Abstract
Background
Myasthenia gravis (MG) affects individuals as a chronic autoimmune disease for many years. Commonly, chronic diseases significantly reduce the patients’ quality of life. Aiming to improve the future quality of life in MG, this study assessed the factors impacting quality of life. As gender-specific medicine is becoming increasingly important, this study also focused on understanding gender differences in the outcome of MG.
Methods
The study is a combined monocentric, retrospective and prospective database analysis of patient records based on 2,370 presentations of 165 patients with clinically, serologically and/or electrophysiologically confirmed MG over an observation period of up to 47 years. The data collection included the following parameters: antibody status, disease severity, age, medication use, gender, and disease duration. In addition, a prospective survey was conducted on the quality of life using the Myasthenia gravis-specific 15-item Quality of Life scale (MG-QoL15) and on the activities of daily living using the MG-specific Activities of Daily Living scale (MG-ADL).
Results
Of the 165 patients, 85 were male (51.5%) and 80 were female (48.5%). The remaining baseline characteristics (e.g. age and antibody status) were consistent with other myasthenia gravis cohorts. A high body mass index (BMI) (p = 0.005) and a high disease severity (p < 0.001) were significantly associated with lower disease-specific quality of life. Additionally, the quality of life in women with MG was significantly reduced compared to male patients (19.7 vs. 13.0 points in the MG-QoL15, p = 0.024). Gender differences were also observable in terms of the period between initial manifestation and initial diagnosis and women were significantly more impaired in their activities of daily living (MG-ADL) than men (4.8 vs. 3.0 points, p = 0.032).
Conclusion
Women with MG had significantly poorer disease specific quality of life compared to men as well as patients with a higher BMI. In order to improve the quality of life, gender-specific medicine and further investigation regarding a modification of the quality of life by lowering the BMI are essential and necessary.
Trial registration
Study approval by the Ethics Committee of the University Medical Center Göttingen was granted (number 6/5/18).
Keywords: Myasthenia gravis, Quality of life, Gender medicine, MG-ADL
Background
Autoimmune myasthenia gravis (MG) is a chronic neuromuscular disease. The global prevalence is 12.4 people per 100,000 [1] making MG a rare disease. MG can manifest at any age [2]. MG with early onset affects mainly women, whereas the proportion of men with late onset predominates [2–4]. Overall, more women than men develop the disease, the ratio being three to two [5].The common clinical symptom of MG is a premature, physical strain-dependent and fluctuating muscle weakness [6], which can be highly heterogeneous in individuals with regard to severity and muscle involvement. MG is caused by different autoantibodies (AB) against the postsynaptic membrane of the neuromuscular junction [7–11].
The treatment regimens usually contain a combination of acetylcholinesterase inhibitors and different immunosuppressant drugs depending on the disease severity and comorbidities (8, 12–14). Some patients may require intravenous immunoglobulins (IVIG) and plasmapheresis (15). Thymectomy is currently one of the regular treatments for thymic hyperplasia or thymoma (16). Recently, several new targeted immunotherapies for the treatment of refractory and/or “active” MG have been developed and partially entered the market [17–19]. It is noteworthy, that MG-specific Activities of Daily Living (MG-ADL) and quality of life measured by the Myasthenia gravis-specific 15-item Quality of Life revised scale (MG-QoL15r) were among the primary and secondary outcome measures in clinical trials for the complement inhibitors and neonatal Fc receptor (FcRn) inhibitors [20–22].
Recently, the examination of the quality of life has become increasingly important in medicine, particularly in the field of neurology [23]. Chronic diseases such as MG can affect the quality of life negatively. Thus, the recording of the quality of life can help to identify possible influences on it and can also be used as a measurement for therapeutic effects on disease progression [24]. Therefore, the MG-QoL15 is available for disease-specific assessment of quality of life in MG. It consists of 15 questions and includes the categories mobility, symptoms, general satisfaction, and psychological well-being [25]. Previous studies have unanimously concluded that the quality of life of patients with MG is impaired. Gender, impairment in activities of daily living, pre-existing conditions, and disease severity were identified as influencing factors for disease specific quality of life [26–29].
The aim of the study was to identify the risk factors associated with reduced quality of life in MG and to examine gender-specific differences in quality of life, as well as in disease progression.
Methods
Patients and study design
The study can be classified as a mixed prospective-retrospective study. All patients, who were treated for MG at the University Medical Center Göttingen (UMG) between their initial diagnosis and November 30, 2019, and who gave their written informed consent to participate in the study were included. All the methods and procedures carried out in this study were in accordance with relevant guidelines and regulation. Starting point of the database analysis was the initial diagnosis of MG or – if the diagnosis was not made at UMG – the initial presentation at the neuromuscular outpatient clinic. End point was the last presentation in the outpatient clinic until 11/30/2019.
The retrospective database analysis included general disease information as well as specific information on the disease course and progression and medical history. This information was extracted from physicians’ letters, results of serological examinations, results of myasthenia gravis-specific tests (e.g. Quantitative Myasthenia Gravis Score (QMG)), evaluations of electrophysiological examinations, radiological findings, and classifications according to the Myasthenia gravis Foundation Association (MGFA) classification. All available documents of a patient since the initial diagnosis until the end of this study (11/30/2019) were considered for the database analysis.
Assessment of the quality of life and impairments in activities of daily living was prospectively collected during the data collection period (02/01/2018–11/30/2019) using the MG-QoL15 and the Myasthenia gravis-specific Activities of Daily Living scale (MG-ADL). In this study, the German translation of the MG-QoL15 questionnaire according to Fitzthum et al. (2015) was used (30). The questionnaires were filled in either during a presentation at the Department of Neurology at UMG or after postal delivery in the patient’s home. The disease severity was analyzed with the help of the QMG. There have been various modifications of the QMG in the last decades and the observation period of this study was long. For harmonization, the QMG version according to Barohn et al. has been chosen [31]. In order to adapt for historically missing values and to compare the QMG score between the patients, in this study the sum of QMG was divided by the number of tests performed, resulting in a total score between 0 and 3 points [32]. Since MG-Qol-15 was standard in our clinic during the years studied, we used it instead of MG-Qol-15r.
