Abstract
Objective:
Clinical trials of cannabinoids for chronic pain have mixed and often inconclusive results. In contrast, many prospective observational studies show analgesic effects of cannabinoids. This survey study aimed to examine the experiences/attitudes of individuals with chronic pain who are currently taking, have previously taken, or never taken cannabinoids for chronic pain to inform future research.
Methods:
This study is based on a cross-sectional, web-based survey of individuals with self-reported chronic pain. Participants were invited to participate via an email that was distributed to the listservs of patient advocacy groups and foundations that engage individuals with chronic pain.
Results:
Of the 969 respondents, 444 (46%) respondents reported currently taking, 213 (22%) previously taking, and 312 (32%) never taking cannabinoids for pain. Participants reported using cannabinoids to treat a wide variety of chronic pain conditions. Those currently taking cannabinoids (vs. previously) more frequently reported: (1) large improvements from cannabinoids in all pain types, including particularly difficult to treat chronic overlapping pain conditions (e.g., pelvic pain), (2) improvements in comorbid symptoms (e.g., sleep), and (3) lower interference from side effects. Those currently taking cannabinoids reported more frequent and satisfied communication with clinicians regarding cannabinoid use. Those never taking cannabinoids reported lack of suggestion/approval of a clinician (40%), illegality (25%) and lack of FDA regulation (19%) as reasons for never trying cannabinoids.
Discussion:
These findings underscore the importance of conducting high-quality clinical trials that include diverse pain populations and clinically relevant outcomes that if successful, could support FDA approval of cannabinoid products. Clinicians could then prescribe and monitor these treatments similarly to other chronic pain medications.
Keywords: Cannabinoids, chronic pain, patient attitudes, trial design
Introduction
Randomized clinical trials investigating the efficacy of cannabinoids for chronic pain have mixed and often inconclusive results and do not provide sufficient evidence that cannabinoids are effective for treating chronic pain.1 These trials are mostly small, likely underpowered and primarily focused on a narrow subset of chronic pain types. In contrast, multiple prospective observational studies suggest improvements in pain and opioid-sparing effects for medicinal cannabinoid use.2–9 As of December 2022, medical cannabis is approved in 38 states and the District of Columbia, allowing many patients access to various cannabinoid-based therapies for pain. Medical cannabis use continues to increase; the estimated retail expenditure on medical cannabis in the United States in 2020 was 7.1 billion dollars.10 Although not all medical cannabis is used to treat pain, studies suggest pain relief is the most common use.10, 11 Collectively, these observations suggest that interventions targeting the cannabinoid pathway may be effective at least for some patients with chronic pain; however, it is important to note that perceived cannabinoid efficacy could largely be due to placebo effects.12
At present, no cannabinoid therapeutics are FDA-approved for the treatment of chronic pain and medical cannabis, when used to treat pain, is not pharmaceutical grade. Furthermore, the extent to which use of the currently available products is guided by clinicians is not known.13 All of these factors could decrease the effectiveness and safety of current cannabinoid products and limit their utilization. It is clear that more research is needed to determine the most appropriate pain conditions for cannabinoid-targeting therapeutics, to inform the design of clinical trials of pharmaceutical grade cannabinoids, and to optimize the clinical implementation of currently-available and future formulations of cannabinoids for individuals with chronic pain.
This study was prompted by the discrepancy between the contradictory and mostly negative or inconclusive results of clinical trials and the generally promising results of observational research as well as increasing utilization of cannabinoids for pain in the general population. This research had 2 main goals. First, we aimed to investigate whether certain types of pain and/or comorbid symptoms may be more responsive to cannabinoids to help inform design of future clinical trials examining the efficacy of novel cannabinoids for pain management. Second, we aimed to survey the landscape of demand for pharmaceutical-grade cannabinoids among individuals with pain who had and had not previously taken cannabinoids. To that end, we investigated the self-reported experiences with and attitudes towards cannabinoids among individuals who are currently taking them to treat pain, those who have stopped taking them, and those who have never taken them. Explored domains include types and source of cannabinoids taken, types of pain treated, involvement of clinicians in cannabinoid treatment plans, sources of information regarding cannabinoids, and attitudes toward clinical trials of novel cannabinoids. The overall goal of this study is to inform future development of novel cannabinoids and research related to optimization of medicinal cannabis implementation and patient care.
Methods
Study design and participants
This study is based on a cross-sectional survey of individuals with self-reported chronic pain that was administered using QualtricsXM, a web-based survey platform. Data were collected between June and August 2021. Participants were invited to participate via an email that was distributed to the listservs of patient advocacy groups and foundations that engage individuals with chronic pain, including the American Chronic Pain Association, the Chronic Pain Research Alliance, the Peripheral Nerve Society, the Alliance to Advance Comprehensive Integrative Pain Medicine, the TMJ Association, and the US Pain Foundation. Because these listservs include individuals without pain (e.g., researchers, policy makers, and allies), it is impossible to calculate a response rate for the survey. The invitation email asked individuals to complete the survey if they were living with chronic pain of any kind and were at least 18 years old. The email stated in bold “If you have chronic pain, no matter how you feel about cannabinoids, we want to hear from you!” in order to promote responses from individuals who had a range of experiences with cannabinoids, including never having tried them. No identifying information was collected to promote honesty in answers to potentially sensitive questions. The study was declared exempt by the Sterling IRB, an independent institutional review board.
Survey
The survey was designed with input from clinician co-authors who treat chronic pain and have expertise in cannabinoids (RF, KPH, AW) as well as a member of the American Chronic Pain Association (PC). The full survey can be found in Appendix 1. In short, participants reported on chronic pain conditions, current pain intensity levels, duration of pain, current pharmacologic and non-pharmacologic treatments used for pain, age, gender, employment status, and level of education. Participants were asked if they were currently using or had ever used cannabinoids for chronic pain. Participants who reported currently or previously taking cannabinoids for chronic pain were asked questions related to the following domains (in either present or past tense as applicable): (1) types and sources of cannabinoids used, (2) whether use was purely medicinal vs. recreational and medicinal, (3) types of pain treated, (4) pain improvement (or worsening) for each type of pain in response to cannabinoids, (5) effect of cannabinoids on co-occurring symptoms, (6) side effects and perceived impact of those side effects, (6) communication with clinicians regarding cannabinoids, (7) sources of information regarding cannabinoid treatment for pain. Individuals reporting previously taking cannabinoids were asked why they discontinued use and individuals reporting never having tried cannabinoids for pain were asked why they had never tried them. Themes in free-text responses from “other boxes” were identified and discussed by co-authors JSG, JD, SB, JEH, ECL. Appendix 2 describes new options generated from “other” responses or types of responses that were batched with existing response options.
