline or mortality in patients with IPF, which themselves may be distinct outcomes from IPF risk. Recent GWASs of FVC decline and mortality in individuals with IPF (46) may provide a future opportunity to improve prediction of poor outcomes in IPF and ILA. The LTRC and Denver cohorts contained different smoking data (LTRC: current vs. former smokers; Denver: ever- vs. never-smokers), so smoking effect estimates for IPF could not be derived in LTRC and applied to the Denver cohort. Incorporation of other clinical risk factors in our models, like gastroesophageal reflux/hiatal hernia, pollution, and socioeconomic stressors, were not included because of heterogeneity of how these variables were assessed or were missing. PRSs have been used to determine whether polygenic risk is modified by environmental effects, such as smoking (47, 48), as they can increase power; however, heterogeneous effects of the included variants and sample size may still have impacted our ability to identify these interactions. The contexts and populations (e.g., smokers) in which IPF genetic risk prediction would be useful for screening and early detection of pulmonary fibrosis require further investigation. Finally, the predictive value of the PRS, trained in Europeans, did not translate to non-European populations. These results highlight the need for improved cross-ancestry genetic prediction methodologies and to enhance current understanding of IPF and ILA genetic risk in individuals of non-European ancestry.
In conclusion, the addition of genetic risk outside the MUC5B region gave a significant prediction improvement when combined with the MUC5B rs35705950-T variant and known clinical risk factors. This PRS was also associated with ILA and ILA progression and may prove useful in the clinical setting. Our work demonstrates that common variants in aggregate outside of the MUC5B region in combination with the MUC5B promoter variant have a substantial contribution to both early and late stages of pulmonary fibrosis.
Acknowledgments
Acknowledgment
The authors thank the patients who offered their time and blood samples for these projects as well as the Global IPF Collaborative (see online supplement). They also thank the studies and participants who provided biological samples and data for TOPMed.
Footnotes
Supported by NHLBI grants T32HL007427 and K08HL159318 (M.M.); NHLBI grant K23 HL150301 (J.S.K.); NHLBI grants K08 HL136928 and U01 HL089856 and an Alpha-1 Foundation Research Grant (B.D.H.); National Institute of Arthritis and Musculoskeletal and Skin Diseases grant T32AR007530 (G.M.); NIH grants R01 HL147148, U01 HL089856, R01 HL133135, R01 HL152728, P01 HL132825, and P01 HL114501 (E.K.S.); National Cancer Institute grants R01CA203636 and 5U01CA209414, NIH/NHLBI grants 2R01HL111024 and 1R01HL130974, and NIH grant R01HL135142 (H.H.); grants R01CA203636, U01CA209414, and R01HL111024 (M.N.); grant P01 HL132825 (J.H.); NIH grants R01 HL111024, R01 HL130974, and R0135142 (G.M.H.); NHLBI grants R01-HL149836, R01-HL158668, P01-HL092870, UG3/UH3-HL151865, and X01-HL134585 (D.A.S. and A.L.P.); U.S. Department of Veterans Affairs VA Merit Review grant IO1BX005295 and U.S. Department of Defense grant W81XWH-17-1-0597 (D.A.S. and A.L.P.); grants R01HL142992 and R01HL111527 (V.E.O.); grants R01-HL077612, R01-HL093081, and R01-HL121270 (R.G.B.); NIH grants R01HL137927, R01HL135142, HL147148, and HL089856 (M.H.C.); GlaxoSmithKline Asthma + Lung UK Chair in Respiratory Research C17-1 (L.V.W.); and NIH grant R01HL153248 (A.M.). The COPDGene (Genetic Epidemiology of Chronic Obstructive Pulmonary Disease [COPD]) project described was supported by NHLBI award numbers U01 HL089897 and U01 HL089856. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH. COPDGene is also supported by the COPD Foundation through contributions made to an Industry Advisory Board that has included AstraZeneca, Bayer Pharmaceuticals, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, and Sunovion. The MESA (Multi-Ethnic Study of Atherosclerosis) Lung Study was supported by NHLBI (NIH) grants R01-HL077612, R01-HL093081, and RC1-HL100543 and contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169. MESA was also funded by National Center for Advancing Translational Sciences (NIH) grants UL1-TR-000040, UL1-TR-001079, UL1-TR-001420, UL1-TR-001881, and DK063491. Funding for MESA SNP Health Association Resource (SHARe) genotyping was provided by NHLBI