Abstract
Background
Gestational diabetes mellitus (GDM) has major short‐ and long‐term implications for both the mother and her baby. GDM is defined as a carbohydrate intolerance resulting in hyperglycaemia or any degree of glucose intolerance with onset or first recognition during pregnancy from 24 weeks' gestation onwards and which resolves following the birth of the baby. Rates for GDM can be as high as 25% depending on the population and diagnostic criteria used, and overall rates are increasing globally. There is wide variation internationally in glycaemic treatment target recommendations for women with GDM that are based on consensus rather than high‐quality trials.
Objectives
To assess the effect of different intensities of glycaemic control in pregnant women with GDM on maternal and infant health outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (26 September 2022), and reference lists of the retrieved studies.
Selection criteria
We included randomised controlled trials (RCTs), cluster‐RCTs, and quasi‐RCTs. Trials were eligible for inclusion if women were diagnosed with GDM during pregnancy and the trial compared tighter and less‐tight glycaemic targets during management. We defined tighter glycaemic targets as lower numerical glycaemic concentrations, and less‐tight glycaemic targets as higher numerical glycaemic concentrations.
Data collection and analysis
We used standard Cochrane methods for carrying out data collection, assessing risk of bias, and analysing results. Two review authors independently assessed trial eligibility for inclusion, evaluated risk of bias, and extracted data for the four included studies. We assessed the certainty of evidence for selected outcomes using the GRADE approach. Primary maternal outcomes included hypertensive disorders of pregnancy and subsequent development of type 2 diabetes. Primary infant outcomes included perinatal mortality, large‐for‐gestational‐age, composite of mortality or serious morbidity, and neurosensory disability.
Main results
This was an update of a previous review completed in 2016. We included four RCTs (reporting on 1731 women) that compared a tighter glycaemic control with less‐tight glycaemic control in women diagnosed with GDM. Three studies were parallel RCTs, and one study was a stepped‐wedged cluster‐RCT. The trials took place in Canada, New Zealand, Russia, and the USA. We judged the overall risk of bias to be unclear. Two trials were only published in abstract form. Tight glycaemic targets used in the trials ranged between ≤ 5.0 and 5.1 mmol/L for fasting plasma glucose and ≤ 6.7 and 7.4 mmol/L postprandial. Less‐tight targets for glycaemic control used in the included trials ranged between < 5.3 and 5.8 mmol/L for fasting plasma glucose and < 7.8 and 8.0 mmol/L postprandial.
For the maternal outcomes, compared with less‐tight glycaemic control, the evidence suggests a possible increase in hypertensive disorders of pregnancy with tighter glycaemic control (risk ratio (RR) 1.16, 95% confidence interval (CI) 0.80 to 1.69, 2 trials, 1491 women; low certainty evidence); however, the 95% CI is compatible with a wide range of effects that encompass both benefit and harm. Tighter glycaemic control likely results in little to no difference in caesarean section rates (RR 0.98, 95% CI 0.82 to 1.17, 3 studies, 1662 women; moderate certainty evidence) or induction of labour rates (RR 0.96, 95% CI 0.78 to 1.18, 1 study, 1096 women; moderate certainty evidence) compared with less‐tight control. No data were reported for the outcomes of subsequent development of type 2 diabetes, perineal trauma, return to pre‐pregnancy weight, and postnatal depression.
For the infant outcomes, it was difficult to determine if there was a difference in perinatal mortality (RR not estimable, 2 studies, 1499 infants; low certainty evidence), and there was likely no difference in being large‐for‐gestational‐age (RR 0.96, 95% CI 0.72 to 1.29, 3 studies, 1556 infants; moderate certainty evidence). The evidence suggests a possible reduction in the composite of mortality or serious morbidity with tighter glycaemic control (RR 0.84, 95% CI 0.55 to 1.29, 3 trials, 1559 infants; low certainty evidence); however, the 95% CI is compatible with a wide range of effects that encompass both benefit and harm. There is probably little difference between groups in infant hypoglycaemia (RR 0.92, 95% CI 0.72 to 1.18, 3 studies, 1556 infants; moderate certainty evidence). Tighter glycaemic control may not reduce adiposity in infants of women with GDM compared with less‐tight control (mean difference −0.62%, 95% CI −3.23 to 1.99, 1 study, 60 infants; low certainty evidence), but the wide CI suggests significant uncertainty. We found no data for the long‐term outcomes of diabetes or neurosensory disability.
Women assigned to tighter glycaemic control experienced an increase in the use of pharmacological therapy compared with women assigned to less‐tight glycaemic control (RR 1.37, 95% CI 1.17 to 1.59, 4 trials, 1718 women). Tighter glycaemic control reduced adherence with treatment compared with less‐tight glycaemic control (RR 0.41, 95% CI 0.32 to 0.51, 1 trial, 395 women).
Overall the certainty of evidence assessed using GRADE ranged from low to moderate, downgraded primarily due to risk of bias and imprecision.
Authors' conclusions
This review is based on four trials (1731 women) with an overall unclear risk of bias. The trials provided data on most primary outcomes and suggest that tighter glycaemic control may increase the risk of hypertensive disorders of pregnancy. The risk of birth of a large‐for‐gestational‐age infant and perinatal mortality may be similar between groups, and tighter glycaemic targets may result in a possible reduction in composite of death or severe infant morbidity. However, the CIs for these outcomes are wide, suggesting both benefit and harm.
There remains limited evidence regarding the benefit of different glycaemic targets for women with GDM to minimise adverse effects on maternal and infant health. Glycaemic target recommendations from international professional organisations vary widely and are currently reliant on consensus given the lack of high‐certainty evidence.
Further high‐quality trials are needed, and these should assess both short‐ and long‐term health outcomes for women and their babies; include women's experiences; and assess health services costs in order to confirm the current findings. Two trials are ongoing.
Keywords: Female; Humans; Infant; Pregnancy; Blood Glucose; Diabetes Mellitus, Type 2; Diabetes, Gestational; Diabetes, Gestational/therapy; Glycemic Control; Hypertension, Pregnancy-Induced
Plain language summary
What is the most effective blood sugar range to guide treatment for women who develop gestational diabetes mellitus in their pregnancy?
What is the issue?
Up to a quarter of pregnant women develop gestational diabetes mellitus (GDM), depending on their ethnicity and the diagnostic criteria used. GDM is defined as high blood sugar levels (hyperglycaemia) during pregnancy and is associated with an increased risk of developing high blood pressure (hypertension) and protein in the urine during pregnancy (pre‐eclampsia). These women are more likely to have a caesarean birth and postnatal depression, and to develop type 2 diabetes and cardiovascular disease later in life. The high blood sugar levels that are associated with GDM usually return to normal after the birth, but women with GDM are at risk of developing GDM in future pregnancies. Babies whose mothers have been diagnosed with GDM are at an increased risk of having a birthweight greater than 4000 g, increased risk of birth trauma because of their size, and development of breathing difficulties after birth. These babies are also at higher risk of future obesity and type 2 diabetes.
Why is this important?
Women with GDM are treated with the aim of controlling high maternal blood sugar levels and reducing the risks of GDM for the mother and the baby. Blood sugar control is monitored by measuring blood sugar concentrations to ensure they are maintained within a predefined level or range. The blood sugar results are usually obtained by the mother using a finger prick to collect a drop of her blood on a test strip, which is inserted into a small machine (a glucometer) that reads the sugar level of the blood on the test strip. The glucometer reading alerts the pregnant woman to her current blood sugar level and is used to guide her treatment, for example how many units of insulin she requires before eating. However, the most effective blood sugar range to aim for and guide treatment in pregnant women with newly diagnosed GDM is currently unclear.
What evidence did we find?
This is an update on a review completed in 2016. We searched for evidence on 26 September 2022 for randomised controlled trials (a type of study where participants are randomly assigned to one of two or more treatment groups) that compared different blood sugar ranges in women with GDM and assessed the impact on mother and infant health. We found nine reports from three different trials in this updated review, amounting to a total of four included studies. Each trial compared two blood sugar ranges, one tighter (lower blood sugar targets), and the other less‐tight (higher blood sugar targets), and reported on health outcomes for the pregnant woman and her baby.
We found that there may be an increase in the risk of the mother developing high blood pressure and protein in the urine during pregnancy with a lower blood sugar target. We found that there is unlikely to be a difference between blood sugar ranges in rates of caesarean birth or induction of labour. The trials did not report any data for the following outcomes for mothers: subsequent development of type 2 diabetes for the mother, trauma to the perineum, return to pre‐pregnancy weight, and postnatal depression.
We are uncertain whether there is any difference in the risk of death for the baby as there were very few deaths in the studies. The evidence shows there is likely no change in the blood sugar levels of the baby and may be no change in the body fat percentage of the baby. The trials did not provide any data for the other main outcomes: long‐term risk of diabetes in the baby and risk of disability in the baby.
Lower blood sugar targets likely result in an increase in the use of drug therapy (insulin, metformin, or glyburide) and may result in a large decrease in adherence to treatment.
Limitations of the evidence
There is some uncertainty in the findings due to a lack of information about how some studies were designed and reported and because for some outcomes there was information from only one study.
What does this mean?
This review found that there is not yet enough evidence from randomised trials to determine the best blood sugar range for improving health for pregnant women with GDM and their babies. The evidence currently points towards no increased benefit when using lower blood sugar targets. Two trials are ongoing. More high‐quality studies are needed that compare different targets for blood sugar levels and assess both short‐ and long‐term health outcomes for women and their babies to guide treatment. Studies should include women's experiences and assess health services costs.
Summary of findings
Summary of findings 1. Intensity of glycaemic control for women with gestational diabetes mellitus: tight glycaemic targets versus less‐tight glycaemic targets (maternal outcomes).
| Intensity of glycaemic control for women with gestational diabetes mellitus: tight glycaemic targets versus less‐tight glycaemic targets (maternal outcomes) | ||||||
|
Patient or population: women with gestational diabetes mellitus Setting: Canada; New Zealand; Oklahoma, USA; St Petersburg, Russia Intervention: tight intensity of glycaemic control Comparison: less‐tight intensity of glycaemic control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|
Risk with less‐tight glycaemic control |
Risk with tight glycaemic control |
|||||
| Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia) | 72 per 1000 |
83 per 1000 (58 to 122) |
RR 1.16 (0.80 to 1.69) |
1491 (2 studies) |
⨁⨁◯◯ LOW a,b | Strict glycaemic targets may increase hypertensive disorders of pregnancy; however, the 95% CI is compatible with a wide range of effects encompassing both benefit and harm. |
| Subsequent development of type 2 diabetes | No data reported for this outcome. | |||||
| Caesarean section | 325 per 1000 |
319 per 1000 (2 to 390) |
RR0.98 (0.82 to 1.17) |
1662 (3 RCTs) | ⨁⨁⨁◯ MODERATE a | Strict glycaemic targets likely result in little to no difference in caesarean section rates. |
| Return to pre‐pregnancy weight | No data reported for this outcome. | |||||
| Induction of labour | 517 per 1000 |
496 per 1000 (403 to 610) |
RR 0.96 (0.78 to 1.18) |
1096 (1 RCT) | ⨁⨁⨁◯ MODERATEc | Strict glycaemic targets likely result in little to no difference in induction of labour. It is difficult to apply GRADE criteria for indirectness and publication bias as only one trial assessed this outcome, therefore we have downgraded for serious imprecision. |
| Perineal trauma | No data reported for this outcome. | |||||
| Postnatal depression | No data reported for this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk was calculated using GRADEpro GDT. CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded one level for serious risk of bias due to insufficient detail to permit a judgement about random sequence generation, allocation concealment, attrition bias, and reporting bias. bWe downgraded one level for serious imprecision due to wide 95% CI. cWe downgraded one level for serious imprecision due to only one trial reporting this outcome.
Summary of findings 2. Intensity of glycaemic control for women with gestational diabetes mellitus: tight glycaemic targets versus less‐tight glycaemic targets (child (as neonate, child, adult) outcomes).
| Intensity of glycaemic control for women with gestational diabetes mellitus: tight glycaemic targets versus less‐tight glycaemic targets (child (as neonate, child, adult) outcomes) | ||||||
|
Patient or population: children (as neonate, child, adult) of women with gestational diabetes mellitus
Setting: Canada; New Zealand; Oklahoma, USA; St Petersburg, Russia Intervention: tight intensity of maternal glycaemic control Comparison: less‐tight intensity of maternal glycaemic control | ||||||
| Outcomes | Anticipated absolute effects*(95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|
Risk with less‐tight glycaemic control |
Risk with tight glycaemic control |
|||||
| Perinatal (fetal and neonatal) mortality | 4 per 1000 | 0 per 1000 | Not estimable | 1499 (2 RCTs) |
⨁⨁◯◯ LOWa | There were a total of 3 deaths in the less‐tight glycaemic control group and no deaths in the tight glycaemic control group. Both trials reported an incidence of zero in at least 1 group and therefore could not be meta‐analysed. |
| Large‐for‐gestational‐age | 157 per 1000 |
151 per 1000 (113 to 203) |
RR 0.96 (0.72 to 1.29) | 1556 (3 RCTs) |
⨁⨁⨁◯ MODERATEb | The evidence shows that strict glycaemic targets likely result in little to no difference in large‐for‐gestational‐age. |
| Composite of mortality or serious morbidity (as defined by trial) | 45 per 1000 |
38 per 1000 (25 to 58) |
RR 0.84 (0.55 to 1.29) |
1559 (3 RCTs) |
⨁⨁◯◯ LOWc | The evidence suggests that strict glycaemic targets may result in a possible reduction in the composite of mortality or serious morbidity; however, the 95% CI is compatible with a wide range of effects encompassing both benefit and harm. |
| Neurosensory disability | No data reported for this outcome. | |||||
| Infant hypoglycaemia | 209 per 1000 |
193 per 1000 (151 to 247) |
RR 0.92 (0.72 to 1.18) | 1556 (3 RCTs) |
⨁⨁⨁◯ MODERATEb | Strict glycaemic targets likely result in little to no difference in infant hypoglycaemia. |
| Adiposity (% fat mass) | The mean adiposity (% fat mass) was 12.10%. | MD 0.62% lower (3.23 lower to 1.99 higher) | ‐ | 60 (1 RCT) |
⨁⨁◯◯ LOWd | Strict glycaemic targets may result in little to no difference in adiposity; however, the wide 95% CI suggests significant uncertainty. |
| Diabetes | No data reported for this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk was calculated using GRADEpro GDT. CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded two levels for very serious imprecision as data were provided from one study with very few events. bWe downgraded one level for serious risk of bias due to insufficient detail to permit a judgement about random sequence generation, allocation concealment, attrition bias, and reporting bias. cWe downgraded one level for serious risk of bias due to insufficient detail to permit a judgement about random sequence generation, allocation concealment, attrition bias, and reporting bias. We further downgraded one level for serious imprecision due to wide 95% CI. dWe downgraded two levels for very serious imprecision as data were provided from a single, small study and 95% CI was wide. We defined a 5% difference in adiposity as constituting a clinically meaningful difference.
Background
Description of the condition
Gestational diabetes mellitus (GDM) is a carbohydrate intolerance resulting in hyperglycaemia, or any degree of glucose intolerance with onset or first recognition during pregnancy from 24 weeks' gestation onwards and which resolves following the birth of the baby (NICE 2015; WHO 2013). The global prevalence of GDM is reported to be between 1% and 25.5%, depending on the diagnostic criteria used and women's ethnicity (ACOG 2018; Bottalico 2007; Cheung 2003; Ferrara 2007; NICE 2015; Sacks 2012). Obesity has been identified as a significant risk factor for GDM (Boney 2005; Chu 2007; Mokdad 2003; Oteng‐Ntim 2012; Rosenberg 2005; Torloni 2009), and rates are likely to increase with the reported global obesity epidemic (Athukorala 2010; Kim 2010; Rowlands 2010; Zhang 2010).
During pregnancy, hormones released by the placenta cause an increase in maternal insulin resistance to ensure a constant supply of glucose and other nutrients to the growing fetus (McCance 2011; Wilcox 2005). The maternal pancreas compensates for the pregnancy‐induced insulin resistance by secreting more insulin. GDM occurs when this compensatory mechanism fails and not enough insulin is available to metabolise glucose (McCurdy 2010; Wilcox 2005). The maternal blood glucose concentration then increases, resulting in hyperglycaemia. Increased amounts of glucose cross the placenta, overnourishing the fetus, with increased fetal insulin secretion in response (Evans 2009; Ragnarsdottir 2010; Suman Rao 2013). Increased fetal insulin may act as a growth‐stimulating factor (Pedersen 1954).
Recognised risk factors for developing GDM include obesity, advanced maternal age, weight gain in pregnancy, and a family history of type 2 diabetes (Athukorala 2010; Chu 2007; Kim 2010; Torloni 2009; Zhang 2010). Women of certain ethnicities, such as Asian, African‐American, Native American, Hispanic, and Pacific Island have an increased risk (Carolan 2012; Chamberlain 2013; Kim 2013; Schneider 2012).