Statistical analyses
Descriptive statistics were used to characterize responses. Chi square tests were used to compare differences in proportions between those currently, previously, and never taking cannabinoids. A p-value of 0.05 was considered significant and no adjustments were made for multiplicity considering the exploratory nature of the study. All analyses were performed in JMP 16 (Cary, NC). The number of participants who responded to each question is included in the tables as (N= number responded).
Results
Respondent demographics
A total of 1179 participants started the survey; 210 did not submit the survey. Because the information letter stated that by completing the survey, the participants were consenting to participate, we did not use any data from participants who did not hit the submit button on the survey. Thus, data from 969 respondents were included in the analyses. The majority of respondents were between 50 and 79 years old. Approximately two-thirds of respondents were women. 92% of respondents self-identified as White and 97% self-identified as non-Hispanic. 75% of the sample had at least a college degree. Almost half of all respondents reported being retired and a quarter of all respondents were receiving disability benefits. A little over one-third reported seeing a mental health provider (Table 1). Almost two-thirds of the sample reported living with pain for over 10 years. Peripheral neuropathy pain, chronic low back pain (CLBP), osteoarthritis, fibromyalgia, and headache were reported most frequently. The most commonly reported pharmacologic treatments were opioids and gabapentinoids and the most commonly reported non-pharmacologic treatment was physical therapy (Table 2).
Table 1.
Demographics
| All participants N (%) | Currently taking N (%) | Previously taking N (%) | Never taking N (%) | p-value | |
|---|---|---|---|---|---|
| Age (N = 967) | 0.0096 | ||||
| 18–39 | 14 (1.3%) | 8 (1.8%) | 1 (0.5%) | 5 (1.6%) | |
| 30–39 | 23 (2.4%) | 15 (3.3%) | 3 (1.4%) | 5 (1.6%) | |
| 40–49 | 97 (10.3%) | 53 (12.0%) | 17 (8.0%) | 27 (8.7%) | |
| 50–59 | 224 (23.2%) | 103 (23.3%) | 38 (17.8%) | 83 (26.6%) | |
| 60–69 | 318 (32.9%) | 145 (32.8%) | 77 (36.2%) | 96 (30.8%) | |
| 70–79 | 226 (23.4%) | 101 (22.9%) | 55 (25.8%) | 70 (22.4%) | |
| 80+ | 65 (6.7%) | 17 (3.8%) | 22 (10.3%) | 26 (8.3%) | |
| Race (N = 966) | 0.761 | ||||
| White | 889 (92.0%) | 408 (92.3%) | 197 (92.5%) | 284 (91.3%) | |
| Black /AA | 23 (2.4%) | 9 (2.0%) | 3 (1.4%) | 11 (3.5%) | |
| American Indian/Native Alaskan | 10 (1.0%) | 5 (1.1%) | 1 (0.5%) | 4 (1.3%) | |
| Mixed races | 10 (1.0%) | 5 (1.1%) | 2 (0.9%) | 3 (1.0%) | |
| Choose not to answer/ Unknown/Other | 31 (3.2%) | 13 (2.9%) | 9 (4.2%) | 9 (2.9%) | |
| Ethnicity (N = 901) | 0.912 | ||||
| Non-Hispanic | 877 (97.3%) | 402 (97.1%) | 188 (97.4%) | 287 (97.6%) | |
| Hispanic | 24 (2.7%) | 12 (2.9%) | 5 (2.6%) | 7 (2.4%) | |
| Gender (N= 965) | 0.380 | ||||
| Female | 609 (63%) | 266 (60.1%) | 132 (62.2%) | 211 (67.8%) | |
| Male | 350 (36.2%) | 172 (38.9%) | 79 (37.3%) | 99 (31.8%) | |
| Non-binary/ gender fluid | 6 (0.6%) | 3 (0.7%) | 1 (0.5%) | 1 (0.3%) | |
| Education (N=968) | 0.244 | ||||
| Less than High School | 6 (0.6%) | 2 (0.5%) | 1 (0.5%) | 3 (0.9%) | |
| High School graduate | 203 (20.1%) | 91 (20.5%) | 49 (23.0%) | 63 (20.2%) | |
| College Degree | 430 (44.4%) | 206 (46.5%) | 93 (43.7%) | 131 (42.0%) | |
| Graduate Degree | 298 (30.8%) | 125 (28.2%) | 68 (31.9%) | 105 (33.7%) | |
| Choose not to answer | 31 (3.2%) | 19 (4.3%) | 2 (0.9%) | 10 (3.2%) | |
| Employment status (N=966) | 0.0003 * | ||||
| Employed full time | 134 (13.9%) | 64 (14.5%) | 23 (10.8%) | 47 (15.1%) | |
| Employed part time | 75 (7.8%) | 38 (8.6%) | 10 (4.7%) | 27 (8.7%) | |
| Retired | 429 (44.4%) | 180 (40.7%) | 121 (56.8%) | 128 (41.1%) | |
| On disability | 247 (25.6%) | 120 (27.1%) | 50 (23.5%) | 77 (24.8%) | |
| Home maker | 25 (2.6%) | 11 (2.5%) | 0 (0%) | 14 (4.5%) | |
| Seeking work | 14 (1.4%) | 7 (1.6%) | 2 (0.9%) | 5 (1.6%) | |
| Disabled; Not receiving disability benefits | 20 (2.1%) | 13 (2.9%) | 1 (0.5%) | 6 (1.9%) | |
| Other | 22 (2.3%) | 9 (2.0%) | 6 (2.8%) | 7 (2.2%) | |
| Mental health provider (N=966) | 0.116 | ||||
| Yes | 336 (34.7%) | 169 (38.2) | 67 (31.5%) | 100 (32.2%) | |
| No | 630 (65.2%) | 273 (62%) | 146 (68.5%) | 211 (67.8%) |
Participants in other category excluded from the statistical analysis.
Table 2.
