Abstract
Substance use disorder (SUD) is a growing health problem that affects several millions of people worldwide, resulting in negative socioeconomic impacts and increased health care costs. Emerging evidence suggests that extracellular vesicles (EVs) play a crucial role in SUD pathogenesis. EVs, including exosomes and microvesicles, are membrane-encapsulated particles that are released into the extracellular space by most types of cells. EVs are important players in mediating cell-to-cell communication through transfer of cargo such as proteins, lipids and nucleic acids. The EV cargo can alter the status of recipient cells, thereby contributing to both physiological and pathological processes; some of these play critical roles in SUD. Although the functions of EVs under several pathological conditions have been extensively reviewed, EV functions and potential applications in SUD remain less studied. In this review, we provide an overview of the current knowledge of the role of EVs in SUD, including alcohol, cocaine, heroin, marijuana, nicotine and opiate abuse. The review will focus on the biogenesis and cargo composition of EVs as well as the potential use of EVs as biomarkers of SUD or therapeutic targets in SUD.
Keywords: Microvesicles, Exosomes, Alcohol, Cocaine, Heroin, Marijuana, Nicotine, Opiates
Introduction
Substance use disorder (SUD) is a chronic, relapsing disease caused by the persistent use of drugs such as alcohol, cocaine, tobacco, and opioids. SUD is now a significant public health problem that results in increased morbidity, mortality, loss of productivity, and increased health care costs [1]. The underlying mechanisms of SUD, however, have yet to be fully explored. While there are several cellular processes linked to causing SUD, emerging evidence suggests that alterations in the quantity and the biological content of extracellular vesicles (EVs) play an essential role in SUD. Therefore, understanding how EVs are involved in the development of SUD could lead to the discovery of novel biomarkers and treatment options for this disease.
Extracellular vesicles are a heterogeneous group of membrane-bound vesicles that are released by various types of cells [2]. EVs carry cargo of nucleic acids, proteins and lipids that can be exchanged between cells [3–5]. As per the International Society of Extracellular Vesicles (ISEV) classification, EVs, which range in diameter from 20 to 1000 nm, consist of several subclasses, including exosomes, microparticles (also termed ectosomes, microvesicles, shedding vesicles, exosome-like vesicles, nanoparticles), and apoptotic bodies [6–8]. Historically, EVs were identified as early as the 1970s when Aaronson et al. found that Ochromonas danica synthesized a variety of large and small intra and extra-cellular membrane-bounded structures derived from membranes associated with the flagella, mitochondria, chloroplasts and plasma membrane [9]. Work in the 1980s identified that the transferrin receptors located within reticulocytes were also linked with 50-nm-sized vesicles that were released into the extracellular space as the reticulocytes matured [10–12]. Since then, EVs have been purified and characterized from several mammalian as well as prokaryotic cells.
The importance of EVs lies in their ability to mediate cell-to-cell communication and their significant roles in various normal physiological processes as well as in pathological conditions such as cardiovascular disease (CVD) [13–15], cancer [16, 17], inflammation [18], and SUD [19–21]. Literature that describes the role of EVs and their cargo in the biogenesis and functional outputs related to drug abuse and addiction is reviewed here. Additionally, we provide a detailed analysis of how EVs could be used as biomarkers and therapeutic targets in SUD.
Biogenesis of EVs in SUD
The biogenesis of EVs occurs either dependent or independent of the endosomal sorting complex required for transport (ESCRT) pathway [22–24]. In the ESCRT dependent pathway, intraluminal vesicles (ILVs) are formed within large multivesicular bodies (MVBs) by invagination of late endosomal membranes that then accumulate proteins and cytosolic components or are trafficked to lysosomes for degradation [24]. The formation of ILVs is regulated by the ESCRT pathway which has been shown to facilitate MVB formation, vesicle budding, and protein cargo sorting [25]. The ESCRT machinery has four functional units known as ESCRT-0, I, II, and III that act together with other proteins to recruit cargo into the ILVs. Evidence also suggests that MVBs and ILVs can form independently of ESCRT function, instead involving proteins of the tetraspanin family (that include CD9, CD63, CD81, CD82, and CD151) [5]. For example, sorting of pre-melanosomal protein (PMEL) to the ILVs of MVBs in melanocytic cells is independent of ESCRT mechanisms [26] but requires the tetraspanin CD63 [27]. Similarly, CD63 can be instrumental in the formation of small (< 40 nm) ILVs in MVBs of HeLa cells, which form independently of the hepatocyte growth factor regulated tyrosine kinase substrate that acts in association with ESCRT-I [28]. The ESCRT-independent pathway has been shown to be mediated via raft-based microdomains that are highly enriched in sphingomyelinases [29]. Two lipid metabolism enzymes (neutral sphingomyelinase and phospholipase D2) have been shown to generate lipids in the limiting membrane of MVBs, which induce inward budding and, thus, formation of ILVs in an ESCRT-independent manner [22, 23]. These studies demonstrate that EVs can be formed by both ESCRT-dependent and independent mechanisms. In this section we will describe how the biogenesis of EVs is modulated by SUD based on the available literature.
Alcohol impairs glial and astrocytic function in the brain, and exposure to alcohol in prenatal stages alters the development of several brain regions such as the cerebellum, cortex, and hippocampus [30, 31]. Additionally, alcohol interferes with communication between nerve cells and suppresses excitatory nerve pathways [32]. Crenshaw B. et al. demonstrated that alcohol, increased heat shock protein-90 (HSP90) and decreased CD18 in the exosomes derived from BV-2 microglial cells [33]. Similarly, increased levels of HSP60, HSP70 and apoptotic proteins FAS and caspase 9 in EVs released from alcohol-stimulated HeLa cells have been observed [34]. In HIV-infected patients, the proteins hemopexin and properdin were decreased in the plasma EVs in HIV+ smokers and HIV+ drinkers compared to HIV+ patients that did not smoke or drink alcohol [35]. These findings indicate that HIV and drug abuse could alter the biogenesis of EVs through the tetraspanins such as CD63.
Cocaine use has also been shown to alter EV characteristics and content. Exposure of human glioblastoma cells to a low concentration of cocaine (150 nM) significantly increased the number of vesicles with 61–80 nm diameter, whereas exposure of these cells to higher concentrations of cocaine (300 nM and 150 μM) resulted in increased release of smaller vesicles (30–40 nm diameter) [36]. In another study, exposure to cocaine increased EV release from neuroblastoma cells through the dissociation of the sigma-1 receptor (Sig-1R) from ADP-ribosylation factor (ARF6), a G-protein regulating EV trafficking, leading to activation of myosin light chain kinase (MLCK) [37]. Trubetckaia et al. showed that cocaine exposure in mice resulted in an increase in EVs release in the serum and the brain [38]. Cocaine-mediated increase of Alix and CD63 in the brain was blocked in α-syn knockout mice, demonstrating the crucial role of α-syn in Alix-mediated formation of MVB ILVs [38]. In line with these findings, a recent study also demonstrated that the use of substances such as cocaine, psychostimulants, marijuana, opiates, and alcohol promoted the secretion of semen EVs in people living with HIV that enhanced actin reorganization, chemotactic migration and adhesion of monocytes [39]. These findings have established that substance abuse alters both the number and composition of EVs in various cells, although the exact underlying mechanisms warrant further investigation.
Composition of EVs in SUD
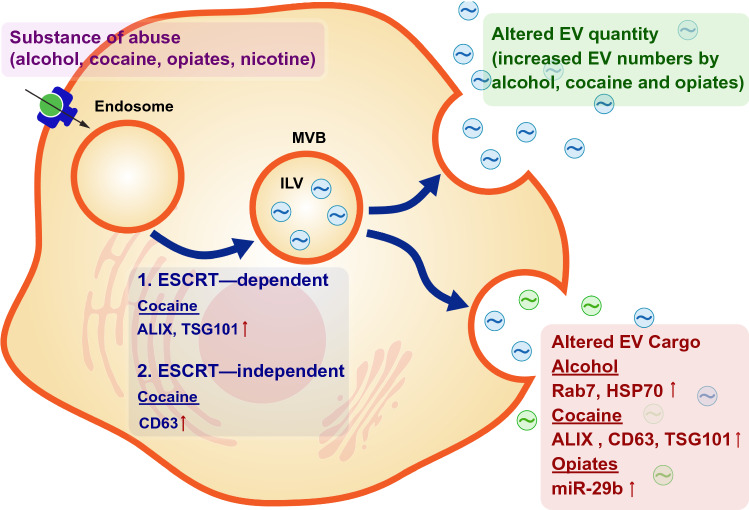
It is well established that EV cargo can include nucleic acids (messenger RNAs (mRNAs) and microRNAs (miRNA)), cytokines, organelles (mitochondria), bioactive lipids, peptides, ions, growth factors, proteins and transcription factors [3–5]. This diverse and vast cargo can be exchanged between cells, thereby contributing to intercellular communication in a multitude of physiological and pathological conditions, including those seen in SUD. In the context of SUD, several studies have focused on characterizing miRNA cargo; however, much less is known about other EV cargos. Consequently, there is a rapidly growing interest aimed at understanding EV composition and function in the context of SUD. In this section, we discuss the composition of EVs in the context of SUD (Fig. 1).
Fig. 1.
Substance abuse affects the biogenesis of Extracellular Vesicles (EVs). Drugs of abuse are taken up either by receptor-mediated mechanisms or by diffusion and are encapsulated as endosomes that can fuse with late endosomes to form multivesicular bodies (MVBs) containing intra-luminal vesicles (ILVs). In this process, drugs of abuse alter ESCRT and non-ESCRT components during biogenesis, ultimately resulting in altered EV cargo and/or release
Table 1 RNA and protein content of EVs and potential for use as biomarkers for SUD
Table 1.