GDM has major short‐ and long‐term implications for both the mother and her baby. Women with GDM are at higher risk of developing gestational hypertension and pre‐eclampsia, and are at increased risk of having a caesarean section (Crowther 2005; HAPO 2008; McCance 2011; NICE 2015). In the long term, these women are at significantly increased risk of developing cardiovascular disease, and over half will develop type 2 diabetes within five to 10 years (Bellamy 2009; Vounzoulaki 2020). Infants of women with GDM have a greater incidence of being born large‐for‐gestational‐age and macrosomic (variously defined as birthweight greater than 4000 g to 4500 g) (Young 2013), which increases the risk of shoulder dystocia and associated birth trauma such as bone fractures and nerve palsy (Athukorala 2006). Macrosomia has been associated with developmental delay in childhood (Ornoy 2005; Slining 2010). In the neonatal period, these infants are at higher risk of hypoglycaemia due to fetal hyperinsulinaemia and need to adjust to not having the high maternal glucose supply (Devlieger 2008). Neonatal hypoglycaemia is associated with developmental delay in childhood (Lucas 1988; Shah 2019). There are lifelong health risks to the infants of mothers with GDM such as higher rates of obesity and type 2 diabetes in childhood (Page 2014), and an increased risk of diabetes, hypertension, and cardiovascular disease in later life (Ornoy 2011), and evidence from published cohort studies indicate an increased risk of postpartum depression (Kozhimannil 2009; Nicklas 2013). Observational neurodevelopmental studies of children of mothers with diabetes (including women with GDM) report a higher rate of neurosensory disability (including gross and fine motor abnormalities, attention deficit hyperactivity disorder (ADHD), learning difficulties, and possibly autism spectrum disorder (ASD)) (Gardener 2009; Krakowiak 2012; Nomura 2012; Ornoy 2015; Rowland 2021).
Screening and diagnosis of GDM remain controversial, with some countries recommending universal screening of all pregnant women between 24 and 28 weeks' gestation (Li‐zhen 2019), and others only recommending selective screening (NICE 2015). The amount of glucose recommended for the diagnostic oral glucose tolerance test (OGTT) differs between countries (75 g/2.6 ounces and 100 g/3.5 ounces), and there is significant variation in the fasting, one‐, two‐, and three‐hour postprandial plasma glucose concentrations above which GDM is diagnosed (ACOG 2018; Feig 2018; Nankervis 2013; New Zealand Ministry of Health 2014; NICE 2015; SIGN 2017; WHO 2013).
Similarly, there is wide variation internationally in glycaemic treatment targets recommended for optimal outcomes for women with GDM and their babies (see Table 3). As evidence emerges that current target thresholds may need to be lower than previously thought to reduce morbidity (Hernandez 2011; Hernandez 2015; Metzger 2008), professional organisations are increasingly advocating lower treatment targets that are closer to observed blood glucose concentrations in pregnant women without GDM (HSE 2010; Nankervis 2013). However, concerns have been raised that lower glycaemic targets may be associated with an increased risk of infants being born small‐for‐gestational‐age (Garner 1997; Langer 1989; Langer 1994), and a potential increased risk of hypoglycaemia in the mother (DCCT 1996), and therefore in the fetus.
1. GDM treatment targets for glycaemic control from Clinical Practice Guidelines.
|
Fasting plasma glucose mmol/L (mg/dL)1 |
1‐hour postprandial mmol/L (mg/dL)1 |
2‐hours postprandial mmol/L (mg/dL)1 |
|
|
Australasian Diabetes in Pregnancy Society (ADIPS) Nankervis 2013(p. 5) and New Zealand Ministry of Health (NZMOH) New Zealand Ministry of Health 2014(p. 32) |
≤ 5.0 (90) | ≤ 7.4 (133) | ≤ 6.7 (120) |
|
American Diabetes Association (ADA) ADA 2020(S21) Canadian Diabetes Association (CDA) Feig 2018(S178) |
≤ 5.3 (95) | ≤ 7.8 (140) | ≤ 6.7 (120) |
|
National Institute of Health and Clinical Excellence (NICE) NICE 2015(p. 21) |
< 5.3 (95) | < 7.8 (140) | < 6.4 (115) |
|
5th International Workshop on GDM Metzger 2007(S254) |
5.0 (90) to 5.5 (99) | < 7.8 (140) | < 6.7 (120) to 7.1 (127) |
|
Scottish Intercollegiate Guidelines Network SIGN 2017(p. 59) |
4.0 (72) to 6.0 (108) | < 8.0 (144) | < 7.0 (126) |
|
German Diabetes Association (DDA) Kleinwechter 2014(p. 404) |
3.6 (65) to 5.3 (95) | < 7.8 (140) | < 6.7 (120) |
| Abbreviations: GDM: gestational diabetes mellitus | |||
1 We converted all published glycaemic values for GDM treatment into both mmol/L or mg/dL.
Description of the intervention
Treatment of GDM aims to reduce the associated risks of gestational diabetes for the mother and baby by controlling the high maternal blood glucose concentrations (Alwan 2009). Glycaemic control is usually measured by monitoring capillary blood glucose concentrations to ensure blood glucose concentrations are maintained within a predefined threshold (Metzger 2008). This may be achieved through the use of diet and lifestyle modifications (ADA 2020; New Zealand Ministry of Health 2014; NICE 2015; SIGN 2017), or with the addition, if necessary, of pharmacological interventions such as oral hypoglycaemic medications or subcutaneous insulin (ACOG 2018; New Zealand Ministry of Health 2014; NICE 2015; SIGN 2017). Trials of interventions for GDM usually compare different treatment strategies with glycaemic control as an outcome, not an intervention (Middleton 2012). The focus of this review is to compare different treatment targets of glycaemic control in women with GDM and their impact on maternal and fetal health. We defined tight glycaemic targets as lower numerical glycaemic concentrations that women are advised to adhere to through lifestyle and pharmacological interventions, and less‐tight glycaemic targets as higher numerical glycaemic concentrations that woman are advised to adhere to through lifestyle and pharmacological interventions. The two interventions provide numerical values for fasting and postprandial glycaemic targets which are measured through blood glucose monitoring. Trials may express their interventions additionally in non‐numerical terms, for example: ‘loose’, 'standard care', 'low(er)', 'less tight', ‘moderate’, ‘tight’, 'tighter', ‘very tight’, 'strict(er)', 'intensive therapy', and 'liberal'.
How the intervention might work
There is a continuous relationship between increasing maternal blood glucose concentrations and detrimental maternal and fetal outcomes (Langer 1994; Metzger 2008). Treatment of GDM aims to maintain maternal blood glucose concentrations within certain glycaemic target thresholds, reducing the physiological response of the fetus to elevated maternal blood glucose concentrations, and has been shown to be beneficial in reducing perinatal morbidity (Crowther 2005; Landon 2009). The Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) trial, Crowther 2005, and the Maternal‐Fetal Medicine Units Network (MFMU) trial, Landon 2009, both compared treatment of GDM with no treatment. The MFMU Network trial had tighter glycaemic control targets (fasting plasma glucose < 5.3 mmol/L (95 mg/dL) and two‐hour postprandial < 6.7 mmol/L (120 mg/dL)) than the ACHOIS trial (fasting plasma glucose < 5.5 mmol/L (99 mg/dL) and two‐hour postprandial < 7.0 mmol/L (126 mg/dL)), and demonstrated a reduction in the risk of caesarean section (risk ratio (RR) 0.79, 97% confidence interval (CI) 0.64 to 0.99) not shown in the ACHOIS trial (RR 0.97, 95% CI 0.81 to 1.16), although both trials demonstrated reductions in birthweight and large‐for‐gestational‐age infants in women with GDM who received treatment compared with women who were not treated (Crowther 2005; Landon 2009; Ornoy 2015). Such evidence suggests lower glycaemic targets may be of benefit.
Why it is important to do this review
The evidence for optimal glycaemic targets for women with GDM is limited and of varying certainty (Hernandez 2015). It appears that women who have better controlled blood glucose concentrations in pregnancy have a lower incidence of pre‐eclampsia and large‐for‐gestational‐age babies (Crowther 2005; Landon 2009). The infants of these women have a reduced incidence of neonatal hypoglycaemia and perinatal mortality (Landon 2009). Target recommendations from international professional organisations for maternal glycaemic control vary widely, all relying on consensus given the lack of high‐certainty evidence (ADA 2020; Feig 2018; Metzger 2007; Nankervis 2013; New Zealand Ministry of Health 2014; NICE 2015; SIGN 2017).
In assessing evidence related to determining the optimal degree of glycaemic targets, this review will contribute to knowledge that can be used to minimise the risk of adverse birth outcomes and diabetic complications for pregnant women and their babies. This is an update of a review last published in 2016 (Martis 2016).
Objectives
To assess the effect of different intensities of glycaemic control in pregnant women with GDM on maternal and infant health outcomes.
Methods
Criteria for considering studies for this review
Types of studies
This is the first update of a review first published in 2016. We included all published and unpublished randomised controlled trials (RCTs), cluster‐RCTs, and quasi‐RCTs, including conference abstracts, assessing different intensities of glycaemic control for women with GDM (Higgins 2020). Cross‐over trials were not eligible for inclusion, as changes in insulin sensitivity throughout pregnancy make cross‐over trials an inappropriate methodology for this review, and women with GDM are usually advised of only one glycaemic target range to guide treatment in their pregnancy.
Types of participants
All pregnant women diagnosed with GDM. Due to varying diagnostic methods and criteria used internationally, we defined screening and subsequent diagnosis and diagnostic criteria as identified in the individual trials. We excluded women with known pre‐existing type 1 or type 2 diabetes.
Types of interventions
The type of intervention included any glycaemic treatment targets (blood glucose concentration) used for glycaemic control for women with GDM to guide treatment. To clarify further, we converted blood glucose values into both mmol/L and mg/dL, as different countries express glucose values in either mmol/L or mg/dL. For example, mmol/L is typically used in most European countries, New Zealand, Australia, and North America, while mg/dL is most often used in South America, China, and Germany. Trials often express their interventions additionally in non‐numerical terms, for example: ‘loose’, 'standard care', 'low(er)', 'less tight', ‘moderate’, ‘tight’, 'tighter', ‘very tight’, 'strict(er)', 'intensive therapy', and 'liberal'. For clarity, we planned to use the trial definitions when discussing the results instead of using the numerical ranges repeatedly.
Types of outcome measures
The primary and secondary maternal and infant outcome measures are based on the Core Outcome Set from Bain 2016 and consensus between the review authors and all other review authors of Cochrane Reviews for treatment of GDM.
Primary outcomes
Maternal
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia).
Subsequent development of type 2 diabetes.
Infant
Perinatal (fetal and neonatal) mortality.
Large‐for‐gestational‐age (birthweight greater than the 90th centile, or as defined by individual trial).
Composite of mortality or serious morbidity (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture, or nerve palsy).
Neurosensory disability (as defined by individual trials).
Secondary outcomes
Maternal
Caesarean section.
Maternal mortality.
Weight gain during pregnancy.
Placental abruption.
Induction of labour.
Perineal trauma.
Postpartum haemorrhage.
Postpartum infection requiring the use of antibiotics (as defined by trialists).
Maternal hypoglycaemia.
Glycaemic control during/end of intervention (as defined by trialists).
Use of pharmacological treatment (insulin, oral hypoglycaemics).
Relevant biomarker changes associated with the intervention (including adiponectin, free fatty acids, triglycerides, high‐density lipoproteins, low‐density lipoproteins, insulin).
Breastfeeding.
Adherence with treatment/management.
Sense of well‐being and quality of life.
Views of the intervention.
Behaviour change associated with the intervention.
Composite of serious morbidity (variously defined by trialists).
Long‐term maternal outcomes
Postnatal depression.
Postnatal weight retention or return to pre‐pregnancy weight.
Body mass index (BMI).
GDM in a subsequent pregnancy.
Type 1 diabetes mellitus.
Impaired glucose tolerance.
Cardiovascular health (as defined by trialists, including blood pressure, hypertension, cardiovascular disease, metabolic syndrome).
Infant
Stillbirth.
Neonatal death.
Macrosomia (birthweight ≥ 4000 g, or as defined by individual trial).
Small‐for‐gestational‐age (birthweight less than the 10th centile, or as defined by individual trial).
Shoulder dystocia.
Bone fracture.
Nerve palsy.
Preterm birth (< 37 weeks' gestation; < 32 weeks' gestation).
Gestational age at birth.
Birthweight and z score.
Head circumference and z score.
Length and z score.
Ponderal index.
Hypoglycaemia (variously defined).
Respiratory distress syndrome.
Neonatal jaundice (hyperbilirubinaemia).
Hypocalcaemia.
Adiposity (variously defined by trials, e.g. skinfold thickness, fat mass).
Polycythaemia.
Apgar score < seven at 5 minutes.
Relevant biomarker changes associated with the intervention (including cord C‐peptide, cord insulin).
Later childhood
Weight and z score.
Height and z score.
Head circumference and z score.
Adiposity (including BMI, skinfold thickness).
Blood pressure.
Type 1 diabetes mellitus.
Type 2 diabetes mellitus.
Impaired glucose tolerance.
Dyslipidaemia or metabolic syndrome.
Educational achievement.
Adulthood outcomes
Weight.
Height.
Adiposity (including BMI, skinfold thickness, fat mass).
Cardiovascular health (as defined by trialists, including blood pressure, hypertension, cardiovascular disease, metabolic syndrome).
Type 1 diabetes mellitus.
Type 2 diabetes mellitus.
Impaired glucose tolerance.
Dyslipidaemia or metabolic syndrome.
Employment, education, and social status/achievement.
Health services
Number of antenatal visits or admissions.
Number of hospital or health professional visits (including midwife, obstetrician, physician, dietician, diabetic nurse).
Admission to neonatal intensive care unit/nursery.
Length of antenatal stay.
Length of postnatal stay (maternal).
Length of postnatal stay (baby).
Cost of maternal care.
Cost of offspring care.
Costs associated with the intervention.
Costs to families associated with the management provided.
Search methods for identification of studies
The following section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register in collaboration with their Information Specialist (26 September 2022).
The Register is a database containing over 34,000 reports of controlled trials in the field of pregnancy and childbirth, and represents over 30 years of searching. For full current search methods used to populate Cochrane Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase, and CINAHL; the list of handsearched journals and conference proceedings; and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (Cumulative Index to Nursing and Allied Health Literature) (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Based on the intervention described, each trial report is assigned a number that corresponds to a specific Cochrane Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov (clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (trialsearch.who.int/) (26 September 2022) for unpublished, planned, and ongoing trial reports (see Appendix 1 for search terms used).
Searching other resources
We searched the reference lists of retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
We used the following methods for assessing the eight reports identified as a result of the search.
The following section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently screened the titles and abstracts of records identified by the search. We retrieved the full texts of all studies deemed potentially relevant, and two review authors independently assessed these for inclusion in the review, and listed the reasons for exclusion of excluded studies. There were no disagreements, hence no need to consult with a third review author.
A study flow diagram is shown in Figure 1.
1.
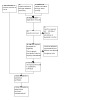
Study flow diagram.
Data extraction and management
We used the Cochrane Pregnancy and Childbirth Group data extraction form. Two review authors (OH and CAC) independently extracted data from two of the identified studies, and one review author (OH) and Dr Luling Lin independently extracted data from the third identified study. We entered the data into Review Manager Web software and checked the data for accuracy (RevMan Web 2022).
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for the four included studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreements by discussion or by involving a third review author. We received statistical advice regarding intracluster correlations from cluster‐randomised trials as outlined in our protocol.
(1) Random sequence generation (checking for possible selection bias)
We described for the included studies the method used to generate the allocation sequence in sufficient detail to permit an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for the included studies the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the method as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for the included studies the methods used, if any, to blind study participants and personnel from the knowledge of which intervention a participant received. We considered studies to be at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high, or unclear risk of bias for participants;
low, high, or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for the included studies the methods used to blind outcome assessors from the knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high, or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data)
We described for the included studies, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We attempted to contact the trial authors for further information and planned to include any relevant missing data in the analyses we undertook. For cluster‐RCTs, we also considered the loss of a cluster from the trial as missing outcome data that could lead to risk of bias.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation); or
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for the included studies how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all of the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; the study fails to include the results of a key outcome that would be expected to have been reported); or
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for the included studies any important concerns we had about other possible sources of bias.
We assessed whether the study was free of other problems that could put it at risk of bias. We assessed the methods as:
low risk of other bias;
high risk of other bias; or
unclear risk of bias.
For cluster‐RCTs, we assessed the risk of recruitment bias. We assessed the methods as:
low risk of bias (where there was no evidence of risk of recruitment bias due to participants being recruited after cluster randomisation and thus at risk of being aware of whether the cluster is an 'intervention' or 'control' cluster);
high risk of bias (where there was evidence of recruitment after cluster randomisation and knowledge of whether the cluster is an 'intervention' or 'control' cluster); or
unclear risk of bias (where it is unclear if there was a risk of recruitment bias).
For cluster‐RCTs, we also assessed for bias due to baseline imbalance. We assessed the methods as:
low risk of bias (where there was evidence showing a reduction of the risk of baseline imbalance through stratified or pair‐matched randomisation of clusters or reporting of baseline comparability of clusters to reduce uncertainty of this risk);
high risk of bias (where there was evidence of baseline imbalance or no attempt to minimise this); or
unclear risk of bias (where it was unclear if there is a risk of baseline imbalance).