Pain characteristics and current treatments (other than cannabinoids)
| All participants N (%) or Median [IQR] | Currently taking N (%) or Median [IQR] | Previously taking N (%) or Median [IQR] | Never taking N (%) or Median [IQR] | p-value | |
|---|---|---|---|---|---|
| Current pain intensity (N=966) | 7 [5, 8] | 7 [5,8] | 7 [5,8] | 7 [5,8] | 0.53 |
| Duration living with pain (N=966) | 0.005 | ||||
| Less than 1 year | 13 (1.3%) | 7 (1.6%) | 0 (0%) | 6 (2.0%) | |
| 1–2 years | 38 (3.9%) | 23 (5.2%) | 1 (0.5%) | 14 (4.5%) | |
| 3–4 years | 68 (7.0%) | 34 (7.7%) | 16 (7.5%) | 18 (5.8%) | |
| 5–6 years | 78 (8.1%) | 33 (7.5%) | 18 (8.5%) | 27 (8.6%) | |
| 6 – 10 years | 143 (14.8%) | 75 (17.0%) | 27 (12.7%) | 41 (13.2%) | |
| More than 10 years | 626 (64.8%) | 270 (61.1%) | 151 (70.8%) | 205 (65.9%) | |
| Pain conditions* (N=968) | |||||
| PN pain | 514 (53%) | 257 (57.9%) | 120 (56.3%) | 137 (43.9%) | 0.0004 |
| CLBP | 492 (51%) | 218 (49.1%) | 106 (49.8%) | 168 (53.9%) | 0.414 |
| Osteoarthritis | 391 (40%) | 165 (37.2%) | 84 (39.4%) | 142 (45.5%) | 0.068 |
| Fibromyalgia | 292 (30%) | 125 (28.2%) | 62 (29.1%) | 105 (33.7%) | 0.254 |
| Headache | 252 (26%) | 112 (25.3%) | 57 (26.8%) | 83 (26.6%) | 0.877 |
| Abdominal pain | 181 (19%) | 87 (19.6%) | 36 (16.9%) | 58 (18.6%) | 0.705 |
| PS/TNP | 154 (15.9%) | 79 (17.8%) | 39 (18.3%) | 36 (11.54%) | 0.032 |
| Orofacial pain | 129 (13.3%) | 51 (11.5%) | 31 (14.6%) | 47 (15.1%) | 0.308 |
| CRPS | 104 (10.7%) | 45 (10.1%) | 18 (8.5%) | 41 (13.1%) | 0.204 |
| Number of pain conditions (N=968) | 0.908 | ||||
| 1 | 284 (29.3%) | 131 (29.6%) | 62 (29.1%) | 91 (29.2%) | |
| 2–3 | 382 (39.5%) | 180 (40.6%) | 84 (39.4%) | 118 (37.8%) | |
| 4 or more | 302 (31.2%) | 132 (29.8%) | 67 (31.5%) | 103 (33.0%) | |
| Pharmacologic treatments* (N=939) | |||||
| Acetaminophen | 388 (40.0%) | 158 (35.6%) | 87 (40.8%) | 143 (45.8%) | 0.018 |
| NSAID | 375 (38.7%) | 165 (37.2%) | 80 (37.6%) | 130 (41.7%) | 0.425 |
| Opioids | 454 (46.9%) | 187 (42.1%) | 117 (54.9%) | 150 (48.1%) | 0.0075 |
| Gabapentinoid | 452 (46.6%) | 209 (47.1%) | 109 (51.2%) | 143 (42.9%) | 0.174 |
| Antidepressant | 345 (35.6%) | 156 (35.1%) | 73 (34.3%) | 116 (37.2%) | 0.762 |
| Amitriptyline | 103 (10.6%) | 60 (13.5%) | 15 (7.0%) | 28 (9.0%) | 0.020 |
| Topical drug | 267 (27.6%) | 114 (25.7%) | 60 (28.2%) | 93 (29.8%) | 0.445 |
| Non-pharmacologic treatments** (N=915) | |||||
| Non-invasive device (e.g., TENS) | 303 (31.3%) | 149 (33.6%) | 62 (29.1%) | 92 (29.5%) | 0.367 |
| Invasive device (e.g., SCS) | 108 (11.1%) | 49 (11.0%) | 25 (11.7%) | 34 (10.9%) | 0.952 |
| Physical therapy | 622 (64.2%) | 300 (67.6%) | 134 (62.9%) | 188 (60.3%) | 0.108 |
| Psychological treatments (e.g., CBT, mindfulness) | 331 (34.2%) | 168 (37.8%) | 66 (31.0%) | 97 (31.1%) | 0.085 |
| Acupuncture | 176 (18.2%) | 95 (21.4%) | 41 (19.3%) | 40 (12.8%) | 0.008 |
less than 10% reported other pain conditions, including but not limited to, cancer pain, pelvic pain, visceral pain, connective tissue disorders, self-reported central pain
Less than 10% of respondents reported other pharmacologics in the following categories: Anesthetics (local injection, of IV), Aspirin, anticonvulsants (other than gapabentinoids), Biologics (including CGRP inhibitors), Botox, low dose naltrexone, muscle relaxants, steroids, triptans.
Less than 10% of respondents reported utilizing a chiropractor, heat, ice, or massage.
Note, for questions with answers with check all that apply answers, the full sample was used as the denominator even if some participants did not select any options to represent the proportion of the total sample that endorsed each option.
Participant cannabinoid use characteristics
Of the 969 respondents, 444 (46%) reported currently taking cannabinoids for chronic pain; 213 (22%) reported previously taking cannabinoids for chronic pain; and 312 (32%) reported never having tried cannabinoids to treat chronic pain. Table 3 illustrates the percentage of respondents who took cannabinoids for medicinal only vs. medicinal and recreational purposes and the length of time that they took cannabinoids for pain, types of cannabinoids taken, and how the cannabinoids were obtained. The most common types of cannabinoids reported were ingested CBD, ingested cannabis, smoked or vaped cannabis, or topical CBD. Pharmaceutical cannabinoids were reported infrequently (i.e., 0–1.5% of respondents) (Table 3). The most common sources of cannabinoids reported by respondents were medical dispensary, online, and family or friends. A higher percentage of those currently taking cannabinoids than those previously taking them obtained cannabinoids from a medical dispensary (59.9% current vs. 45.1% previous), with a higher percentage of those previously than currently taking cannabinoids obtaining them from a family or friend (26.3% previous vs. 13.3% current) or non-licensed dealer (12.7% previous vs. 5.0% current) (Table 3).
Table 3.