Statistical analyses
Statistical analyses were performed with the statistical software R (version 3.4.0; (R Core Team 2018)) using the R packages lmerTest (version 3.1.1 [33]) and lme4 (version 1.1.21 [34]) for the mixed linear models, and survival (version 3.2.7 [35]) for the Cox regression model. For all statistical analyses, the significance level was set at α = 5% (p ≤ 0.05). No correction for multiple testing was made because the present study was exploratory.
At the outset, descriptive data analysis was performed to highlight the characteristics of the patient cohort. In order to prevent a disproportionate influence of patients with many clinical presentations compared with patients with only a few presentations, the numerical data were always averaged per patient over the number of presentations. On this basis the mean, standard deviation, median and the minimum and maximum were calculated. The p-values for metric variables were derived using the Mann-Whitney U test. In order to calculate the p-values for categorical variables, Fisher’s exact test was applied. For thymic hyperplasia, thymomas, thymectomy, glucocorticoids, ciclosporine, cyclophosphamide and comorbidities we analyzed the number of patients with at least one reported occurrence during the patient’s observation period.
In a further step, correlations were tested using regression analyses. In order to include repeated measurements per patient into the evaluation, univariate and multivariate linear mixed models with a random intercept for each patient were fitted for this purpose. In the results section, the estimate and the standard error for each independent variable as well as for the intercept were given for the linear mixed models in addition to the p-value. The dependent variable MG-ADL was log(x + 1)-transformed for the regression analysis. For the analysis of association between BMI and the scores QMG, MG-QoL15 and MG-ADL, we faced the problem that there were only few cases where BMI and one of the scores were measured at the same day. Thus, we also considered BMI measurements from visits being closest to the day of the evaluations of the scores allowing a maximum time difference of one year.
As a sensitivity analysis we additionally included sex and age in all mixed linear models and obtained similar results.
In order to examine gender differences in the risk of myasthenic crisis Cox regression was used for the analysis of the time to the first crisis.
Results
Basic characteristics
The patient population consisted of a total of 165 individuals ranging from the age of 12 to 91 years throughout the entire observation period. 2,370 individual presentations were included in the database analysis. On average, patients were presented to the hospital 14 times during the study period, with a minimum of one and a maximum of 65 presentations per patient. The retrospective observation period per patient comprised up to 47 years. With regard to the prospective surveys of the MG-ADL, 82 questionnaires were completed and evaluated by 55 patients. In addition, 101 MG-QoL15 questionnaires from 64 patients were analyzed.
Over the entire observation period, the average mean age at the patients’ visit was 59.8 years. At first onset of the symptoms, the patients were on average 54.4 years old, and at the time of initial diagnosis, the average age was 55.4 years (Table 1). The majority of patients (42.4%) suffered from mild generalized disease (MGFA class II). The mean QMG score was 0.49 (SD 0.36), whereas the survey of disease-specific quality of life showed a mean score of 16.7 (SD 13.0) and of activities of daily living a mean score of 3.99 (SD 3.5). A summary of patients’ demographics, clinical status and disease activity is listed in Table 1 (see Table 1).
Table 1.
Basis characteristics
| Characteristic | value | Characteristic | value |
|---|---|---|---|
| Gender (Number of patients (%)) | Scores | ||
| Male | 85 (51.5%) | QMG modified (value ± SD) | 0.49 ± 0.4 |
| Female | 80 (48.5%) | Female | 0.56 ± 0.4 |
| Age at symptom onset (years ± SD) | 54.4 ± 19.3 | Male | 0.42 ± 0.3 |
| Female | 50.1 ± 20.8 | Missing data (number of patients) | 0 |
| Male | 58.4 ± 17.0 | ||
| Age at diagnosis (years ± SD) | 55.4 ± 18.9 | MG-ADL (value ± SD) | 3.99 ± 3.5 |
| Female | 51.9 ± 20.2 | Female | 4.8 ± 3.7 |
| Male | 58.7 ± 17.1 | Male | 3.0 ± 3.0 |
| BMI (kg/m2 ± SD) | 28.0 ± 5.5 | Missing data (number of patients) | 110 |
| Female | 27.0 ± 5.8 | ||
| Male | 29.1 ± 5.0 | MG-QoL15 (value ± SD) | 16.7 ± 13.0 |
| Missing data (number of patients) | 41 | Female | 19.7 ± 12.4 |
| Male | 13.0 ± 13.0 | ||
| MGFA (number of patients (%)) | Missing data (number of patients) | 101 | |
| I | 18 (10.9%) | ||
| II | 70 (42.4%) | Treatment (number of patients (%)) | |
| IIA | 34 (20.6%) | Pyridostigmine | 164 (99.4%) |
| IIB | 14 (8.5%) | Pyridostigmine extended release | 139 (84.2%) |
| Not class. | 22(13.3%) | Glucocorticosteroids | 143 (86.7% |
| III | 31 (18.8%) | IVIG | 44 (26.7%) |
| IIIA | 17 (10.3%) | AZA | 120 (72.7%) |
| IIIB | 10 (6.1%) | MMF | 47 (28.5%) |
| Not class. | 4 (2.4%) | Thymectomy (number of patients (%)) | 52 (31.5%) |
| IV | 27 (16.4%) | ||
| IVA | 2 (1.2%) | Thymoma | 17 (37.8%) |
| IVB | 24 (14.5%) | Thymic hyperplasia | 12 (26.7%) |
| Not class. | 1 (0.6%) | No pathology | 16 (35.6) |
| V | 19 (11.5%) | Myasthenic crisis in the past | |
| Serological status (number of patients (%)) | (number of patients (%)) | ||
| No | 123 (74.5%) | ||
| Anti-AChR-AB (+) (missing data n = 1) | 137 (83.5%) | Yes | 42 (25.5%) |
| Anti-MuSK-AB (+) (missing data n = 75) | 5 (5.6%) | - 1 crisis | 29 (17.6%) |
| Anti-Titin-AB (+) (missing data n = 63) | 36 (35.3%) | - 2 crisis | 5 (3.0%) |
| Anti-LRP4-AB (+) (missing data n = 161) | 0 (0%) | - > 2 crisis | 8 (4.8%) |
Effects on MG-QoL15
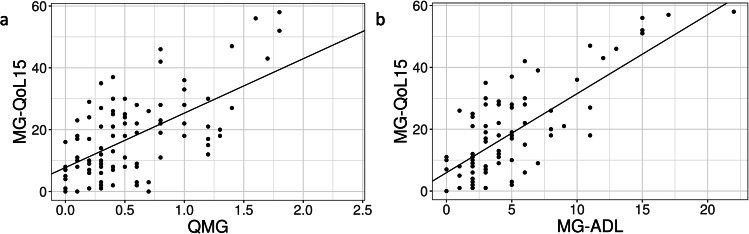
Linear mixed models identified a significant positive correlation between the QMG and the MG-QoL15: The higher the disease severity, the more severely the disease-specific quality of life was impaired. The estimated mean increase in the MG-QoL15 per one point increase in the QMG was 17.6 points (see Fig. 1a).
