Abstract
Monocytes can differentiate into tissue-resident pleural macrophages, but the mechanisms underlying this process are not yet fully understood. In this issue of Immunity, Finlay et al.1 show that Th2 cytokines promote this differentiation in resistant mice infected with Litomosoides sigmodontis.
In parallel to the T helper 1 (Th1), T helper 2 (Th2) cell paradigm,2 in vitro cytokine stimulation of monocytes can result in their differentiation into different macrophage subsets. Specifically, exposure to the Th1 cell cytokine interferon-γ (IFN-γ) leads to differentiation into classically activated M1-like macrophages, while the Th2 cell cytokines interleukin-4 (IL-4) and IL-13 induce differentiation into alternatively activated M2-like macrophages. In vivo, macrophages are broadly classified as either tissue-resident or recruited macrophages. Tissue-resident macrophages are terminally differentiated cells with a relatively stable transcriptional profile.3 Certain types of tissue-resident macrophages are found exclusively in one organ, such as alveolar macrophages (airways), Kupffer cells (liver), Langerhans cells (skin), and peritoneal macrophages, and they exhibit unique transcriptional signatures due to the specific environment they occupy.3 In contrast, another type of tissue-resident macrophages, interstitial macrophages, are transcriptionally conserved across most organs.4 While macrophages in vivo may exhibit some aspects of an M1-like or M2-like phenotype, showing proinflammatory or resolving functions, respectively, M1-like and M2-like macrophages generally arise from recruited monocytes that enter the environment as M0-like cells during inflammation, such as that caused by Litomosoides sigmodontis infection. These monocytes can differentiate into various cell types, including tissue-resident macrophages, recruited macrophages, or migratory monocyte-derived dendritic cells.1,5 Studies have shown that recruited monocytes acquire residency during the transition from acute to chronic infection, which coincides with a shift from an M1-like to an M2-like phenotype, thereby safeguarding against overwhelming and uncontrolled inflammation.6 This finding is further supported by the current study by Finlay et al.1
In their study of comparative infection in resistant C57BL/6 and susceptible BALB/c mice with the filarial parasitic nematode L. sigmodontis, Finlay and colleagues demonstrate that protection from L. sigmodontis in the resistant strain is mediated in part by the ability of C57BL/6 mice to develop mature Th2 cell responses to infection. In contrast, susceptible BALB/c mice develop a more suppressive immune response to the nematode.1,7 Mature Th2 cell responses in the C57BL/6 mice were found to support the differentiation of recruited monocytes into protective, terminally differentiated, IL-4- and IL-4Rα-dependent, tissue-resident large-cavity macrophages (LCMs), referred to as M(IL-4) LCMs, while monocytes from BALB/c mice were arrested at an intermediate, converting cavity macrophage (CCM) non-protective phenotypic state (Figure 1).1 These findings help explain the differential outcome of infection between the two mouse strains.
Figure 1. Uncovering the mechanism behind Litomosoides sigmodontis resistance: Type 2 cytokine-induced pleural macrophages and the transition from monocytes to M(IL-4) LCMs in C57BL/6 mice.