Characteristics of cannabinoid use
| All participants N (%) | Currently taking N (%) | Previously taking N (%) | p-value | |
|---|---|---|---|---|
| Reason for use (N=650) | 0.0005 | |||
| Medical only | 542 (83.3%) | 352 (80%) | 190 (90.5%) | |
| Medical and Recreational | 108 (16.6%) | 88 (20%) | 20 (9.5%) | |
| Duration of cannabinoid use (N=656) | <0.0001 | |||
| < 1 year | 243 (37%) | 92 (20.8%) | 151 (70.9%) | |
| 1–2 years | 121 (18.4%) | 89 (20.0%) | 32 (15%) | |
| 2–4 years | 122 (18.6%) | 106 (23.9%) | 16 (7.5%) | |
| 4–6 years | 42 (6.4%) | 38 (8.6%) | 4 (1.8%) | |
| 6–10 years | 33 (5.0%) | 32 (7.2%) | 1 (0.4%) | |
| >10 years | 95 (14.5%) | 86 (19.4%) | 9 (4.2%) | |
| Type of cannabinoid used (N=656) | ||||
| Cannabis smoked | 255 (34.2%) | 160 (36.0%) | 65 (30.5%) | 0.167 |
| Cannabis vaped | 180 (27.4%) | 147 (33.1%) | 33 (15.5%) | <0.0001 |
| Cannabis ingested | 301 (45.8%) | 228 (51.4%) | 73 (34.3%) | <0.0001 |
| CBD ingested | 358 (54.5%) | 228 (51.4%) | 130 (61.0%) | 0.019 |
| CBD topical | 249 (37.9%) | 166 (37.4%) | 83 (39.0%) | 0.696 |
| CBD smoked/vaped | 4 (0.6%) | 2 (0.5%) | 2 (0.9%) | 0.466 |
| CBD unspecified | 3 (0.5%) | 2 (0.5%) | 1 (0.5%) | 0.973 |
| THC topical | 12 (1.8%) | 10 (2.3%) | 2 (0.9%) | 0.213 |
| THC unspecified | 6 (0.9%) | 3 (0.7%) | 3 (1.4%) | 0.371 |
| Sativex | 10 (1.5%) | 4 (0.9%) | 6 (2.8%) | 0.072 |
| Epidiolex | 8 (1.2%) | 5 (1.1%) | 3 (1.4%) | 0.760 |
| Marinol | 11 (1.7%) | 5 (1.1%) | 6 (2.8%) | 0.128 |
| Cesamet | 3 (0.5%) | 0 (0%) | 3 (1.4%) | 0.009 |
| Cannabinoid source (N=654) | ||||
| Medical dispensary | 362 (55.1%) | 266 (59.9%) | 96 (45.1%) | 0.0003 |
| Online | 119 (18.1%) | 84 (18.9%) | 35 (16.4%) | 0.435 |
| Family or friend | 115 (17.5%) | 59 (13.3%) | 56 (26.3%) | <0.0001 |
| Homegrown | 43 (6.5%) | 35 (7.9%) | 8 (3.8%) | 0.036 |
| Recreational dispensary | 36 (5.5%) | 27 (6.1%) | 9 (4.2%) | 0.317 |
| Pharmacy | 44 (6.7%) | 26 (5.9%) | 18 (8.5%) | 0.221 |
| Non-licensed dealer | 49 (7.5%) | 22 (5.0%) | 27 (12.7%) | 0.0007 |
| Store | 26 (4.0%) | 16 (3.6%) | 10 (4.7%) | 0.508 |
CBD; cannabidiol
Note, for questions with answers with check all that apply answers, the full sample size for each group was used as the denominator to represent the proportion of the total sample that endorsed each option.
Respondent-reported cannabinoid benefits and adverse effects
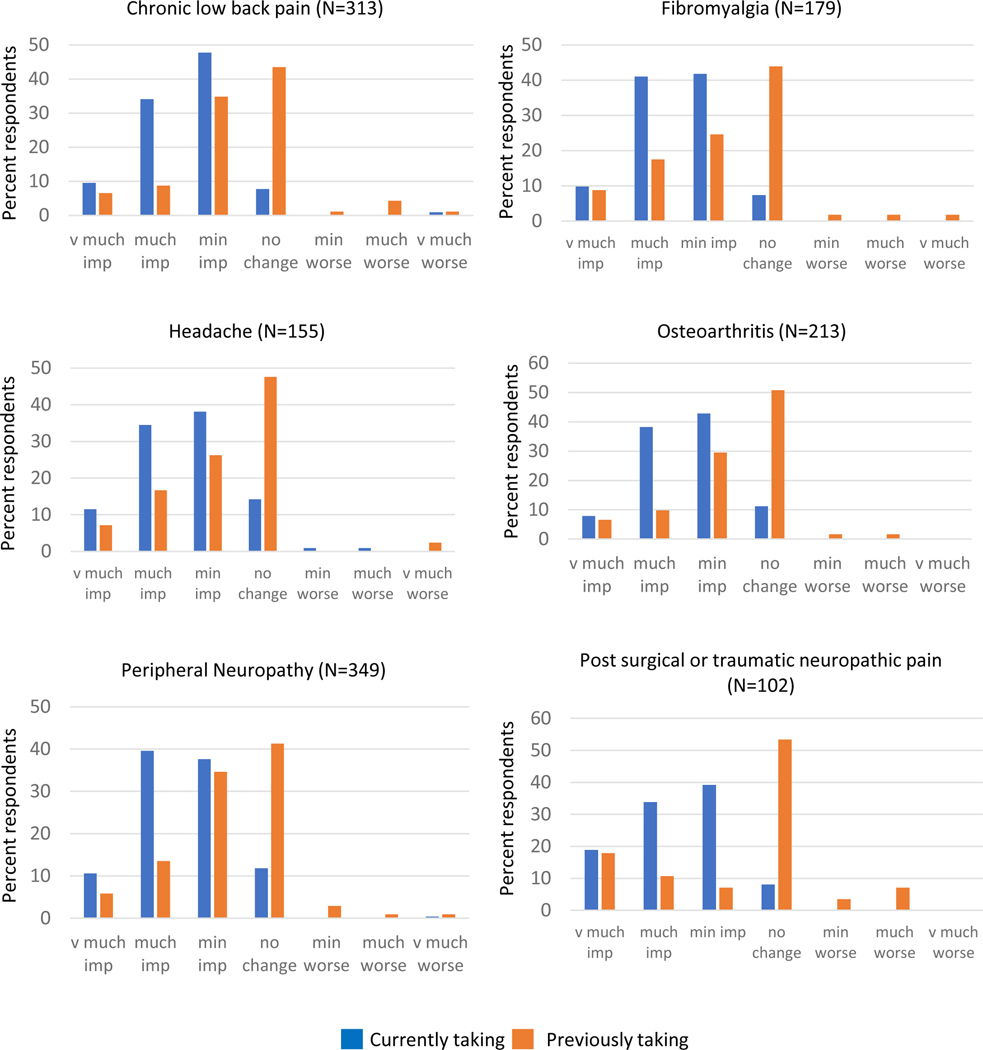
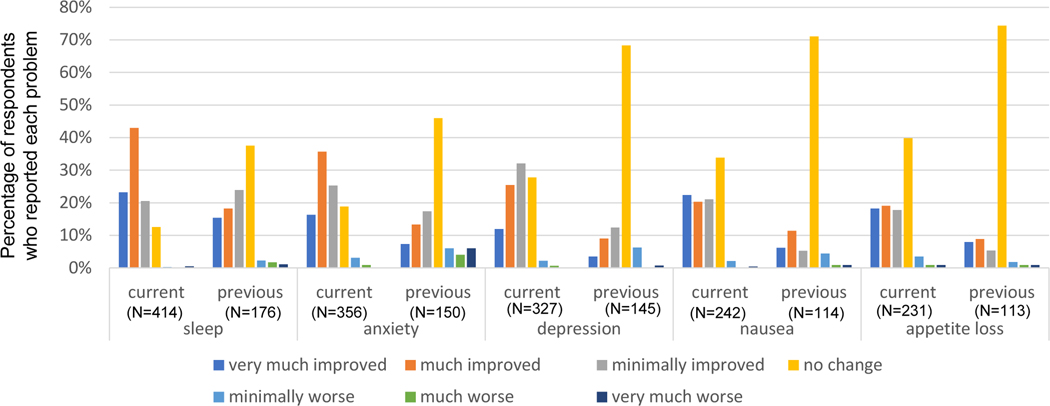
Respondents were asked to report the degree to which cannabinoids impacted their pain for each type of pain condition that they had treated with cannabinoids. For every type of pain queried, those currently taking cannabinoids more commonly reported higher-magnitude improvements in pain with cannabinoid use than those previously taking it. However, almost 50% of those previously taking cannabinoids reported at least minimally-improved pain for all types of chronic pain. Figure 1 illustrates the distribution of the magnitude of pain change in response to cannabinoids for the 6 most common conditions for which respondents reported trying cannabinoids. These distributions are similar to those conditions with smaller sample sizes (see Supplemental Figure 1 for remaining conditions queried). In addition to improvements in pain, respondents frequently endorsed improvements in symptoms that often co-occur with chronic pain. Similar to pain, a higher percentage of those currently taking cannabinoids reported higher-magnitude improvements in these symptoms than those previously taking them. The following percentages of those currently taking cannabinoids who reported having each symptom reported the symptoms as very much improved or much improved: sleep (66%), anxiety (52%), nausea (43%), depression (37%), and appetite loss (37%) (Figure 2). Notably, 34% of those previously taking cannabinoids reported that cannabinoids very much or much improved their sleep (Figure 2). Approximately half of respondents reported taking opioids to control their chronic pain when they started taking cannabinoids. Of those who reported using opioids, 144 (65%) of those currently taking cannabinoids and 34 (30%) of those previously taking them reported being able to reduce their opioid usage after starting cannabinoids.