Fig. 1.
Correlations of quality of life and functional status. The linear mixed model shows a significant positive correlation between the QMG (modified) and the MG-QoL15 and the MG-ADL and the MG-QoL15. (a) Scatter-plot: significant positive correlation between QMG (modified) and MG-QoL15. A 1-point increase in QMG results in an estimated mean 17.6-point increase in MG-QoL15. Note: number of presentations: 99; number of patients: 63. Intercept: coefficient 7.75; SE 1.90; p < 0.001. QMG: coefficient 17.60; SE 2.80; p < 0.001. (b) Scatter-plot: significant positive correlation between MG-ADL and MG-QoL15. Note: number of presentations: 81; number of patients: 54. Intercept: coefficient 5.95; SE 1.76; p = 0.001. MG-ADL: coefficient 2.55; SE 0.30; p < 0.001
Abbreviations: MG-ADL = Myasthenia Gravis-Activities of Daily Living scale, MG-QoL15 = 15-item Myasthenia Gravis-Quality of Life scale, QMG = Quantitative Myasthenia Gravis Score
In addition, the extent of impairment in activities of daily living and the limitations in disease-specific quality of life correlated significantly positively with each other (see Fig. 1b).
A significant correlation was also detected between the QMG and the MG-ADL: The higher the severity of the disease, the more impaired were the patients’ MG-specific activities of daily living. An increase in disease severity (QMG) by one point led to an estimated increase in MG-ADL by the factor 2.7.
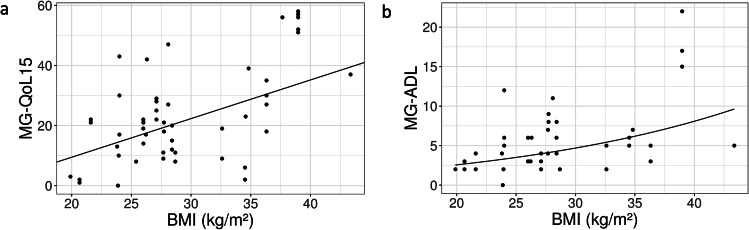
The BMI level also showed significant positive correlation with the degree of impairment of disease-specific quality of life and MG-specific activities of daily living (Fig. 2a and b). An increase in BMI by one point was associated with an estimated mean worsening of quality of life by 1.29 points or activities of daily living by about 5%. In contrast, no significant correlation between BMI and QMG could be found in the cohort (coefficient for BMI − 0.004, p = 0.414).
Fig. 2.
Correlation of BMI with quality of life and functional status. A higher BMI has a negative influence on quality of life as well as on functional disease specific status. (a) Scatter plot: significant positive correlation between BMI and MG-QoL15. Note: number of presentations: 53; number of patients: 27. Intercept: coefficient − 16.28; SE 12.01; p = 0.187. BMI: coefficient 1.29; SE 0.42; p = 0.005. (b) Scatter plot: significant positive correlation between BMI and MG-ADL. Note: number of presentations: 45; number of patients: 25. Intercept: coefficient 0.336; SE 0.527; p = 0.530. BMI: coefficient 0.05; SE 0.02; p = 0.017. The dependent variable MG-ADL was log(x + 1)-transformed
Abbreviations: BMI = body mass index, MG-ADL = Myasthenia Gravis-Activities of Daily Living scale, MG-QoL15 = 15-item Myasthenia Gravis-Quality of Life scale
The MG-QoL15 score is not only related to the disease severity, activities of daily living and the BMI, but also correlates to the gender. Whereas the mean MG-QoL15 of the females was 19.68 points (SD 12.43, median 18.75), the mean MG-QoL15 of men was 13.00 points (SD 13.03, median 10.00). Thus, the mean MG-QoL15 of females was almost 6.7 points higher than that of males (p = 0.024).
Furthermore, a myasthenic crisis had significant negative influence on patients’ quality of life. Patients who had not experienced a myasthenic crisis – compared with those with having experienced a myasthenic crisis – stated a significantly better disease-specific quality of life and a significant lower impairment in MG-specific activities of daily living. After a myasthenic crisis, patients scored an average of 23.8 points on the MG-QoL15. In contrast, the MG-QoL15 of patients who had never experienced a myasthenic crisis averaged 14.5 points, meaning that they perceived their quality of life to be less impaired.
