Abstract
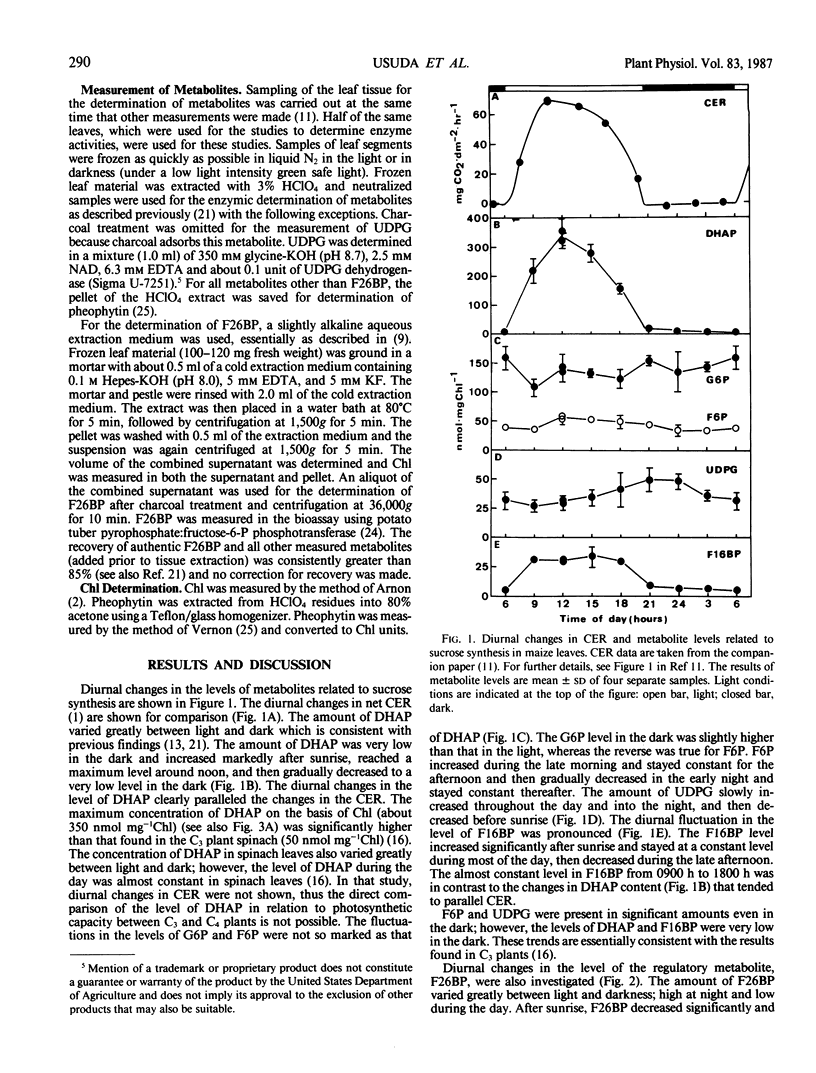
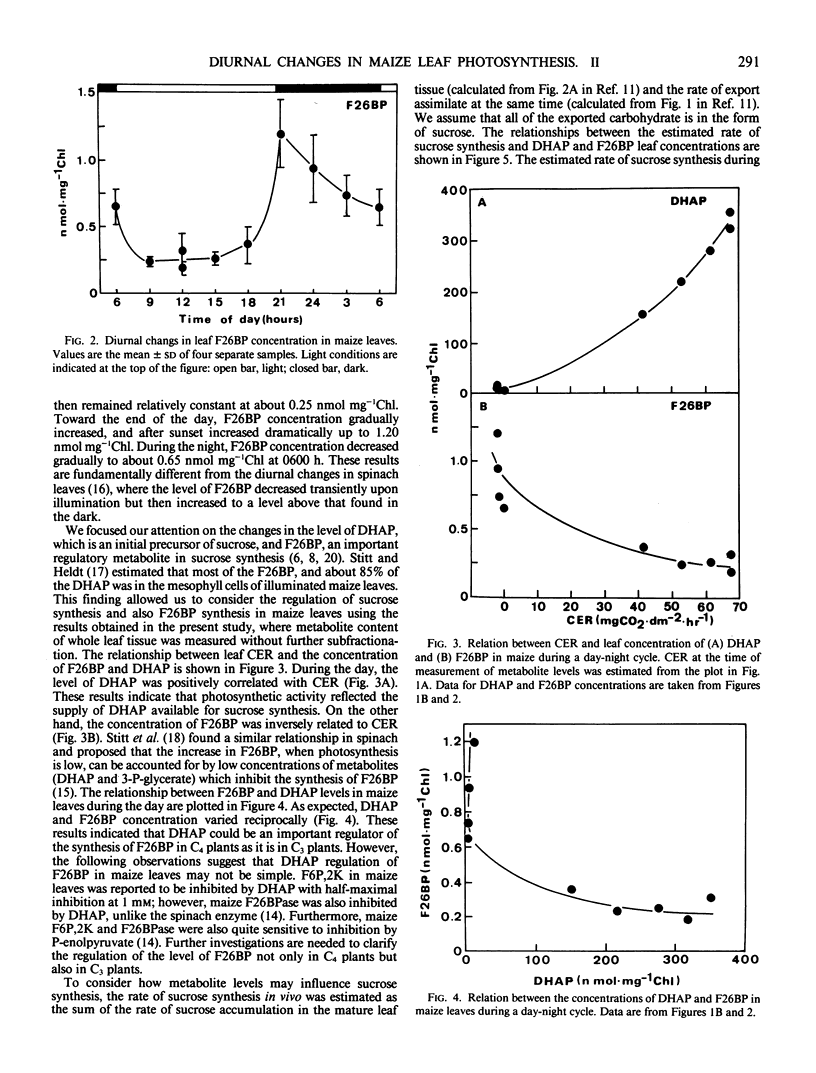
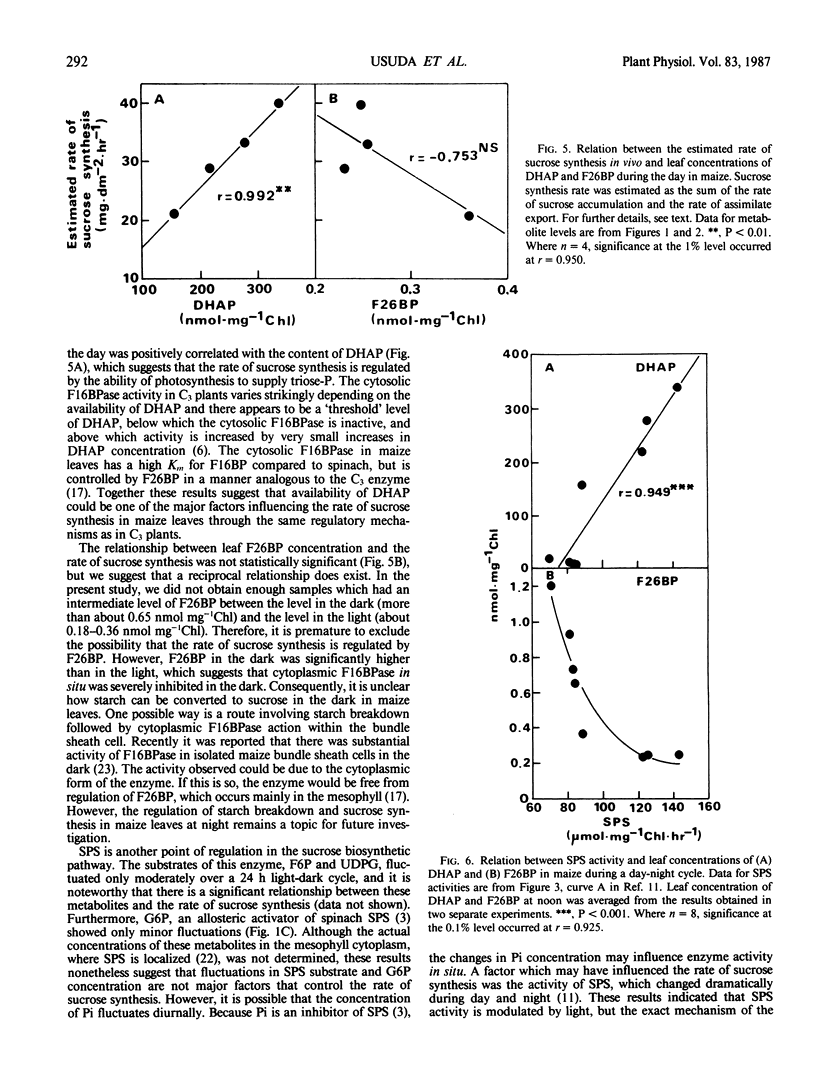
Diurnal changes in the regulatory metabolite, fructose-2,6-bisphosphate (F26BP), and key metabolic intermediates of sucrose biosynthesis were studied in maize (Zea mays L. cv Pioneer 3184) during a day-night cycle. Whole leaf concentrations of dihydroxyacetonephosphate (DHAP) and fructose 1,6-bisphosphate changed markedly during the photoperiod. DHAP concentration was correlated positively with the rate of sucrose formation in vivo (assimilate export plus sucrose accumulation) and extractable activity of sucrose phosphate synthase (SPS). The changes closely followed net photosynthetic rate, which tracked irradiance. The other metabolic intermediates measured (glucose 6-phosphate, fructose 6-phosphate, and UDP-glucose) were either relatively constant over the 24 hour period or changed in a different pattern. Diurnal changes in leaf F26BP concentrations were pronounced, and fundamentally different than the pattern reported with other species. F26BP concentration decreased at the beginning of the day and remained low and constant; a 3- to 4-fold increase occurred with darkness, and slowly declined thereafter. In general, leaf F26BP concentration was negatively correlated with net photosynthetic rate, and also leaf DHAP concentration. Consequently, co-ordination of the regulation of cytosolic fructose 1,6-bisphosphatase and SPS was apparent. The results support the postulate that in maize leaves the activation state of SPS may