Abstract
New metrics for assessing glycemic control beyond HbA1c have recently emerged due to the increasing use of continuous glucose monitoring (CGM) in diabetes clinical practice. Among them, time in range (TIR) has appeared as a simple and intuitive metric that correlates inversely with HbA1c and has also been newly linked to the risk of long-term diabetes complications. The International Consensus on Time in Range established a series of target glucose ranges (TIR, time below range and time above range) and recommendations for time spent within these ranges for different diabetes populations. These parameters should be evaluated together with the ambulatory glucose profile (AGP). Using standardized visual reporting may help people with diabetes and healthcare professionals in the evaluation of glucose control in frequent clinical situations. The objective of the present review is to provide practical insights to quick interpretation of patient-centered metrics based on flash glucose monitoring data, as well as showing some visual examples of common clinical situations and giving practical recommendations for their management.
Keywords: continuous glucose monitoring, flash glucose monitoring, time in range, hypoglycemia, glycemic variability, ambulatory glucose profile
Introduction
Glycated hemoglobin (HbA1c) has been considered the gold standard for assessing glycemic control in people with diabetes (PWD), at least since the landmark Diabetes Control and Complications Trial (DCCT) was published. 1 However, the systematic use of HbA1c in the diabetes clinic has some limitations. The influence of nonglycemic factors (eg, anemia, kidney disease, and any condition associated with variability in red-blood cell glycation kinetics), 2 the lack of data on hypoglycemic and hyperglycemic events, and no information on glycemic variability make HbA1c inadequate to help in improving glycemic control. Alternatively, continuous glucose monitoring (CGM) using a glucose sensor, which measures glucose in the interstitial fluid, generates large amounts of evaluable data and new metrics to assess short-term glycemic control and glycemic variability. Therefore, CGM has emerged as a powerful tool in helping people with diabetes achieve better glucose control. CGM systems are currently available in two categories: real-time CGM (rtCGM) and intermittently scanned CGM (isCGM), also called flash glucose monitoring (FLASH). rtCGM systems measure glucose every few minutes and actively transmit data wirelessly from the sensor to a reader or smartphone app. These devices include alarms for hypoglycemia and hyperglycemia, rapid rates of glycemic change, and predicted hypoglycemia and hyperglycemia. Smart devices send data to the cloud so that followers (parents, caregivers, etc.) can track glycemic trends in real time on their smart device remotely. FLASH systems collect measurements each minute and transmit data only when the user scans the sensor with a reader or smartphone app. FreeStyle Libre® 2 include alarms for hypo- and hyperglycemia.
Amongst the different parameters derived from the systematic analysis of rtCGM and FLASH data, time in range (TIR) is considered a major metric in the evaluation of glycemic control. 3 TIR is defined as the percentage of time that a person can maintain their glucose levels within the established target glucose range. It provides information on the level of glucose exposure and also, to some extent, on glycemic variability. TIR provides adequate information on short-term changes in glucose levels, resulting in a simple and intuitive parameter for glucose management by healthcare professionals (HCPs) and PWD. 4 TIR correlates inversely with HbA1c: a 50% TIR corresponds to a HbA1c of approximately 7.9%, and a 70% TIR corresponds to a HbA1c of 7%. 5 That is, each variation of 10% in TIR corresponds to a change in HbA1c of ~0.5%. However, it should be considered that for a given TIR percentage, there is a wide range of possible HbA1c levels. 5 Importantly, there is increasing evidence to link lower TIR values with microvascular and macrovascular complications.6-8
The most recent International Consensus 9 on the management of hyperglycemia in diabetes recommends using additional parameters besides HbA1c to evaluate glycemic control. In addition to TIR, at least one indicator of the frequency and duration of hypoglycemia should be included (time below range, TBR) and a measure of glycemic variability (coefficient of variation, CV), which is the standard deviation divided by arithmetic mean glucose. 9 These parameters should be evaluated together with the ambulatory glucose profile (AGP) to obtain a complete picture of the glycemic control for each person with diabetes and then be used as a key element for clinical decision-making.10,11
In 2019, the International Consensus on Time in Range 9 recommended several glycemic control targets for different clinical situations. These recommendations include adults and children with type 1 diabetes mellitus (T1D) and adults with type 2 diabetes (T2D). Additionally, distinct recommendations were included for people with T1D or T2D at increased risk of hypoglycemia or with frailty, and also for targets during pregnancy. The objective of this manuscript, which has been developed by a group of Spanish expert diabetologists, is to help clinicians interested in the implementation of this consensus in clinical practice through a compendium of practical ideas based on flash glucose monitoring data.
Defining Objectives of Glycemic Control According To Patient Profiles
Objectives of Glycemic Control in People With T1D
T1D without complications
The general goal for people with T1D (PWT1D) without additional complications is to try to achieve a TIR (70-180 mg/dL) higher than 70%, with a time in hyperglycemia (TAR) level 1 (>180 mg/dL) lower than 25% and level 2 (>250 mg/dL) lower than 5%. Targets for time below range (TBR) in hypoglycemia are for TBR level 1 (<70 mg/dL) lower than 4% and TBR level 2 (<54 mg/dL) lower than 1%. 9 Achieving a TIR percentage higher than 70% is equivalent to reach an HbA1c target of ~7%, as has been proposed by the American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) (Figure 1).9,11 Recent studies indicates a correlation between TIR and microvascular complications. In a post hoc analysis from the DCCT, TIR was derived from 7-point self-monitoring of blood glucose (SMBG) and it was found that every 10% decrease in TIR was associated with a 64% increase in the risk of retinopathy progression and a 40% increase in microalbuminuria. 6
Figure 1.