In order to consider a mutual influence of the parameters in the evaluation, the observation regarding the quality of life and the QMG was also carried out in a multivariate mixed model.
Analogous to the results of the univariate linear mixed model, disease severity correlated significantly positively with disease-specific quality of life in the multivariate model. Here, an one-point increase in QMG resulted in an estimated mean increase in MG-QoL15 of 16.92 points (see Table 2).
Table 2.
Multivariate linear mixed model on factors influencing the MG-QoL15
| Coefficient | SE | p | ||
|---|---|---|---|---|
| Experienced myasthenic crisis | 0.04 | 3.23 | 0.989 | |
| Patient age at disease onset | 0.10 | 0.08 | 0.233 | |
| Time between disease onset and initial diagnosis (in years) | 0.25 | 0.32 | 0.452 | |
| Time since initial diagnosis (in years) | -0.22 | 0.14 | 0.133 | |
| Gender (female) | 8.20 | 2.76 | 0.005 | |
| QMG (modified) | 15.52 | 2.98 | < 0.001 | |
Note: Number of presentations: 98, number of patients: 62.
Intercept: Coefficient = 0.44, SE = 5.56, p = 0.937
Abbreviations: MG-QoL15 = 15-item Myasthenia Gravis-Quality of Life scale, QMG = Quantitative Myasthenia Gravis Score
The occurrence of a myasthenic crisis did not significantly affect the MG-QoL15 score in multivariate analysis (see Table 2). Hence, the detected association between myasthenic crisis and poor quality of life is most probably due to the higher disease severity, expressed as QMG, after a myasthenic crisis and to the higher proportion of women experiencing a myasthenic crisis.
The MG-QoL15 score was not significantly influenced by the time between disease onset and initial diagnosis, and the time since initial diagnosis (see Table 2).
Gender differences
There were not only found significant repercussions on the female gender’s quality of life, but also evidence for additional differences between men and women in disease progression.
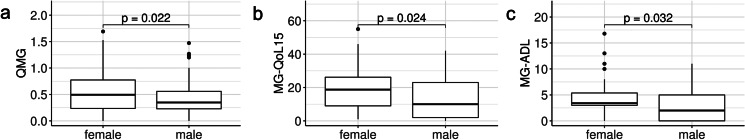
At disease onset and initial diagnosis, females were significantly younger than males (median 53 vs. 63 years, p = 0.036; median 56 vs. 63 years, p = 0.011, respectively). In women, the time between disease onset and initial diagnosis was significantly longer (mean 2.15 vs. 0.87 years, median 0.5 vs. 0.2 years, p = 0.034). Moreover, women were affected more severely. Thus, the QMG as well as the MG-ADL were significantly higher in women (Fig. 3a-c).
Fig. 3.
Gender differences regarding functional status and quality of life. The comparison of gender differences shows a significant higher disease activity, more strongly impaired functional status and a lower quality of life in women. (a) Boxplot on gender differences in QMG (modified). Note: women n = 80; mean 0.56; SD 0.40; median 0.49; [Min; Max] [0; 1.69]. Men n = 85; mean 0.42; SD 0.30; median 0.35; [Min; Max] [0; 1.47]. (b) Boxplot on gender differences in MG-QoL15. Note: women n = 35; mean 19.68; SD 12.43; median 10.00; [Min; Max] [1; 55]. Men n = 29; mean 13.00; SD 13.00; median 18.75; [Min; Max] [0; 42]. (c) Boxplot on gender differences in MG-ADL Note: women n = 30; mean 4.80; SD 3.74; median 3.41; [Min; Max] [0; 16.8]. Men n = 25; mean 3.01; SD 2.99; median 2.00; [Min; Max] [0; 11.0]
Abbreviations: QMG = Quantitative Myasthenia Gravis Score, MG-QoL15 = 15-item Myasthenia Gravis-Quality of Life scale, MG-ADL = Myasthenia Gravis-Activities of Daily Living scale
With regard to thymic pathologies, gender-specific differences were also found. While thymic hyperplasia occurred more frequently in women (32% vs. 20%, missing data 120), thymomas were more frequently detected in men (45% vs. 32%, missing data 120). However, these differences were statistically not significant (p = 0.502 and p = 0.537, respectively). In contrast, the number of thymectomies performed differed significantly between the sexes. While 40% of women underwent thymectomy, only 23.5% of men underwent thymectomy (p = 0.029).
All but one patient in the cohort (n = 164, 99.4%) took pyridostigmine at least once during the observation period. While women were less likely to be treated with glucocorticoids than men (80% vs. 92.9%, p = 0.015), they were significantly more likely to receive IVIG (35% vs. 18.8%, p = 0.019). In contrast, the dose of pyridostigmine and of glucocorticoids did not differ significantly between genders (pyridostigmine: median 180.0 for both, p = 0.734; glucocorticoids: median 20.0 for women and median 17.6 for men, p = 0.717). Ciclosporine and cyclophosphamide were taken only by male patients, whereas only female patients were treated with tacrolimus and eculizumab.
Regarding concomitant diseases, female patients were significantly more frequently affected by depression (f = 28.7% vs. m = 15.3%, p = 0.040), autoimmune diseases (f = 27.5% vs. m = 9.4%, p = 0.004), in particular hypo-/hyperthyroidism and neurological diseases, than male patients. In contrast, men were significantly more likely to be affected by concomitant cardiovascular disease (f = 56.2% vs. m = 76.5%, p = 0.008).
The risk of myasthenic crisis was not significantly increased for females compared to males in univariate Cox regression analysis (hazard ratio 1.6, p = 0.145).