be dependent on availability of DHAP and possibly other metabolites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog B., Stitt M., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : III. Properties of the Cytosolic Fructose 1,6-Bisphosphatase. Plant Physiol. 1984 Jul;75(3):561–565. doi: 10.1104/pp.75.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Bickett D. M. Evidence for control of carbon partitioning by fructose 2,6-bisphosphate in spinach leaves. Plant Physiol. 1984 Feb;74(2):445–447. doi: 10.1104/pp.74.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalt-Torres W., Kerr P. S., Usuda H., Huber S. C. Diurnal changes in maize leaf photosynthesis : I. Carbon exchange rate, assimilate export rate, and enzyme activities. Plant Physiol. 1987 Feb;83(2):283–288. doi: 10.1104/pp.83.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr P. S., Rufty T. W., Huber S. C. Endogenous Rhythms in Photosynthesis, Sucrose Phosphate Synthase Activity, and Stomatal Resistance in Leaves of Soybean (Glycine max [L.] Merr.). Plant Physiol. 1985 Feb;77(2):275–280. doi: 10.1104/pp.77.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J., Wötzel C., Buchanan B. B. Enzyme regulation in c(4) photosynthesis : identification and localization of activities catalyzing the synthesis and hydrolysis of fructose-2,6-bisphosphate in corn leaves. Plant Physiol. 1985 Apr;77(4):999–1003. doi: 10.1104/pp.77.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Cseke C., Buchanan B. B. Regulation of fructose 2,6-bisphosphate concentration in spinach leaves. Eur J Biochem. 1984 Aug 15;143(1):89–93. doi: 10.1111/j.1432-1033.1984.tb08345.x. [DOI] [PubMed] [Google Scholar]