Figure 1.
Respondent-reported cannabinoid-induced changes in pain.
Figure 2.
Cannabinoid effects on co-occurring symptoms
Table 4 summarizes the side effects reported by those who were currently or previously taking cannabinoids for chronic pain. Those previously taking cannabinoids indicated that, with the exception of headache, all of the side effects interfered on average to a greater extent in their daily activities than those currently taking cannabinoids (Table 4). Similar trends were observed when participants were asked how distressing the side effects were (Supplemental Table 1). Those previously taking cannabinoids rated many of the side effects as having a high degree of interference. For example, the median score on the interference scale (out of 10) was 10 for irrational fears; 8 for nausea; and 7 for fatigue, dizziness, and difficulty thinking straight for those previously taking cannabinoids for pain (Table 4).
Table 4.
Respondent-reported side effects (N=650)
| Currently taking | Previously taking | p-value Interference | |||
|---|---|---|---|---|---|
| N (%) reporting side effect | Interference* Median [IQR] | N (%) reporting side effect | Interference* Median [IQR] | ||
| Dry mouth | 194 (43.7%) | 0 [0, 1] | 54 (25.3%) | 1 [0, 3] | 0.015 |
| Drowsiness | 178 (40.1%) | 2 [0, 4] | 62 (29.1%) | 4 [1, 8] | 0.0004 |
| Increased Appetite | 135 (30.4%) | 0 [0, 1] | 36 (16.9%) | 3 [0, 5] | 0.0009 |
| Difficulty thinking straight | 80 (18.0%) | 4 [2, 6] | 46 (21.6%) | 7 [4.8, 9] | <0.0001 |
| Dizziness | 69 (15.5%) | 2 [1, 5] | 26 (12.2%) | 7 [3.8, 8.3] | <0.0001 |
| Fatigue | 58 (13.1%) | 3 [1, 6] | 22 (10.2%) | 7 [6, 9] | <0.0001 |
| Increased heart rate | 38 (8.6%) | 1 [0, 2] | 20 (9.4%) | 5 [2, 8] | <0.0001 |
| Headache | 25 (5.6%) | 2 [1, 4.5] | 9 (4.2%) | 5 [4, 7] | 0.063 |
| Irrational fears | 18 (4.1%) | 2.5 [1, 5.3] | 13 (6.1%) | 10 [6, 10] | 0.0005 |
| Nausea | 16 (3.6%) | 2 [0, 6] | 7 (3.2%) | 8 [3, 10] | 0.043 |
| None | 122 (27.5%) | 96 (45.1%) | |||
If a participant indicated that they experienced a side effect, they were asked to rate how much that side effect interfered with their daily activities.
The full sample for each group was used as the denominator for calculating percentages to represent the proportion of the total sample that endorsed each side effect.
Clinician communication and other information sources regarding cannabinoid use for pain
Seventy-three percent of those currently taking cannabinoids and 60% of those previously taking cannabinoids reported discussing their cannabinoid use for pain with a clinician (Table 5). Those currently taking cannabinoids tended to be more satisfied with that communication than those previously taking them, with 60% of those currently taking them reporting being extremely or very satisfied with the communication (vs. 49% of those previously taking them), and 18% of those currently taking them and 28% of those previously taking them reporting being minimally or not at all satisfied (Table 6). Those currently and previously taking cannabinoids reported similar opinions regarding how knowledgeable their clinicians were regarding cannabinoid use for chronic pain, with only 39% of all respondents reporting that their clinician was very or extremely knowledgeable (Table 5). The most common reported sources of information related to cannabinoid use for chronic pain were the internet (59.2%), clinicians (41.6%), foundations (34.6%), family or friends (33.6%), and social media (12.5%) (Table 5).
Table 5.