Cluster‐RCTs are assessed for their risk for biased estimate effects due to not accounting for clustering in their analyses. We therefore judged whether trials considered this in their analyses, and if not, made adjustments in our meta‐analysis to allow for the clustering effect. We also considered the comparability of a cluster‐randomised trial to standard RCTs due to the risk of 'herd effect'. We considered whether a potential 'herd effect' or 'contamination' may lead to underestimation of the intervention effect.
(7) Overall risk of bias
We made explicit judgements about whether the study was at high risk of bias, according to the criteria in the Cochrane Handbook (Higgins 2017). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether it impacted on the findings. We planned to explore the impact of the level of bias through the undertaking of sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI). Where required, we calculated risk ratios using the number of events and the number assessed for each group. Additionally, we calculated standard error for the generic inverse variance approach using the provided CIs or P values.
Continuous data
For continuous data, we used the mean difference (MD) or risk difference (RD), depending on the results provided. For count outcomes, we used ratios of means. In future updates, if needed, we will use the standardised mean difference (SMD) to combine trials that measure the same outcome but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We identified one stepped‐wedge cluster‐RCT (Crowther 2022). We received advice from a Cochrane statistician, and under their guidance, deemed the analytical adjustments performed by Crowther 2022 to be appropriate. We therefore analysed outcomes that included data from the cluster‐RCT using the generic inverse variance approach, which enabled us to directly include the adjusted treatments effects. We considered it reasonable to combine the results from both the cluster‐randomised trial and individually randomised trials, as there was little heterogeneity between the study designs, and interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely. In future, if we include a cluster‐randomised trial that did not make the appropriate adjustments, we will adjust the standard errors using the methods described in Section 23.1 of the Cochrane Handbook using an estimate of the intracluster correlation coefficient (ICC) as provided by the trial authors and the mean cluster size (M) in order to determine the design effect (1 + (M − 1)ICC) (Higgins 2020). We will then apply the design effect to the number of events and sample size for dichotomous outcomes and the sample size only for continuous outcomes. This reduces the cluster‐randomised data to their effect sample size.
Multiple pregnancy
One trial reported on multiple pregnancy (eight women). For this trial we presented maternal data as per woman randomised and neonatal data per infant. This trial also adjusted for clustering due to multiple pregnancy, and these adjusted treatment effects were included using the generic inverse variance method.
Multiple‐arm studies
No study included in this review was a multiple‐arm trial. In future updates of the review, where a trial has multiple intervention arms, we will avoid 'double‐counting' of participants by combining groups to create a single pair‐wise comparison if possible. Where this is not possible, we will split the 'shared' group into two or more groups with smaller sample size and include two or more (reasonably independent) comparisons.
Dealing with missing data
The levels of attrition in the included studies did not exceed 20%. In future updates of this review, we will explore the impact of including studies with high levels of missing data (> 20%) in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, to the greatest degree possible, on an intention‐to‐treat basis, that is we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the groups to which they had been allocated, regardless of whether they had received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I², and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30%, and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity (Higgins 2020).
Assessment of reporting biases
We included four studies in the review. In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager Web software (RevMan Web 2022). The data from the four studies included in this review were combined in meta‐analysis. We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that the studies were estimating the same underlying treatment effect, that is where trials were examining the same intervention, and the trials’ populations and methods were judged to be sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered to be clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects, and we have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was considered not clinically meaningful, we did not combine trials.
If we used random‐effects analyses, we presented the results as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We did not identify substantial heterogeneity between the four included trials, therefore we have not explored heterogeneity or subgroup analyses. If in future updates we identify substantial heterogeneity, we will investigate it using subgroup analyses. We will consider whether an overall summary is meaningful, and if it is, use a random‐effects model for analysis.
We planned not to combine trials based on the individual trial definition of intensity of glycaemic control. We planned to use the mmol/L (mg/dL) thresholds employed in the trials and subgroups based on these if there was significant heterogeneity.
The following are the clinical subgroups of interest.
-
Types of strategies used to target or achieve glycaemic control, or both
Diet and lifestyle changes alone
Oral hypoglycaemics +\− diet and lifestyle changes
Insulin therapy +\− diet and lifestyle changes
-
Criteria used for diagnosis of GDM
International Association of Diabetes in Pregnancy Study Group (IADPSG 2010), Australasian Diabetes in Pregnancy Society (ADIPS) (Nankervis 2013); World Health Organization (WHO) (WHO 2013); American Diabetes Association (ADA 2020); Scottish Intercollegiate Guidelines Network (SIGN 2017) versus
New Zealand Ministry of Health (New Zealand Ministry of Health 2014) versus
National Institute of Health and Clinical Excellence (NICE) (NICE 2015) versus
Canadian Diabetes Association (Feig 2018) versus
American College of Obstetricians and Gynecologists (ACOG 2013) versus
Carpenter and colleagues (Carpenter 1982) versus
National Diabetes Data Group (National Data Group 1979) versus
Hoffmann and colleagues (ADIPS) (Hoffman 1998), NICE (NICE 2008), WHO (WHO 1999) versus
Any others identified by individual trial
-
Gestational age at diagnosis
< 24 weeks versus
24 to < 28 weeks versus
≥ 28 weeks
Gestation unknown/not specified
-
Woman’s ethnicity as identified in the trials
White
Black
Asian or Pacific Islander
Indigenous peoples
Other
-
Women who are primiparas versus multiparas
Primiparas women only
Multiparas women
Parity unknown/not specified
-
Twin pregnancies versus singleton pregnancies
Singletons only
Twins only
Plurality unknown/not specified
We will conduct subgroup analyses using primary outcomes only. We will assess subgroup differences by interaction tests available within Review Manager Web and report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We performed a sensitivity analysis to investigate the effect of the randomisation unit, as we included a cluster‐randomised trial along with individually randomised trials. We did not require any additional sensitivity analyses. In future, if required, we will also carry out sensitivity analyses to explore the impact of including studies assessed as at high risk of bias due to randomisation method (e.g. quasi‐randomisation versus true randomisation) and allocation concealment on the primary outcomes to assess whether this makes any difference to the overall results. In addition, we will perform sensitivity analysis by excluding trials assessed as at high risk of bias due to missing data.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach as outlined in Chapter 5 of the GRADE Handbook (Schünemann 2013), employing GRADEpro GDT software to produce two summary of findings tables (GRADEpro GDT). We generated a summary of the intervention effect and a measure of certainty of the body of evidence for each of the outcomes listed below using the GRADE approach, based on the five GRADE domains (study limitations, consistency of effect, imprecision, indirectness, and publication bias). We chose seven maternal and seven child (as neonate, child, adult) outcomes (seven are the maximum of outcomes permitted with this software). These are based on the core outcome set used for selecting outcomes for this review, which is based on consensus between the review authors and all other review authors of Cochrane Reviews for treatment of GDM (Bain 2016). See Table 1 and Table 2. We assessed the certainty of the evidence for each outcome as high, moderate, low, or very low. Given that outcomes were assessed by randomised trials, the certainty of the evidence was considered to be initially high before being graded.
Maternal
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia)
Subsequent development of type 2 diabetes
Caesarean section
Weight gain during pregnancy
Induction of labour
Perineal trauma
Postnatal depression
Child (as neonate, child, adult)
Perinatal mortality
Large‐for‐gestational‐age
Composite of mortality or serious morbidity
Neurosensory disability
Neonatal hypoglycaemia
Adiposity
Type 1 or type 2 diabetes mellitus
Results
Description of studies
Results of the search
We identified 10 new reports and reassessed the four ongoing studies from the previous version of the review (Ardilouze 2015; Crowther 2022; Hague 2014; Scifres 2019).
We included three new trials (10 reports) (Crowther 2022; Popova 2018; Scifres 2019). Three trials are ongoing (Hague 2014; NCT03610178; NCT04672031).
We excluded Ardilouze 2015 due to termination of the trial.
See Figure 1.
Included studies
We included four trials in this updated review (Crowther 2022; Popova 2018; Scifres 2019; Snyder 1998). See Characteristics of included studies.
Crowther 2022, a multicentre, stepped‐wedge, cluster‐randomised trial, was conducted in New Zealand from 2015 and involved 1096 women. Women were diagnosed with GDM by an oral glucose tolerance test in mid‐pregnancy and were included in the study if they were receiving pregnancy care at one of the 10 participating hospitals in New Zealand. Women were excluded from the study if they had a known major fetal anomaly. The study compared tighter targets for glycaemic control versus less‐tight targets for glycaemic control. Tighter targets for glycaemic control were defined as fasting plasma glucose ≤ 5.0 mmol/L (90 mg/dL); 1‐hour postprandial ≤ 7.4 mmol/L (133.2 mg/dL); 2‐hour postprandial ≤ 6.7 mmol/L (120.6 mg/dL). Less‐tight targets for glycaemic control were defined as fasting plasma glucose < 5.5 mmol/L (99 mg/dL); 1‐hour postprandial < 8.0 mmol/L (144 mg/dL); 2‐hour postprandial < 7.0 mmol/L (126 mg/dL). The primary outcome was the incidence of large‐for‐gestational‐age infants. The trial was funded through a four‐year project grant from the Health Research Council in New Zealand (No. 14/449). The funding body had no role in the study design or writing of the manuscript. The authors declared no competing interests.
Popova 2018, an RCT, was conducted in St Petersburg, Russia at the Almazov National Medical Research Centre from 2015. The preliminary results of the main trial were only available in abstract form. However, two associated publications were identified, one in abstract form and one as a complete report. Women were diagnosed with GDM in accordance with WHO 2013 criteria and included 395 women. The trial compared very tight glycaemic targets (GDM1) versus less‐tight glycaemic targets (GDM2). GDM1 was defined as fasting blood glucose (FBG) < 5.1 mmol/L (91.8 mg/dL) and < 7.0 mmol/L (126 mg/dL) postprandial. GDM2 was defined as FBG < 5.3 mmol/L (95.4 mg/dL) and < 7.8 mmol/L (140.4 mg/dL) postprandial. The primary outcome was the incidence of large‐for‐gestational‐age infants. No additional inclusion criteria were specified, nor were any exclusion criteria detailed. The study was partly funded by the Russian Science Foundation, and the authors had no declarations of interest to disclose. In response to our query for further information, the primary author let us know of their intention to publish the final results when further data will be available. We will include the results of their published trial data in future updates of this review.
Scifres 2019, a randomised controlled clinical feasibility trial, was performed in the USA at the University of Oklahoma Medical Center. Women were recruited between December 2015 and December 2017 and were diagnosed with GDM by the Carpenter‐Coustan Criteria between 12 and 32 weeks’ gestation. Women were either overweight (BMI 25 to 29.9 kg/m2) or obese (BMI ≥ 30 kg/m2) pre‐pregnancy and were enrolled from prenatal clinics at the University of Oklahoma. Inclusion criteria included singleton pregnancy, age 18 to 45 years, and planned birth at the University of Oklahoma Medical Center. Women were excluded from the trial if they had maternal tobacco use, birth was planned prior to 34 weeks’ gestation, chronic hypertension requiring medical therapy, vascular disease, serum creatinine > 1.5 mg/dL, rheumatological disorders, or oral steroid use within 30 days of enrolment. A total of 60 women were included in the feasibility trial in order to ensure 85% power. Women were randomised to either standard (fasting glucose < 5.3 mmol/L (95 mg/dL), 1‐hour postprandial glucose < 7.8 mmol/L (140 mg/dL)) or intensive (fasting < 5.0 mmol/L (90 mg/dL), 1‐hour postprandial glucose < 6.7 mmol/L (120 mg/dL)) glycaemic targets. The primary outcome was the feasibility of lowering mean glycaemic concentrations as assessed by continuous glucose monitoring, but study authors also assessed other maternal and infant health outcomes. The trial was funded in part by the University of Oklahoma College of Medicine Alumni Association and the National Institutes of Health, National Institute of General Medical Sciences (Grant 1 U54GM104938, PI James). The authors reported no conflict of interest. We emailed the primary author regarding any additional data and information but received no response.
Snyder 1998, an RCT from Canada, was available in abstract form only. We identified no full‐text publication. We emailed co‐author Meltzer for further information, as Meltzer was the only author with a contact email address found via the internet. Synder, the main author, and co‐authors Morin and Nadeau were not contactable. We had received no response from Meltzer at time of submission. The trial involved 180 women who were diagnosed with GDM between 20 and 32 weeks' gestation, and were recruited over a 12‐month period (1996 to 1997). The study compared strict versus liberal glycaemic targets for glycaemic control for women treated with insulin. Strict glycaemic targets for insulin treatment were defined as pre‐prandial: 5.0 mmol/L (90 mg/dL) and 1‐hour postprandial: 6.7 mmol/L (120 mg/dL); liberal glycaemic targets were defined as pre‐prandial: 5.8 mmol/L (104 mg/dL) and 1‐hour postprandial: 7.8 mmol/L (140 mg/dL). No other inclusion criteria were detailed. The study assessed caesarean section rates, insulin use, infant birthweight, macrosomia, and small‐for‐gestational‐age infants. Data for other characteristics (pre‐pregnancy BMI, maternal age, gestational age at diagnosis, and length of treatment) and the criteria used to diagnose GDM were not reported. No funding sources or declarations of interest were identified.
Excluded studies
We excluded one study (Garner 1997), as it was a study of intensification of treatment, not of comparing different intensities of glycaemic control targets in women diagnosed with GDM. We excluded Ardilouze 2015 due to termination of the trial. See Characteristics of excluded studies.
Risk of bias in included studies
We assessed the overall risk of bias for Popova 2018 and Snyder 1998 to be unclear, as they were only published as abstracts and provided limited information regarding the methods used. We assessed Crowther 2022 and Scifres 2019 to be at low risk of bias, as the methods of the trial and the outcomes were clearly described.
The risk of bias summaries present the review authors' judgements about each risk of bias item for each included study (Figure 2) and each risk of bias item presented as percentages across all included studies (Figure 3).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We assessed Crowther 2022 to be at low risk of allocation bias, as the allocation of hospitals was prepared by the trial statistician using a computer‐generated random number table.
The method of random sequence generation was not described in detail, and allocation concealment was not described in Popova 2018 and Snyder 1998. We therefore judged these trials to be at unclear risk of bias.
We judged Scifres 2019 to be at low risk of bias for allocation and randomisation sequence generation, as randomisation occurred via a computer‐based platform programmed in REDCap.
Blinding
It remains unclear whether participants in Crowther 2022 were blinded to their allocation, and the potential impact of this on risk of bias.
Given that Popova 2018 remains in abstract form and is yet to be published, it is unclear if participants were blinded to their assignment and the potential impact this may have had on outcomes.
In Snyder 1998, the blinding of women, their clinical carers, and the researcher to group allocation was most likely not feasible. A lack of blinding may have influenced the study outcomes. No details were provided regarding blinding of outcome assessors.
We assessed Scifres 2019 as at high risk of performance bias, as the authors stated that it not being feasible to blind participants and personnel to the assigned targets was a limitation of the trial. However, detection bias was likely low risk, as both women and their providers were blinded to the results.
Incomplete outcome data
We judged Crowther 2022 to be at low risk of attrition bias, as although some women and infants were not assessed for some outcomes, this was likely due to incomplete hospital records and therefore unlikely to introduce bias.
We judged Popova 2018 to be at low risk for attrition bias, as it appears there were no incomplete data at the preliminary results stage.
We judged Scifres 2019 to be at unclear risk of attrition bias due to four participants not completing the second visit during the trial, the impact of which on the results is unclear.
We judged Snyder 1998 to be at unclear risk of attrition bias. Data were reported for 171 of 180 women who were recruited to the trial. No data for the missing nine women were provided. Intention‐to‐treat analysis was not reported.
Selective reporting
Crowther 2022 and Popova 2018 reported on the outcomes prespecified in the protocol and were therefore judged to be at low risk of reporting bias.
The results reported in Scifres 2019 matched those described in the methods and materials section, therefore we judged this study to be at low risk of reporting bias.
Snyder 1998 was only published in abstract form for a conference, and it is likely that data were not reported for all outcomes as the abstract only stated there were no differences in outcomes. We therefore judged this study to be at high risk of selective reporting bias.
Other potential sources of bias
It was not possible to judge if there were other sources of bias in Popova 2018 and Snyder 1998, as minimal information was provided in the abstracts. The statement "the groups were comparable for pre‐pregnancy body mass, maternal age, gestational age at diagnosis and length of treatment" in Snyder 1998 is not substantiated with any data.
Crowther 2022 had likely minimal recruitment bias, and baseline characteristics suggested no evidence of baseline imbalance. The trial adjusted for the impact of cluster effect in their analyses, which were also likely minimally impacted by the 'herd effect', resulting in the trial being comparable to other parallel RCTs. As a result there were no other obvious concerns with Crowther 2022.
There were no obvious concerns in Scifres 2019.
Effects of interventions
See: Table 1: Intensity of glycaemic control for women with GDM and Table 2: for their children.