RNA and protein content of EVs and potential for use as biomarkers for SUD
| Molecule and/or potential biomarker | Drug | Model | Potential source of biomarker | Methods | Change | Function | Ref |
|---|---|---|---|---|---|---|---|
| RNA species | |||||||
| miR-27a | Alcohol | Monocytes | Serum | qPCR | Up | M2 monocyte polarization | [20] |
| Let-7f, miR-29a, and miR-340 | Alcohol | Mouse hepatocytes | Serum | RNA-seq and qPCR | Up | Inflammation, liver injury | [43] |
| miR-122 and miR-155 | Alcohol | Mice | Serum | qPCR | Up | Liver damage and inflammation | [44], [44] |
| miR-122, miR-192 and miR-30a | Alcohol | Mice and humans | Serum | miRNA microarray and qPCR | Up | Liver damage and inflammation | [46] |
| miR-130a | Cocaine | Macrophages | Serum | RNA-seq and qPCR | Up | Pulmonary smooth muscle proliferation | [47] |
| miR-146a, miR-126, miR-21 and let-7a | Heroin | HIV+ heroin users | Serum | miRNA microarray and qPCR | Up | Immune regulation and inflammation | [48] |
| miR-145-3p and miR-181a-5p | Methamphetamine | Rat | Serum | Gene-chip sequencing and qPCR | Up | Neural plasticity and reward circuits | [49] |
| miR-29b | Morphine | Macaques | CSF, brain | miRNA microarray and qPCR | Up | Regulated PDGF-B and neuronal viability | [50] |
| Linc00355 | Opiates | Human | Urine | qPCR | Up | Cell proliferation | [51] |
| Malat 1 | Opiates | Human | Urine | qPCR | Down | Cell proliferation | [51] |
| Protein | |||||||
| CYP2E1 | Alcohol | Mice and humans | Serum | Immunoblot | Up | Oxidative hepatocyte injury | [52] |
| CD40L | Alcohol | Mice hepatocytes | Serum | Chemokine/cytokine array, immunoblot and immunogold EM | Up | Macrophage activation, inflammation | [53] |
RNA composition of EVs
Seminal studies have demonstrated that EVs contain functional RNA species [40, 41]. Specifically, EVs have been shown to contain mRNAs [40, 42], long non-coding RNAs (lncRNA) [54], miRNAs [40, 50], piwi-interacting RNAs, and ribosomal RNAs (rRNA) [54]. miRNA processing components such as Dicer and AGO1 have also been found within EVs [55–59]. Several miRNAs have been identified as being altered in EVs in animal models or humans affected by SUD (Table 1). For example, miR-27a, let-7f, miR-29a, miR-340, miR-122, miR-155, miR-122, miR-192 and miR-30a were found to be elevated in EVs from rodents exposed to alcohol [19, 43–46]. These miRNAs were implicated in alcohol-mediated polarization of monocytes into M2 proinflammatory status, liver injury and inflammation. In HIV-infected and cocaine-treated human monocyte-derived macrophages, Sharma et al., observed a significant increase in miR-130a levels in the EVs derived from these cells. Following the addition of these EVs to primary human pulmonary arterial smooth muscle cells, a decrease in the expression of miR-130a targeted molecules such as phosphatase and tensin homolog and tuberous sclerosis 1 and 2, and concomitant activation of PI3K/protein kinase B signaling was observed [47]. In HIV+ heroin users, Wang et al., showed that the levels of four neuroinflammation-related miRNAs (146a, 126, 21, and let-7a) in plasma exosomes were higher in HIV-infected heroin users as compared with the control individuals [48]. Similarly, opiates such as morphine have been shown to enhance HIV transactivator of transcription (Tat)-mediated toxicity in both human neurons and neuroblastoma cells [50]. Morphine and HIV Tat increased the release of miR-29b in EVs from astrocytes and exposure of neuronal SH-SY5Y cells to EVs from morphine-treated astrocytes showed a decrease in the expression of platelet-derived growth factor-B (PDGF-B), with a concomitant decrease in viability of neurons [50]. Interestingly, HIV infection and heroin also upregulated the majority (98%) of a panel of plasma exosomal miRNAs associated with immune regulation and inflammation [48].
Increased expression of miR-145-3p and miR-181a-5p has also been reported in serum exosomes from rats exposed to methamphetamine [49]. While reports of lncRNAs in SUD are still scarce, in one study the expression of LINC00355 and MALAT1 was found to be significantly lower in urinary exosomes isolated from cigarette smokers and opium-addicted patients with transitional cell carcinoma (TCC) when compared with controls. On the other hand, the expression of LINC00355 tended to be higher in opium-addicted TCC patients that did not smoke cigarettes compared to opium-addicted smokers [51].
Protein composition of EVs
Proteomic analyses have revealed a set of proteins commonly found in EVs that are routinely used to characterize EVs [3]. Due to their endosomal origin, exosomes contain classical membrane transport and fusion proteins (GTPases, annexins and flotillin), tetraspanins (CD9, CD63, CD81 and CD82), specific stress proteins (Hsc70 and Hsp90), protein members of the ESCRT (Alix and TSG101), and proteins involved in membrane fusion (Rabs and ARF6) [24, 60, 61]. EVs have also been described to contain ADAM10, ACE, EHD4, and major histocompatibility complex [3].
In the context of SUD, Cho et al. reported the increased expression of CYP2E1 in plasma EVs obtained from rats exposed to oral doses of binge ethanol or dextrose controls and also in humans with alcoholism [52]. These EVs from alcohol-exposed rats and patients with alcoholism were shown to be functional and could promote cell death in naïve cells [52]. Verma et al. found that exposure of hepatocytes to alcohol resulted in the release of EVs that contain CD40L in a caspase-dependent manner, which, in turn, led to macrophage activation and inflammation [53]. As of now, the role of EVs containing other types of cargos such as organelles, bioactive lipids, peptides and ions in SUD has not been well studied and deserves attention in the future.
Mechanisms and functions of EVs in SUD
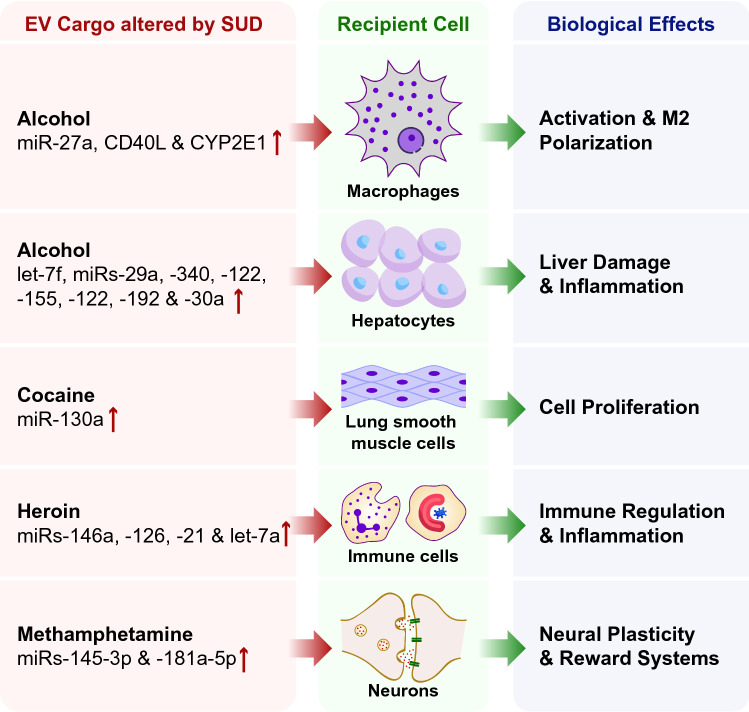
Due to their rich and unique composition and the inherent ability to interact with other cells, EVs play functional roles in many biological processes in the context of SUD, as shown in Fig. 2. EVs from macrophages exposed to alcohol are readily taken up by naïve macrophages leading, in turn, to cellular activation and polarization towards an inflammatory (M2) phenotype [20]. Hepatocytes exposed to alcohol released EVs that contain miRNA cargo that contributes to liver injury and inflammation [43]. In the context of cocaine exposure, macrophage-derived EVs contributed to a significant increase in the proliferation of primary human pulmonary arterial smooth muscle cells (HPASMCs) [47]. Plasma exosomes from HIV-infected heroin users have high levels of neuroinflammation-related miRNAs such as miRs-146a, -126, -21, and -let-7a that contribute to immune regulation and inflammation [48]. Plasma EVs released in the context of methamphetamine in rats are involved in the regulation of neural plasticity, reward circuits and the development of addiction [49]. These examples demonstrate the functional roles of EVs in inflammation, immune regulation, cell proliferation, as well as organ injury and damage (Fig. 2). In the following sub-sections, we discuss the current literature on the functional roles of EVs in alcohol, cocaine, marijuana, methamphetamine, nicotine and opioids.
Fig. 2.
Extracellular vesicles (EVs) released from cells in the context of substance abuse can exert various biological effects. EVs can activate macrophages and polarize these cells towards an M2 inflammatory phenotype, regulate immune function and inflammation in immune cells, induce liver damage in hepatocytes and modulate neural plasticity and reward circuits in neurons
Alcohol
According to the World Health Organization (WHO), alcohol abuse and its related complications contribute to 5% of the global health burden and 6% of total deaths worldwide (WHO 2014) [62]. Alcohol is known to cause liver damage and damage to other organs, including the central and peripheral nervous system, gastrointestinal tract, heart and vascular systems, and endocrine and immune systems [63]. More recently, it has been reported that alcohol intake accelerates several disease conditions such as HIV, tuberculosis and pneumonia (WHO 2014) [62]. Consequently, multiple studies have been carried out to investigate different alcohol-induced hepatic and extrahepatic complications [64, 65]. However, the detailed molecular mechanisms are poorly understood. Interestingly, several reports have identified the effects of alcohol abuse on EV release and altered EV functions, which may be associated with extrahepatic complications [66]. Exposure of human monocytes to alcohol led to increased release of EVs from these cells, which in turn stimulated naive monocytes to polarize into M2 macrophages [66]. These activated macrophages increased secretion of IL-10, TGF-1β and phagocytic activity. Further studies demonstrated that these effects were mediated by the upregulation of M2-polarizing miR-27a in EVs released from alcohol-exposed monocytes [66]. Another study showed that plasma exosomes that contain substantial amounts of CYP2E1 aggravated alcohol-induced toxicity in both hepatic and monocytic cells [21]. Inhibition of CYP2E1 enzyme activity abrogated the toxic effects in these cells. These authors also validated the induction of plasma exosomal CYP2E1 in a murine alcohol binge drinking model [21].