Experiences regarding communication with and impression of expertise of clinicians regarding cannabinoids and other sources of information regarding cannabinoids.
| All participants N (%) | Currently taking N (%) | Previously taking N (%) | p-value | |
|---|---|---|---|---|
| Discuss with doc (y) (N=656) | 454 (69.2%) | 326 (73) | 128 (60.4) | 0.0008 |
| Satisfied with communication (N=452) | 0.017 | |||
| Completely | 145 (32.1%) | 106 (33.3%) | 39 (30.7%) | |
| Very | 110 (24.3%) | 87 (26.8%) | 23 (18.1%) | |
| Somewhat | 101 (22.3%) | 72 (22.2%) | 29 (22.8%) | |
| Minimally | 54 (11.9%) | 39 (12.0%) | 15 (11.8%) | |
| Not at all | 42 (9.2%) | 21 (6.4%) | 21 (16.5%) | |
| Respondent rating of clinician knowledge (N=450) | 0.999 | |||
| Extremely | 72 (16.0%) | 51 (15.9%) | 21 (16.4%) | |
| Very | 104 (23.1%) | 75 (23.3%) | 29 (22.7%) | |
| Somewhat | 145 (32.2%) | 104 (32.2%) | 41 (32.0%) | |
| Minimally | 89 (19.8%) | 63 (19.6%) | 26 (20.3%) | |
| Not at all | 40 (8.9%) | 29 (9.0%) | 11 (8.6%) | |
| Respondent-endorsed “sources of information regarding cannabinoids” (N=657) | ||||
| Internet | 389 (59.2%) | 279 (62.8%) | 110 (51.6%) | 0.007 |
| Clinician | 273 (41.6%) | 189 (42.6%) | 84 (39.4%) | 0.445 |
| Foundations | 227 (34.6%) | 185 (41.7%) | 42 (19.7%) | <0.0001 |
| Family of friend | 221 (33.6%) | 121 (27.3%) | 100 (46.9%) | <0.0001 |
| Social media | 82 (12.5%) | 61 (13.7%) | 21 (9.9%) | 0.0042 |
| Journals/print sources | 35 (5.3%) | 34 (7.7%) | 1 (0.5%) | <0.001 |
| Personal experience | 32 (4.9%) | 29 (6.5%) | 3 (1.4%) | 0.0016 |
| Dispensary staff/store employees | 29 (4.4%) | 22 (5.0%) | 7 (3.3%) | 0.318 |
Table 6.
Reasons for discontinuation or never trying cannabinoids.
| N (%) | |
|---|---|
| Reason for stopping cannabinoids use (previously taking) (N=209) | |
| Side effects | 63 (29.6%) |
| No efficacy | 62 (29%) |
| Efficacy waned | 37 (17.4%) |
| Expense | 27 (12.7%) |
| Illegal or prohibited | 22 (10.3%) |
| Stigma | 7 (3.2%) |
| Other method worked better | 7 (3.3%) |
| Fear of addiction | 2 (0.9%) |
| Reasons for not starting cannabinoid use (never taking)* (N=311) | |
| Doctor never suggested it | 126 (40.4%) |
| Illegal | 79 (25.3%) |
| View that cannabinoids not FDA regulated | 58 (18.6%) |
| Stigma | 47 (15.1%) |
| Doctor discouraged it | 42 (13.5%) |
| Pain well controlled | 40 (12.8%) |
| Fear of addiction | 27 (8.7%) |
| Unaware that people use cannabinoids for pain | 22 (7.1%) |
Fewer than 5% wrote in the following reasons for never trying cannabinoids for chronic pain: Expense, anticipated side effects, general anti-drug attitude, lack of information, prohibited by job, prohibited medically while getting other treatments.
Note, the full sample size was used as the denominator to represent the proportion of the total sample that endorsed each option.
Reasons for discontinuation or never trying cannabinoids for chronic pain.
The most common reasons respondents reported stopping cannabinoid therapy for chronic pain were side effects (29.6%) and either lack of efficacy (29%) or waning efficacy (17.4%). Additional reasons cited were expense (12.7%) and that it was illegal or prohibited either by work or clinician (10.3%). Stigma (3.2%) or fear of addiction (0.9%) were rarely cited as reasons for stopping cannabinoids (Table 6). The most common reasons for which respondents reported not trying cannabinoids for chronic pain were that their doctor never suggested it (40.4%) and that it is illegal (25.3%). Other common concerns included the view that cannabinoids are not FDA-regulated (18.6%), their doctor discouraged using cannabinoids (13.5%), or that their pain was well controlled with other medications (12.8%). Stigma (15.1%) and fear of addiction (8.7%) were also cited by those who had never tried cannabinoids as reasons for not trying cannabinoids. Finally, 7.1% of those who had never tried cannabinoids for pain reported not being aware that people use cannabinoids to treat chronic pain (Table 6).
Interest in participating in clinical trials of novel cannabinoids.
A high proportion of respondents reported that they would consider joining a clinical trial, with more individuals willing to join a trial without a placebo group. Specifically, 58% of those currently taking cannabinoids, 65% of those previously taking cannabinoids, and 72% of those who had never tried cannabinoids for pain reported “yes” or “maybe” they would be willing to join a clinical trial of a novel cannabinoid that included a placebo group, while 40%, 33%, and 24% of those currently, previously, and never taking cannabinoids for chronic pain answered “no”. For trials of a novel cannabinoid that did NOT include a placebo group, 79%, 76%, and 78% of those currently, previously, and never taking cannabinoids for chronic pain, respectively answered “yes” or “maybe” they would be willing to join, while only 20%, 23%, and 19%, respectively answered “no.” The difference in proportions between groups to the question regarding trials that included a placebo was significant (p<0.0001), whereas it was not for the question regarding trials that did not include a placebo group (p=0.328) (Supplemental Table 2).
Discussion
To our knowledge, this is the first large survey study to compare the attitudes and experiences of those currently, previously, and never taking cannabinoids for chronic pain. Consistent with previous observational research,2, 3, 11 our study found that many individuals, especially those currently using cannabinoids, frequently perceive large benefits and are able to reduce opioid medications.3–5, 9 Thus, even though RCT data demonstrating efficacy are limited and these reported effects could be at least partially due to placebo effects, it is likely that currently available cannabinoids will continue to be used to treat chronic pain. Better understanding of who is using these substances to control pain, which substances they are using, the resulting benefits and adverse effects, and the roles that clinicians play in cannabinoid pain therapy are important to inform future research that aims to optimize clinical outcomes for patients.