Discussion
It is well-known that chronic diseases such as multiple sclerosis or amyotrophic lateral sclerosis have a negative impact on a patient’s quality of life [36–40]. In our study, we identified the quality of life – measured prospectively by the MG-QoL15 – in patients with MG to be impaired. One of the negative influencing factors is a high disease severity measured by the QMG. Studies that used the SF-36 questionnaire instead of the MG-QoL15 [41] or the MG composite score instead of the QMG also found a significant correlation between the quality of life and the disease severity [42, 43]. Another factor influencing disease-specific quality of life was the degree of limitation in activities of daily living, as measured by the MG-ADL. The higher the MG-ADL was in the present cohort, the more restricted was the disease-specific quality of life. These results are also consistent with those of other studies [28, 29, 44].
Based on the results of the present study, it can be concluded that severe myasthenic symptomatology measured by objective (e.g. QMG) or subjective (e.g. MG-ADL) parameters has a negative impact on the disease-specific quality of life. Therefore, adequate therapy appears to be essential not only to minimize the physical limitations caused by the disease, but also to improve the patients’ quality of life. Mendoza et al. (2020) found that a score of ≤ 8 points in the MG-QoL15 is – on average – considered acceptable by patients [45]. Since both in the present study and in the majority of studies available in the literature the average MG-QoL15 is considerably higher than this value [26–28, 46, 47], it seems that a relevant proportion of patients with MG receiving the therapeutic options available was not sufficient controlled to achieve a satisfactory quality of life [48].
Moreover, patients with MG are more frequently obese compared to the normal population [49], caused by low physical activity due to muscle weakness and therapy side effects (esp. by glucocorticoids) [50]. The average BMI of the cohort studied was also higher than the average BMI of the German population [51]. A high BMI may negatively affect the quality of life of patients with MG [39, 46]. The association of a high BMI with worse disease-specific quality of life as well as with MG-specific activities of daily living could be verified in our cohort.
Analogous to previous studies [48, 52, 53], women in the studied cohort were significantly more impaired in their disease-specific quality of life and in their disease-specific activities of daily living compared with men. Furthermore, the disease severity of the women was higher compared with the men. Additionally, the time between disease onset and initial diagnosis was significantly longer in women than in men. One reason for the higher disease severity in women or the higher impairment in MG-ADL could be hormonal influences. Previous studies described that sex hormones can influence antibody production in patients with MG. Interacting with the autoimmune regulator of the thymus, testosterone and estrogen may influence the production of anti-AChR-AB and thus have a crucial impact on the development and severity of MG [54]. The hypothesis that hormonal influences play a crucial role in disease progression is reinforced by the fact that postpartum women are at increased risk for the onset or worsening of MG [55]. In addition, exacerbations of the disease occur more frequently in women in association with menstruation [56]. The gender differences in pathophysiology could also lead to unequal efficacy of disease-specific medications in men and women. In the present study, significant gender differences in QMG and MG-ADL occurred despite comparable ingested doses of disease-specific medication.
As already mentioned, the study showed longer time elapses between the first symptoms and diagnosis in women. The longer period of uncertainty and the increased suffering associated with this can have a negative effect and can cause a later start of therapy. The delay in initiating targeted therapy may explain the greater severity of the disease in women. The significantly higher proportion of female patients receiving IVIG therapy also supports the observation that the severity is higher in women. In addition, the side effect profile of glucocorticoid medication seems to be more pronounced in women [57], which may explain both the reduced quality of life and the frequency of therapy escalation to IVIGs.
There are limitations of the study, because – due to its retrospective study design – data on certain characteristics, such as antibody status or concomitant diseases, could not be found in the files of some patients. Nevertheless, the overall willingness to participate in the prospective part of the study was low. The monocentric approach must also be regarded as a limitation. Furthermore, concerning the analysis of the influence of the BMI on the quality of life and the functional status, reverse causality cannot be ruled out, and our results rely on the assumption that BMI changes over short time periods are negligible.
However, we clearly show that the time to diagnosis needs to be shortened, especially with respect to gender aspect, and that the use of patient reported outcome is an important tool in the definition of treatment goals. Complimentary, lifestyle counselling should be provided. This means that treatment goals for MG patients will become even more patient-centered as has already been incorporated in the newly published guidelines of the German Society for Neurology [58].
Conclusion
Despite many available drug treatment options, the results of the study confirm that patients with MG are limited in their quality of life. Since the quality of life is significantly influenced by disease severity, the development of new therapeutic approaches represents an important element in improving the quality of life. In light of approval of new therapies such as FcRn inhibitors and complement inhibitors, MG patient care is currently entering a phase of paradigm shift [20, 59–61]. To meet the new therapeutic concepts, e.g. “hit hard and early” and “minimal symptom expression”, the need for a timely diagnosis has to be emphasized, especially for women who are typically faced with a prolonged diagnosis. Our data underscore the need to conduct further studies that aim to delineate reasons for gender differences and how to overcome them.
Furthermore, patients with myasthenia gravis should be informed that a high BMI can have a negative impact on their disease-specific quality of life. With a holistic view to future, dietary interventions, given an appropriate benefit/risk ratio, are an interesting option for further studies involving patients in the design from the beginning.
Acknowledgements
JZ, JS and SG are members of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO-NMD). SG is supported by the Deutsche Forschungsgemeinschaft (DFG) within the Clinician Scientist Program “Cell Dynamics in Disease and Therapy” at the University Medical Center Göttingen (project number 413501650). JZ is supported by Horizon-2020: IMI2-2020-23-05 (Grantnumber: 101034427-2) The funding body had no influence on writing the manuscript. Revision of English style and grammar has been kindly provided by Christopher Nadorf.