In a model of alcoholic liver disease (ALD), exposure to alcohol was shown to dysregulate the autophagy pathway and lysosomal function that was accompanied by increased exosome production [67]. In this study, the authors also demonstrated that the release of exosomes in the context of alcohol was regulated by miR-155 [67]. Exposure to alcohol not only affects EV release in peripheral cells but also in the central nervous system (CNS). In line with these findings, ethanol administration to astrocytes increased the number of secreted nanovesicles containing increased amounts of TLR-4, NF-κB-p65, IL-1R, caspase 1, NLRP3, and miR-146a and -182, and reduced amounts of miR-200b [68]. The authors further demonstrated that these EVs were taken-up by neurons, which increased the neuronal levels of the inflammatory protein Cox-2 and miR-146a, compromising the viability of the neuronal cells [68]. A recent report has also shown that exposure of microglia to alcohol resulted in increased exosome biogenesis as well as significantly impacted the morphology, viability and protein content of the microglia [33]. A recent study conducted on humanized mice demonstrated that HIV-infection and ethanol administration increased secretion of human hepatocyte-derived EVs into the serum and the increase in EVs secretion was associated with lysosomal dysfunction [69]. Overall, these studies showed that alcohol abuse impacted several cellular functions by altering the function and content of EVs, which could be considered important targets for abrogating the effects of alcohol abuse.
Cocaine
Cocaine is a naturally occurring and highly addictive stimulant drug. As per the National Survey on Drug Use and Health (NSDUH), there is relatively stable use of cocaine since 2009 [70]. The action of cocaine has been shown to block the functions of the dopamine transporter, thus increasing concentrations of synaptic dopamine in the reward pathways of the brain. In addition, it is well known that exposure to cocaine results in dysregulation of miRNA expression and synaptic plasticity, which, in turn, leads to an increased propensity for the consumption of cocaine. The miRNAs that have been reported to be altered by cocaine include miR-132 [71], miR-181a [72], miR-134 [73], miR-22 [73] and miR-124 [74]. As an example, exposure to cocaine has been shown to decrease the expression of miR-124 in the brain of cocaine-administered rodents [72, 75–77].
Delivery of these miRNAs into the recipient cells could be facilitated by EVs. In this regard, Jarvis et al. demonstrated that cocaine-induced downregulation of astroglial internalization of neuronal CD63-GFP+ exosomes resulted in decreased transferred neuron-derived miR-124-3p into astrocytes, which, in turn, lead to decreased GLT1 expression [30]. GLT1 is a protein that regulates synaptic plasticity in the nucleus accumbens (NAc) and is associated with cocaine-seeking behavior [78]. This work thus suggests that EV-miRNA-mediated interaction between neurons and astrocytes could contribute to cocaine addiction. In addition, emerging evidence suggested that cocaine could induce synthesis of the endocannabinoid 2-arachidonoylglycerol (2-AG) in the midbrain, which, in turn, resulted in increased activity of dopaminergic neurons that contribute to cocaine addiction [37]. Nakamura et al. [37] examined a novel pathway by which cocaine induces the release of 2-AG. The authors demonstrated that cocaine increased EV release in a Sig-1R dependent manner. Furthermore, cocaine can also induce the secretion of 2-AG via its interactions with the Sig-1R. This in turn led to the release of 2-AG in EVs, consequently engaging type-1 cannabinoid receptors (CB1) that contribute to cocaine addiction [37]. Another study revealed that cocaine exposure could also increase release of EVs by glioblastoma cells [36]. Sharma et al., demonstrated that HIV–infected and cocaine-treated human monocyte derived macrophages released a higher number of EVs compared to HIV-infected or uninfected cocaine-treated macrophages, with a significant increase in the particle size range to 100–150 nm. These EVs also had increased levels of miR-130a [47]. Overall, these studies showed that exposure to cocaine could increase not only the release of EVs but also the delivery of EV-miRNAs, which, in turn, contributes to cocaine addiction.
Marijuana
A recent study has shown that cannabidiol (CBD) is a potent inhibitor of the release of EVs in cancer cell lines such as prostate cancer (PC3), hepatocellular carcinoma (HEPG2),and breast adenocarcinoma (MDA-MB-231) [79]. It was also shown that cannabinoids sensitize cancer cells to chemotherapy. This study concluded that the anti-cancer effects of CBD are partly due to its effects on EV biogenesis, suggesting that CBD could be considered as a therapeutic agent for targeting EV-mediated pathological events [79]. In another study, exposure of glioblastoma cells to CBD resulted in released EVs containing reduced levels of pro-oncogenic miR-21 and increased levels of anti-oncogenic miR-126, compared to that of controls [80]. In addition, it was also observed that exposure of glioblastoma cells to CBD resulted in reduced expression of prohibitin, a multifunctional protein with mitochondrial protective properties and chemoresistant functions, suggesting that CBD has implications for the treatment of glioblastoma [80]. Of note, it was observed that EVs released from microglia serve as transporters of endocannabinoids. These endocannabinoids were associated on the surface of the EVs, leading to activation of CB1 and inhibit presynaptic transmission in target GABAergic neurons [81]. Only a few studies have been conducted on the effects of marijuana and its compounds on EVs. However, these reports show a strong effect of CBD on EV biogenesis, which open promising avenues for future research.
Methamphetamine
Methamphetamine is a potent psychostimulant that is among the most commonly used illicit drugs. There are over 35 million users worldwide, thus making methamphetamine abuse a significant global health crisis [82]. Emerging studies have demonstrated that acute and chronic doses of methamphetamine exposure resulted in long-term damage in many brain regions, leading to neurocognitive impairment. However, the mechanisms by which methamphetamine mediates neurotoxicity are still largely unknown.
The effect of methamphetamine on miRNA delivery via EVs has not been examined in detail. In one study, increased expression of miR-145-3p and miR-181a-5p was observed in the serum exosomes from methamphetamine exposed rats [49]. Another study in humans with methamphetamine use disorder demonstrated that the level of miR-9-3p was significantly increased in methamphetamine abusers compared with normal controls [83]. Furthermore, an in vivo study demonstrated that methamphetamine treatment increased the release of endothelial cell-derived EVs with Annexinv+/CD144+/CD41−/CD31+ phenotype [84]. These studies support the idea that EVs could serve as an efficient carrier of miRNAs contributing to methamphetamine-mediated neurotoxicity.
Studies have shown that methamphetamine use can exacerbate HIV-1 infection and HIV-associated neuropathogenesis [85–89]. Since methamphetamine exposure can facilitate the release of EVs [84] with HIV-1 components such as Nef proteins [90, 91] and TAR RNA [92, 93], EVs could play an essential role in the development of neuropathogenesis in HIV-1 + methamphetamine users [94].
Nicotine
Smoking of cigarettes is known as a leading cause of preventable disease and premature death all over the world. In the United States, approximately 435,000 people die prematurely from smoking-related diseases each year; overall, there is approximately a 50% chance that a lifelong smoker will die from a complication of smoking [95]. Cytokine profiling analysis revealed that the levels of plasma EV IL-8 and IL-6 expression was significantly upregulated in HIV-positive smokers compared with HIV-positive non-smokers and HIV-negative subjects, respectively [96]. The cytochromes P450 (CYPs)-mediated metabolites of Benzo[a]pyrene (BaP), a major carcinogen in cigarette smoke, have been shown to induce HIV-1 replication [97]. The levels of CYPs 1A1, 1B1, 3A4 were significantly upregulated in EVs derived from HIV-infected U1 cells treated with cigarette smoke condensate (CSC) compared with EVs derived from uninfected U937 cells treated with CSC [98], suggesting upregulated CYPs in EVs could contribute to the enhancement of HIV replication in macrophages. Interestingly, EVs released from CSC-exposed monocytic cells exhibited a protective effect against cytotoxicity [99], indicating a clinical value of EVs as proposed previously [100].
A recent study has demonstrated that nicotine exposure could result in the release of atherogenic exosomes from macrophages. These miRNA-containing exosomes mediate cellular crosstalk which, in turn, leads to proatherogenic phenotypes of vascular smooth muscle cells (VSMCs) [101]. The nicotine-mediated development of atherosclerosis is driven via macrophages-derived miR-21-3p inducing migration and proliferation of VSMC through its target phosphatase and tensin homolog (PTEN) [101]. In addition, nicotine has been shown to increase levels of circulating endothelial cell-derived and platelet-derived EVs, which could be the mechanism by which nicotine induces cardiovascular disease [102]. Although not many studies have been conducted on the effects of nicotine on EVs, the few reports show that EVs may serve as potential carriers of behavior-altering miRNAs that underly the mechanism(s) by which nicotine mediates the pathogenesis of several chronic diseases.
Opiates
Opiates are analgesics extensively used in clinical settings as well as drugs of abuse [103]. Chronic exposure leads to several complications leading to addiction, tolerance and cognitive impairment etc. [104]. EVs derived from morphine-stimulated astrocytes were shown to be taken up by microglial cells which caused activation of the TLR-7-lincRNA-Cox2 axis resulting in impaired microglial phagocytosis [105]. Additionally, intranasal delivery of EVs loaded with lincRNA-Cox2 siRNA restored microglial phagocytic activity in mice administered morphine, suggesting a role for EVs in morphine mediated dysregulation of microglial phagocytosis [105]. In another study, EVs derived from astrocytes that were exposed to morphine and HIV protein Tat were shown to contain miR-29b. When neuronal SH-SY5Y cells were exposed to these EVs, there was decreased expression of PDGF-B along with decreased viability of neurons. miR-29b was identified to target PDGF-B mRNA resulting in translational repression in SH-SY5Y cells. This study demonstrated the important role of miR-29b in the EVs and its regulation of PDGF-B in HIV-infected opiate addicts [50]. Moreover, morphine has also been shown to induce the expression of miR-138 in morphine-stimulated astrocyte-derived EVs, which can be taken up by microglial cells and, in turn, activates the TLR7-NF-kB axis and ultimately leading to microglial activation [106].