- Stitt M., Gerhardt R., Kürzel B., Heldt H. W. A role for fructose 2,6-bisphosphate in the regulation of sucrose synthesis in spinach leaves. Plant Physiol. 1983 Aug;72(4):1139–1141. doi: 10.1104/pp.72.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Herzog B., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : I. Coordination of CO(2) Fixation and Sucrose Synthesis. Plant Physiol. 1984 Jul;75(3):548–553. doi: 10.1104/pp.75.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Kürzel B., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : II. Partitioning between Sucrose and Starch. Plant Physiol. 1984 Jul;75(3):554–560. doi: 10.1104/pp.75.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H. Changes in Levels of Intermediates of the C(4) Cycle and Reductive Pentose Phosphate Pathway during Induction of Photosynthesis in Maize Leaves. Plant Physiol. 1985 Aug;78(4):859–864. doi: 10.1104/pp.78.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H., Edwards G. E. Localization of glycerate kinase and some enzymes for sucrose synthesis in c(3) and c(4) plants. Plant Physiol. 1980 May;65(5):1017–1022. doi: 10.1104/pp.65.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H., Ku M. S., Edwards G. E. Activation of NADP-Malate Dehydrogenase, Pyruvate,Pi Dikinase, and Fructose 1,6-Bisphosphatase in Relation to Photosynthetic Rate in Maize. Plant Physiol. 1984 Sep;76(1):238–243. doi: 10.1104/pp.76.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]