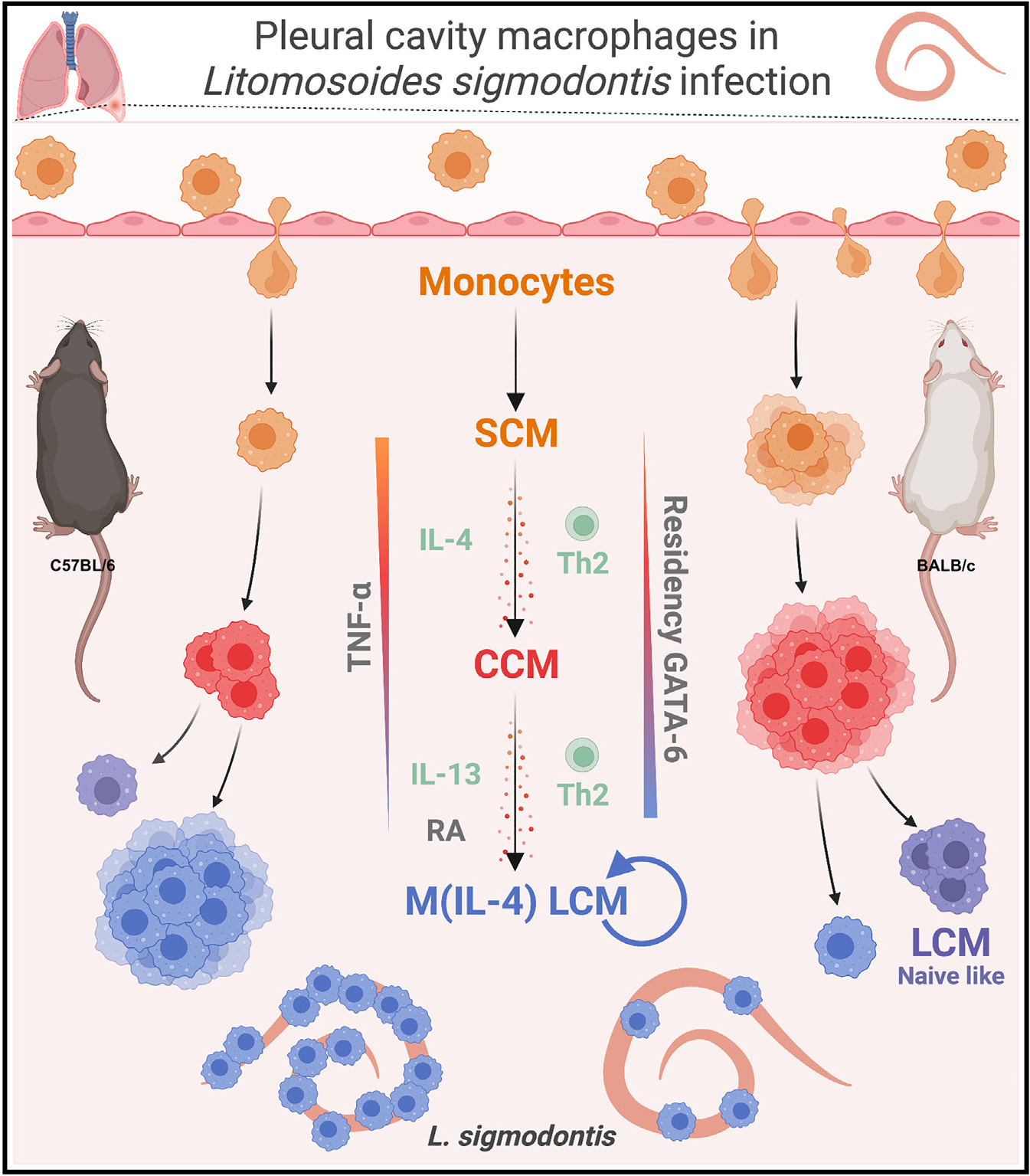
Small-cavity macrophages (SCMs); converting cavity macrophages (CCMs); large-cavity macrophages (LCMs); IL-4Rα-dependent large-cavity macrophages (M[IL-4] LCMs); type 2 helper T cells (Th2); retinoic acid (RA). Created with BioRender.com.
Using mass cytometry, the study reveals intrinsic differences in immune cell abundance between L. sigmodontis-resistant C57BL/6 and susceptible BALB/c mice following infection. They observed a discrepancy in CD11b+ mononuclear phagocytes (MNPs), with a greater expansion in C57BL/6 mice compared with BALB/c mice after infection. Flow cytometry identified four distinct subpopulations of CD11b+ MNPs including recruited Ly6Chi monocytes, MHCIIhiF4/80lo small-cavity macrophages (SCMs), PD-L2+ CD206+FRβ+Lyve1+ CCMs, and CD102+ CD73+F4/80hi LCMs. After L. sigmodontis infection, C57BL/6 mice showed a higher proportion of LCMs, whereas BALB/c mice exhibited stronger recruitment of monocytes and more CCMs. The high ratio of LCMs with other MNPs was negatively correlated with worm recovery, revealing the underlying mechanism for the susceptibility observed in BALB/c mice (i.e., fewer LCMs).