Several miRNAs, namely miR-15b, 181, 125b, and the let-7 family, have been implicated in morphine-induced tolerance as well as expression of the µ-opioid receptor. Chronic morphine treatment led to time-dependent increased expression of let-7 both in in vitro and in vivo models, which was associated with tolerance [107]. It has also been shown that exosomes loaded with µ-opioid receptor siRNA can effectively be used as treatment for morphine relapse [108]. Detailed studies on EVs from opiate-exposed cells as well as addicts will be necessary for developing strategies to cope with opioid tolerance leading to addiction.
EVs as potential biomarkers for SUD
The literature reviewed here clearly shows that several substances of abuse such as alcohol, cocaine, marijuana, methamphetamine, nicotine and opiates modulate the release of EVs and alter the constituents of these EVs. Besides their roles in cell-to-cell communication, EVs have the potential to serve as potential biomarkers since their counts, content, and origin might provide useful information about pathophysiology. Consequently, several research groups are interested and focused on examining the role of these EVs as potential biomarkers. The potential to use EVs as biomarkers for the diagnosis and prognosis of diseases is supported in part by the stability of exosomal cargo in plasma [109, 110]. In addition, EVs can easily be obtained from blood and urine. In fact, EVs have long been considered as sources of potential molecular biomarkers for the early detection, monitoring and evaluation of drug response in various diseases [111]. In this section, we discuss the potential of EVs as biomarkers of SUD.
Several types of biomarkers can be used in liquid biopsies. Table 1 summarizes potential biomarkers based on the altered composition of EVs in several SUD involving alcohol, cocaine, marijuana, methamphetamine, nicotine and opioids. To our knowledge, there are currently no universal biomarkers associated with SUD; however, the difference in EV composition may serve as potential biomarkers. Given that EVs can cross the blood–brain barrier, brain-derived EVs in the plasma could serve as biomarkers of neuropathogenesis [112–115]. For example, the numbers of neuron-derived EVs in the plasma of neuropsychologically impaired individuals were decreased compared with normal controls [115]. The levels of high-mobility group box 1 (HMGB1), NF-L, and amyloid β proteins were upregulated in the plasma neuron-derived EVs from neuropsychologically impaired individuals were decreased compared with normal controls [115]. Additionally, astrocytic and neuronal-specific proteins—GFAP and L1CAM—are elevated in the plasma EVs from HIV-positive alcohol or tobacco users compared to HIV-positive nonsubstance users [112].
A study on ethanol-fed mice showed that increased CYP2E1 levels in EVs could serve as a general marker of liver injury [52]. Likewise, increased levels of three miRNAs (let-7f, miR-29a, and miR-340) in the blood EVs are associated with alcoholic steatohepatitis (ASH) in mice [43]. Four miRNAs (miR-146a, miR-126, miR-21, and let-7a) were also found elevated in the plasma of HIV-1 infected heroin users, which make them potential biomarkers for diagnosis and prognosis of the neuroinflammatory disease [48]. High specificity and sensitivity of lncRNAs UCA1-201, UCA1-203, MALAT1, and LINC00355 have been reported previously to have potential for biomarkers in the diagnosis of bladder cancer in opium-addicted and cigarette smokers [51]. Presence of elevated levels of miR-145-3p and miR-181a-5p in serum EVs has been associated with methamphetamine addiction [49]. Three miRNAs, including let-7b-5p, miR-206, and miR-486-5p, were verified to be significantly and steadily increased in heroin abusers [53] and miR-9-3p was significantly increased in methamphetamine abusers compared with normal controls, demonstrating their ability as biomarkers [83]. It is interesting to note that in most of these studies listed in Table 1, not only one miRNA is altered, but also several of them. This suggests the need to develop panels of miRNAs as biomarkers that also need validation in large cohorts of study participants. Although EVs demonstrate promise as potential biomarkers, their clinical applicability is currently limited by lack of well-powered clinical studies investigating the correlation between EV biomarkers and SUD or SUD-related organ injury.
EVs as potential therapeutic vehicles for SUD
Substance abuse has been demonstrated to increase the release of endogenous EVs and alter the composition of the EVs that are released [36], demonstrating a reliance of the host system on EV signaling in response to drug exposure. Several studies have evaluated EVs as therapeutic vehicles because of their ability to carry diverse payloads, their favorable immunogenic profiles, stability in circulation, biocompatibility, and low toxicity [105, 116]. Though there are many benefits to using EVs as therapies, potential side effects should also be considered. For example, EVs and their cargo have been shown to induce inflammation [117]. Full characterization and evaluation of EV properties such as cargo and source are required to better understand the promise of EV-based therapies. Moreover, optimizing tissue-targeted delivery of EVs remains one of the major challenges in the field. Therapeutic administration of engineered EVs could regulate cellular signals in the brain that perpetuate substance use and addiction as well as decrease the end-organ injury caused by substance use. Despite multiple studies addressing the miRNA and protein signaling involved in the abuse of nicotine [118], alcohol [119], opiates [120], cocaine [121], and cannabinoids [122] (Table 2), there are still minimal data directly demonstrating the EV-mediated shuttling of these molecules. Those that have been published are described below.
Table 2.
Selected miRNAs and proteins associated with substance addiction, withdrawal, and relapse that may be targeted by therapeutic EV-mediated delivery
| Molecule | Drug | Function | Reference |
|---|---|---|---|
| miRNA | |||
| miR-27a | Alcohol | M2 monocyte polarization | [20] |
| miR-124 | Alcohol | BDNF downregulation | [125] |
| miR-206 | Alcohol | BDNF downregulation | [126] |
| miR-9 | Alcohol | Ca2+ and K+ channel expression | [127] |
| miR-431 | Cocaine | Arc expression | [128] |
| miR-212 | Cocaine | CREB activation | [129, 130] |
| miR-101b | Cocaine | [128] | |
| miR-132 | Cocaine | CREB and BDNF-mediated synaptic plasticity | [128, 130] |
| miR-137 | Cocaine | [128] | |
| miR-190 | Fentanyl | µ opioid receptor expression | [131] |
| miR-218 | Heroin | Gabrb3, GluR2, Ube3a, Nrxn1, Gng3, and Mecp2 expression | [132] |
| Let-7d | Marijuana | CB1 receptor signaling | [122] |
| Let-7a/c/g | Morphine | µ opioid receptor expression | [107] |
| miR-27a | Morphine | Serpini1 expression | [133, 134] |
| miR-29b | Morphine | [50] | |
| miR-140-5p | Nicotine | Inhibits Dynamin-1 expression | [135] |
| miR-504 | Nicotine | Upregulates dopamine D1 receptors | [136] |
| miR-542-3p | Nicotine | Increased nicotinic acetylcholine receptors | [137] |
| Protein | |||
| GLT-1 | Alcohol | Glutamate transport | [138, 139] |
| mTORC1 | Alcohol | Protein synthesis/translation | [140] |
| LGALS3 | Cocaine (+ HIV) | Neuronal migration | [141] |
| GLUL | Cocaine (+ HIV) | Glutamate detoxification | [141] |
| HBB/HBD | Cocaine (+ HIV) | Learning and memory | [141] |
| MCP-5 | Methamphetamine | Chemokine | [142] |
| sTNFR1 | Methamphetamine | Chemokine | [142] |
| NMDAR1 | Morphine | Glutamate receptor | [143] |
| p-CREB | Morphine | Cellular transcription | [144] |
| Arc/Arg3.1 | Morphine | Synaptic plasticity/memory | [145, 146] |
Decreasing substance dependence and relapse
Some of the earliest research into EV therapy in substance dependence and relapse focused on alcohol exposure. Chronic alcohol consumption is known to cause neuroinflammation resulting in CNS toxicity. This proinflammatory state appears to play a role in propagating additional voluntary alcohol consumption in animals [123]. As such, it has been hypothesized that the anti-inflammatory effects of mesenchymal stem cell (MSC)-derived EVs may decrease chronic alcohol consumption. A study performed in rats that were chronically consuming alcohol demonstrated that intranasal administration of MSC-derived exosomes inhibited alcohol intake by 84%, decreased relapses, and fully reversed neuroinflammation and hippocampal oxidative stress [124].
Since the study described above, much of the literature assessing the use of EVs as therapeutics for dependence and relapse has focused on opioid use. Exosomes from SH-SY5Y neuroblastoma cells have been pre-treated with sinomenine, an alkaloid used to prevent morphine dependence. When sinomenine pre-treated exosomes are administered to morphine-treated SH-SY5Y cells, the cells demonstrate a decrease in cAMP expression, intracellular Ca+, and expression of p-CREB/CREB compared to exosomes pre-treated with saline [143]. In our previous work, we demonstrated that morphine treatment is associated with an increase in exosomal miR-29b expression from astrocytes. When administering astrocyte-derived exosomes containing miR-29b to Tat protein-treated SH-SY5Y cells, we demonstrated attenuation of PDGF-BB expression and increased neuronal cytotoxicity [50]. Although this study focused on opioid effects in the context of HIV infection, it successfully demonstrated the impact of morphine on the exosomal delivery of miRNAs with a correlation to neuronal protein expression and cell survival.
Extracellular vesicles-mediated therapy may also be able to target substance use relapse. A study by Liu et al. demonstrated that engineering the membrane surface of EVs to express the rabies virus glycoprotein (RVG) peptide effectively delivers µ-opioid receptor siRNA into the brain, leading in turn to downregulation of µ-opioid receptor expression [108]. Importantly, delivery of µ-opioid receptor siRNA loaded EV to the brain prevented relapse in a mouse model of morphine addiction [108].
Additionally, other non-EV carriers have been used for suppression of drug addiction. For instance, the administration of glial cell line-derived neurotrophic factor (GDNF)-conjugated nanoparticles has been shown to decrease the amount of cocaine self-administration in rats [147]. More recently, exosomes have been found to carry pathogen antigens known to evoke immune response, and have, therefore, been examined as carriers for vaccination against various disease processes [148]. Although EVs have not yet been used for vaccination against addiction, nanoparticle-delivered toll-like receptor-based adjuvants have been shown to reduce the level of nicotine entering the brain and may therefore be a promising approach for treating nicotine addiction [149].