Primary outcomes
Maternal outcomes
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia)
Two studies reported on the incidence of pre‐eclampsia (Crowther 2022; Popova 2018). Sixty‐five of 796 (8.2%) women in the tighter glycaemic control group had pre‐eclampsia compared to 50 of 695 (7.2%) women in the less‐tight glycaemic control group. Tighter glycaemic control may result in an increase in pre‐eclampsia (risk ratio (RR) 1.16, 95% confidence interval (CI) 0.80 to 1.69, 2 trials, 1491 women; Analysis 1.1); however, the 95% CI is compatible with a wide range of effects that encompass both benefit and also significant harm, so we cannot be certain of the true effect. We assessed the certainty of evidence for hypertensive disorders of pregnancy to be low due to unclear risk of bias in the studies and substantial imprecision in the results.
1.1. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 1: Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia)
No data were reported for subsequent development of type 2 diabetes.
Infant outcomes
Perinatal (fetal and neonatal) mortality
Two studies reported on perinatal mortality (Crowther 2022; Popova 2018). There were a total of three deaths in the less‐tight glycaemic control group and no deaths in the tighter glycaemic control group. Both trials reported an incidence of zero in at least one group and therefore could not be meta‐analysed to produce an overall risk ratio (Analysis 1.2). It is difficult to determine whether there is a difference between groups due to the very low number of events. Each trial individually reported no difference in risk of perinatal mortality. We assessed the certainty of evidence to be low due to a high risk of bias and imprecision in the results.
1.2. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 2: Perinatal (foetal and neonatal) mortality
Large‐for‐gestational‐age (LGA)
One hundred and twenty‐six of 830 (15.2%) infants in the tighter glycaemic control group were LGA compared to 114 of 729 (15.6%) infants in the less‐tight glycaemic control group. The evidence suggests that tighter glycaemic control may result in no difference in risk of being born LGA (RR 0.96, 95% CI 0.72 to 1.29, 3 studies, 1556 infants; Analysis 1.3). We assessed the certainty of evidence to be moderate due to serious risk of bias in the results.
1.3. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 3: Large‐for‐gestational‐age
Composite mortality or serious morbidity (as defined by trial)
Sixteen of 830 (1.9%) infants in the tighter glycaemic control group experienced composite mortality or serious morbidity compared to 33 of 720 (4.6%) infants in the less‐tight glycaemic control group. The evidence suggests a possible reduction in the composite of mortality or serious morbidity in the tighter glycaemic control group (RR 0.84, 95% CI 0.55 to 1.29, 3 studies, 1559 infants; Analysis 1.4); however, the 95% CI is compatible with a wide range of effects that encompass both appreciable benefit and also harm. We assessed the certainty of evidence to be low due to high risk of bias in the studies and imprecision in the results.
1.4. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 4: Composite of mortality or serious morbidity (as defined by trial)
No data were reported for the primary infant outcome of neurosensory disability.
Secondary outcomes
Maternal outcomes
Caesarean section
Three hundred and three of 881 (34.3%) women in the tighter glycaemic control group had a caesarean section compared with 254 of 781 women (32.5%) in the less‐tight glycaemic control group. Tighter glycaemic control likely results in little to no difference in risk of birth by caesarean section (RR 0.98, 95% CI 0.82 to 1.17, 3 studies, 1662 women; Analysis 1.5). We assessed the certainty of evidence to be moderate, downgraded for risk of bias.
1.5. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 5: Caesarean section
Weight gain during pregnancy
There was a mean weight gain of 9.9 kg in women in the tighter glycaemic control group (n = 20) compared to a mean weight gain of 9.5 kg in the less‐tight glycaemic control group (n = 21). There was little or no difference in gestational weight gain between groups (mean difference (MD) 0.40, 95% CI −2.91 to 3.71, 1 study, 41 participants; Analysis 1.6).
1.6. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 6: Gestational weight gain
Induction of labour
Three hundred and twelve of 595 women had an induction of labour in the tighter glycaemic control group compared to 259 of 501 women in the less‐tight glycaemic control group. There was likely no difference in rates of induction of labour between groups (RR 0.96, 95% CI 0.78 to 1.18, 1 study, 1096 women; Analysis 1.7). We assessed the certainty of evidence to be moderate, downgraded for serious imprecision.
1.7. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 7: Induction of labour
Maternal hypoglycaemia
Five of 595 women in the tighter glycaemic control group experienced hypoglycaemia compared to six of 501 women in the less‐tight glycaemic control group. There was likely no evidence of a difference in maternal hypoglycaemia between groups (RR 1.23, 95% CI 0.31 to 4.91, 1 study, 1096 women; Analysis 1.8).
1.8. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 8: Maternal hypoglycaemia
Glycaemic control during/end of intervention (as defined by trialists)
One trial assessed glycaemic control through measuring mean daily fasting blood glucose (mmol/L) and mean daily postprandial blood glucose (mmol/L) (Popova 2018). The mean daily fasting blood glucose was 4.8 mmol/L (86.4 mg/dL) in the tighter glycaemic control group (201 women) compared to 4.9 mmol/L (88.2 mg/dL) in the less‐tight glycaemic control group (194 women). The evidence suggests the tighter group achieved slightly lower fasting blood glucose concentrations (MD −0.10, 95% CI −0.18 to −0.02, 1 study, 395 women; Analysis 1.9). The mean daily postprandial blood glucose was 6.2 mmol/L (111.6 mg/dL) in the tighter glycaemic control group (201 women) compared to 6.4 mmol/L (115.2 mg/dL) in the less‐tight glycaemic control group (194 women). The tighter glycaemic control group achieved slightly lower postprandial glucose levels (MD −0.20, 95% CI −0.31 to −0.09, 1 study, 395 women; Analysis 1.10). One trial also assessed glycated haemoglobin as a measure of glycaemic control (Scifres 2019). Women in the tighter glycaemic control group (29 women) achieved an average haemoglobin of 5.6% compared to women in the less‐tight glycaemic group (27 women) who achieved an average of 5.49%. Tighter glycaemic control may result in little to no difference in glycated haemoglobin (MD 0.11, 95% CI −0.31 to 0.53, 1 study, 56 women; Analysis 1.11).
1.9. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 9: Mean daily fasting blood glucose mmol/L
1.10. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 10: Mean daily postprandial blood glucose
1.11. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 11: Glycated haemoglobin
Use of pharmacological therapy
Tighter glycaemic targets likely result in an increase in the use of pharmacological therapy (identified as the use of insulin, metformin, or glyburide in the four included studies) (558/910; 61.3%) compared with less‐tight glycaemic targets (379/808; 46.9%) (RR 1.37, 95% CI 1.17 to 1.59; 4 trials, 1718 women; Analysis 1.12).
1.12. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 12: Use of pharmacological therapy
Relevant biomarker changes associated with the intervention
One trial reported on triglycerides (mg/dL), non‐esterified fatty acids (NEFA) (mmol/L), total cholesterol (mg/dL), low‐density lipoprotein (LDL) cholesterol (mg/dL), and high‐density lipoprotein (HDL) cholesterol (mg/dL) (Scifres 2019).
Triglycerides (mg/dL) MD −7.90, 95% CI −57.54 to 41.74, 1 study, 56 women, Analysis 1.13.
NEFA (mmol/L) MD 0.03, 95% CI −0.06 to 0.12, 1 study, 56 women, Analysis 1.14.
Total cholesterol (mg/dL) MD −14.50, 95% CI −41.72 to 12.72, 1 study, 56 women, Analysis 1.15.
LDL (mg/dL) MD −4.40, 95% CI −26.37 to 17.57, 1 study, 56 women, Analysis 1.16.
HDL (mg/dL) MD −0.46, 95% CI −7.28 to 6.36, 1 study, 56 women, Analysis 1.17.
1.13. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 13: Triglycerides mg/dL
1.14. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 14: NEFA mmol/L
1.15. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 15: Total cholesterol mg/dL
1.16. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 16: LDL mg/dL
1.17. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 17: HDL mg/dL
Breastfeeding
Tighter glycaemic targets were likely not associated with any difference in rates of breastfeeding (561/595; 94.3%) compared with less‐tight glycaemic control (479/498; 96.2%) (RR 0.99, 95% CI 0.85 to 1.15, 1 study, 1093 women; Analysis 1.18).
1.18. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 18: Breastfeeding
Adherence with treatment
One study reported on adherence to treatment (Popova 2018). Tighter glycaemic control may result in a large decrease in adherence to treatment (58/ 201; 28.9%) compared with less‐tight glycaemic control (137/194; 70.6%) (RR 0.41, 95% CI 0.32 to 0.52, 1 study, 395 women; Analysis 1.19).
1.19. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 19: Adherence with treatment
No data were reported for any of our other maternal secondary outcomes (maternal mortality, placental abruption, perineal trauma, postpartum haemorrhage, postpartum infection requiring use of antibiotics (variously defined), sense of well‐being and quality of life, views of the intervention; behaviour change associated with the intervention).
Long‐term maternal outcomes
No data were reported for any of our prespecified long‐term maternal outcomes (postnatal depression, postnatal weight retention or return to pre‐pregnancy weight, BMI, GDM in a subsequent pregnancy, type 1 diabetes mellitus, impaired glucose tolerance, cardiovascular health (as defined by trialists, including blood pressure, hypertension, cardiovascular disease, metabolic syndrome)).
Maternal health outcomes not prespecified in this review
Serious maternal health outcomes (as defined by trialists)
One trial reported a composite of serious maternal health outcomes (Crowther 2022). While this outcome was not prespecified in the review, we deemed it to be of high importance. Serious maternal health outcomes were defined as one or more of maternal death, pulmonary oedema, eclampsia, stroke, adult respiratory distress syndrome, cardiac arrest, respiratory arrest, placental abruption, haemolysis, coagulopathy, major postpartum haemorrhage, deep vein thrombosis, or pulmonary embolus requiring anticoagulant therapy. Strict glycaemic targets were likely associated with an increased risk of serious maternal health outcomes (35/595; 5.55%) when compared to liberal glycaemic control (15/501; 2.99%) (RR 2.29, 95% CI 1.14 to 4.60, 1 study, 1096 women; Analysis 1.20).
1.20. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 20: Composite of serious maternal health outcomes
Infant outcomes
There is no evidence of a difference in health outcomes for babies born to women assigned to tight glycaemic targets when compared to babies born to women assigned to liberal glycaemic targets for:
stillbirth RR 0.12, 95% CI 0.01 to 2.33, 2 trials, 1499 infants, Analysis 1.21;
neonatal death RR not estimable as no deaths occurred, 2 trials, 1499 infants, Analysis 1.22;
macrosomia (birthweight > 4000 g) RR 1.12, 95% CI 0.81 to 1.56, 3 trials, 1667 infants, Analysis 1.23;
small‐for‐gestational‐age RR 0.86, 95% CI 0.58 to 1.28, 4 trials, 1727 infants, Analysis 1.24;
shoulder dystocia RR 0.44, 95% CI 0.13 to 1.50, 1 trial, 1101 infants, Analysis 1.25;
gestational age at birth MD −0.11 weeks, 95% CI −0.24 to 0.02, 4 trials, 1727 infants, Analysis 1.26;
birthweight MD 2.42 g, 95% CI −48.00 to 52.83, 4 trials, 1727 infants, Analysis 1.27;
birthweight z score RD 0.11, 95% CI −0.04 to 0.26, 1 trial, 1101 infants, Analysis 1.28;
head circumference RD 0.02 cm, 95% CI −0.22 to 0.26, 1 trial, 1101 infants, Analysis 1.29;
head circumference z score RD 0.07, 95% CI −0.09 to 0.23, 1 trial, 1101 infants, Analysis 1.30;
length RD 0.12 cm, 95% CI −0.28 to 0.52, 1 trial, 1101 infants, Analysis 1.31;
length z score RD 0.12, 95% CI −0.03 to 0.27, 1 trial, 1101 infants, Analysis 1.32;
infant hypoglycaemia RR 0.92, 95% CI 0.72 to 1.18, 3 trials, 1556 infants, Analysis 1.33;
neonatal jaundice (hyperbilirubinaemia) RR 0.71, 95% CI 0.45 to 1.10, 2 trials, 1161 infants, Analysis 1.34;
adiposity (% fat mass) MD −0.62, 95% CI −3.23 to 1.99, 1 trial, 60 infants, Analysis 1.35;
-
relevant biomarkers associated with intervention:
C‐peptide ng/mL MD −0.20, 95% CI −0.54 to 0.14, 1 trial, 41 infants, Analysis 1.36;
cord leptin/adiponectin ratio MD −0.73, 95% CI −1.64 to 0.18, 1 trial, 41 infants, Analysis 1.37;
ANGPTL4 in cord serum ng/mL MD 5.80, 95% CI −1.05 to 12.65, 1 trial, 41 infants, Analysis 1.38;
cord adiponectin (ng/mL) MD −0.40, 95% CI −8.36 to 7.56, 1 study, 41 women, Analysis 1.39.
1.21. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 21: Stillbirth
1.22. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 22: Neonatal death
1.23. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 23: Macrosomia
1.24. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 24: Small‐for‐gestational‐age
1.25. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 25: Shoulder dystocia
1.26. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 26: Gestational age at birth (weeks)
1.27. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 27: Birthweight
1.28. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 28: Birthweight z score
1.29. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 29: Head circumference
1.30. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 30: Head circumference z score
1.31. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 31: Length
1.32. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 32: Length z score
1.33. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 33: Infant hypoglycaemia
1.34. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 34: Neonatal jaundice (hyperbilirubinaemia)
1.35. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 35: Adiposity (% fat mass)
1.36. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 36: C‐peptide ng/mL
1.37. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 37: Cord leptin/adiponectin ratio
1.38. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 38: ANGPTL4 in cord serum ng/mL
1.39. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 39: Cord adiponectin ng/mL
However, tight glycaemic control may reduce cord leptin (ng/mL) concentrations in infants (MD −9.50, 95% CI −16.97 to −2.03, 1 study, 41 women; Analysis 1.40).
1.40. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 40: Cord leptin ng/mL
No data were reported for our other infant outcomes (bone fracture, nerve palsy, preterm birth (< 37 weeks' gestation; < 32 weeks' gestation), ponderal index, respiratory distress syndrome, hypocalcaemia, polycythaemia, Apgar score < seven at 5 minutes).
Later‐childhood outcomes
No data were reported for any of our later‐childhood outcomes (weight and z score, height and z score, head circumference and z score, adiposity (including BMI, skinfold thickness), blood pressure, type 1 diabetes mellitus, type 2 diabetes mellitus, impaired glucose tolerance, dyslipidaemia or metabolic syndrome, educational achievement).
Adulthood outcomes
No data were reported for any of our adulthood outcomes (weight, height, adiposity (including skinfold thickness, fat mass), cardiovascular health (as defined by trialists, including blood pressure, hypertension, cardiovascular disease, metabolic syndrome), type 1 diabetes mellitus, type 2 diabetes mellitus, impaired glucose tolerance, dyslipidaemia or metabolic syndrome, employment, education and social status/achievement).
Health services outcomes
Admission to neonatal intensive care unit/nursery
Twenty‐eight of 629 infants (4.5%) in the strict glycaemic control group required admission to the neonatal intensive care unit/nursery compared with 28 of 532 infants (5.3%) in the liberal glycaemic control group. Strict glycaemic control likely does not affect the risk of neonatal intensive care unit admission (RR 0.59, 95% CI 0.33 to 1.04, 2 trials, 1161 infants; Analysis 1.41).
1.41. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 41: Admission to neonatal intensive care unit/nursery
Length of antenatal stay
Women in the strict glycaemic control group (595 women) had an average antenatal stay of 3.85 days compared to women in the liberal glycaemic control group, who had an average antenatal stay of 3.8 days. Strict glycaemic targets do not increase the length of antenatal stay (mean ratio (MR) 0.96, 95% CI 0.71 to 1.21, 1 trial, 1096 women; Analysis 1.42).
1.42. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 42: Length of antenatal stay
Length of postnatal stay (maternal)
Women in the strict glycaemic control group (595 women) had an average postnatal stay of 3.30 days compared to women in the liberal glycaemic control group, who had an average postnatal stay of 2.66 days. Women in the strict glycaemic control group likely experienced a longer postnatal stay (MR 1.41, 95% CI 1.24 to 1.58, 1 trial, 1096 women; Analysis 1.43).
1.43. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 43: Length of postnatal stay (maternal)
Length of postnatal stay (infant)
Infants of mothers assigned to the strict glycaemic control group (599 infants) had an average postnatal stay of 4.11 days compared to infants of mothers assigned to the liberal glycaemic control group, who had an average postnatal stay of 4.18 days. There was little to no difference reported between groups (MR 0.95, 95% CI 0.83 to 1.07, 1 trial, 1101 infants; Analysis 1.44).
1.44. Analysis.

Comparison 1: Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets, Outcome 44: Length of postnatal stay (infant)
No data were reported for our other health service outcomes (number of antenatal visits or admissions, number of hospital or health professional visits (including midwife, obstetrician, physician, dietician, diabetic nurse), cost of maternal care, cost of offspring care, costs associated with the intervention, costs to families associated with the management provided).
Sensitivity analysis
We undertook sensitivity analysis to evaluate the effect of tight glycaemic targets on each outcome by excluding cluster‐RCTs from the analyses (Crowther 2022). This exclusion did not significantly affect the findings for the outcomes.