contract N02-HL-64278. Genotyping was performed at Affymetrix (Santa Clara, CA) and the Broad Institute of Harvard and MIT (Boston, MA) using the Affymetrix Genome-Wide Human SNP Array 6.0. The Framingham Heart Study is funded by NIH contracts N01-HC25195, HHSN268201500001I, and 75N92019D00031. The research was partially supported by the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre; the views expressed are those of the author(s) and not necessarily those of the National Health Service, the NIHR, or the Department of Health. This research was funded in part by the Wellcome Trust. For the purpose of open access, the author has applied a creative commons by public copyright license to any Author Accepted Manuscript version arising from this submission. SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) was supported by NHLBI grants R01 HL137880 and NHLBI contracts HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, and HSN268200900020C, which were supplemented by contributions made through the Foundation for the NIH from AstraZeneca; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc; Chiesi Farmaceutici SpA; Forest Research Institute, Inc; GSK; Grifols Therapeutics, Inc; Ikaria, Inc; Nycomed GmbH; Takeda Pharmaceutical Company; Novartis Pharmaceuticals Corporation; Regeneron Pharmaceuticals, Inc; and Sanofi. Molecular data for the Trans-Omics in Precision Medicine (TOPMed) program were supported by the NHLBI. Whole-genome sequencing for NHLBI TOPMed: Pulmonary Fibrosis Whole Genome Sequencing (phs001607) was provided by Broad Genomics grant HHSN268201600034I and MGI grant HHSN268201600037I. Core support, including centralized genomic read mapping and genotype calling, together with variant quality metrics and filtering, were provided by the TOPMed Informatics Research Center (3R01HL-117626-02S1; contract HHSN268201800002I). Core support, including phenotype harmonization, data management, sample-identity QC, and general program coordination were provided by the TOPMed Data Coordinating Center R01HL-120393; U01HL-120393; and contract HHSN268201800001I).
Author Contributions: Study design: M.M., A.L.P., D.A.S., B.D.H., T.E.F., M.H.C., and G.M.H. Acquisition, analysis, or interpretation of the data: M.M., A.L.P., J.S.K., C.L.D., H.X., A.M., B.D.H., H.H., M.N., R.K.P., T.E.F., D.A.S., G.M.H., and M.H.C. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: M.M., A.L.P., J.S.K., C.L.D., A.M., H.X., J.D., D.A.S., T.E.F., G.M.H., and M.H.C. Obtained funding: D.A.S., A.M., G.T.O’C., E.K.S., M.H.C., and G.M.H.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202212-2257OC on July 31, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med . 2018;378:1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 2.Hewson T, McKeever TM, Gibson JE, Navaratnam V, Hubbard RB, Hutchinson JP.Timing of onset of symptoms in people with idiopathic pulmonary fibrosis. 2017. [DOI] [PubMed]
- 3. Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med . 2014;2:566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 4. Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA . 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet . 2013;45:613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, Lane KB, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax . 2004;59:977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allen RJ, Guillen-Guio B, Oldham JM, Ma SF, Dressen A, Paynton ML, et al. Genome-wide association study of susceptibility to idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2020;201:564–574. doi: 10.1164/rccm.201905-1017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. COPDGene Investigators Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med . 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med . 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hobbs BD, Putman RK, Araki T, Nishino M, Gudmundsson G, Gudnason V, et al. Overlap of genetic risk between interstitial lung abnormalities and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2019;200:1402–1413. doi: 10.1164/rccm.201903-0511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moll M, Hobbs BD, Menon A, Ghosh AJ, Putman RK, Hino T, et al. Blood gene expression risk profiles and interstitial lung abnormalities: COPDGene and ECLIPSE cohort studies. Respir Res . 2022;23:157. doi: 10.1186/s12931-022-02077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khera AV, Chaffin M, Wade KH, Zahid S, Brancale J, Xia R, et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell . 2019;177:587–596.e9. doi: 10.1016/j.cell.2019.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khera AV, Chaffin M, Zekavat SM, Collins RL, Roselli C, Natarajan P, et al. Whole-genome sequencing to characterize monogenic and polygenic contributions in patients hospitalized with early-onset myocardial infarction. Circulation . 2019;139:1593–1602. doi: 10.1161/CIRCULATIONAHA.118.035658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet . 2018;50:1219–1224. doi: 10.1038/s41588-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shrine N, Guyatt AL, Erzurumluoglu AM, Jackson VE, Hobbs BD, Melbourne CA, et al. Understanding Society Scientific Group New genetic signals for lung function highlight pathways and chronic obstructive pulmonary disease associations across multiple ancestries. Nat Genet . 2019;51:481–493. doi: 10.1038/s41588-018-0321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moll M, Sakornsakolpat P, Shrine N, Hobbs BD, DeMeo DL, John C, et al. International COPD Genetics Consortium; SpiroMeta Consortium Chronic obstructive pulmonary disease and related phenotypes: polygenic risk scores in population-based and case-control cohorts. Lancet Respir Med . 2020;8:696–708. doi: 10.1016/S2213-2600(20)30101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Namba S, Lopera E, Kerminen S, Tsuo K, Läll K, et al. Global biobank analyses provide lessons for computing polygenic risk scores across diverse cohorts [preprint] 2021. https://www.medrxiv.org/content/10.1101/2021.11.18.21266545v3 [DOI] [PMC free article] [PubMed]
- 18. Belsky DW, Sears MR, Hancox RJ, Harrington H, Houts R, Moffitt TE, et al. Polygenic risk and the development and course of asthma: an analysis of data from a four-decade longitudinal study. Lancet Respir Med . 2013;1:453–461. doi: 10.1016/S2213-2600(13)70101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Demenais F, Margaritte-Jeannin P, Barnes KC, Cookson WOC, Altmüller J, Ang W, et al. Australian Asthma Genetics Consortium (AAGC) collaborators Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat Genet . 2018;50:42–53. doi: 10.1038/s41588-017-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W, Kanai M, Wu KHW, Humaira R, Tsuo K, Hirbo JB, et al. Global Biobank Meta-analysis Initiative: powering genetic discovery across human diseases [preprint] 2021. https://www.medrxiv.org/content/10.1101/2021.11.19.21266436v1 [DOI] [PMC free article] [PubMed]
- 21. Zhu Q-Q, Zhang XL, Zhang SM, Tang SW, Min HY, Yi L, et al. Association between the MUC5B promoter polymorphism rs35705950 and idiopathic pulmonary fibrosis: a meta-analysis and trial sequential analysis in Caucasian and Asian populations. Medicine (Baltimore) . 2015;94:e1901. doi: 10.1097/MD.0000000000001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allen RJ, Stockwell A, Oldham JM, Guillen-Guio B, Schwartz DA, Maher TM, et al. International IPF Genetics Consortium Genome-wide association study across five cohorts identifies five novel loci associated with idiopathic pulmonary fibrosis. Thorax . 2022;77:829–833. doi: 10.1136/thoraxjnl-2021-218577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Putman RK, Gudmundsson G, Araki T, Nishino M, Sigurdsson S, Gudmundsson EF, et al. The MUC5B promoter polymorphism is associated with specific interstitial lung abnormality subtypes. Eur Respir J . 2017;50:1700537. doi: 10.1183/13993003.00537-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leavy OC, Ma SF, Molyneaux PL, Maher TM, Oldham JM, Flores C, et al. Proportion of idiopathic pulmonary fibrosis risk explained by known common genetic loci in European populations. Am J Respir Crit Care Med . 2021;203:775–778. doi: 10.1164/rccm.202008-3211LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leonenko G, Baker E, Stevenson-Hoare J, Sierksma A, Fiers M, Williams J, et al. Identifying individuals with high risk of Alzheimer’s disease using polygenic risk scores. Nat Commun . 2021;12:4506. doi: 10.1038/s41467-021-24082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang IV, Pedersen BS, Rabinovich E, Hennessy CE, Davidson EJ, Murphy E, et al. Relationship of DNA methylation and gene expression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2014;190:1263–1272. doi: 10.1164/rccm.201408-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med . 