Role in repairing end-organ injury induced by SUD
Extracellular vesicles are known to play a major role in the inflammatory response of alcohol-induced liver disease (ALD) through several signaling pathways, including activation of Hsp90, Bax, and caspase-3 [19, 52]. Thus, the administration of exogenous engineered EVs or targeted modulation of endogenous EVs could result in decreased inflammation and fibrosis after ALD. Hepatic stellate cells are liver-specific mesenchymal cells that facilitate repair of the injured liver through deposition of fibrillar collagens. The continued activation of these cells in chronic disease processes such as ALD results in fibrosis, in part due to over-expression of the CCN2 protein [150]. Delivery of miR-214 enriched hepatic stellate cell-derived exosomes to either activated stellate cells or hepatocytes decreases the expression of CCN2 and may protect against fibrosis [151]. Stem cell-mediated recovery of liver injury may be mediated by glutathione peroxidase 1 (GPX1) [152].
Exosomes or exosome-mimetic nanovesicles from hepatocytes can also be used to aid in liver regeneration after ALD. The use of exosome-mimetic nanovesicles generated through serial extrusion of primary hepatocytes through polycarbonate membranes enhanced sphingosine kinase 2 (SK2) after delivery to hepatocytes, resulting in hepatocyte proliferation and liver regeneration [153]. A similar study used exosomes derived from primary murine hepatocytes and also demonstrated transfer of ceramidase and SK2 to injured hepatocytes, resulting in increased cell proliferation and liver regeneration both in vitro and in vivo [154].
Of note, the origin cell for EVs appears to be important in providing the regenerative effects in ALD. The promotion of hepatocyte proliferation was seen with administration of stellate cell- and hepatocyte-derived exosomes but was not demonstrated with exosomes derived from other liver cells such as Kupffer or sinusoidal endothelial cells [154]. Exosomes derived from non-liver stem cells may also provide benefit to the injured liver [155], however, and have the added benefit of potentially providing benefit to other non-liver organs when administered systemically.
Other organs have also been targeted for protection or repair by nanoparticle formulation, including using cerium oxide nanoparticles to inhibit reactive oxygen species production and cell death in cardiomyocytes after cigarette smoke exposure [156].
The future of EV-mediated therapies for SUD
There are no active clinical trials of EV-mediated therapies in drug abuse currently registered in Clinicaltrials.gov, though there are currently more than 20 active NIH-funded projects addressing this question. Most of these studies will provide additional pathophysiological insight into the effects of drug abuse on endogenous EV release and content. Three of the studies specifically focus on using EVs as potential therapeutic avenues. Given the widespread interest in EV signaling, it is likely that the literature in this field will continue to rapidly expand over the next several years.
Conclusions and perspectives
The literature reviewed and summarized here demonstrate the variety of cargo transported by EVs and their effects on biological functions in the context of SUD. The research to date has clearly highlighted the role of EV-mediated transfer of RNA (miRNAs and lncRNAs) and proteins that play important roles in immune regulation, inflammation, cell proliferation and organ injury. Additionally, EV features (number, size distribution, charge, etc.) and cargo (RNAs, DNAs, proteins) could serve as biomarkers and indicators for various human diseases, including SUD. The development of high-sensitivity single EV analysis techniques would significantly advance the potential to use EVs as biomarkers for diseases. Finally, the unique ability of EVs to cross biological barriers, such as the blood–brain barrier, makes EVs ideal for the delivery of therapeutics. Indeed, some studies have demonstrated this possibility, and studies on specific organ and cell type delivery of EVs are underway. All in all, the functional and application roles of EVs in the context of SUD open exciting possibilities for diagnostic and therapeutic advances.
Acknowledgments
We would like to thank the support from the Nebraska Centre for Substance Abuse Research (NCSAR).
Author contributions
Conceptualization, GH, EC, SB; writing-original draft preparation, EC, RD, EP, SS, LC, KL, and GH; writing-review and editing, EC, EP, RM, CBG, SB and GH. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NIH grant DA046831 (E.C., C.B.G and G.H.), DA042704 (G.H.), DA043138 (S.B. and G.H.), R35HG010719 (C.B.G), MH112848 (S.B. and G.H.), R01DA050545-02S1 (PI: S.B. and E.C. as Research Supplement recipient). L.C. acknowledges the support of The National Natural Science Foundation of China (62002212) and 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (2020LKSFG07D, 2020LKSFG04D). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ernest T. Chivero, Email: ernest.chivero@unmc.edu
Guoku Hu, Email: guoku.hu@unmc.edu.
References
- 1.Tenegra JC, Leebold B. Substance abuse screening and treatment. Prim Care. 2016;43(2):217–227. doi: 10.1016/j.pop.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113(8):E968–977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee TH, D'Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer–the emerging science of cellular 'debris'. Semin Immunol. 2011;33(5):455–467. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- 5.Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular Vesicles: composition, biological relevance, and methods of study. Bioscience. 2015;65(8):783–797. doi: 10.1093/biosci/biv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gamez-Valero A, Gardiner C, Gartner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Gorgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ, 2nd, Kornek M, Kosanovic MM, Kovacs AF, Kramer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lasser C, Laurent LC, Lavieu G, Lazaro-Ibanez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Line A, Linnemannstons K, Llorente A, Lombard CA, Lorenowicz MJ, Lorincz AM, Lotvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG, Jr, Meehan KL, Mertens I, Minciacchi VR, Moller A, Moller Jorgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saa P, Sahoo S, Salas-Huenuleo E, Sanchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schoyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL, 2nd, Soares RP, Sodar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ, Jr, Veit TD, Vella LJ, Velot E, Verweij FJ, Vestad B, Vinas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yanez-Mo M, Yin H, Yuana Y, Zappulli V, Zarubova J, Zekas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zappulli V, Friis KP, Fitzpatrick Z, Maguire CA, Breakefield XO. Extracellular vesicles and intercellular communication within the nervous system. J Clin Invest. 2016;126(4):1198–1207. doi: 10.1172/JCI81134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aaronson S, Behrens U, Orner R, Haines TH. Ultrastructure of intracellular and extracellular vesicles, membranes, and myelin figures produced by Ochromonas danica. J Ultrastruct Res. 1971;35(5):418–430. doi: 10.1016/s0022-5320(71)80003-5. [DOI] [PubMed] [Google Scholar]
- 10.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 11.Harding C, Stahl P. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem Biophys Res Commun. 1983;113(2):650–658. doi: 10.1016/0006-291x(83)91776-x. [DOI] [PubMed] [Google Scholar]
- 12.Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol. 2013;200(4):367–371. doi: 10.1083/jcb.201212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osteikoetxea X, Nemeth A, Sodar BW, Vukman KV, Buzas EI. Extracellular vesicles in cardiovascular disease: are they Jedi or Sith? J Physiol. 2016;594(11):2881–2894. doi: 10.1113/JP271336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hafiane A, Daskalopoulou SS. Extracellular vesicles characteristics and emerging roles in atherosclerotic cardiovascular disease. Metabolism. 2018;85:213–222. doi: 10.1016/j.metabol.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Rezaie J, Rahbarghazi R, Pezeshki M, Mazhar M, Yekani F, Khaksar M, Shokrollahi E, Amini H, Hashemzadeh S, Sokullu SE, Tokac M. Cardioprotective role of extracellular vesicles: a highlight on exosome beneficial effects in cardiovascular diseases. J Cell Physiol. 2019;234(12):21732–21745. doi: 10.1002/jcp.28894. [DOI] [PubMed] [Google Scholar]
- 16.D'Asti E, Chennakrishnaiah S, Lee TH, Rak J. Extracellular vesicles in brain tumor progression. Cell Mol Neurobiol. 2016;36(3):383–407. doi: 10.1007/s10571-015-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi D, Montermini L, Kim DK, Meehan B, Roth FP, Rak J. The impact of oncogenic EGFRvIII on the proteome of extracellular vesicles released from Glioblastoma cells. Mol Cell Proteomics. 2018;17(10):1948–1964. doi: 10.1074/mcp.RA118.000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JP, Mackman N. Monocytic microparticles activate endothelial cells in an IL-1beta-dependent manner. Blood. 2011;118(8):2366–2374. doi: 10.1182/blood-2011-01-330878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha B, Momen-Heravi F, Furi I, Kodys K, Catalano D, Gangopadhyay A, Haraszti R, Satishchandran A, Iracheta-Vellve A, Adejumo A. Extracellular vesicles from mice with alcoholic liver disease carry a distinct protein cargo and induce macrophage activation through heat shock protein 90. Hepatology. 2018;67(5):1986–2000. doi: 10.1002/hep.29732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha B, Momen-Heravi F, Kodys K, Szabo G. MicroRNA cargo of extracellular vesicles from alcohol-exposed monocytes signals naive monocytes to differentiate into M2 macrophages. J Biol Chem. 2016;291(1):149–159. doi: 10.1074/jbc.M115.694133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman MA, Kodidela S, Sinha N, Haque S, Shukla PK, Rao R, Kumar S. Plasma exosomes exacerbate alcohol- and acetaminophen-induced toxicity via CYP2E1 pathway. Sci Rep. 2019;9(1):6571. doi: 10.1038/s41598-019-43064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, Slavik J, Machala M, Zimmermann P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014;5:3477. doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- 23.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 24.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurley JH. ESCRTs are everywhere. EMBO J. 2015;34(19):2398–2407. doi: 10.15252/embj.201592484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell. 2006;10(3):343–354. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The tetraspanin CD63 regulates ESCRT-independent and dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edgar JR, Eden ER, Futter CE. Hrs and CD63-dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic. 2014;15(2):197–211. doi: 10.1111/tra.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Airola MV, Hannun YA. Sphingolipid metabolism and neutral sphingomyelinases. Handb Exp Pharmacol. 2013;215:57–76. doi: 10.1007/978-3-7091-1368-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarvis R, Tamashiro-Orrego A, Promes V, Tu L, Shi J, Yang Y. Cocaine self-administration and extinction inversely alter neuron to glia exosomal dynamics in the nucleus accumbens. Front Cell Neurosci. 2019;13:581. doi: 10.3389/fncel.2019.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore EM, Migliorini R, Infante MA, Riley EP. Fetal alcohol spectrum disorders: recent neuroimaging findings. Curr Dev Disord Rep. 2014;1(3):161–172. doi: 10.1007/s40474-014-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherjee S. Alcoholism and its effects on the central nervous system. Curr Neurovasc Res. 2013;10(3):256–262. doi: 10.2174/15672026113109990004. [DOI] [PubMed] [Google Scholar]