Discussion
Summary of main results
In this updated review, we identified and included four RCTs reporting on a total of 1731 women (Crowther 2022; Popova 2018; Scifres 2019; Snyder 1998).
We included pregnant women who were diagnosed with GDM as defined by the trial. Each trial randomised women to either tight glycaemic control or less‐tight glycaemic control, and women were instructed to adhere to the different glycaemic targets as described by the trial. Tighter glycaemic targets may be associated with an increase in the incidence of the primary outcome hypertensive disorders of pregnancy; however, the evidence is uncertain due to wide CIs. Tighter glycaemic targets likely do not affect the rate of caesarean section birth. No data were reported for the outcomes of subsequent development of type 2 diabetes, perineal trauma, return to pre‐pregnancy weight, or postnatal depression.
The evidence suggests no difference between groups in perinatal mortality or large‐for‐gestational‐age infants, but tighter glycaemic targets may be associated with a reduction in the composite of mortality or serious infant morbidity. However, the evidence remains uncertain due to wide CIs. Tighter glycaemic targets likely do not result in a difference in infant hypoglycaemia and may not result in a difference in adiposity. No data were reported for the long‐term infant outcomes of development of diabetes and neurosensory disability.
Tighter glycaemic targets may be associated with an increase in the use of insulin and oral hypoglycaemics and decreased adherence to glycaemic targets.
Overall, we assessed the certainty of the evidence according to GRADE as low to moderate, downgrading primarily due to high risk of bias and imprecision.
A sensitivity analysis assessing the impact of the cluster‐randomised trial showed no change in the previously described outcomes. Based on the current evidence, it remains unclear whether tighter glycaemic targets compared with less‐tight targets improve maternal and infant health.
Overall completeness and applicability of evidence
There remains limited evidence on the effectiveness of different intensities of glycaemic control in women with GDM. The available data come from four trials: a small (n = 180 women) Canadian study published only as a conference abstract (Snyder 1998); a small feasibility study on overweight and obese women (n = 60) from Oklahoma, USA (Scifres 2019); a study from St Petersburg, Russia (n = 395) with preliminary results only published in abstract form (Popova 2018); and a stepped‐wedged cluster‐RCT (n = 1096) (Crowther 2022). The trials reported data for most primary and secondary outcomes, but with insufficient power to determine effects with narrow CIs to enable a clear conclusion. No data were available for maternal and child long‐term outcomes.
Tight glycaemic control was associated with an increased use of pharmacological treatment, and Crowther 2022 report an increased adverse effect on serious maternal health outcomes. The trials recruited women who were diagnosed with GDM and treated with a variety of treatments such as lifestyle interventions, insulin, and oral hypoglycaemic agents such as metformin and glyburide.
The evidence from Popova 2018 suggests a reduction in adherence to glycaemic targets in the tighter glycaemic control group. This low rate of adherence may have contributed to the small differences between groups observed in some analyses.
Significantly more women were included in this updated review, but due to several of the results only being available in abstract form, the risk of bias remains unclear, and the strength of the evidence is generally low. Given that the available evidence is from a range of countries and includes a variety of treatment options, the generalisability of the current evidence is greater.
We identified two ongoing trials and a 4.5‐year follow‐up of Crowther 2022; when data from these studies are published, they will be included in future updates of this systematic review (see Ongoing studies).
Quality of the evidence
The overall risk of bias of the included trials was generally unclear. Primary results from two trials were only available in abstract form, thus random sequence generation and allocation concealment were judged to be unclear due to insufficient detail. We judged most trials to be at high or unclear risk of performance bias, as they were unlikely to have been blinded due to the nature of the intervention. We deemed most trials to be at unclear risk of detection and attrition bias due to limited information provided on how outcomes were assessed and the reasons for loss of follow‐up for some women. We judged the risk of selective reporting to be high in Snyder 1998, as results presented in abstract form are likely to be selected rather than providing all prespecified outcomes.
We assessed the certainty of the evidence using GRADE as low to moderate for most outcomes. Outcomes were generally downgraded due to risk of bias associated with the included trials, such as randomisation, allocation bias, and participant blinding (which is likely unavoidable in a trial assessing an intervention such as this), and only being in abstract form. We also downgraded outcomes due to imprecision of results, as most outcomes failed to meet the optimal information size, and wide CIs indicated significant benefit or harm. Statistical heterogeneity was low for most outcomes assessed using GRADE. See Table 1; Table 2.
Potential biases in the review process
The Information Specialist for the Cochrane Pregnancy and Childbirth Group and the authors of this review carried out systematic searches for all potentially eligible trials. We searched the Cochrane Pregnancy and Childbirth Group's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform, and the reference lists of identified trials. We contacted three authors of included studies via email for further information but received no response. No evidence of potential bias was identified through these systematic searches for published and unpublished studies. If we identify any studies in future searches, we will assess them for potential inclusion in the review. Given the unclear risk of bias in the included trials and the missing outcome data, the potential for bias is present. Three out of the four review authors are also associated with the TARGET Trial by Crowther 2022, which is a potential source of bias in this review. In order to mitigate this risk of bias, review author OH and non‐author Dr Luling Lin, neither of whom is associated with the TARGET Trial, performed the data extraction and risk of bias assessment for the Crowther 2022 trial. Additionally, OH completed the data entry for all trials to reduce this risk.
Agreements and disagreements with other studies or reviews
This review did not provide sufficient evidence to fully evaluate which intensity of glycaemic control is most effective for improving the health outcomes of women with GDM and their babies. Results from only four trials were available (Crowther 2022; Popova 2018; Scifres 2019; Snyder 1998), but the overall risk of bias from these trials remain unclear (Figure 2 ; Figure 3).
There is limited evidence to guide clinical practice for glycaemic control targets for women with GDM to minimise adverse effects on maternal and fetal health. Glycaemic target recommendations from international professional organisations for maternal glycaemic control vary widely and are reliant on consensus given the lack of high‐certainty evidence (Table 3) (ACOG 2018; ADA 2020; Feig 2018; Metzger 2007; Nankervis 2013; New Zealand Ministry of Health 2014; NICE 2015; SIGN 2017). The evidence on which these recommendations have been made is generally unclear.
Prutsky and colleagues published a systematic review that included 34 observational studies involving a total of 9433 women (Prutsky 2013), which summarised the evidence for glycaemic targets in pregnant women with GDM, type 1 diabetes, and type 2 diabetes. No relevant RCTs were identified at that time. Twenty‐six of the 34 observational studies included women with GDM. Overall, the certainty of the evidence of the included observational studies was judged to be low, with the literature limited and heterogeneity among studies high. The results of Prutsky's systematic review showed that a fasting glucose target of < 5.0 mmol/L was associated with a significant reduction in macrosomia (P < 0.01), large‐for‐gestational‐age infants (P = 0.01), neonatal hypoglycaemia (P = 0.01), and neonatal jaundice (P = 0.01). For the mother, there was a significant reduction in pre‐eclampsia during the third trimester of pregnancy (P = 0.01) (Prutsky 2013). Based on the results of these observational studies, the authors concluded that it remains unclear whether glucose targets above or below a fasting glucose threshold of < 5.0 mmol/L offer a better balance of benefits and risks. There was insufficient evidence on postprandial measures to assess different cut‐off points and health outcomes. Prutsky and colleagues highlighted that there have been no well‐conducted large RCTs comparing any two glycaemic thresholds that report on benefits and harms for the mother and her baby. In light of the current evidence assessed in our review, we have identified four randomised trials together with two ongoing trials. The findings from this updated review are in contrast to Prutsky 2013 and suggest that tighter glycaemic control is not associated with improved infant and maternal health outcomes. The results of ongoing trials will be essential in establishing a strong evidence base to develop treatment recommendations.
Authors' conclusions
Implications for practice.
This review is based on four trials involving a total of 1731 women. We assessed the overall risk of bias as unclear, as half of the included trials were reported in abstract form that provided limited information about the study methods used.
The trials provided data on most of our primary outcomes, and there may be an increase in the risk of hypertensive disorders of pregnancy with tighter glycaemic control compared to less‐tight glycaemic control; however, the uncertainty of the evidence suggests both possible significant benefit and harm. The evidence shows that use of tighter glycaemic targets likely has no impact on rates of caesarean section birth or induction of labour. No data were reported for the outcomes of subsequent development of type 2 diabetes, perineal trauma, return to pre‐pregnancy weight, or postnatal depression.
For infants, there is an unclear difference in the risk of perinatal mortality, and likely no difference in being large‐for‐gestational‐age. The evidence suggests a possible reduction in the composite of death or severe morbidity with tighter glycaemic control, but the wide confidence interval is compatible with a range of effects that includes significant benefit and harm. Tighter glycaemic control probably results in little to no difference in infant hypoglycaemia and may result in little to no difference in adiposity when compared with less‐tight glycaemic targets. No data were reported for the long‐term infant outcomes of development of diabetes and neurosensory disability.
Tighter glycaemic targets may be associated with an increase in the use of insulin and oral hypoglycaemics and decreased adherence to glycaemic targets.
It is important to note that these findings are based on data from trials that are at overall unclear risk of bias with small numbers of participants. There remains insufficient evidence to support tighter over less‐tight glycaemic treatment targets for women with gestational diabetes mellitus (GDM) in order to minimise adverse effects on maternal and infant health.
Implications for research.
We identified two ongoing randomised controlled trials (see Characteristics of ongoing studies) (Hague 2014; NCT03610178). When data become available from these studies, in addition to the 4.5‐year follow‐up of Crowther 2022, they will be included in future updates of this review. All trials are comparing glycaemic control targets for women with GDM.
Further large, high‐quality trials with long‐term follow‐up are needed that compare different intensities of glycaemic control targets to guide treatment of women with GDM. High‐quality trials should evaluate different blood glycaemic targets to guide treatment; assess both short‐ and long‐term health outcomes for women and their babies; include women's experiences; and assess health services costs. It is also important to continue to evaluate women’s views of adhering to different glycaemic intensities and how this affects their daily lives to understand and overcome impracticalities and inconveniences such as hospital clinic attendances and the effect of blood glucose monitoring (Martis 2017).
What's new
| Date | Event | Description |
|---|---|---|
| 10 October 2023 | New citation required but conclusions have not changed | In this updated review we included three new trials and one previously identified trial. It remains unclear as to whether tight glycaemic targets affects maternal and infant health when compared to less‐tight glycaemic targets due to the current evidence being of an unclear risk of bias and significant imprecision in the results. Further research from high quality randomised controlled trials may change this. |
| 10 October 2023 | New search has been performed | The search was updated and 10 new trial reports identified. New reports from 3 different trials were included. In this updated review 4 trials were included (1731 women), 1 trial was excluded and 3 trials are ongoing. |
History
Protocol first published: Issue 4, 2015 Review first published: Issue 4, 2016
Acknowledgements
We acknowledge the support from the Cochrane Pregnancy and Childbirth editorial team in Liverpool, and the Research Synthesis Group for Pregnancy, Childbirth and Neonatal Reviews based at the Liggins Institute, University of Auckland, New Zealand. We acknowledge Lynn Hampson's support for the updated literature search and the help of Kerry Dwan, Cochrane’s Methods Support Unit Lead and Statistical Editor, for advice on the adjustments required in the statistical analyses.
We also acknowledge the Aotearoa Foundation for supporting Olivia Hofer's research internship at the Liggins institute.
We thank Julie Brown and Tineke Crawford for their contributions as authors on the 2016 version of this review. We thank Dr Luling Lin, research fellow at the Liggins Institute, for her contribution in extracting the data and undertaking the risk of bias assessment for Crowther 2022.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, the National Health Service (NHS), or the Department of Health and Social Care.
Appendices
Appendix 1. Search methods for trial registry searches
ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
glycemic control AND pregnancy
glycemic control AND pregnant
glycaemic control AND pregnancy
glycaemic control AND pregnant
glycaemic control AND gestational
glycemic control AND gestational
gestational diabetes mellitus AND treatment thresholds
gestational diabetes mellitus AND treatment targets
Data and analyses
Comparison 1. Intensity of glycaemic control: tight glycaemic targets versus less‐tight glycaemic targets.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia) | 2 | 1491 | Risk Ratio (IV, Fixed, 95% CI) | 1.16 [0.80, 1.69] |
| 1.2 Perinatal (foetal and neonatal) mortality | 2 | 1499 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 1.3 Large‐for‐gestational‐age | 3 | 1556 | Risk Ratio (IV, Fixed, 95% CI) | 0.96 [0.72, 1.29] |
| 1.4 Composite of mortality or serious morbidity (as defined by trial) | 3 | 1559 | Risk Ratio (IV, Fixed, 95% CI) | 0.84 [0.55, 1.29] |
| 1.5 Caesarean section | 3 | 1662 | Risk Ratio (IV, Fixed, 95% CI) | 0.98 [0.82, 1.17] |
| 1.6 Gestational weight gain | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.91, 3.71] |
| 1.7 Induction of labour | 1 | 1096 | Risk Ratio (IV, Fixed, 95% CI) | 0.96 [0.78, 1.18] |
| 1.8 Maternal hypoglycaemia | 1 | 1096 | Risk Ratio (IV, Fixed, 95% CI) | 1.23 [0.31, 4.91] |
| 1.9 Mean daily fasting blood glucose mmol/L | 1 | 395 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.18, ‐0.02] |
| 1.10 Mean daily postprandial blood glucose | 1 | 395 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.31, ‐0.09] |
| 1.11 Glycated haemoglobin | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.31, 0.53] |
| 1.12 Use of pharmacological therapy | 4 | 1718 | Risk Ratio (IV, Fixed, 95% CI) | 1.37 [1.17, 1.59] |
| 1.13 Triglycerides mg/dL | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐7.90 [‐57.54, 41.74] |
| 1.14 NEFA mmol/L | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.06, 0.12] |
| 1.15 Total cholesterol mg/dL | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐14.50 [‐41.72, 12.72] |
| 1.16 LDL mg/dL | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐4.40 [‐26.37, 17.57] |
| 1.17 HDL mg/dL | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐7.28, 6.36] |
| 1.18 Breastfeeding | 1 | 1093 | Risk Ratio (IV, Fixed, 95% CI) | 0.99 [0.85, 1.15] |
| 1.19 Adherence with treatment | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.32, 0.52] |
| 1.20 Composite of serious maternal health outcomes | 1 | 1096 | Risk Ratio (IV, Fixed, 95% CI) | 2.29 [1.14, 4.60] |
| 1.21 Stillbirth | 2 | 1499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.33] |
| 1.22 Neonatal death | 2 | 1499 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.23 Macrosomia | 3 | 1667 | Risk Ratio (IV, Fixed, 95% CI) | 1.12 [0.81, 1.56] |
| 1.24 Small‐for‐gestational‐age | 4 | 1727 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.58, 1.28] |
| 1.25 Shoulder dystocia | 1 | 1101 | Risk Ratio (IV, Fixed, 95% CI) | 0.44 [0.13, 1.50] |
| 1.26 Gestational age at birth (weeks) | 4 | 1727 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.24, 0.02] |
| 1.27 Birthweight | 4 | 1727 | Mean Difference (IV, Fixed, 95% CI) | 2.42 [‐48.00, 52.83] |
| 1.28 Birthweight z score | 1 | 1101 | Risk Difference (IV, Fixed, 95% CI) | 0.11 [‐0.04, 0.26] |
| 1.29 Head circumference | 1 | 1101 | Risk Difference (IV, Fixed, 95% CI) | 0.02 [‐0.22, 0.26] |
| 1.30 Head circumference z score | 1 | 1101 | Risk Difference (IV, Fixed, 95% CI) | 0.07 [‐0.09, 0.23] |
| 1.31 Length | 1 | 1101 | Risk Difference (IV, Fixed, 95% CI) | 0.12 [‐0.28, 0.52] |
| 1.32 Length z score | 1 | 1101 | Risk Difference (IV, Fixed, 95% CI) | 0.12 [‐0.03, 0.27] |
| 1.33 Infant hypoglycaemia | 3 | 1556 | Risk Ratio (IV, Fixed, 95% CI) | 0.92 [0.72, 1.18] |
| 1.34 Neonatal jaundice (hyperbilirubinaemia) | 2 | 1161 | Risk Ratio (IV, Fixed, 95% CI) | 0.71 [0.45, 1.10] |
| 1.35 Adiposity (% fat mass) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐3.23, 1.99] |
| 1.36 C‐peptide ng/mL | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.54, 0.14] |
| 1.37 Cord leptin/adiponectin ratio | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.64, 0.18] |
| 1.38 ANGPTL4 in cord serum ng/mL | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 5.80 [‐1.05, 12.65] |
| 1.39 Cord adiponectin ng/mL | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐8.36, 7.56] |
| 1.40 Cord leptin ng/mL | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐9.50 [‐16.97, ‐2.03] |
| 1.41 Admission to neonatal intensive care unit/nursery | 2 | 1161 | Risk Ratio (IV, Fixed, 95% CI) | 0.59 [0.33, 1.04] |
| 1.42 Length of antenatal stay | 1 | 1096 | Mean ratio (IV, Fixed, 95% CI) | 0.96 [0.71, 1.21] |
| 1.43 Length of postnatal stay (maternal) | 1 | 1096 | Mean ratio (IV, Fixed, 95% CI) | 1.41 [1.24, 1.58] |
| 1.44 Length of postnatal stay (infant) | 1 | 1101 | Mean ratio (IV, Fixed, 95% CI) | 0.95 [0.83, 1.07] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Crowther 2022.