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghosh AJ, Hobbs BD, Yun JH, Saferali A, Moll M, Xu Z, et al. NHLBI Trans-Omics for Precision Medicine (TOPMed) Consortium Lung tissue shows divergent gene expression between chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Respir Res . 2022;23:97. doi: 10.1186/s12931-022-02013-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic Epidemiology of COPD (COPDGene) study design. COPD . 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham offspring study. Am J Epidemiol . 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 31. Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, et al. The third generation cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol . 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 32. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol . 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 33. Choi SW, Mak TS-H, O’Reilly PF. Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc . 2020;15:2759–2772. doi: 10.1038/s41596-020-0353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mak TSH, Porsch RM, Choi SW, Zhou X, Sham PC. Polygenic scores via penalized regression on summary statistics. Genet Epidemiol . 2017;41:469–480. doi: 10.1002/gepi.22050. [DOI] [PubMed] [Google Scholar]
- 35. Sakornsakolpat P, Prokopenko D, Lamontagne M, Reeve NF, Guyatt AL, Jackson VE, et al. SpiroMeta Consortium; International COPD Genetics Consortium Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat Genet . 2019;51:494–505. doi: 10.1038/s41588-018-0342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hatabu H, Hunninghake GM, Richeldi L, Brown KK, Wells AU, Remy-Jardin M, et al. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner Society. Lancet Respir Med . 2020;8:726–737. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med . 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Putman RK, Gudmundsson G, Axelsson GT, Hida T, Honda O, Araki T, et al. Imaging patterns are associated with interstitial lung abnormality progression and mortality. Am J Respir Crit Care Med . 2019;200:175–183. doi: 10.1164/rccm.201809-1652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wray NR, Yang J, Goddard ME, Visscher PM. The genetic interpretation of area under the ROC curve in genomic profiling. PLoS Genet . 2010;6:e1000864. doi: 10.1371/journal.pgen.1000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics . 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics . 1988;44:837–845. [PubMed] [Google Scholar]
- 42. Schupp JC, Kaminski N. Toward early detection of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2019;200:1339–1340. doi: 10.1164/rccm.201908-1530ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jenkins RG. Three steps to cure pulmonary fibrosis. Step 1: the runaway train or Groundhog Day? Am J Respir Crit Care Med . 2020;201:1172–1174. doi: 10.1164/rccm.202002-0260ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hunninghake GM, Quesada-Arias LD, Carmichael NE, Martinez Manzano JM, Poli De Frías S, Baumgartner MA, et al. Interstitial lung disease in relatives of patients with pulmonary fibrosis. Am J Respir Crit Care Med . 2020;201:1240–1248. doi: 10.1164/rccm.201908-1571OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Axelsson GT, Gudmundsson G, Pratte KA, Aspelund T, Putman RK, Sanders JL, et al. The proteomic profile of interstitial lung abnormalities. Am J Respir Crit Care Med . 2022;206:337–346. doi: 10.1164/rccm.202110-2296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Allen RJ, Oldham JM, Jenkins DA, Leavy OC, Guillen-Guio B, Melbourne CA, et al. CleanUP-IPF Investigators of the Pulmonary Trials Cooperative Longitudinal lung function and gas transfer in individuals with idiopathic pulmonary fibrosis: a genome-wide association study. Lancet Respir Med . 2023;11:65–73. doi: 10.1016/S2213-2600(22)00251-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aschard H, Tobin MD, Hancock DB, Skurnik D, Sood A, James A, et al. Understanding Society Scientific Group Evidence for large-scale gene-by-smoking interaction effects on pulmonary function. Int J Epidemiol . 2017;46:894–904. doi: 10.1093/ije/dyw318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim W, Moll M, Qiao D, Hobbs BD, Shrine N, Sakornsakolpat P, et al. Interaction of cigarette smoking and polygenic risk score on reduced lung function. JAMA Netw Open . 2021;4:e2139525. doi: 10.1001/jamanetworkopen.2021.39525. [DOI] [PMC free article] [PubMed] [Google Scholar]