- 33.Crenshaw BJ, Kumar S, Bell CR, Jones LB, Williams SD, Saldanha SN, Joshi S, Sahu R, Sims B, Matthews QL. Alcohol modulates the biogenesis and composition of microglia-derived exosomes. Biology. 2019 doi: 10.3390/biology8020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones LB, Kumar S, Curry AJ, Price JS, Krendelchtchikov A, Crenshaw BJ, Bell CR, Williams SD, Tolliver TA, Saldanha SN, Sims B, Matthews QL. Alcohol exposure impacts the composition of hela-derived extracellular vesicles. Biomedicines. 2019 doi: 10.3390/biomedicines7040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kodidela S, Wang Y, Patters BJ, Gong Y, Sinha N, Ranjit S, Gerth K, Haque S, Cory T, McArthur C, Kumar A, Wan JY, Kumar S. Proteomic profiling of exosomes derived from plasma of hiv-infected alcohol drinkers and cigarette smokers. J Neuroimmune Pharmacol. 2019 doi: 10.1007/s11481-019-09853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carone C, Genedani S, Leo G, Filaferro M, Fuxe K, Agnati LF. In vitro effects of cocaine on tunneling nanotube formation and extracellular vesicle release in glioblastoma cell cultures. J Mol Neurosci. 2015;55(1):42–50. doi: 10.1007/s12031-014-0365-9. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura Y, Dryanovski DI, Kimura Y, Jackson SN, Woods AS, Yasui Y, Tsai SY, Patel S, Covey DP, Su TP, Lupica CR. Cocaine-induced endocannabinoid signaling mediated by sigma-1 receptors and extracellular vesicle secretion. Elife. 2019 doi: 10.7554/eLife.47209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trubetckaia O, Lane AE, Qian L, Zhou P, Lane DA. Alpha-synuclein is strategically positioned for afferent modulation of midbrain dopamine neurons and is essential for cocaine preference. Commun Biol. 2019;2:418. doi: 10.1038/s42003-019-0651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyu Y, Kaddour H, Kopcho S, Panzner TD, Shouman N, Kim EY, Martinson J, McKay H, Martinez-Maza O, Margolick JB, Stapleton JT, Okeoma CM. Human immunodeficiency virus (HIV) infection and use of illicit substances promote secretion of semen exosomes that enhance monocyte adhesion and induce actin reorganization and chemotactic migration. Cells. 2019 doi: 10.3390/cells8091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 41.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 42.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, Branski P, Ratajczak MZ, Zembala M. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006;55(7):808–818. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eguchi A, Lazaro RG, Wang J, Kim J, Povero D, Willliams B, Ho SB, Starkel P, Schnabl B, Ohno-Machado L, Tsukamoto H, Feldstein AE. Extracellular vesicles released by hepatocytes from gastric infusion model of alcoholic liver disease contain a MicroRNA barcode that can be detected in blood. Hepatology. 2017;65(2):475–490. doi: 10.1002/hep.28838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, Alao H, Kodys K, Szabo G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56(5):1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Momen-Heravi F, Bala S, Kodys K, Szabo G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci Rep. 2015;5:9991. doi: 10.1038/srep09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Momen-Heravi F, Saha B, Kodys K, Catalano D, Satishchandran A, Szabo G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J Transl Med. 2015;13:261. doi: 10.1186/s12967-015-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma H, Chinnappan M, Agarwal S, Dalvi P, Gunewardena S, O'Brien-Ladner A, Dhillon NK. Macrophage-derived extracellular vesicles mediate smooth muscle hyperplasia: role of altered miRNA cargo in response to HIV infection and substance abuse. FASEB J. 2018;32(9):5174–5185. doi: 10.1096/fj.201701558R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Sun L, Zhou Y, Su QJ, Li JL, Ye L, Liu MQ, Zhou W, Ho WZ. Heroin abuse and/or HIV infection dysregulate plasma exosomal miRNAs. J Neuroimmune Pharmacol. 2019 doi: 10.1007/s11481-019-09892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li HC, Lin YB, Li C, Luo CH, Zhou YT, Ou JY, Li J, Mo ZX. Expression of miRNAs in serum exosomes versus hippocampus in methamphetamine-induced rats and intervention of rhynchophylline. Evid Based Complement Alternat Med. 2018;2018:8025062. doi: 10.1155/2018/8025062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu G, Yao H, Chaudhuri AD, Duan M, Yelamanchili SV, Wen H, Cheney PD, Fox HS, Buch S. Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction. Cell Death Dis. 2012;3:e381. doi: 10.1038/cddis.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yazarlou F, Modarressi MH, Mowla SJ, Oskooei VK, Motevaseli E, Tooli LF, Nekoohesh L, Eghbali M, Ghafouri-Fard S, Afsharpad M. Urinary exosomal expression of long non-coding RNAs as diagnostic marker in bladder cancer. Cancer Manag Res. 2018;10:6357–6365. doi: 10.2147/CMAR.S186108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho YE, Mezey E, Hardwick JP, Salem N, Jr, Clemens DL, Song BJ. Increased ethanol-inducible cytochrome P450–2E1 and cytochrome P450 isoforms in exosomes of alcohol-exposed rodents and patients with alcoholism through oxidative and endoplasmic reticulum stress. Hepatol Commun. 2017;1(7):675–690. doi: 10.1002/hep4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, Cao S, Contreras PC, Malhi H, Kamath PS, Gores GJ, Shah VH. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2016;64(3):651–660. doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, Liang M, Dittmar RL, Liu Y, Liang M, Kohli M, Thibodeau SN, Boardman L, Wang L. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bukong TN, Momen-Heravi F, Kodys K, Bala S, Szabo G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 2014;10(10):e1004424. doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mantel PY, Hjelmqvist D, Walch M, Kharoubi-Hess S, Nilsson S, Ravel D, Ribeiro M, Gruring C, Ma S, Padmanabhan P, Trachtenberg A, Ankarklev J, Brancucci NM, Huttenhower C, Duraisingh MT, Ghiran I, Kuo WP, Filgueira L, Martinelli R, Marti M. Infected erythrocyte-derived extracellular vesicles alter vascular function via regulatory Ago2-miRNA complexes in malaria. Nat Commun. 2016;7:12727. doi: 10.1038/ncomms12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKenzie AJ, Hoshino D, Hong NH, Cha DJ, Franklin JL, Coffey RJ, Patton JG, Weaver AM. KRAS-MEK signaling controls Ago2 sorting into exosomes. Cell Rep. 2016;15(5):978–987. doi: 10.1016/j.celrep.2016.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, Lucci A, Ivan C, Calin GA, Kalluri R. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39(16):7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147(3):599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferguson SW, Nguyen J. Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J Control Release. 2016;228:179–190. doi: 10.1016/j.jconrel.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 62.Organization WH (2014) Global status report on alcohol and health 2014.:1–390. https://www.who.int/substance_abuse/publications/alcohol_2014/en/
- 63.Rusyn I, Bataller R. Alcohol and toxicity. J Hepatol. 2013;59(2):387–388. doi: 10.1016/j.jhep.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Res Health. 2007;30(1):38–41. [PMC free article] [PubMed] [Google Scholar]
- 65.Chan C, Levitsky J. Infection and alcoholic liver disease. Clin Liver Dis. 2016;20(3):595–606. doi: 10.1016/j.cld.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 66.Rahman MA, Patters BJ, Kodidela S, Kumar S. Extracellular vesicles: intercellular mediators in alcohol-induced pathologies. J Neuroimmune Pharmacol. 2019 doi: 10.1007/s11481-019-09848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Babuta M, Furi I, Bala S, Bukong TN, Lowe P, Catalano D, Calenda C, Kodys K, Szabo G. Dysregulated autophagy and lysosome function are linked to exosome production by micro-RNA 155 in alcoholic liver disease. Hepatology. 2019;70(6):2123–2141. doi: 10.1002/hep.30766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ibanez F, Montesinos J, Urena-Peralta JR, Guerri C, Pascual M. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte-derived extracellular vesicles. J Neuroinflammation. 2019;16(1):136. doi: 10.1186/s12974-019-1529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dagur RS, New-Aaron M, Ganesan M, Wang W, Romanova S, Kidambi S, Kharbanda KK, Poluektova LY, Osna NA. Alcohol-and-HIV-induced lysosomal dysfunction regulates extracellular vesicles secretion in vitro and in liver-humanized mice. Biology. 2021 doi: 10.3390/biology10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Health USDoHaHSSAaMHSAOoASNSoDUa (2009) Ann Arbor. MI: Inter-university Consortium for Political and Social Research 2015-11-23. https://doi.org/10.3886/ICPSR29621.v6