Abbreviations
- AB
Antibody
- AChR
Acetylcholine receptor
- AZA
Azathioprine
- BMI
Body mass index
- F
Female
- FcRn
Neonatal Fc receptor
- IVIG
Intravenous immunoglobulin
- LRP4
Lipoprotein receptor-related protein-4
- M
Male
- MG-ADL
Myasthenia gravis-specific Activities of Daily Living scale
- MG-QoL15
Myasthenia gravis-specific 15-item Quality of Life scale
- MG-QoL15r
Myasthenia gravis-specific 15-item Quality of Life revised scale
- MG-QoL60
Myasthenia gravis-specific 60-item Quality of Life scale
- MG
Myasthenia gravis
- MGFA
Myasthenia gravis Foundation Association
- MMF
Mycophenolate mofetil
- MuSK
Muscle-specific kinase
- QMG
Quantitative Myasthenia Gravis Score
- SD
Standard deviation
- SE
Standard error
- SF-36
Short form-36
- UMG
University Medical Center Göttingen
Authors’ contributions
JZ had the initial idea for the study and submitted the ethics application. HW performed the retrospective and prospective data collection according to instructions. FK and CA performed the statistical analysis. SG was a major contributor in writing the manuscript. All authors, particularly HW, analyzed and interpreted the results. Therefore, HW and SG contributed equally to this work. DL contributed to the conception of the study. JZ and JS proofread the manuscript carefully. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
The publication was financially supported by UCB. We acknowledge support by the Open Access Publication Funds/transformative agreements of the Göttingen University.
Data Availability
data are available on request from the corresponding author.
Declarations
Ethics approval and consent to participate
approval of the study by the Ethics Committee of the University Hospital Göttingen was granted on October 16, 2017 (application number: 5/2/16) and on October 08, 2018 (application number: 6/5/18). All patients gave their written informed consent for the study participation.
Consent for publication
not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hannah Wilcke and Stefanie Glaubitz contributed equally to this study.
References
- 1.Salari N, Fatahi B, Bartina Y, Kazeminia M, Fatahian R, Mohammadi P, et al. Global prevalence of myasthenia gravis and the effectiveness of common drugs in its treatment: a systematic review and meta-analysis. J Transl Med. 2021;19(1):516. 10.1186/s12967-021-03185-7 [DOI] [PMC free article] [PubMed]
- 2.Hellmann MA, Mosberg-Galili R, Steiner I. Myasthenia gravis in the elderly. J Neurol Sci. 2013;325(1–2):1–5. doi: 10.1016/j.jns.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Compston DA, Vincent A, Newsom-Davis J, Batchelor JR. Clinical, pathological, HLA antigen and immunological evidence for disease heterogeneity in myasthenia gravis. Brain J Neurol. 1980;103(3):579–601. doi: 10.1093/brain/103.3.579. [DOI] [PubMed] [Google Scholar]
- 4.Aarli JA, Romi F, Skeie GO, Gilhus NE. Myasthenia gravis in individuals over 40. Ann N Y Acad Sci. 2003;998:424–31. doi: 10.1196/annals.1254.055. [DOI] [PubMed] [Google Scholar]
- 5.Schneider-Gold C, Hartung H-P. Myasthenia gravis: pathogenese, diagnostik und therapie. Fortschritte Neurol Psychiatr. 2004;72(1):45–57. 10.1055/s-2003-812457 [DOI] [PubMed]
- 6.Gilhus NE. Myasthenia Gravis. N Engl J Med. 2016;375(26):2570–81. doi: 10.1056/NEJMra1602678. [DOI] [PubMed] [Google Scholar]
- 7.Almon RR, Andrew CG, Appel SH. Serum globulin in myasthenia gravis: inhibition of alpha-bungarotoxin binding to acetylcholine receptors. Science. 1974;186(4158):55–7. doi: 10.1126/science.186.4158.55. [DOI] [PubMed] [Google Scholar]
- 8.Melzer N, Ruck T, Fuhr P, Gold R, Hohlfeld R, Marx A, et al. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German neurological society. J Neurol. 2016;263(8):1473–94. doi: 10.1007/s00415-016-8045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gasperi C, Melms A, Schoser B, Zhang Y, Meltoranta J, Risson V, et al. Anti-agrin autoantibodies in myasthenia gravis. Neurology. 2014;82(22):1976–83. doi: 10.1212/WNL.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 10.Cordts I, Bodart N, Hartmann K, Karagiorgou K, Tzartos JS, Mei L, et al. Screening for lipoprotein receptor-related protein 4-, agrin-, and titin-antibodies and exploring the autoimmune spectrum in myasthenia gravis. J Neurol. 2017;264(6):1193–203. doi: 10.1007/s00415-017-8514-z. [DOI] [PubMed] [Google Scholar]
- 11.Kim KH, Kim SW, Cho J, Chung HY, Shin HY. Anti-titin antibody is associated with more frequent hospitalization to manage thymoma-associated myasthenia gravis. Front Neurol. 2022;13:978997. 10.3389/fneur.2022.978997/full [DOI] [PMC free article] [PubMed]