To comprehensively investigate the heterogeneity and dynamics of different MNPs during nematode infection, single-cell RNA sequencing (scRNA-seq) was conducted. RNA velocity analyses revealed two distinct routes of monocyte differentiation: (1) from monocyte-like SCMs to DC-like SCMs and (2) from monocyte-like SCMs to CCMs, and then LCMs, with an increased dependence on the transcription factor GATA6 (Figure 1). However, in C57BL/6 and BALB/c mice, the L. sigmodontis infection resulted in differential transcriptional profiles of LCMs. In L. sigmodontis-resistant C57BL/6 mice, pathway analysis predicted signal transducing activator of transcription-6 (STAT6) and IL-4 as upstream regulators in post-infection M(IL-4) LCMs compared with naive LCMs. Moreover, the post-infection CCM population was predicted to be an intermediate between monocyte-like cells and M(IL-4) LCMs. However, in infected BALB/c mice, these CCMs accumulated and only a small fraction differentiated, with those differentiating more into the naive-like LCMs instead of the L. sigmodontis-protective M(IL-4) LCM phenotype (Figure 1), supporting the concept that tissue-resident macrophage differentiation influences the susceptibility of different mouse strains to nematode infection.
Through CellRank analysis, the distinct differentiation patterns of MNPs in the pleural cavity during L. sigmodontis infection were evaluated. Monocyte-like SCMs in infected C57BL/6 mice had a higher probability of differentiating into M(IL-4) LCMs, whereas those in BALB/c mice were more likely to differentiate into dendritic cell (DC)-like cells and naive-like LCMs. In addition, adoptive transfer of congenic monocytes showed their different conversion capability in both infected BALB/c and C57BL/6 mice. Specifically, BALB/c mice exhibited an accumulation of CCMs, while C57BL/6 mice showed an accumulation of LCMs.
To investigate differences in monocyte-macrophage residency between strains, bone marrow chimera mice were generated using BALB/c and B10.D2 mice. The latter shares the H2d haplotype with BALB/c mice and has a resistance phenotype to L. sigmodontis, similar to C57BL/6 mice. Various chimeras were examined, leading to the conclusion that hematopoietic cells play a primary role in the abundance of LCMs. This abundance was accompanied by the expansion of Th2 cells, suggesting their role in regulating LCM residence. The importance of Th2 cells was supported by studies using a T cell and conventional DC 2-deficient mouse strain, as well as tools to deplete T cells (anti-CD4 antibody) and inhibit lymphocyte migration. After illustrating the importance of Th2 cells, the involvement of type 2 cytokines in the conversion process of LCMs was examined using Il13eGFP (IL-13-deficient) mice and Il4ra−/− (receptor-subunit deficient for both IL-4 and IL-13) mice in a C57BL/6 background. Both mouse strains exhibited varying degrees of stalled monocyte-to-LCM transition, indicating distinct stages at which different Th2 cell cytokines impact the process. Specifically, IL-13 predominantly affected the CCM-to-LCM transition, while IL-4 affected the monocyte-CCM transition, resulting in fewer CCMs and LCMs in the Il4ra−/− compared with control mice, illustrating a functional difference between IL-4 and IL-13 during the pleural cavity macrophage residency.