- 71.Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, Storm DR. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus. 2010;20(4):492–498. doi: 10.1002/hipo.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandrasekar V, Dreyer JL. Regulation of MiR-124, Let-7d, and MiR-181a in the accumbens affects the expression, extinction, and reinstatement of cocaine-induced conditioned place preference. Neuropsychopharmacology. 2011;36(6):1149–1164. doi: 10.1038/npp.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen CL, Liu H, Guan X. Changes in microRNA expression profile in hippocampus during the acquisition and extinction of cocaine-induced conditioned place preference in rats. J Biomed Sci. 2013;20:96. doi: 10.1186/1423-0127-20-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang K, Jing X, Wang G. MicroRNAs as regulators of drug abuse and immunity. Cent Eur J Immunol. 2016;41(4):426–434. doi: 10.5114/ceji.2016.65142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo ML, Periyasamy P, Liao K, Kook YH, Niu F, Callen SE, Buch S. Cocaine-mediated downregulation of microglial miR-124 expression involves promoter DNA methylation. Epigenetics. 2016 doi: 10.1080/15592294.2016.1232233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Periyasamy P, Liao K, Kook YH, Niu F, Callen SE, Guo ML, Buch S. Cocaine-mediated downregulation of miR-124 activates microglia by targeting KLF4 and TLR4 signaling. Mol Neurobiol. 2018;55(4):3196–3210. doi: 10.1007/s12035-017-0584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chandrasekar V, Dreyer JL. microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Mol Cell Neurosci. 2009;42(4):350–362. doi: 10.1016/j.mcn.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 78.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 79.Kosgodage US, Mould R, Henley AB, Nunn AV, Guy GW, Thomas EL, Inal JM, Bell JD, Lange S. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front Pharmacol. 2018;9:889. doi: 10.3389/fphar.2018.00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kosgodage US, Uysal-Onganer P, MacLatchy A, Mould R, Nunn AV, Guy GW, Kraev I, Chatterton NP, Thomas EL, Inal JM, Bell JD, Lange S. Cannabidiol affects extracellular vesicle release, miR21 and miR126, and reduces prohibitin protein in glioblastoma multiforme cells. Transl Oncol. 2019;12(3):513–522. doi: 10.1016/j.tranon.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gabrielli M, Battista N, Riganti L, Prada I, Antonucci F, Cantone L, Matteoli M, Maccarrone M, Verderio C. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep. 2015;16(2):213–220. doi: 10.15252/embr.201439668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roehr B. Half a million Americans use methamphetamine every week. BMJ. 2005;331(7515):476. doi: 10.1136/bmj.331.7515.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gu WJ, Zhang C, Zhong Y, Luo J, Zhang CY, Zhang C, Wang C. Altered serum microRNA expression profile in subjects with heroin and methamphetamine use disorder. Biomed Pharmacother. 2020;125:109918. doi: 10.1016/j.biopha.2020.109918. [DOI] [PubMed] [Google Scholar]
- 84.Nazari A, Zahmatkesh M, Mortaz E, Hosseinzadeh S. Effect of methamphetamine exposure on the plasma levels of endothelial-derived microparticles. Drug Alcohol Depend. 2018;186:219–225. doi: 10.1016/j.drugalcdep.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 85.Borgmann K, Ghorpade A. HIV-1, methamphetamine and astrocytes at neuroinflammatory crossroads. Front Microbiol. 2015;6:1143. doi: 10.3389/fmicb.2015.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gupta S, Bousman CA, Chana G, Cherner M, Heaton RK, Deutsch R, Ellis RJ, Grant I, Everall IP. Dopamine receptor D3 genetic polymorphism (rs6280TC) is associated with rates of cognitive impairment in methamphetamine-dependent men with HIV: preliminary findings. J Neurovirol. 2011;17(3):239–247. doi: 10.1007/s13365-011-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu G, Yelamanchili S, Kashanchi F, Haughey N, Bond VC, Witwer KW, Pulliam L, Buch S. Proceedings of the 2017 ISEV symposium on "HIV, NeuroHIV, drug abuse, and EVs". J Neurovirol. 2017;23(6):935–940. doi: 10.1007/s13365-017-0599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I, Group H Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- 89.Skowronska M, McDonald M, Velichkovska M, Leda AR, Park M, Toborek M. Methamphetamine increases HIV infectivity in neural progenitor cells. J Biol Chem. 2018;293(1):296–311. doi: 10.1074/jbc.RA117.000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McNamara RP, Costantini LM, Myers TA, Schouest B, Maness NJ, Griffith JD, Damania BA, MacLean AG, Dittmer DP. Nef secretion into extracellular vesicles or exosomes is conserved across human and simian immunodeficiency viruses. MBio. 2018 doi: 10.1128/mBio.02344-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sami Saribas A, Cicalese S, Ahooyi TM, Khalili K, Amini S, Sariyer IK. HIV-1 Nef is released in extracellular vesicles derived from astrocytes: evidence for Nef-mediated neurotoxicity. Cell Death Dis. 2017;8(1):e2542. doi: 10.1038/cddis.2016.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DeMarino C, Pleet ML, Cowen M, Barclay RA, Akpamagbo Y, Erickson J, Ndembi N, Charurat M, Jumare J, Bwala S, Alabi P, Hogan M, Gupta A, Noren Hooten N, Evans MK, Lepene B, Zhou W, Caputi M, Romerio F, Royal W, 3rd, El-Hage N, Liotta LA, Kashanchi F. Antiretroviral drugs alter the content of extracellular vesicles from HIV-1-infected cells. Sci Rep. 2018;8(1):7653. doi: 10.1038/s41598-018-25943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sampey GC, Saifuddin M, Schwab A, Barclay R, Punya S, Chung MC, Hakami RM, Zadeh MA, Lepene B, Klase ZA, El-Hage N, Young M, Iordanskiy S, Kashanchi F. Exosomes from HIV-1-infected cells stimulate production of pro-inflammatory cytokines through trans-activating response (TAR) RNA. J Biol Chem. 2016;291(3):1251–1266. doi: 10.1074/jbc.M115.662171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Odegaard KE, Chand S, Wheeler S, Tiwari S, Flores A, Hernandez J, Savine M, Gowen A, Pendyala G, Yelamanchili SV. Role of extracellular vesicles in substance abuse and HIV-related neurological pathologies. Int J Mol Sci. 2020 doi: 10.3390/ijms21186765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kodidela S, Ranjit S, Sinha N, McArthur C, Kumar A, Kumar S. Cytokine profiling of exosomes derived from the plasma of HIV-infected alcohol drinkers and cigarette smokers. PLoS ONE. 2018;13(7):e0201144. doi: 10.1371/journal.pone.0201144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ranjit S, Sinha N, Kodidela S, Kumar S. Benzo(a)pyrene in cigarette smoke enhances HIV-1 replication through NF-kappaB activation via CYP-mediated oxidative stress pathway. Sci Rep. 2018;8(1):10394. doi: 10.1038/s41598-018-28500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haque S, Kodidela S, Sinha N, Kumar P, Cory TJ, Kumar S. Differential packaging of inflammatory cytokines/ chemokines and oxidative stress modulators in U937 and U1 macrophages-derived extracellular vesicles upon exposure to tobacco constituents. PLoS ONE. 2020;15(5):e0233054. doi: 10.1371/journal.pone.0233054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haque S, Sinha N, Ranjit S, Midde NM, Kashanchi F, Kumar S. Monocyte-derived exosomes upon exposure to cigarette smoke condensate alter their characteristics and show protective effect against cytotoxicity and HIV-1 replication. Sci Rep. 2017;7(1):16120. doi: 10.1038/s41598-017-16301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ranjit S, Patters BJ, Gerth KA, Haque S, Choudhary S, Kumar S. Potential neuroprotective role of astroglial exosomes against smoking-induced oxidative stress and HIV-1 replication in the central nervous system. Expert Opin Ther Targets. 2018;22(8):703–714. doi: 10.1080/14728222.2018.1501473. [DOI] [PubMed] [Google Scholar]
- 101.Zhu J, Liu B, Wang Z, Wang D, Ni H, Zhang L, Wang Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9(23):6901–6919. doi: 10.7150/thno.37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mobarrez F, Antoniewicz L, Hedman L, Bosson JA, Lundback M. Electronic cigarettes containing nicotine increase endothelial and platelet derived extracellular vesicles in healthy volunteers. Atherosclerosis. 2020;301:93–100. doi: 10.1016/j.atherosclerosis.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 103.Johnson F, Setnik B. Morphine sulfate and naltrexone hydrochloride extended-release capsules: naltrexone release, pharmacodynamics, and tolerability. Pain Physician. 2011;14(4):391–406. doi: 10.36076/ppj.2011/14/391. [DOI] [PubMed] [Google Scholar]
- 104.Sil S, Periyasamy P, Guo ML, Callen S, Buch S. Morphine-mediated brain region-specific astrocytosis involves the ER stress-autophagy axis. Mol Neurobiol. 2018;55(8):6713–6733. doi: 10.1007/s12035-018-0878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu G, Liao K, Niu F, Yang L, Dallon BW, Callen S, Tian C, Shu J, Cui J, Sun Z, Lyubchenko YL, Ka M, Chen XM, Buch S. Astrocyte EV-induced lincRNA-cox2 regulates microglial phagocytosis: implications for morphine-mediated neurodegeneration. Mol Ther Nucleic Acids. 2018;13:450–463. doi: 10.1016/j.omtn.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liao K, Niu F, Hu G, Yang L, Dallon B, Villarreal D, Buch S. Morphine-mediated release of miR-138 in astrocyte-derived extracellular vesicles promotes microglial activation. J Extracell Vesicles. 2020;10(1):e12027. doi: 10.1002/jev2.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He Y, Yang C, Kirkmire CM, Wang ZJ. Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci. 2010;30(30):10251–10258. doi: 10.1523/JNEUROSCI.2419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X, Jiang X, Hou D, Chen X, Chen Y. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543. doi: 10.1038/srep17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cho YE, Song BJ, Akbar M, Baek MC. Extracellular vesicles as potential biomarkers for alcohol and drug-induced liver injury and their therapeutic applications. Pharmacol Ther. 2018;187:180–194. doi: 10.1016/j.pharmthera.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14(8):455–466. doi: 10.1038/nrgastro.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;7:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 112.Kodidela S, Gerth K, Sinha N, Kumar A, Kumar P, Kumar S. Circulatory astrocyte and neuronal EVs as potential biomarkers of neurological dysfunction in HIV-infected subjects and alcohol/tobacco users. Diagnostics. 2020 doi: 10.3390/diagnostics10060349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dagur RS, Liao K, Sil S, Niu F, Sun Z, Lyubchenko YL, Peeples ES, Hu G, Buch S. Neuronal-derived extracellular vesicles are enriched in the brain and serum of HIV-1 transgenic rats. J Extracell Vesicles. 