- 12.Schneider-Gold C, Gajdos P, Toyka KV, Hohlfeld RR. Corticosteroids for myasthenia gravis. Cochrane Database Syst Rev. 2005;2005(2):CD002828. doi: 10.1002/14651858.CD002828.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bromberg MB, Wald JJ, Forshew DA, Feldman EL, Albers JW. Randomized trial of azathioprine or prednisone for initial immunosuppressive treatment of myasthenia gravis. J Neurol Sci. 1997;150(1):59–62. doi: 10.1016/S0022-510X(97)05370-7. [DOI] [PubMed] [Google Scholar]
- 14.Piehl F, Eriksson-Dufva A, Budzianowska A, Feresiadou A, Hansson W, Hietala MA, et al. Efficacy and safety of rituximab for new-onset generalized myasthenia gravis: the RINOMAX randomized clinical trial. JAMA Neurol. 2022;79(11):1105. https://jamanetwork.com/journals/jamaneurology/fullarticle/2796552 [DOI] [PMC free article] [PubMed]
- 15.Gajdos P, Chevret S, Toyka K. Plasma exchange for myasthenia gravis. Cochrane Database Syst Rev. 2002;2002(4):CD002275. doi: 10.1002/14651858.CD002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med. 2016;375(6):511–22. doi: 10.1056/NEJMoa1602489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalakas MC. Progress in the therapy of myasthenia gravis: getting closer to effective targeted immunotherapies. Curr Opin Neurol. 2020;33(5):545–52. doi: 10.1097/WCO.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 18.Dalakas MC. Role of complement, anti-complement therapeutics, and other targeted immunotherapies in myasthenia gravis. Expert Rev Clin Immunol. 2022;18(7):691–701. doi: 10.1080/1744666X.2022.2082946. [DOI] [PubMed] [Google Scholar]
- 19.Menon D, Bril V. Pharmacotherapy of generalized myasthenia gravis with special emphasis on newer biologicals. Drugs. 2022;82(8):865–87. doi: 10.1007/s40265-022-01726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard JF, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16(12):976–86. doi: 10.1016/S1474-4422(17)30369-1. [DOI] [PubMed] [Google Scholar]
- 21.Muppidi S, Silvestri NJ, Tan R, Riggs K, Leighton T, Phillips GA. Utilization of MG-ADL in myasthenia gravis clinical research and care. Muscle Nerve. 2022;65(6):630–9. 10.1002/mus.27476 [DOI] [PMC free article] [PubMed]
- 22.Howard JF, Bril V, Burns TM, Mantegazza R, Bilinska M, Szczudlik A, et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. 2019;92(23):e2661–73. doi: 10.1212/WNL.0000000000007600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pöllmann W, Busch C, Voltz R. Lebensqualität bei multipler sklerose: messinstrumente, bedeutung, probleme und perspektiven. Nervenarzt. 2005;76(2):154–69. 10.1007/s00115-004-1790-8 [DOI] [PubMed]
- 24.Bullinger M. [Health related quality of life and subjective health. Overview of the status of research for new evaluation criteria in medicine] Psychother Psychosom Med Psychol. 1997;47(3–4):76–91. [PubMed] [Google Scholar]
- 25.Burns TM, Conaway MR, Cutter GR, Sanders DB, Muscle Study Group Less is more, or almost as much: a 15-item quality-of-life instrument for myasthenia gravis. Muscle Nerve. 2008;38(2):957–63. doi: 10.1002/mus.21053. [DOI] [PubMed] [Google Scholar]
- 26.Burns TM. History of outcome measures for myasthenia gravis. Muscle Nerve. 2010;42(1):5–13. doi: 10.1002/mus.21713. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Lapiscina EH, Erro ME, Ayuso T, Jericó I. Myasthenia gravis: sleep quality, quality of life, and disease severity. Muscle Nerve. 2012;46(2):174–80. doi: 10.1002/mus.23296. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann S, Ramm J, Grittner U, Kohler S, Siedler J, Meisel A. Fatigue in myasthenia gravis: risk factors and impact on quality of life. Brain Behav. 2016;6(10):e00538. doi: 10.1002/brb3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutter G, Xin H, Aban I, Burns TM, Allman PH, Farzaneh-Far R, et al. Cross-sectional analysis of the Myasthenia Gravis Patient Registry: disability and treatment. Muscle Nerve. 2019;60(6):707–15. doi: 10.1002/mus.26695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzthum K. Lebensqualität bei myasthenia gravis. Klinische einflussfaktoren und langfristiger verlauf. Berlin, Germany: Charité - Universitätsmedizin Berlin; 2015.
- 31.Barohn RJ, McIntire D, Herbelin L, Wolfe GI, Nations S, Bryan WW. Reliability testing of the quantitative myasthenia gravis score. Ann N Y Acad Sci. 1998;841:769–72. doi: 10.1111/j.1749-6632.1998.tb11015.x. [DOI] [PubMed] [Google Scholar]
- 32.Besinger UA, Toyka KV, Heininger K, Fateh-Moghadam A, Schumm F, Sandel P, Long-term correlation of clinical course and acetylcholine receptor antibody in patients with myasthenia gravis. Ann N Y Acad Sci. 1981;377(1 Myasthenia Gr):812–5. 10.1111/j.1749-6632.1981.tb33781.x
- 33.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82(13). http://www.jstatsoft.org/v82/i13/
- 34.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme. J Stat Softw. 2015;67(1). http://www.jstatsoft.org/v67/i01/
- 35.Therneau TM. A Package for survival analysis in S; 2015.