| Study characteristics | ||
| Methods | Multicentre, stepped‐wedge, cluster‐randomised controlled trial | |
| Participants | A total sample size of 1096 participants from 10 participating hospitals. Inclusion criteria: women with GDM, diagnosed by oral glucose tolerance test in between 24 and 34 weeks' gestation, receiving pregnancy care at 1 of the 10 participating hospitals in New Zealand Exclusion criteria: pregnant women with GDM with a known major fetal anomaly. |
|
| Interventions | Less‐tight glycaemic target period: fasting plasma glucose < 5.5 mmol/L ; 1‐hour postprandial < 8.0 mmol/L; 2‐hours postprandial < 7.0 mmol/L Tighter glycaemic target period: fasting plasma glucose ≤ 5.0 mmol/L, 1‐hour postprandial ≤ 7.4 mmol/L; 2‐hours postprandial ≤ 6.7 mmol/L Women received standard care for GDM by their lead maternity carer and the local diabetes service with appropriate advice about their diet and lifestyle, blood glucose monitoring, and pharmacological medication as needed. |
|
| Outcomes | Primary outcome: large‐for‐gestational‐age infant (birthweight > 90th centile using growth charts adjusted for gestational age and infant sex) Secondary outcomes: up to the time of hospital discharge after birth: For the woman: composite of serious maternal health outcome, pre‐eclampsia, induction of labour, caesarean section, use of pharmacological treatment for GDM, maternal hypoglycaemia, length of postnatal stay, and breastfeeding at discharge For the baby: composite serious health outcomes (defined as perinatal death, birth trauma, nerve palsy, bone fracture or shoulder dystocia); gestational age at birth, birthweight, macrosomia, small‐for‐gestational‐age, length, head circumference, respiratory support, hypoglycaemia, hyperbilirubinaemia, neonatal intensive care unit admission, length of postnatal stay |
|
| Notes | Funding: funded through a 4‐year project grant from the Health Research Council (HRC) in New Zealand (No. 14/449). The funding body had no role in the study design or writing of the manuscript. Dates of trial: 29 May 2015 Declaration of interest of trial authors: the authors declare they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Hospitals will be randomised, in clusters of two, to the timing of the change from use of the less tight glycaemic target period to use of the tighter glycaemic target period." (Protocol page 3) |
| Allocation concealment (selection bias) | Low risk | "The sequence of allocation of the hospitals to the sequential implementation of the tighter glycaemic target period will be prepared by the trial statistician using a computer generated random number table" (Protocol page 3) "Participating sites will be blind to their randomised time point until training for the implementation of the tighter glycaemic targets begins at their site, not more than two weeks prior to the change." (Protocol page 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear as to whether participants were aware of their allocation and therefore the impact this would have on outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "At each of the participating sites a research assistant collected the study outcome data from the case records of the women enrolled and their infants up to the time of primary hospital discharge after the birth." (Protocol page 4) No enough information to make judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although some infants and mother data are missing for some outcomes, this is likely to be due to incomplete hospital records and unlikely to introduce bias. |
| Selective reporting (reporting bias) | Low risk | Outcomes stated in protocol match those reported in results. |
| Other bias | Low risk | No other concerns identified. Trial adjusted appropriately for cluster and stepped‐wedge properties. |
Popova 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Women were included if they were in the 8th to 31st week of gestation and met the World Health Organization criteria for GDM (WHO 2013). GDM1 n = 201, GDM2 n = 194 Exclusion criteria: nil reported Setting: Almazov National Medical Research Centre (Saint Petersburg, Russian Federation) |
|
| Interventions | GDM1/very tight glycaemic targets: FBG < 5.1 mmol/L and < 7.0 mmol/L postprandial GDM2/less‐tight glycaemic targets: FBG < 5.3 mmol/L and < 7.8 mmol/L postprandial All participants were instructed on diet and lifestyle changes. In case of exceeding the target blood glucose levels (in 2 or more measurements per week in group 1 and in more than 1/3 of measurements per week in group 2), insulin therapy was started. |
|
| Outcomes | Infant outcomes: large‐for‐gestational‐age infants, perinatal death, composite of neonatal death or severe morbidity (nerve palsy, bone fracture, and shoulder dystocia), gestational age at birth, birthweight, macrosomia (birthweight > 4000 g), small‐for gestational‐age, and hypoglycaemia Maternal outcomes: pre‐eclampsia, mode of birth, mean daily fasting and postprandial capillary glucose concentration during treatment, proportion of glucose values within target, proportion of women requiring insulin therapy Subanalyses outcomes: gestational weight gain (kg), blood glucose average (mmol/L), fasting BG (mmol/L), 1‐hour postprandial blood glucose (mmol/L), number of blood glucose measurements, percentage treated with insulin, gestational age at delivery (weeks), caesarean section, birthweight (g), height (cm), large‐for‐gestational‐age, small‐for‐gestational‐age, Apgar score 1 min, Apgar score 5 min, glucose (mmol/L), C‐peptide (ng/mL), leptin (ng/mL), adiponectin (ng/mL), leptin/adiponectin ratio, ANGPTL4 in cord serum (ng/mL), ANGPTL4 relative expression in HUVECS |
|
| Notes | Abstract only. Subanalyses performed. Funding: partly funded by the Russian Science Foundation (project no. 1514‐30012) Dates of trial: start July 2015 Declaration of interest: none to disclose |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were 'randomly assigned' but unclear as to how (materials and methods) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent incomplete data at preliminary results stage |
| Selective reporting (reporting bias) | Low risk | Outcomes pre‐specified in protocol match study outcomes |
| Other bias | Unclear risk | Primarily in abstract form so information is described about the study methods and processes |
Scifres 2019.
| Study characteristics | ||
| Methods | Randomised controlled clinical feasibility trial Randomisation occurred via a computer‐based platform programmed in REDCap. Randomisation was stratified based on whether GDM was diagnosed before or after 24 weeks' gestation. Neither patients nor their providers will be blinded to patient study group. |
|
| Participants | 60 women (standard n = 30, intensive n = 30) Inclusion criteria: women with GDM diagnosed by the Carpenter‐Coustan criteria between 12 and 32 weeks' gestation who were either overweight (BMI 25 to 29.9 kg/m2) or obese (BMI > 30 kg/m2) pre‐pregnancy were enrolled from prenatal clinics at the University of Oklahoma. Singleton pregnancies, age 18 to 45 years, and planned delivery at the University of Oklahoma Medical Center Exclusion criteria: maternal tobacco use, planned delivery prior to 34 weeks' gestation, chronic hypertension requiring medical therapy, vascular disease, serum creatinine > 1.5 mg/dL, rheumatologic disorders, or oral steroid use within 30 days of enrolment Setting: University of Oklahoma Medical Center, USA, conducted over a 2‐year period from December 2015 to December 2017 |
|
| Interventions | Standard: fasting glucose < 95 mg/dL, 1‐hour postprandial glucose < 140 mg/dL Intensive: fasting glucose < 90 mg/dL, 1‐hour postprandial glucose < 120 mg/dL |
|
| Outcomes | Mean glycaemic levels as assessed by continuous glucose monitoring, mean daytime, nocturnal, 1‐hour preprandial, and 1‐hour and 2‐hours postprandial glucose values, percentage of time maternal glucose was < 60 mg/dL or > 140 mg/dL, maternal lipids, cytokines, adipokines, serum triglycerides, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, non‐esterified fatty acids, interleukin‐6, tumour necrosis factor‐alpha, leptin, adiponectin, maternal adverse events, gestational age at delivery, large‐for‐gestational‐age birthweight, small‐for‐gestational‐age birthweight, mean birthweight, neonatal adiposity, infant weight at birth, infant length at birth, infant head circumference at birth, composite neonatal morbidity (NICU admission, supplemental oxygen, hyperbilirubinaemia, hypoglycaemia) | |
| Notes | Sample size calculation: to achieve 85% power to detect a difference in means of 10 mg/dL, assuming a standard deviation of 11 mg/dL, it was estimated 23 participants would be required per group. To account for a 20% loss to follow‐up rate, it was estimated a total of 30 women per group would be required. Funding: supported in part by the University of Oklahoma College of Medicine Alumni Association and by the National Institutes of Health, National Institute of General Medical Sciences (Grant 1 U54GM104938, PI James). Dates of trial: December 2015 to December 2017 Declaration of interest of trial authors: authors report no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation occurred via a computer‐based platform programmed in REDCap" (Methods and Materials page 2) |
| Allocation concealment (selection bias) | Low risk | "Randomisation occurred via a computer‐based platform programmed in REDCap. Randomisation was stratified based on whether GDM was diagnosed before or after 24 weeks’ gestation.” (Methods and Materials page 2) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “At the time of the first study visit, the prior glucose logs were reviewed, and patients were counselled regarding dietary changes or initiation of medical therapy as needed. Women were then encouraged to report their glucose targets weekly. Throughout the study, providers made the decision to initiate or titrate either insulin or glyburide as necessary to meet assigned glycemic targets.” (Methods and Materials page 2) “One limitation is that because of the nature of this study, we were unable to blind clinicians and participants to the treatment groups.” (Strengths and Limitations page 8) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Both patients and providers were blinded to the results" (Materials and Methods page 2) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four participants did not complete visit two (tables 2 and 3 on pages 6‐8) |
| Selective reporting (reporting bias) | Low risk | Methods and results matched procedures and pre‐specified outcomes (Methods and materials page 2 and results pages 5‐7) |
| Other bias | Low risk | No obvious concerns |
Snyder 1998.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 180 women Inclusion criteria: pregnant women, 20 to 37 weeks' gestation, referred for GDM (no details of diagnostic criteria) Exclusion criteria: no details Setting: Royal Victoria Hospital, McGill University, Montreal, Canada Timing: 1996 to 1997 |
|
| Interventions | Liberal glycaemic control criteria: before meal 5.8 mmol/L (104 mg/dL) and 1‐hour postprandial 7.8 mmol/L (140 mg/dL). Monitored weekly and twice a week after 32 weeks' gestation. Birth planned before 40 weeks' gestation (n = 86). Treated with insulin if outside liberal glycaemic targets. Strict glycaemic control criteria: before meal 5.0 mmol/L (90 mg/dL) and 1‐hour postprandial 6.7 mmol/L (120 mg/dL). Monitored weekly and twice a week after 32 weeks' gestation. Birth planned before 40 weeks' gestation (n = 85). Treated with insulin if outside strict glycaemic targets. |
|
| Outcomes | Insulin therapy, caesarean section, gestational age at birth, birthweight, birthweight > 4 kg, small‐for‐gestational‐age, induction of labour, neonatal birth trauma, neonatal metabolic disturbances | |
| Notes | Sample size calculation: not reported ITT analysis: not clear, data reported for 171/180 women Conference abstract only. One of the authors, Sara Meltzer, was contacted via email for further information, e.g. study protocol or any further unpublished papers. No response was received at time of submission. No funding sources or declarations of interest were identified. Dates of trial: 1996 to 1997 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomised' no other details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details but blinding unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details as to whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data reported on 171 of 180 women enrolled. No details on loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | Conference abstract only. Data not reported for all outcomes, only stated that no differences. |
| Other bias | Unclear risk | Authors state that there was no difference between groups at baseline but no data provided. No protocol has been identified for this trial and no full publication has been identified. |
BMI: body mass index FBG: fasting blood glucose GDM: gestational diabetes mellitus ITT: intention‐to‐treat NICU: neonatal intensive care unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ardilouze 2015 | Terminated (very difficult recruitment) |
| Garner 1997 | In this Canadian study, 300 women diagnosed with GDM were randomised to receive either "intensive" follow‐up care or "routine care". Intensive follow‐up care took place with an obstetrician and an endocrinologist in a tertiary setting, and after receiving dietary counselling women were placed on a calorie‐restricted diet. Daily blood glucose estimations were obtained; women were seen bi‐weekly at the hospital where biophysical profiles were performed at each visit and ultrasonographic assessments for fetal growth, amniotic fluid volume, and cardiac size were performed. In the routine care group, women were not seen by a dietician, and were advised stay on an unrestricted healthy diet, performed only 2 glucose levels weekly at home, and returned for follow‐up care to their primary obstetric care provider in the community. No high‐risk monitoring of the fetus unless there was an indication. All women in the trial were advised to maintain their fasting glucose level < 4.4 mmol/L (79 mg/dL) and 1‐hour postprandial < 7.8 mmol/L (140 mg/dL). The intensification of the treatment was compared between the 2 groups, not different intensities of glycaemic control. |
GDM: gestational diabetes mellitus
Characteristics of ongoing studies [ordered by study ID]
Hague 2014.
| Study name | An evaluation of the safety of very tight glycaemic control versus tight glycaemic control in women with gestational diabetes ‐ GluT pilot |
| Methods | Randomised controlled trial Funding: Women's and Children's Hospital, Adelaide, SA, Australia; Robinson Research Institute University of Adelaide, SA, Australia; Novo Nordisk Regional Support Scheme for 2013, Baulkham Hills, NSW, Australia |
| Participants | 40 women with GDM diagnosed on 75 g OGTT: fasting glucose ≥ 5.5 mmol/L (99 mg/dL) and 2 hours glucose ≥ 8.5 mmol/L (153 mg/dL), between 12 and 30 weeks' gestation, with a singleton or twin pregnancy, not previously diagnosed as diabetic, attending antenatal care at collaborating hospitals, and giving informed written consent. Minimum age 18 years. Exclusion criteria > 30 + 0 weeks' gestation, or with triplets or higher‐order gravidity, or with major active medical disorders (including psychiatric disease requiring antipsychotic medication and inflammatory disorders requiring corticosteroid therapy, but not including chronic hypertension) |
| Interventions | Very tight glycaemic control as monitored by self‐monitoring of blood glucose with a memory glucometer, aiming to keep fasting capillary blood glucose < 5.0 mmol/L (90 mg/dL) and 2‐hour postprandial capillary blood glucose < 6.7 mmol/L (120 mg/dL) until birth, using diet, exercise, insulin, other drugs, as necessary, and at appropriate doses to maintain the control, under the supervision of an obstetric physician and a diabetes nurse educator Tight glycaemic* control as monitored by self‐monitoring blood glucose with a memory glucometer, aiming to keep fasting capillary blood glucose < 5.5 mmol/L (99 mg/dL) and 2‐hour postprandial < 7.0 mmol/L (126 mg/dL) until birth, using diet, exercise, insulin, other drugs, as necessary, and at appropriate doses to maintain the control, under the supervision of an obstetric physician and a diabetes nurse educator *Email correspondence confirmed the tight glycaemic targets as stated above. On the ANZCTR very tight and tight glycaemic targets are listed as the same glycaemic targets, which according to Hague is a typo and will be rectified soon. At time of submission of this review it had not been corrected. |
| Outcomes | Primary outcomes: maternal hypoglycaemia: self‐monitoring capillary blood glucose < 3.0 mmol/L (54 mg/dL) – number of episodes, symptomatic or not and severe maternal hypoglycaemia: self‐monitoring capillary blood glucose < 2.5 mmol/L (45 mg/dL) – number of episodes Secondary outcomes: birthweight; neonatal hypoglycaemia in whole blood from heel prick < 2.6 mmol/L (47 mg/dL); severe neonatal hypoglycaemia in whole blood from heel prick < 2.0 mmol/L (36 mg/dL) |
| Starting date | 23 December 2014 |
| Contact information | Professor William Hague, Women's and Children's Hospital, 72 King William Road, North Adelaide, SA 5006, Australia, Ph. +61 4 11114575; bill.hague@adelaide.edu.au |
| Notes | Clinical Trial Identifier: ACTRN12614001250628 |
NCT03610178.
| Study name | Genetic and epigenetic mechanisms of developing gestational diabetes mellitus and its effects on the fetus |
| Methods | A randomised controlled trial of different glycaemic targets during treatment of women with GDM with assessment of epigenetic aspect of their effects on the fetus and pregnancy outcomes. This study is an interventional, randomised controlled trial, open‐label. Masking: single (Investigator) |
| Participants | 850 enrolled. Inclusion criteria: pregnant women with GDM diagnosed according to the Russian national consensus and the recommendations of the International Association of Diabetes and Pregnancy Study Groups (fasting glucose of > 5.1 mmol/L, and/or > 10.0 mmol/L after 1 h, and/or > 8.5 mmol/L after 2 h in OGTT with 75 g of glucose). Gestational ages at time of inclusion in the study 12 weeks 0 days – 31 weeks 6 days. For control group: pregnant women with normal glucose tolerance confirmed by OGTT at 24 to 31 weeks of gestation. Exclusion criteria: diabetes mellitus type 1 and type 2, other diseases that affect metabolism of carbohydrates, use of drugs that affect metabolism of carbohydrates, malformations of the fetus identified prior to inclusion to the study |
| Interventions | Very tight glycaemic targets: all women are assigned to lifestyle modification (diet and physical exercise). If target glucose levels (< 5.1 mmol/L fasting and < 7.0 mmol/L postprandial) are not achieved, insulin therapy is started. Tight‐moderate glycaemic targets: all women are assigned to lifestyle modification (diet and physical exercise). If target glucose levels (< 5.3 mmol/L fasting and < 7.8 mmol/L postprandial) are not achieved, insulin therapy is started. Control group: no intervention |
| Outcomes | Primary outcomes: large‐for‐gestational‐age newborns, TRIB1, LEP, ADIPOQ, ANGPTL4, NR3CL Secondary outcomes: caesarean sections, small‐for‐gestational‐age newborns, methylation of candidate genes |
| Starting date | August 2015 (estimated completion July 2020, primary completion data May 2020) |
| Contact information | Polina Popova, MD, PhD Phone: +78127550595 Email: pvpopova@yandex.ru |
| Notes |
NCT04672031.