Previous studies of cannabinoids have included multiple pain types (e.g.,2, 4, 6); however, these studies rarely report the effects of cannabinoids on each type of pain. This study provides a unique opportunity to compare the perceived effectiveness of cannabinoids for different types of pain. We observed similar distributions in pain relief for different types of pain (Figure 1). This is noteworthy considering many clinical trials of cannabinoids have focused on neuropathic pain (e.g., 13 of 36 trials included in a recent systematic review of cannabinoid efficacy for pain included patients with neuropathic pain1). Multiple sclerosis and cancer pain made up the majority of the remaining studies, with only one trial conducted in CLBP, one of the most common pain conditions. Our results suggest that it would be beneficial to explore other chronic pain types in future clinical trials. The proportion of respondents with each type of pain who are currently vs. previously taking cannabinoids for pain could be considered a surrogate for overall satisfaction with using cannabinoids for pain. The percentage of respondents with each pain condition who reported currently taking cannabinoids ranged from 69%−74% for most conditions. In contrast, between 85% and 87% of respondents reporting abdominal, pelvic, and visceral pain were currently taking cannabinoids (i.e., at least 10% higher than the other pain types). Although these results should be interpreted with caution given the generally smaller numbers in these groups (N=32 to 91), they generate interesting hypotheses for future research and clinical trials. These conditions are all chronic overlapping pain conditions (COPCs) that tend to co-occur, cause higher pain intensity and more frequent and severe comorbid psychological symptoms, and are particularly difficult to treat.14–16 Better understanding of the efficacy of cannabinoids for pain and comorbid psychological symptoms in COPC populations should be prioritized for future research. Based on a recent systematic review, only one clinical trial has investigated cannabinoids for pelvic pain and no trials of abdominal or visceral pain have been conducted.1
Our study and others found that individuals with chronic pain report improvements in co-morbid psychological symptoms (e.g., anxiety, sleep disturbance,) with cannabinoid use.2, 11 These data have multiple implications for future clinical trials. First, they suggest that trials that include only patients who have chronic pain AND at least one other comorbid psychological symptom could increase the likelihood of identifying a significant treatment effect. Second, using a composite primary outcome that includes both pain and other psychological symptoms could be more likely to yield a significant treatment effect and be more clinically meaningful. The FDA guidance on migraine trials recommends using 2 co-primary outcomes: pain and a second symptom (e.g., nausea, photophobia, phonophobia) that participants identify a priori as most important to them.17 A similar approach could be used for cannabinoid trials that include chronic pain patients with at least one other psychological symptom. The design of such an outcome should consider many issues related to designing and analyzing co-primary or composite primary outcomes (see Gewandter et al. for discussion of pertinent issues18).
Our data suggest that communication between patients and clinicians regarding cannabinoid use for pain is less than optimal (Table 6). Furthermore, when asked why they never tried cannabinoids for pain, 40% of respondents reported that their clinicians never suggested it as an option and 14% reported that their clinicians discouraged cannabinoid use. These data suggest that some clinicians have a negative attitude toward use of cannabinoids for pain. These findings are consistent with prior literature in which physicians often report low perceived knowledge and display limited knowledge about medical cannabinoids,19–21 particularly about appropriate dosages and conditions for which cannabinoids have been found effective.22 Moreover, physicians endorse lack of knowledge as an impediment to discussing cannabinoids with patients (e.g., feeling unprepared to answer patient questions).22,23 Given the current lack of RCT data to support and inform cannabinoid use for pain, especially in the wake of the opioid epidemic for which clinicians who treat chronic pain have at least partly been blamed, clinician hesitancy is understandable. Clinicians may be more comfortable utilizing a medication that is FDA-approved, with dosing that is informed by data from scientifically rigorous clinical trials. Furthermore, the dosing of a FDA-approved product would be more transparent and reliable than many currently available cannabinoid products. Together, these data suggest that investment in high-quality research toward developing FDA-approved cannabinoids for pain could be highly beneficial for increase access to and efficacy of cannabinoid-based medications for many patients. Nevertheless, availability and use of these therapies continues to increase and most patients are informed by non-medical sources, which could lead to worse outcomes. This conclusion is supported by the observation that of those who had a more favorable experience with cannabinoids, as indicated by their current use, more frequently communicated with a clinician regarding cannabinoids.
Finally, our survey suggests that many individuals with chronic pain would be open to participation in clinical trials of a novel cannabinoid. Approximately three-quarters of the respondents who had never tried cannabinoids for chronic pain expressed interest in joining a clinical trial. This estimate may be biased by the fact that respondents could have self-selected based on an interest in cannabinoids, even if they have not yet tried them, and have an interest in research in general. This highly positive response to potential participation in cannabinoid trials suggests that conduct of such trials will be feasible, even in the context of cannabis being legalized in many places.
This study has strengths and limitations. The strengths include the large sample size and recruitment through patient advocacy groups and foundations, allowing us to enroll patients with varying attitudes toward cannabinoids, including those who have never tried them. The limitations include the cross-sectional design and potential recall bias of assessing pain relief and side effects that have occurred in the past. The data are based on self-report and were not confirmed via other methods. Recruitment through listservs that include stakeholders who are not living with chronic pain makes it impossible to calculate a survey response rate. We did not inquire about geographic location to encourage respondents living in places where medical cannabis is illegal to respond truthfully; this limits our ability to assess whether medical cannabis legality in respondents’ locations effected outcomes. Recruitment through listservs of advocacy groups could lead to a selection bias of patients who may be more actively involved in seeking care or managing their health, and generalizability to the entire population of patients needing care for chronic pain maybe limited. Furthermore, the respondents were predominantly white, female, and highly educated, which further limits the generalizability of the data. Inviting people to participate in a survey regarding their experiences with cannabinoids for chronic pain could have led to a sampling bias that overestimates the favorable assessment of cannabinoid effects. However, we tried to minimize this sampling bias and promote responses from individuals who had a range of experiences with cannabinoids by inclusion of the following bolded statement in our invitation email: “If you have chronic pain, no matter how you feel about cannabinoids, we want to hear from you!” Almost half of our sample was individuals who were no longer taking cannabinoids or who had never tried them for pain. Because the questions regarding whether respondents were currently taking, had previously taken, or had never taken cannabinoids was specific to the context of pain, it is possible that respondents classified as having previously or never taken them could have taken cannabinoids for other reasons. However, because the remainder of the survey questions were focused on the effects of these compounds on pain, some misclassification is not likely to have a large effect on the results of the survey.
Finally, we did not collect information regarding cannabinoid dosages because of the high likelihood that such self-report data would not be sufficiently accurate.
Conclusions
These data will be useful to inform future research evaluating cannabinoid efficacy and tolerability in chronic pain patients and should spur educational initiatives for clinicians who treat patients with chronic pain.
Supplementary Material
Respondent-reported cannabinoid-induced changes in pain.
Acknowledgements
We thank the individuals with chronic pain who took the time to answer this survey and the following organizations for helping to distribute the survey invitation: Alliance to Advance Comprehensive Integrative Pain Medicine, American Chronic Pain Association, Chronic Pain Research Alliance, The TMJ Association, US Pain Foundation.