Overall, Finlay et al.1 have uncovered an additional role for type 2 cytokines in regulating pleural macrophage residency, extending beyond previous knowledge that type 2 cytokines promote the proliferation of reparative functions of macrophage. Interestingly, the CCM population described in the study shares signature genes with CD206hi interstitial macrophages found in the lung interstitium, airspace, and pleural membrane,4,8,9 which also express IL-4R, indicating their responsiveness to type 2 cytokines. It would be intriguing to investigate whether the CCM population is a heterogeneous population that contains bona fide interstitial macrophages recruited from the pleural membrane into the pleural space upon infection and provides a distinct functional role in the environment. Additionally, it remains unclear whether interstitial macrophages differentiate into LCMs or other tissue-specific macrophages, or whether monocytes naturally express interstitial macrophage signature genes as they differentiate into tissue-specific macrophages while migrating from the bloodstream through the interstitium and into the luminal space, as previously observed.10 As stated in the study, a lineage-tracing mouse is required to conclusively determine the extent of the contribution of monocyte conversion to LCMs versus local proliferation of tissue-resident macrophages. Determining whether the type 2 cytokines are equally required for other tissue-resident macrophage populations will also be interesting. Furthermore, the next investigation steps should examine the long-term effects of recruited LCMs on subsequent encounters with the same or different pathogens, as recruited experienced macrophages possess distinct transcriptional and functional profiles compared with the original macrophages (e.g., M[IL-4] LCM versus naive LCM). Studies in the lung have shown that recruited alveolar macrophages may have either beneficial or detrimental effects during subsequent infections compared with the original resident alveolar macrophages,3 highlighting the importance of environmental factors in shaping the function of resident macrophages and their response to infections. In summary, this study provides an elegant explanation for the apparent nematode susceptibility paradox between C57BL/6 and BALB/c mice by showing that Th2 cell cytokines not only induce M2-like macrophages and their proliferation, but also help establish their homecoming in the tissues.
ACKNOWLEDGMENTS
This preview was supported by CVJ NIH grant R35 HL155458.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Finlay CM, Parkinson JE, Zhang L, Chan BH, Ajendra J, Chenery A, Morrison A, Kaymak I, Houlder EL, Murtuza Baker S, et al. (2023). T helper 2 cells control monocyte to tissue-resident macrophage differentiation during nematode infection of the pleural cavity. Immunity 56, 1064–1081. 10.1016/j.immuni.2023.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang S, and Dong C (2013). A complex issue on CD4(+) T-cell subsets. Immunol. Rev. 252, 5–11. 10.1111/imr.12041. [DOI] [PubMed] [Google Scholar]
- 3.Aegerter H, Lambrecht BN, and Jakubzick CV (2022). Biology of lung macrophages in health and disease. Immunity 55, 1564–1580. 10.1016/j.immuni.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibbings SL, Thomas SM, Atif SM, McCubbrey AL, Desch AN, Danhorn T, Leach SM, Bratton DL, Henson PM, Janssen WJ, and Jakubzick CV (2017). Three Unique Interstitial Macrophages in the Murine Lung at Steady State. Am. J. Respir. Cell Mol. Biol. 57, 66–76. 10.1165/rcmb.2016-0361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawat K, Tewari A, Li X, Mara AB, King WT, Gibbings SL, Nnam CF, Kolling FW, Lambrecht BN, and Jakubzick CV (2023). CCL5-producing migratory dendritic cells guide CCR5+ monocytes into the draining lymph nodes. J. Exp. Med. 220, e20222129. 10.1084/jem.20222129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sica A, and Mantovani A (2012). Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795. 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finlay CM, and Allen JE (2020). The immune response of inbred laboratory mice to Litomosoides sigmodontis: A route to discovery in myeloid cell biology. Parasite Immunol. 42, e12708. 10.1111/pim.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore PK, Anderson KC, McManus SA, Tu TH, King EM, Mould KJ, Redente EF, Henson PM, Janssen WJ, and McCubbrey AL (2023). Single-Cell RNA Sequencing Reveals Unique Monocyte-derived Interstitial Macrophage Subsets during Lipopolysaccharide-Induced Acute Lung Inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 324, L536–L549. 10.1152/ajplung.00223.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Kolling FW, Aridgides D, Mellinger D, Ashare A, and Jakubzick CV (2022). ScRNA-seq expression of IFI27 and APOC2 identifies four alveolar macrophage superclusters in healthy BALF. Life Sci. Alliance 5, e202201458. 10.26508/lsa.202201458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbings SL, Goyal R, Desch AN, Leach SM, Prabagar M, Atif SM, Bratton DL, Janssen W, and Jakubzick CV (2015). Transcriptome analysis highlights the conserved difference between embryonic and postnatal-derived alveolar macrophages. Blood 126, 1357–1366. 10.1182/blood-2015-01-624809. [DOI] [PMC free article] [PubMed] [Google Scholar]