2020;9(1):1703249. doi: 10.1080/20013078.2019.1703249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease. J Neurovirol. 2019;25(5):702–709. doi: 10.1007/s13365-018-0695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun B, Dalvi P, Abadjian L, Tang N, Pulliam L. Blood neuron-derived exosomes as biomarkers of cognitive impairment in HIV. AIDS. 2017;31(14):F9–F17. doi: 10.1097/QAD.0000000000001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sil S, Dagur RS, Liao K, Peeples ES, Hu G, Periyasamy P, Buch S. Strategies for the use of extracellular vesicles for the delivery of therapeutics. J Neuroimmune Pharmacol. 2020;15(3):422–442. doi: 10.1007/s11481-019-09873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perez PS, Romaniuk MA, Duette GA, Zhao Z, Huang Y, Martin-Jaular L, Witwer KW, Thery C, Ostrowski M. Extracellular vesicles and chronic inflammation during HIV infection. J Extracell Vesicles. 2019;8(1):1687275. doi: 10.1080/20013078.2019.1687275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee S, Woo J, Kim YS, Im H-I. Integrated miRNA-mRNA analysis in the habenula nuclei of mice intravenously self-administering nicotine. Sci Rep. 2015;5:12909. doi: 10.1038/srep12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lewohl JM, Nunez YO, Dodd PR, Tiwari GR, Harris RA, Mayfield RD. Up-regulation of microRNAs in brain of human alcoholics. Alcoholism. 2011;35(11):1928–1937. doi: 10.1111/j.1530-0277.2011.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barbierato M, Zusso M, Skaper D, Giusti P. MicroRNAs: emerging role in the endogenous μ opioid system. CNS Neurol Disord-Drug Targets. 2015;14(2):239–250. doi: 10.2174/1871527314666150116123932. [DOI] [PubMed] [Google Scholar]
- 121.Smith A, Kenny P. MicroRNAs regulate synaptic plasticity underlying drug addiction. Genes Brain Behav. 2018;17(3):e12424. doi: 10.1111/gbb.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chiarlone A, Börner C, Martín-Gómez L, Jiménez-González A, García-Concejo A, García-Bermejo ML, Lorente M, Blázquez C, García-Taboada E, de Haro A. MicroRNA let-7d is a target of cannabinoid CB1 receptor and controls cannabinoid signaling. Neuropharmacology. 2016;108:345–352. doi: 10.1016/j.neuropharm.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 123.Mayfield J, Ferguson L, Harris RA. Neuroimmune signaling: a key component of alcohol abuse. Curr Opin Neurobiol. 2013;23(4):513–520. doi: 10.1016/j.conb.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ezquer F, Quintanilla ME, Morales P, Santapau D, Ezquer M, Kogan MJ, Salas-Huenuleo E, Herrera-Marschitz M, Israel Y. Intranasal delivery of mesenchymal stem cell-derived exosomes reduces oxidative stress and markedly inhibits ethanol consumption and post-deprivation relapse drinking. Addict Biol. 2019;24(5):994–1007. doi: 10.1111/adb.12675. [DOI] [PubMed] [Google Scholar]
- 125.Bahi A, Dreyer JL. Striatal modulation of BDNF expression using micro RNA 124a-expressing lentiviral vectors impairs ethanol-induced conditioned-place preference and voluntary alcohol consumption. Eur J Neurosci. 2013;38(2):2328–2337. doi: 10.1111/ejn.12228. [DOI] [PubMed] [Google Scholar]
- 126.Tapocik JD, Barbier E, Flanigan M, Solomon M, Pincus A, Pilling A, Sun H, Schank JR, King C, Heilig M. microRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. J Neurosci. 2014;34(13):4581–4588. doi: 10.1523/JNEUROSCI.0445-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, Siegelmann HT, Treistman SN. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59(2):274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Quinn R, Brown A, Goldie B, Levi E, Dickson PW, Smith D, Cairns MJ, Dayas CV. Distinct miRNA expression in dorsal striatal subregions is associated with risk for addiction in rats. Transl Psychiatry. 2015;5(2):e503. doi: 10.1038/tp.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hollander JA, Im H-I, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, Kenny PJ. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466(7303):197. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sadakierska-Chudy A, Frankowska M, Miszkiel J, Wydra K, Jastrzębska J, Filip M. Prolonged induction of miR-212/132 and REST expression in rat striatum following cocaine self-administration. Mol Neurobiol. 2017;54(3):2241–2254. doi: 10.1007/s12035-016-9817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zheng H, Chu J, Zeng Y, Loh HH, Law P-Y. Yin Yang 1 phosphorylation contributes to the differential effects of μ-opioid receptor agonists on microRNA-190 expression. J Biol Chem. 2010;285(29):21994–22002. doi: 10.1074/jbc.M110.112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yan B, Hu Z, Yao W, Le Q, Xu B, Liu X, Ma L. MiR-218 targets MeCP2 and inhibits heroin seeking behavior. Sci Rep. 2017;7:40413. doi: 10.1038/srep40413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Borges VM, Lee TW, Christie DL, Birch NP. Neuroserpin regulates the density of dendritic protrusions and dendritic spine shape in cultured hippocampal neurons. J Neurosci Res. 2010;88(12):2610–2617. doi: 10.1002/jnr.22428. [DOI] [PubMed] [Google Scholar]
- 134.Tapocik JD, Ceniccola K, Mayo CL, Schwandt ML, Solomon M, Wang B-D, Luu TV, Olender J, Harrigan T, Maynard TM. MicroRNAs are involved in the development of morphine-induced analgesic tolerance and regulate functionally relevant changes in Serpini1. Front Mol Neurosci. 2016;9:20. doi: 10.3389/fnmol.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang W, Li MD. Nicotine modulates expression of miR-140*, which targets the 3′-untranslated region of dynamin 1 gene (Dnm1) Int J Neuropsychopharmacol. 2009;12(4):537–546. doi: 10.1017/S1461145708009528. [DOI] [PubMed] [Google Scholar]
- 136.Huang W, Li MD. Differential allelic expression of dopamine D1 receptor gene (DRD1) is modulated by microRNA miR-504. Biol Psychiat. 2009;65(8):702–705. doi: 10.1016/j.biopsych.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hogan EM, Casserly AP, Scofield MD, Mou Z, Zhao-Shea R, Johnson CW, Tapper AR, Gardner PD. miRNAome analysis of the mammalian neuronal nicotinic acetylcholine receptor gene family. RNA. 2014;20(12):1890–1899. doi: 10.1261/rna.034066.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rao P, Saternos H, Goodwani S, Sari Y. Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology. 2015;232(13):2333–2342. doi: 10.1007/s00213-015-3868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barak S, Liu F, Hamida SB, Yowell QV, Neasta J, Kharazia V, Janak PH, Ron D. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat Neurosci. 2013;16(8):1111. doi: 10.1038/nn.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dominy SS, Brown JN, Ryder MI, Gritsenko M, Jacobs JM, Smith RD. Proteomic analysis of saliva in HIV-positive heroin addicts reveals proteins correlated with cognition. PLoS ONE. 2014;9(4):e89366. doi: 10.1371/journal.pone.0089366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Narita M, Miyatake M, Narita M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31(11):2476. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- 143.Ou J, Zhou Y, Li C, Chen Z, Li H, Fang M, Zhu C, Huo C, Yung K, Li J. Sinomenine protects against morphine dependence through the NMDAR1/CAMKII/CREB pathway: a possible role of astrocyte-derived exosomes. Molecules. 2018;23(9):2370. doi: 10.3390/molecules23092370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang WS, Chen ZG, Liu WT, Chi ZQ, He L, Liu JG. Dorsal hippocampal NMDA receptor blockade impairs extinction of naloxone-precipitated conditioned place aversion in acute morphine-treated rats by suppressing ERK and CREB phosphorylation in the basolateral amygdala. Br J Pharmacol. 2015;172(2):482–491. doi: 10.1111/bph.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lv X-F, Sun L-L, Cui C-L, Han J-S. NAc shell Arc/Arg3 1. protein mediates reconsolidation of morphine CPP by increased GluR1 cell surface expression: activation of ERK-coupled CREB is required. Int J Neuropsychopharmacol. 2015 doi: 10.1093/ijnp/pyv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hearing MC, Schwendt M, McGinty JF. Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. Int J Neuropsychopharmacol. 2011;14(6):784–795. doi: 10.1017/S1461145710001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Green-Sadan T, Kuttner Y, Lublin-Tennenbaum T, Kinor N, Boguslavsky Y, Margel S, Yadid G. Glial cell line-derived neurotrophic factor-conjugated nanoparticles suppress acquisition of cocaine self-administration in rats. Exp Neurol. 2005;194(1):97–105. doi: 10.1016/j.expneurol.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 148.Devhare PB, Ray RB. A novel role of exosomes in the vaccination approach. Ann Transl Med. 2017;5(1):23. doi: 10.21037/atm.2016.12.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao Z, Harris B, Hu Y, Harmon T, Pentel PR, Ehrich M, Zhang C. Rational incorporation of molecular adjuvants into a hybrid nanoparticle-based nicotine vaccine for immunotherapy against nicotine addiction. Biomaterials. 2018;155:165–175. doi: 10.1016/j.biomaterials.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang G, Brigstock DR. Regulation of hepatic stellate cells by connective tissue growth factor. Front Biosci. 2012;17(2):2495–2507. doi: 10.2741/4067. [DOI] [PubMed] [Google Scholar]
- 151.Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, Tsukamoto H, Lee LJ, Paulaitis ME, Brigstock DR. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59(3):1118–1129. doi: 10.1002/hep.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Gong A, Qian H, Xu W. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther. 2017;25(2):465–479. doi: 10.1016/j.ymthe.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu J-Y, Ji A-L, Wang Z-X, Qiang G-H, Qu Z, Wu J-H, Jiang C-P. Exosome-Mimetic Nanovesicles from Hepatocytes promote hepatocyte proliferation in vitro and liver regeneration in vivo. Sci Rep. 2018;8(1):2471. doi: 10.1038/s41598-018-20505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, Gulbins E, Lentsch AB. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol. 2016;64(1):60–68. doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tan CY, Lai RC, Wong W, Dan YY, Lim S-K, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5(3):76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Niu J, Wang K, Kolattukudy PE. Cerium oxide nanoparticles inhibits oxidative stress and nuclear factor-κB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J Pharmacol Exp Ther. 2011;338(1):53–61. doi: 10.1124/jpet.111.179978. [DOI] [PMC free article] [PubMed] [Google Scholar]