| Study name | Glycemic targets for pregnant women with GDM and T2DM |
| Methods | A randomised parallel controlled trial to determine whether glycaemic targets that are lower than those currently recommended by the American Diabetes Association and the American College of Obstetricians and Gynecologists would improve overall outcomes in pregnant women with diabetes. This study is an interventional, randomised controlled, open‐label, unmasked trial. |
| Participants | Estimated 120 participants – currently recruiting. Inclusion criteria: pregnant women with a singleton gestation, 18 years or older, diagnosis of gestation diabetes (prior to 34 weeks' gestational age) or type 2 diabetes Exclusion criteria: diagnosed with gestational diabetes at or beyond 34 weeks' gestational age, type 1 diabetes, diabetic retinopathy, diabetic nephropathy, diabetic vasculopathy |
| Interventions | Experimental arm (new target): women in the experimental arm will be instructed to check blood sugars 7 times per day: fasting, pre‐prandial, and 1 hour after each meal. Glycaemic targets for the intervention arm will be defined as follows: fasting ≤ 80 mg/dL, pre‐prandial ≤ 80 mg/dL, and 1‐hour postprandial ≤ 110 mg/dL. Women who do not achieve glycaemic goals with diet and exercise will be started on medical therapy (metformin or insulin) at the discretion of a maternal‐fetal medicine subspecialist and endocrinologist. Control arm (standard target): women in the control arm will be instructed to check blood sugars 7 times per day: fasting, pre‐prandial, and 1 hour after each meal. Glycaemic targets for the control arm will be defined as follows: fasting ≤ 95 mg/dL, pre‐prandial ≤ 95 mg/dL, and 1‐hour postprandial ≤ 140 mg/dL (i.e. conventional targets). Women who do not achieve glycaemic goals with diet and exercise will be started on medical therapy (metformin or insulin) at the discretion of a maternal‐fetal medicine subspecialist and endocrinologist. |
| Outcomes | Primary outcomes: difference in birthweight – 250 g difference in birthweight Secondary outcomes: total prenatal care visits, prenatal care visits after enrolment, number of prenatal care visits with log/glucometer available for RN or MD to review, number of prenatal care visits in which participant met blood sugar targets, number of prenatal care visits in which an intervention for blood sugars was recommended (e.g. starting medication or changing medication dose), symptomatic hypoglycaemia, asymptomatic hypoglycaemia, glycated haemoglobin at the time of enrolment, glycated haemoglobin at 36 weeks' gestational age, lowest recorded blood sugar during prenatal care, highest recorded blood sugar during prenatal care, average recorded blood sugar during prenatal care, average number of blood sugar checks actually performed each week, % of blood sugars within goal each week, diabetes medication, intrapartum insulin, gestational weight gain, antepartum admission, corticosteroids, oligohydramnios, polyhydramnios, fetal growth restriction, gestational age at delivery, induction of labour, mode of delivery, caesarean indication, trial of labour after caesarean, blood loss, 3rd‐ or 4th‐degree laceration, pregnancy‐induced hypertension (gestational hypertension, pre‐eclampsia, HELLP syndrome), hypertensive emergency, chorioamnionitis, endometritis, VTE, length of stay (maternal), postpartum readmission, postpartum wound complications, cardiac complications, seizures, macrosomia, LGA, SGA, shoulder dystocia, 5‐minute Apgar score, cord gas pH < 7.0, base excess, neonatal blood glucose, RDS, TTN, hyperbilirubinaemia, neonatal sepsis, NICU admission, length of stay (neonatal), congenital anomaly, IUFD or stillbirth. |
| Starting date | |
| Contact information | |
| Notes | Currently recruiting. Contact: Michelle T Nguyen, MD michelle.nguyen3@med.usc.edu Los Angeles County + University of Southern California Medical Center (LAC+USC), Los Angeles, California, USA, 90033 |
GDM: gestational diabetes mellitus HELLP: haemolysis, elevated liver enzymes, and low platelets IUFD: intrauterine fetal death LGA: large‐for‐gestational‐age NICU: neonatal intensive care unit OGTT: oral glucose tolerance test RDS: respiratory distress syndrome SGA: small‐for‐gestational‐age TTN: transient tachypnoea of the newborn VTE: venous thromboembolism
Differences between protocol and review
Differences between 2016 review and 2016 protocol
The published protocol listed seven maternal and child outcomes together to be assessed for certainty of evidence using the GRADE approach. However, we identified that mother and child outcomes needed to be assessed separately, and this was changed to seven outcomes each for maternal and child (as neonate, child, adult).
We modified some of the outcomes for this review based on consensus between the review authors and other review authors of Cochrane Reviews for treatment of gestational diabetes mellitus (GDM) and the core outcome set published by Bain 2016. The outcomes are now in line with the updated outcomes across the Cochrane GDM reviews.
Primary outcomes
For the mother: caesarean section was changed from a primary outcome to a secondary outcome. Pre‐eclampsia was amended to hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, and eclampsia). Subsequent development of type 2 diabetes was moved from a long‐term maternal outcome to a primary outcome.
For the infant: death or severe morbidity (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture, or nerve palsy) was amended from a secondary outcome to a primary outcome.
Secondary outcomes
Deleted outcomes
The following maternal secondary outcomes were deleted: mode of birth (normal vaginal birth, operative vaginal birth, caesarean section); hyperglycaemia requiring changes in management during pregnancy; diabetic ketoacidosis; anxiety.
The following long‐term maternal outcomes were deleted: postnatal glucose tolerance; development of type 2 diabetes mellitus; hypertension; blood lipids.
The following neonatal secondary outcomes were deleted: death in infancy or childhood; congenital fetal anomaly; Z scores of birthweight, head circumference, length; neonatal infection; neonatal hyperglycaemia.
The following later‐childhood outcomes were deleted: appropriate weight for age; anthropometry (weight, height, head circumference, adiposity, skinfold thickness, fat mass); developmental delay (variously defined by individual trials).
The following health service outcome was deleted: length of stay in neonatal intensive care unit/nursery.
Amended outcomes
The following maternal secondary outcomes were amended:
hypoglycaemia requiring treatment during pregnancy amended to maternal hypoglycaemia;
glycaemic control achieved (e.g. blood glucose or glycated haemoglobin concentrations) (proportion of blood glucose concentrations within target) amended to glycaemic control during/end of intervention (as defined by trialists);
satisfaction with treatment/management amended to views of the intervention;
postnatal weight retention amended to postnatal weight retention or return to pre‐pregnancy weight;
postnatal depression was moved to long‐term maternal outcomes.
The following neonatal secondary outcomes were amended:
preterm birth amended to preterm birth (< 37 weeks' gestation; < 32 weeks' gestation);
birthweight, head circumference, and length amended to birthweight and z score, head circumference and z score, and length and z score;
fetal adiposity amended to adiposity;
neonatal hypoglycaemia amended to hypoglycaemia (variously defined).
The following adulthood outcomes were amended:
metabolic syndrome was amended to dyslipidaemia or metabolic syndrome;
glucose tolerance/type 2 diabetes mellitus was amended to type 1 diabetes, type 2 diabetes, impaired glucose tolerance;
blood pressure and blood lipids were amended to cardiovascular health (as defined by trialists, including blood pressure, hypertension, cardiovascular disease, metabolic syndrome).
The following health service outcomes were amended:
maternal antenatal admission amended to length of antenatal stay;
additional requirements for families (such as change of diet, exercise, extra antenatal visits, glucose monitoring and strips) amended to costs to families associated with the management provided;
use of healthcare services in pregnancy (consultations, blood glucose monitoring, length and number of antenatal visits, and to whom – midwife/obstetrician/physician) amended to number of antenatal visits or admissions and number of hospital or health professional visits (including midwife, obstetrician, physician, dietician, diabetic nurse).
Additional outcomes
The following maternal secondary outcomes were added: behaviour change associated with the intervention; relevant biomarker changes associated with the intervention (including adiponectin, free fatty acids, triglycerides, high‐density lipoproteins, low‐density lipoproteins, insulin); sense of well‐being and quality of life.
The following long‐term maternal outcomes were added: GDM in a subsequent pregnancy; type 1 diabetes mellitus; impaired glucose tolerance; cardiovascular health (as defined by trialists, including blood pressure, hypertension, cardiovascular disease, metabolic syndrome).
The following neonatal secondary outcomes were added: Apgar score < seven at 5 minutes; polycythaemia; relevant biomarker changes associated with the intervention (including cord C‐peptide, cord insulin).
The following later‐childhood outcomes were added: weight and z score; height and z score; head circumference and z score; adiposity (including body mass index (BMI), skinfold thickness); blood pressure; type 1 diabetes mellitus; type 2 diabetes mellitus; impaired glucose tolerance; dyslipidaemia or metabolic syndrome; educational achievement.
The following adulthood outcomes were added: weight, height, adiposity (including BMI, skinfold thickness); employment, education, and social status/achievement.
The following health service outcomes were added: costs associated with the intervention; length of postnatal stay (baby).
Subgroup analysis and investigation of heterogeneity
We added the following subgroup analysis in the Methods: woman's ethnicity as identified from the trials.
Differences between updated review 2023 and 2016 review
We included an additional composite outcome of serious maternal health outcomes (variously defined by trial authors) in this review.
Contributions of authors
Caroline Crowther (CC) initiated the update of this review. Olivia Hofer (OH), Ruth Martis (RM), and CC were responsible for the preparation of this review update, including data extraction and risk of bias assessments. OH entered the data into Review Manager Web and prepared the initial draft of the text. All authors provided feedback and comments throughout the preparation of the review and approved the final submitted version.
Contributions of authors to the 2016 version of this review: Tineke Crawford (TC), Julie Brown (JB), and CC were involved in conceiving the review. TC was responsible for preparing the initial draft of the protocol and designing the search strategies. JB and CC assisted in the preparation of the protocol. Jane Alsweiler (JA) and RM provided additional comments and feedback for the protocol and the first published version of this review.
Sources of support
Internal sources
-
Liggins Institute, The University of Auckland, New Zealand
Institutional support
External sources
-
No external source of support, Other
No external source of support
Declarations of interest
Caroline Crowther and Jane Alsweiler are principal investigators on the TARGET randomised controlled trial examining optimal glycaemic targets for women with gestational diabetes mellitus. None of the authors associated with the TARGET randomised controlled trial were involved in data extraction or risk of bias assessment. Olivia Hofer and Luling Lin, a research fellow at the Liggins Institute, undertook the data extraction for the TARGET Trial.
Olivia Hofer: is a medical student at the University of Auckland and has no conflicts of interest to declare.
Ruth Martis: was involved in the qualitative side studies for the TARGET Trial as a PhD student but was not involved with the main randomised trial data analysis. She was not involved in the assessment of this trial for inclusion in the review, or risk of bias assessment and data extraction for this trial. Ruth is a locum midwife at a rural maternity hospital in New Zealand.
Jane Alsweiler: an investigator for the TARGET randomised controlled trial assessing optimal glycaemic targets for women with gestational diabetes mellitus. Not involved in the assessment of this trial for inclusion in the review, or risk of bias assessment and data extraction for this trial.
Caroline Crowther: lead investigator for the TARGET randomised controlled trial assessing optimal glycaemic targets for women with gestational diabetes mellitus. Not involved in the assessment of this trial for inclusion in the review, or risk of bias assessment and data extraction for this trial.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Crowther 2022 {unpublished data only}
- ACTRN12615000282583. Optimal glycaemic targets for women with gestational diabetes: the randomised trial – TARGET. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365403 (first received 2015). [CENTRAL: CN-02137449]
- Crowther CA, Alsweiler JM, Hughes R, Brown J. Tight or less tight glycaemic targets for women with gestational diabetes mellitus for reducing maternal and perinatal morbidity? (TARGET): study protocol for a stepped wedge randomised trial. BMC Pregnancy and Childbirth 2018;18(1):425. [CENTRAL: CN-01668619] [EMBASE: 624611435] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther CA, Hughes R, Brown J, Tran T, Alsweiler J. Tight or less tight glycaemic targets for women with gestational diabetes mellitus for reducing maternal and perinatal morbidity? the TARGET Trial. Journal of Paediatrics and Child Health 2019;55:15. [CENTRAL: CN-01937661] [EMBASE: 627192656] [Google Scholar]
- Crowther CA, Samuel D, Hughes R, Tran T, Brown J, Alsweiler JM, TARGET Study Group. Tighter or less tight glycaemic targets for women with gestational diabetes mellitus for reducing maternal and perinatal morbidity: a stepped-wedge, cluster-randomised trial. PLOS Medicine 2022;19(9):e1004087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Popova 2018 {published data only}
- Popova P, Tkachuck A, Bolotko Y, Gerasimov A, Pustozerov E, Vasilyeva E, et al. Randomised controlled trial of very tight versus less tight glycaemic targets in women with gestational diabetes: preliminary results. Diabetologia 2018;61(Suppl 1):S27-S28. [CENTRAL: CN-01647130] [EMBASE: 624031104] [Google Scholar]
- Popova P, Vasilyeva L, Tkachuck A, Puzanov M, Golovkin A, Bolotko Y, et al. A randomised, controlled study of different glycaemic targets during gestational diabetes treatment: effect on the level of adipokines in cord blood and ANGPTL4 expression in human umbilical vein endothelial cells. International Journal of Endocrinology 2018;2018:6481658. [CENTRAL: CN-02118491] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova P, Vasilyeva L, Tkachuck A, Puzanov M, Golovkin A, Bolotko Y, et al. The impact of the intrauterine hyperglycaemia on the expression of genes related to cardio-metabolic diseases in human umbilical vein endothelial cells: results from a randomised, controlled study of different glycaemic targets during gestational diabetes treatment. Endocrine Abstracts 2019;63:63 GP213. [Google Scholar]
Scifres 2019 {published data only}
- NCT02530866. Intensive management for gestational diabetes [Randomized Controlled Clinical Pilot Trial of Intensive Management for Gestational Diabetes]. clinicaltrials.gov/show/nct02530866 (first received 2015 Aug 21). [CENTRAL: CN-01491721]
- Scifres C, Mead-Harvey C, Nadeau H, Reid S, Pierce S, Feghali M, et al. 88: intensive glycemic control in gestational diabetes mellitus: a randomized controlled clinical trial. American Journal of Obstetrics and Gynecology 2019;220(1 Suppl):S71. [CENTRAL: CN-01741681] [EMBASE: 2001404912] [DOI] [PubMed] [Google Scholar]
- Scifres CM, Mead-Harvey C, Stoner JA, Nadeau H, Reid S, Pierce S, et al. Intensive glycemic control in gestational diabetes mellitus: a randomized controlled clinical feasibility trial. American Journal of Obstetrics and Gynecology MFM 2019;1(4):10050. [CENTRAL: CN-02086864] [EMBASE: 2003527993] [DOI] [PubMed] [Google Scholar]
Snyder 1998 {published data only}
- Snyder J, Morin I, Meltzer S, Nadeau J. Gestational diabetes and glycaemic control: a randomized clinical trial. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S55. [Google Scholar]
References to studies excluded from this review
Ardilouze 2015 {published data only}
- NCT02478762. Glycemic objectives of women with gestational diabetes mellitus [Reevaluation of the Glycemic Objectives of Women With Gestational Diabetes Mellitus]. clinicaltrials.gov/show/nct02478762 (first received 2015 Jun 16). [CENTRAL: CN-01584563]
Garner 1997 {published data only}
- Garner P, Okun N, Keely E, Wells G, Perkins S, Sylvain J, et al. A randomized controlled trial of strict glycemic control and tertiary level obstetric care versus routine obstetric care in the management of gestational diabetes: a pilot study. American Journal of Obstetrics and Gynecology 1997;177(1):190-5. [DOI] [PubMed] [Google Scholar]
- Keely EJ, Malcolm JC, Hadjiyannakis S, Gaboury I, Lough G, Lawson ML. Prevalence of metabolic markers of insulin resistance in offspring of gestational diabetes pregnancies. Pediatric Diabetes 2008;9(1):53-9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Hague 2014 {published data only}
- ACTRN12614001250628. Glucose targets in gestational diabetes pilot. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367441&isReview=true 2014.