- 36.Murphy N, Confavreux C, Haas J, König N, Roullet E, Sailer M, et al. Quality of life in multiple sclerosis in France, Germany, and the United Kingdom. Cost of multiple sclerosis Study Group. J Neurol Neurosurg Psychiatry. 1998;65(4):460–6. doi: 10.1136/jnnp.65.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15(3):205–18. doi: 10.1002/(SICI)1520-7560(199905/06)15:3<205::AID-DMRR29>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 38.Nelson ND, Trail M, Van JN, Appel SH, Lai EC. Quality of life in patients with amyotrophic lateral sclerosis: perceptions, coping resources, and illness characteristics. J Palliat Med. 2003;6(3):417–24. doi: 10.1089/109662103322144736. [DOI] [PubMed] [Google Scholar]
- 39.Winter Y, Schepelmann K, Spottke AE, Claus D, Grothe C, Schröder R, et al. Health-related quality of life in ALS, myasthenia gravis and facioscapulohumeral muscular dystrophy. J Neurol. 2010;257(9):1473–81. doi: 10.1007/s00415-010-5549-9. [DOI] [PubMed] [Google Scholar]
- 40.Lipka AF, Boldingh MI, van Zwet EW, Schreurs MWJ, Kuks JBM, Tallaksen CM, et al. Long-term follow-up, quality of life, and survival of patients with Lambert-Eaton myasthenic syndrome. Neurology. 2020;94(5):e511–20. doi: 10.1212/WNL.0000000000008747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basta IZ, Pekmezović TD, Perić SZ, Kisić-Tepavčević DB, Rakočević-Stojanović VM, Stević ZD, et al. Assessment of health-related quality of life in patients with myasthenia gravis in Belgrade (Serbia) Neurol Sci off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2012;33(6):1375–81. doi: 10.1007/s10072-012-1170-2. [DOI] [PubMed] [Google Scholar]
- 42.Masuda M, Utsugisawa K, Suzuki S, Nagane Y, Kabasawa C, Suzuki Y, et al. The MG-QOL15 Japanese version: validation and associations with clinical factors. Muscle Nerve. 2012;46(2):166–73. doi: 10.1002/mus.23398. [DOI] [PubMed] [Google Scholar]
- 43.Mourão AM, Gomez RS, Barbosa LSM, Freitas DdaS, Comini-Frota ER, Kummer A, et al. Determinants of quality of life in Brazilian patients with myasthenia gravis. Clin Sao Paulo Braz. 2016;71(7):370–4. doi: 10.6061/clinics/2016(07)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong D, Chong MKC, Wu Y, Kaminski H, Cutter G, Xu X, et al. Gender differences in quality of life among patients with myasthenia gravis in China. Health Qual Life Outcomes. 2020;18(1):296. doi: 10.1186/s12955-020-01549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendoza M, Tran C, Bril V, Katzberg HD, Barnett C. Patient-acceptable symptom states in myasthenia gravis. Neurology. 2020;95(12):e1617–28. doi: 10.1212/WNL.0000000000010574. [DOI] [PubMed] [Google Scholar]
- 46.Szczudlik P, Sobieszczuk E, Szyluk B, Lipowska M, Kubiszewska J, Kostera-Pruszczyk A. Determinants of quality of life in Myasthenia Gravis Patients. Front Neurol. 2020;11:553626. doi: 10.3389/fneur.2020.553626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diez Porras L, Homedes C, Alberti MA, Velez Santamaria V, Casasnovas C. Quality of life in Myasthenia Gravis and correlation of MG-QOL15 with other functional scales. J Clin Med. 2022;11(8):2189. doi: 10.3390/jcm11082189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehnerer S, Jacobi J, Schilling R, Grittner U, Marbin D, Gerischer L, et al. Burden of disease in myasthenia gravis: taking the patient’s perspective. J Neurol. 2022;269(6):3050–63. doi: 10.1007/s00415-021-10891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braz NFT, Rocha NP, Vieira ÉLM, Gomez RS, Kakehasi AM, Teixeira AL. Body composition and adipokines plasma levels in patients with myasthenia gravis treated with high cumulative glucocorticoid dose. J Neurol Sci. 2017;381:169–75. doi: 10.1016/j.jns.2017.08.3250. [DOI] [PubMed] [Google Scholar]
- 50.Hafer-Macko C. Sarcopenic obesity in myasthenia gravis (P5.063). Neurology. 2015;84(14 Supplement):P5.063. Available from: http://n.neurology.org/content/84/14_Supplement/P5.063.abstract
- 51.Mensink GBM, Schienkiewitz A, Haftenberger M, Lampert T, Ziese T, Scheidt-Nave C. Übergewicht und adipositas in Deutschland: ergebnisse der studie zur gesundheit erwachsener in Deutschland (DEGS1). Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2013;56(5–6):786–94. 10.1007/s00103-012-1656-3 [DOI] [PubMed]
- 52.Lee I, Kaminski HJ, Xin H, Cutter G. Gender and quality of life in myasthenia gravis patients from the myasthenia gravis foundation of America registry: gender and QOL in MG patients. Muscle Nerve. 2018;58(1):90–8. 10.1002/mus.26104 [DOI] [PubMed]
- 53.Thomsen JLS, Vinge L, Harbo T, Andersen H. Gender differences in clinical outcomes in myasthenia gravis: a prospective cohort study. Muscle Nerve. 2021;64(5):538–44. doi: 10.1002/mus.27331. [DOI] [PubMed] [Google Scholar]
- 54.Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren JJGM. Myasthenia gravis. Nat Rev Dis Primer. 2019;5(1):30. doi: 10.1038/s41572-019-0079-y. [DOI] [PubMed] [Google Scholar]
- 55.Boldingh MI, Maniaol AH, Brunborg C, Weedon-Fekjær H, Verschuuren JJGM, Tallaksen CME. Increased risk for clinical onset of myasthenia gravis during the postpartum period. Neurology. 2016;87(20):2139–45. doi: 10.1212/WNL.0000000000003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stickler DE, Stickler LL. Single-fiber electromyography during menstrual exacerbation and ovulatory suppression in MuSK antibody-positive myasthenia gravis. Muscle Nerve. 2007;35(6):808–11. doi: 10.1002/mus.20734. [DOI] [PubMed] [Google Scholar]
- 57.Lee I, Kaminski HJ, McPherson T, Feese M, Cutter G. Gender differences in prednisone adverse effects: Survey result from the MG registry. Neurol Neuroimmunol Neuroinflamm. 2018;5(6):e507. doi: 10.1212/NXI.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiendl H. Meisel A. Diagnostik und therapie myasthener syndrome, S2k-Leitlinie. DGN; 2022.
- 59.Vu T, Meisel A, Mantegazza R, Annane D, Katsuno M, Aguzzi R. et al. Terminal complement inhibitor ravulizumab in generalized myasthenia gravis. NEJM Evid. 2022;1(5). 10.1056/EVIDoa2100066 [DOI] [PubMed]
- 60.Nixon AE, Chen J, Sexton DJ, Muruganandam A, Bitonti AJ, Dumont J, et al. Fully human monoclonal antibody inhibitors of the neonatal fc receptor reduce circulating IgG in non-human primates. Front Immunol. 2015;6:176. doi: 10.3389/fimmu.2015.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ward ES, Ober RJ. Targeting FcRn to generate antibody-based therapeutics. Trends Pharmacol Sci. 2018;39(10):892–904. doi: 10.1016/j.tips.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
data are available on request from the corresponding author.