Funding:
GW Pharmaceuticals provided funds to support the conduct of the survey, including consulting fees to. GW Pharmaceuticals did not provide any renumeration for authorship activities. Representatives from GW Pharma did not have any input on the data analyses or writing of the manuscript. The analyses and writing of this manuscript were supported by NIH grant K24NS126861.
Disclosures:
Drs. Freeman, Gewandter, Hill, Edwards, and Wasan received consulting fees from GW to design the survey. Dr. Bernard Le Foll has obtained funding from Pfizer Inc. (GRAND Awards, including salary support) for investigator-initiated projects. Dr Le Foll has obtained funding from Indivior for a clinical trial sponsored by Indivior. Dr. Le Foll has in-kind donations of cannabis products from Aurora Cannabis Enterprises Inc. and study medication donations from Pfizer Inc. (varenicline for smoking cessation) and Bioprojet Pharma. He was also provided a coil for a Transcranial magnetic stimulation (TMS) study from Brainsway. Dr. Le Foll has obtained industry funding from Canopy Growth Corporation (through research grants handled by the Centre for Addiction and Mental Health and the University of Toronto), Bioprojet Pharma, Alcohol Countermeasure Systems (ACS), Alkermes and Universal Ibogaine. He has participated in a session of a National Advisory Board Meeting ( Emerging Trends BUP-XR) for Indivior Canada and has been consultant for Shinogi. He is supported by CAMH, Waypoint Centre for Mental Health Care, a clinician-scientist award from the department of Family and Community Medicine of the University of Toronto and a Chair in Addiction Psychiatry from the department of Psychiatry of University of Toronto. The remaining authors have nothing related to the research to disclose.
References
- 1.Fisher E, Moore RA, Fogarty AE, et al. Cannabinoids, cannabis, and cannabis-based medicine for pain management: a systematic review of randomised controlled trials. Pain 2021;162:S45–S66. [DOI] [PubMed] [Google Scholar]
- 2.Aviram J, Pud D, Gershoni T, et al. Medical cannabis treatment for chronic pain: Outcomes and prediction of response. Eur J Pain 2021;25:359–374. [DOI] [PubMed] [Google Scholar]
- 3.Greis A, Larsen E, Liu C, et al. Perceived Efficacy, Reduced Prescription Drug Use, and Minimal Side Effects of Cannabis in Patients with Chronic Orthopedic Pain. Cannabis and cannabinoid research 2021. Epub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron EP, Lucas P, Eades J, et al. Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. The journal of headache and pain 2018;19:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehnke KF, Litinas E, Clauw DJ. Medical Cannabis Use Is Associated With Decreased Opiate Medication Use in a Retrospective Cross-Sectional Survey of Patients With Chronic Pain. The journal of Pain 2016;17:739–744. [DOI] [PubMed] [Google Scholar]
- 6.Campbell G, Hall WD, Peacock A, et al. Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: findings from a 4-year prospective cohort study. The Lancet Public Health 2018;3:e341–e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noori A, Miroshnychenko A, Shergill Y, et al. Opioid-sparing effects of medical cannabis or cannabinoids for chronic pain: a systematic review and meta-analysis of randomised and observational studies. BMJ Open 2021;11:e047717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Foll B. Opioid-sparing effects of cannabinoids: Myth or reality? Progress in Neuro-Psychopharmacology & Biological Psychiatry 2021;106:110065. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen S, Picco L, Murnion B, et al. Opioid-sparing effect of cannabinoids for analgesia: an updated systematic review and meta-analysis of preclinical and clinical studies. Neuropsychopharmacology 2022;47:1315–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikulik M. Medical marijuana in the U.S. - Statistics & Facts. [Accessed 10-17-2022] https://www.statista.com/topics/3064/medical-marijuana-in-the-us/#topicHeader__wrapper.
- 11.Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: Systematic review and meta-analysis. Social Science & Medicine 2019;233:181–192. [DOI] [PubMed] [Google Scholar]
- 12.Gedin F, Blomé S, Pontén M, et al. Placebo Response and Media Attention in Randomized Clinical Trials Assessing Cannabis-Based Therapies for Pain: A Systematic Review and Meta-analysis. JAMA Netw Open 2022;5:e2243848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill KP, Palastro MD, Johnson B, et al. Cannabis and Pain: A Clinical Review. Cannabis and Cannabinoid Research 2017;2:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fillingim RB, Ohrbach R, Greenspan JD, et al. Associations of Psychologic Factors with Multiple Chronic Overlapping Pain Conditions. J Oral Facial Pain Headache 2020;34:s85–s100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veasley C, Clare D, Clauw DJ, et al. Impact of chronic overlapping pain conditions on public health and the urgent need for safe and effective treatment: 2015 analysis and policy recommendations. [Accessed 10-17-2022] http://www.chronicpainresearch.org/public/CPRA_WhitePaper_2015-FINAL-Digital.pdf.
- 16.Ohrbach R, Sharma S, Fillingim RB, et al. Clinical Characteristics of Pain Among Five Chronic Overlapping Pain Conditions. J Oral Facial Pain Headache 2020;34:s29–s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. Migraine: Developing Drugs for AcuteTreatment Guidance for Industry. 2018; Accessed 10-17-2022 [https://www.fda.gov/media/89829/download].
- 18.Gewandter J, McDermott M, Evans S, et al. Composite outcomes for pain trials: Considerations for design and interpretation. Pain 2021;162:1899–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruger DJ, Mokbel MA, Clauw DJ, et al. Assessing Health Care Providers’ Knowledge of Medical Cannabis. Cannabis and Cannabinoid Research 2022;4:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardiner KM, Singleton JA, Sheridan J, et al. Health professional beliefs, knowledge, and concerns surrounding medicinal cannabis - A systematic review. PloS One 2019;14:e0216556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hordowicz MJ, Jarosz J, Klimkiewicz A, et al. To Treat or Not to Treat? Polish Physicians’ Opinions about the Clinical Aspects of Cannabinoids-An Online Survey. Journal of Clinical Medicine 2022;11, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philpot LM, Ebbert JO, Hurt RT. A survey of the attitudes, beliefs and knowledge about medical cannabis among primary care providers. BMC Family Practice 2019;20:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun IM, Wright A, Peteet J, et al. Medical Oncologists’ Beliefs, Practices, and Knowledge Regarding Marijuana Used Therapeutically: A Nationally Representative Survey Study. JCO 2018;36:1957–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Respondent-reported cannabinoid-induced changes in pain.