NCT03610178 {published data only}
- NCT03610178. Genetic and epigenetic mechanisms of developing gestational diabetes mellitus and its effects on the fetus [Genetic and Epigenetic Mechanisms of Developing Gestational Diabetes Mellitus and Its Effects on the Fetus: a Randomised Controlled Trial of Different Glycemic Targets During Pregnancy]. clinicaltrials.gov/show/nct03610178 2018 Aug 1. [CENTRAL: CN-01661683]
NCT04672031 {published data only}
- NCT04672031. Glycemic targets for pregnant women With GDM and T2DM [Tight versus standard glycemic targets for pregnant women with gestational diabetes and type 2 diabetes]. clinicaltrials.gov/show/NCT04672031 (first received 2020 Dec 17). [CENTRAL: CN-02206521]
Additional references
ACOG 2013
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins – Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 137, August 2013 (replaces Practice Bulletin Number 30, September 2001). Gestational diabetes mellitus. Obstetrics and Gynecology 2013;122(3 Pt 1):406-16. [PubMed] [Google Scholar]
ACOG 2018
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstetrics & Gynecology 2018;131:406-8. [DOI: 10.1097/AOG.0000000000002498] [DOI] [PubMed] [Google Scholar]
ADA 2020
- American Diabetes Association. Standards of medical care in diabetes – 2020. Diabetes Care 2020;43(Suppl 1):S183-S192. [DOI] [PubMed] [Google Scholar]
Alwan 2009
- Alwan N, Tuffnell DJ, West J. Treatments for gestational diabetes. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD003395. [DOI: 10.1002/14651858.CD003395.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Athukorala 2006
- Athukorala C, Middleton P, Crowther CA. Intrapartum interventions for preventing shoulder dystocia. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD005543. [DOI: 10.1002/14651858.CD005543.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Athukorala 2010
- Athukorala C, Rumbold AR, Willson KJ, Crowther CA. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth 2010;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bain 2016
- Bain E, Middleton P, Crowther CA. Progressing towards standard outcomes in gestational diabetes Cochrane reviews and randomised trials. Australian and New Zealand Journal of Obstetrics and Gynaecology 2016;56(1):113-116. [DOI: 10.1111/ajo.12433).] [DOI] [PubMed] [Google Scholar]
Bellamy 2009
- Bellamy L, Casas J, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773-9. [DOI] [PubMed] [Google Scholar]
Boney 2005
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birthweight, maternal obesity and gestational diabetes mellitus. Pediatrics 2005;115(3):e290-6. [DOI] [PubMed] [Google Scholar]
Bottalico 2007
- Bottalico JN. Recurrent gestational diabetes: risk factors, diagnosis, management and implications. Seminars in Perinatology 2007;31:176-84. [DOI] [PubMed] [Google Scholar]
Carolan 2012
- Carolan M, Davey MA, Biro MA, Kealey M. Maternal age, ethnicity and gestational diabetes mellitus. Midwifery 2012;28(6):778-83. [DOI: 10.1016/j.midw.2011.08.014] [DOI] [PubMed] [Google Scholar]
Carpenter 1982
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. American Journal of Obstetrics & Gynecology 1982;144:768-73. [DOI] [PubMed] [Google Scholar]
Chamberlain 2013
- Chamberlain C, McNamara B, Williams E, Yore D, Oldenburg B, Oats J, et al. Diabetes in pregnancy among indigenous women in Australia, Canada, New Zealand and the United States. Diabetes Metabolism Research Review 2013;29(4):241-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 2003
- Cheung NW, Byth K. Population health significance of gestational diabetes. Diabetes Care 2003;26(7):2005-9. [DOI] [PubMed] [Google Scholar]
Chu 2007
- Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus: a meta-analysis. Diabetes Care 2007;30:2070-6. [DOI] [PubMed] [Google Scholar]
Crowther 2005
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005;352(24):2477-86. [DOI] [PubMed] [Google Scholar]
DCCT 1996
- The Diabetes Control and Complications Trial Research Group. Pregnancy outcomes in the Diabetes Control and Complications Trial (DCCT). American Journal of Obstetrics & Gynecology 1996;174:1343-53. [DOI] [PubMed] [Google Scholar]
Devlieger 2008
- Devlieger R, Casteels K, Van Assche FA. Reduced adaptation of the pancreatic B cells during pregnancy is the major causal factor for gestational diabetes: current knowledge and metabolic effects on the offspring. Acta Obstetricia et Gynecologica Scandinavica 2008;87(12):1266-70. [DOI] [PubMed] [Google Scholar]
Evans 2009
- Evans MJ. Diabetes and pregnancy: a review of pathology. British Journal of Diabetes & Vascular Disease 2009;9:201-6. [Google Scholar]
Feig 2018
- Feig DS, Berger H, Donovan L, Godbout A, Kader T, Keely E, et al. Clinical Practice Guidelines: Diabetes and pregnancy. Canadian Journal of Diabetes 2018;42:S255–S282. [DOI] [PubMed] [Google Scholar]
Ferrara 2007
- Farrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30(Suppl 2):S141-S146. [DOI] [PubMed] [Google Scholar]
Gardener 2009
- Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. British Journal of Psychiatry 2009;195:7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 18 July 2023. Hamilton (ON): McMaster University (developed by Evidence Prime), 2023. Available at gradepro.org.
HAPO 2008
- HAPO Study Cooperative Research Group. Hyperglycaemia and adverse pregnancy outcomes. New England Journal of Medicine 2008;358:1991-2002. [DOI: 10.1056/NEJMoa0707943] [DOI] [PubMed] [Google Scholar]
Hernandez 2011
- Hernandez TL, Friedman JE, Van Pelt RE, Barbour LA. Patterns of glycemia in normal pregnancy: should the current therapeutic targets be challenged? Diabetes Care 2011;34(7):1660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernandez 2015
- Hernandez TL. Glycemic targets in pregnancies affected by diabetes: historical perspective and future directions. Current Diabetes Reports 2015;15(1):565-72. [DOI: 10.1007/s11892-014-0565-2] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC. Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2020
- Higgins J, Thomas J, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. The Cochrane Collaboration, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Hoffman 1998
- Hoffman L, Nolan C, Wilson JD, Oats JJN, Simmons D The Australasian Diabetes in Pregnancy Society. Gestational diabetes mellitus management guidelines. Medical Journal of Australia 1998;169:93-7. [DOI] [PubMed] [Google Scholar]
HSE 2010
- Health Services Executive. Guidelines for the Management of Pre-gestational and Gestational Diabetes from Pre-conception to the Post-natal Period. Dublin: Offices of the Nursing and Midwifery Services Director, 2010. [Google Scholar]
IADPSG 2010
- IADPSG Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33(3):676-82. [DOI: 10.2337/dc09-1848] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2010
- Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. American Journal of Public Health 2010;100(6):1047-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2013
- Kim SY, Saraiva C, Curtis M, Wilson HG, Troyan J, Sharma AJ. Fraction of gestational diabetes mellitus attributable to overweight and obesity by race/ethnicity, California, 2007-2009. American Journal of Public Health 2013;103(10):e65-72. [DOI: 10.2105/AJPH.2013.301469] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleinwechter 2014
- Kleinwechter H, Schäfer-Graf U, Bührer C, Hoesli I, Kainer F, Kautzky-Willer A, et al. Gestational Diabetes Mellitus (GDM) diagnosis, therapy and follow-up care practice guideline of the German Diabetes Association (DDG) and the German Association for Gynaecology and Obstetrics (DGGG). Experimental and Clinical Endocrinology & Diabetes Journal 2014;122:395-405. [DOI] [PubMed] [Google Scholar]
Kozhimannil 2009
- Kozhimannil KB, Pereira MA, Harlow BL. Association between diabetes and perinatal depression among low-income mothers. JAMA 2009;301(8):842-7. [DOI: 10.1001/jama.2009.201] [DOI] [PubMed] [Google Scholar]
Krakowiak 2012
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansenm RL, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 2012;129(5):e1121-8. [DOI: 10.1542/peds.2011-2583] [DOI] [PMC free article] [PubMed] [Google Scholar]
Landon 2009
- Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine 2009;361(14):1339-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langer 1989
- Langer O, Levy J, Brustman L, Anyaegbunam A, Merkatz R, Divon M. Glycemic control in gestational diabetes mellitus – how tight is tight enough: small for gestational age versus large for gestational age? American Journal of Obstetrics & Gynecology 1989;161(3):646-53. [DOI] [PubMed] [Google Scholar]
Langer 1994
- Langer O, Rodriguez DA, Xenakis EMJ, McFarland MB, Berkus MD, Arredondo F. Intensified versus conventional management of gestational diabetes. American Journal of Obstetrics & Gynecology 1994;170(4):1036-47. [DOI] [PubMed] [Google Scholar]
Li‐zhen 2019
- Li-zhen L, Yun X, Xiao-Dong Z, Shu-bin H, Zi-lian W, Dobs Adrian S, et al. Evaluation of guidelines on the screening and diagnosis of gestational diabetes mellitus: systematic review. BMJ Open 2019;9(5):e023014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucas 1988
- Lucas A, Morley R, Cole T. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ 1988;297(6659):1304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martis 2017
- Martis R, Brown J, Crowther CA. Views and experiences of New Zealand women with gestational diabetes in achieving glycaemic control targets: the Views Study. Journal of Diabetes Research 2017;2017:2190812. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCance 2011
- McCance DR. Pregnancy and diabetes. Best Practice & Research Clinical Endocrinology & Metabolism 2011;25:945-58. [DOI] [PubMed] [Google Scholar]
McCurdy 2010
- McCurdy C, Friedman J. Mechanisms underlying insulin resistance in human pregnancy and gestational diabetes mellitus. In: Kim K, Ferrara A, editors(s). Gestational Diabetes During and After Pregnancy. London: Springer, 2010:125-38. [Google Scholar]
Metzger 2007
- Metzger BE, Buchanan TA, Coustan DR, Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the 5th International Workshop Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30(Suppl 2):S251-S260. [DOI] [PubMed] [Google Scholar]
Metzger 2008
- Metzger BE, Contreras M, Sacks DA, Watson W, Dooley SL, Foderaro M, et al. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine 2008;358(19):1991-2002. [DOI] [PubMed] [Google Scholar]
Middleton 2012
- Middleton P, Crowther CA, Simmonds L. Different intensities of glycaemic control for pregnant women with pre-existing diabetes. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD008540. [DOI: 10.1002/14651858.CD008540.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mokdad 2003
- Mokdad AH, Ford ES, Bowman BA, Dieta WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes and obesity-related health risk factors. JAMA 2001;289(1):76-9. [DOI] [PubMed] [Google Scholar]
Nankervis 2013
- Nankervis A, McIntyre HD, Moses R, Ross GP, Callaway L, Porter C, et al. Consensus Guidelines for the Testing and Diagnosis of Gestational Giabetes Mellitus in Australia. Sydney: Australasian Diabetes in Pregnancy Society, 2013. [Google Scholar]
National Data Group 1979
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039-57. [DOI] [PubMed] [Google Scholar]
New Zealand Ministry of Health 2014
- New Zealand Ministry of Health. Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A Clinical Practice Guideline. Wellington, New Zealand: Ministry of Health, 2014. [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence (NICE). Diabetes in Pregnancy: Management of Diabetes and its Complications from Pre-Conception to the Postnatal Period. NICE Clinical Guideline 63. NICE, 2008. [Google Scholar]
NICE 2015
- National Institute for Health and Clinical Excellence (NICE) and National Collaborating Centre for Women and Children's Health Project Team. Diabetes in Pregnancy: Management of Diabetes and Its Complications From Pre-conception to the Postnatal Period. NG3. NICE, 2015. [ISBN: 978-1-4731-0993-3] [Google Scholar]
Nicklas 2013
- Nicklas JM, Miller LJ, Zera CA, Davis RB, Levkoff SE, Seely EW. Factors associated with depressive symptoms in the early postpartum period among women with recent gestational diabetes mellitus. Maternal Child Health Journal 2013;17(9):1665-72. [DOI: 10.1007/s10995-012-1180-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nomura 2012
- Nomura Y, Marks DJ, Grossman B, Yoon M, Loudon H, Stone J, et al. Exposure to gestational diabetes mellitus and low socio-economic status: effects on neurocognitive development and risk of attention-deficit hyperactivity disorder in offspring. Archives of Pediatrics and Adolescent Medicine 2012;166:337-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ornoy 2005
- Ornoy A. Growth and neurodevelopment outcome of children born to mothers with pregestational and gestational diabetes. Pediatric Endocrinology Reviews 2005;3(2):104-13. [PubMed] [Google Scholar]
Ornoy 2011
- Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reproductive Toxicology 2011;32:205-12. [DOI] [PubMed] [Google Scholar]
Ornoy 2015
- Ornoy A, Reece EA, Pavlinkova G, Kappen C, Miller RK. Effect of maternal diabetes on the embryo, fetus, and children: Congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Research Part C: Embryo Today: Reviews 2015;105(1):53-72. [DOI: 10.1002/bdrc.21090] [DOI] [PubMed] [Google Scholar]
Oteng‐Ntim 2012
- Oteng-Ntim E, Varma R, Croker H, Poston L. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Medicine 2012;10:47. [DOI: 10.1186/1741-7015-10-47] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2014
- Page KA, Romero A, Buchanan TA, Xiang AH. Gestational diabetes mellitus, maternal obesity and adiposity in offspring. Journal of Pediatrics 2014;164(4):807-10. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedersen 1954
- Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinologica 1954;16(4):330-42. [DOI] [PubMed] [Google Scholar]
Prutsky 2013
- Prutsky GJ, Domecq JP, Wand Z, Carranza Leon BG, Elraiyah T, Nabhan M, et al. Glucose targets in pregnant women with diabetes: a systematic review and meta-analysis. Journal of Clinical Endocrinology and Metabolism 2013;98(11):4319-24. [DOI: 10.121/jc2013-2461] [DOI] [PubMed] [Google Scholar]
Ragnarsdottir 2010
- Ragnarsdottir L, Conroy S. Development of macrosomia resulting from gestational diabetes mellitus: physiology and social determinants of health. Advanced Neonatal Care 2010;10(1):7-12. [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rosenberg 2005
- Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: Differences among four racial/ethnic groups. American Journal of Public Health 2005;95(9):1545-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowland 2021
- Rowland J, Wilson CA. The association between gestational diabetes and ASD and ADHD: a systematic review and meta‑analysis. Scientific Reports 2021;11:5136. [DOI: 10.1038/s41598-021-84573-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowlands 2010
- Rowlands I, Graves N, Jersey S, McIntyre HD, Gallaway L. Obesity in pregnancy: outcomes and economics. Seminars in Neonatal & Fetal Medicine 2010;15:94-9. [DOI] [PubMed] [Google Scholar]
Sacks 2012
- Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care 2012;35(3):526-8. [DOI: 10.2337/dc11-1641] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2012
- Schneider S, Bock C, Wetzel M, Maul H, Loerbroks A. The prevalence of gestational diabetes in advanced economies. Journal of Perinatal Medicine 2012;40(5):511. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shah 2019
- Shah R, Harding J, Brown J, McKinlay C. Neonatal glycaemia and neurodevelopmental outcomes: a systematic review and meta-analysis. Neonatology 2019;115(2):116-26. [DOI] [PubMed] [Google Scholar]
SIGN 2017
- SIGN (Scottish Intercollegiate Guidelines Network). Management of Diabetes: A National Clinical Guideline. Edinburgh, Scotland: SIGN and Healthcare Improvement Scotland, 2017 updated. [URL: www.sign.ac.uk] [Google Scholar]
Slining 2010
- Slining M, Adiar LS, Goldman BD, Borja JB, Bentley M. Infant overweight is associated with delayed motor development. Journal of Pediatrics 2010;157(1):20-5.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suman Rao 2013
- Suman Rao PN, Shashidhar A, Ashok C. In utero fuel homeostasis: Lessons for a clinician. Indian Journal of Endocrinology and Metabolism 2013;17(1):60-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torloni 2009
- Torloni MR, Betran AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obesity Reviews 2009;10(2):194-203. [DOI] [PubMed] [Google Scholar]
Vounzoulaki 2020
- Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 2020;369:m1361:1-11. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Vol. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, Switzerland: World Health Organization, 1999. [Google Scholar]
WHO 2013
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. WHO/NMH/MND/13.2 edition. Geneva, Switzerland: World Health Organization, 2013. [PubMed] [Google Scholar]
Wilcox 2005
- Wilcox G. Insulin and insulin resistance. Clinical Biochemistry Review 2005;26(5):19-39. [PMC free article] [PubMed] [Google Scholar]
Young 2013
- Young BC, Ecker JL. Fetal macrosomia and shoulder dystocia in women with gestational diabetes: risks amenable to treatment? Current Diabetes Reports 2013;13(1):12-8. [DOI] [PubMed] [Google Scholar]
Zhang 2010
- Zhang C. Risk factors for gestational diabetes: from an epidemiological standpoint. In: Kim C, Ferrara A, editors(s). Gestational Diabetes During and After Pregnancy. London: Springer, 2010:71-81. [Google Scholar]
References to other published versions of this review
Martis 2016
- Martis R, Brown J, Alsweiler J, Crawford TJ, Crowther CA. Different intensities of glycaemic control for women with gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD011624. [DOI: 10.1002/14651858.CD011624.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


