Abstract
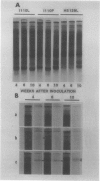
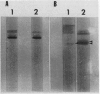
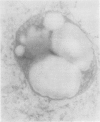
Polyacrylamide gel electrophoresis and electron microscopy revealed that accumulation of iron-protein in soybean nodules is influenced by nodule age, mutation in bradyrhizobia, and rhizobial/bradyrhizobial strain-soybean cultivar interactions. Iron-protein concentrations (micrograms per milligram protein) were inversely related to heme concentrations (nanomoles per milligram protein), with correlation coefficients (r values) ranging from −0.98 in young nodules to −0.83 in mature ones. Bradyrhizobium japonicum symbiotic mutants HS 129 and HS 145 (Nod+ Fix−) produced nodules high in iron-protein. Electrophoresis of homogenate prepared from nodules on Lee 68 produced by B. japonicum HS 129 yielded two different forms of the iron-proteins, 570 and 600 kilodaltons. The 570 kilodalton iron-protein isolated by preparative polyacrylamide gel electrophoresis behaved like horse-spleen-ferritin in responses to iron-stains, heat stability, ultraviolet absorption spectrum, iron unloading and reloading, and characteristic appearance in electron micrographs. These properties led to the conclusion that the 570 kilodalton iron-protein is phytoferritin. The nodule phytoferritin differed from horse-spleen-ferritin in electrophoretic mobility, serological properties, and molecular size and was distinct from most other known phytoferritins in that it was composed of different subunit types.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Bergmann H., Preddie E., Verma D. P. Nodulin-35: a subunit of specific uricase (uricase II) induced and localized in the uninfected cells of soybean nodules. EMBO J. 1983;2(12):2333–2339. doi: 10.1002/j.1460-2075.1983.tb01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Theil E. C. Red cells, ferritin, and iron storage during amphibian development. J Biol Chem. 1978 Apr 25;253(8):2673–2678. [PubMed] [Google Scholar]
- Crichton R. R., Ponce-Ortiz Y., Koch M. H., Parfait R., Stuhrmann H. B. Isolation and characterization of phytoferritin from pea (Pisum sativum) and Lentil (Lens esculenta). Biochem J. 1978 May 1;171(2):349–356. doi: 10.1042/bj1710349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass J., Nunez M. T., Fischer S., Robinson S. H. Transferrin receptors, iron transport and ferritin metabolism in Friend erythroleukemia cells. Biochim Biophys Acta. 1978 Aug 3;542(1):154–162. doi: 10.1016/0304-4165(78)90241-6. [DOI] [PubMed] [Google Scholar]
- Keyser H. H., Bohlool B. B., Hu T. S., Weber D. F. Fast-growing rhizobia isolated from root nodules of soybean. Science. 1982 Mar 26;215(4540):1631–1632. doi: 10.1126/science.215.4540.1631. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- MAZUR A., CARLETON A. Relation of ferritin iron to heme synthesis in marrow and reticulocytes. J Biol Chem. 1963 May;238:1817–1824. [PubMed] [Google Scholar]
- Macara I. G., Hoy T. G., Harrison P. M. The formation of ferritin from apoferritin. Catalytic action of apoferritin. Biochem J. 1973 Oct;135(2):343–348. doi: 10.1042/bj1350343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N., Linder M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol Rev. 1978 Apr;58(2):317–396. doi: 10.1152/physrev.1978.58.2.317. [DOI] [PubMed] [Google Scholar]
- Noel K. D., Stacey G., Tandon S. R., Silver L. E., Brill W. J. Rhizobium japonicum mutants defective in symbiotic nitrogen fixation. J Bacteriol. 1982 Oct;152(1):485–494. doi: 10.1128/jb.152.1.485-494.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, function, and regulation. Adv Inorg Biochem. 1983;5:1–38. [PubMed] [Google Scholar]
- Theil E. C. Red cell ferritin content during the hemoglobin transition of amphibian metamorphosis. Dev Biol. 1973 Oct;34(2):282–288. doi: 10.1016/0012-1606(73)90357-6. [DOI] [PubMed] [Google Scholar]
- Ulvik R., Romslo I. Studies on the utilization of ferritin iron in the ferrochelatase reaction of isolated rat liver mitochondria. Biochim Biophys Acta. 1978 Jun 15;541(2):251–262. doi: 10.1016/0304-4165(78)90398-7. [DOI] [PubMed] [Google Scholar]