Glycemic control objectives in T1D and T2D according to patient profiles. Consensus recommendations for time in range (TIR), time below range (TBR) and time above range (TAR) for: (a) adults with T1D or T2D: (b) older adults or people at high-risk of hypoglycemia because of age, duration of diabetes, duration of insulin therapy or impaired awareness of hypoglycemia (IAH); (c) children/adolescents; (d) TIR in pregnancy. Recommendations are based on limited evidence. (e) No consensus recommendations for TIR in pregnancy in T2D or in gestational diabetes are available.
Newly diagnosed T1D (<5 years history)
Newly diagnosed PWT1D have higher residual pancreatic beta-cell function and usually show lower glycemic variability and a lower risk of hypoglycemia.12,13 Furthermore, tight glycemic control from disease initial diagnosis has been associated with greater preservation of pancreatic beta-cell function. 14 On the basis of these data, stricter targets at T1D onset may be useful, provided they are not associated to advanced age or the presence of serious comorbidities (advanced chronic renal disease, cardiovascular disease, osteoporosis and/or fractures, cancer or nonstabilized psychiatric pathology). In the absence of supporting evidence, we suggest that a TIR (70-180 mg/dL) higher than 80%, if possible, without increasing the risk of hypoglycemia (increased TBR), may be recommended in newly diagnosed T1D.
T1D in older people or those with associated severe comorbidities
For older or high-risk people with T1D (those with complications, comorbidities, or cognitive deficits), the objective for TIR (70-180 mg/dL) should be higher than 50%, with a priority on the prevention of hypoglycemia episodes with a level 1 TBR (<70 mg/dL) target of lower than 1%. There is also a higher tolerance to hyperglycemia with a TAR level 2 (>250 mg/dL) lower than 10% instead of lower than 5%.4,9
The recommended time in range targets in T1D according to time of diagnosis, age, and the presence of comorbidities are shown in Table 1.
Table 1.
Recommended Goals for TIR, TBR, and TAR in T1D.
| T1D | TIR | % | TBR | % | TAR | % |
|---|---|---|---|---|---|---|
| >5 years duration | 70-180 mg/dL | >70 | < 70 mg/dL < 54 mg/dL |
< 4 < 1 |
>180 mg/dL >250 mg/dL |
< 25 < 5 |
| < 5 years duration | 70-180 mg/dL | >80 a | < 70 mg/dL < 54 mg/dL |
< 4 < 1 |
>180 mg/dL >250 mg/dL |
< 15
a
< 5 |
| Older and/or comorbidities | 70-180mg/dL | >50 | < 70 mg/dL | < 1 | >180 mg/dL >250 mg/dL |
< 50 < 10 |
Abbreviations: TIR, time in range; TBR, time below range; TAR, time above range; T1D, Type 1 diabetes.
If possible.
Objectives of Glycemic Control in People With T2D
Scientific evidence supporting the use of CGM systems in T2D is less extensive than in T1D. However, recent clinical trials and real-life studies have shown the potential value of CGM systems in insulin-treated people with T2D (PWT2D). The DIAMOND study, which involved PWT2D on multiple daily injections (MDI) of insulin, showed a reduction of HbA1c of 0.3% with rtCGM compared with a control group using SMBG, without increasing hypoglycemia. 15 HbA1c reduction was greater in subjects with higher HbA1c at baseline (1.4% in the CGM group vs 0.7% in the control group in those with HbA1c >9%). 16 In the REPLACE study, 17 again on PWT2D on MDI, those using FLASH showed a 43% and 53% reduction in TBR, lower than 70 mg/dL and lower than 54 mg/dL, respectively, compared with SMBG. No significant change in HbA1c was evident in the total study population, but there was a reduction in a prespecified subgroup of patients younger than 65 years (–0.53% ± 0.09% vs −0.20% ± 0.12%, P=.0301). 17 In line with these results, a meta-analysis of observational studies that included 363 subjects with T2D on MDI found a mean reduction in HbA1c of −0.9% from baseline at 3 to 6 months after the onset of FLASH. 18 In addition, in PWT2D on basal-insulin therapy, using of CGM systems was associated with reductions in HbA1c.19,20 Finally, recent studies have confirmed the cost-effectiveness of FLASH in T2D, especially in those patients on complex insulin therapy regimens. 21
Despite this growing evidence in T2D, there are no specific recommendations for glycemic variability targets in T2D, and the recommendations for T1D 9 are often adapted in T2D. However, as the risk of hypoglycemia and glycemic variability is often lower in T2D, stricter glycemic control may be reachable in this group of people (Figure 1a). 22
T2D in elderly or frail patients
In contrast to the general T2D population, hypoglycemia risk, impaired awareness of hypoglycemia, and complications from insulin therapy are higher in older PWT2D and in those at higher risk of vulnerability (eg, after a recent hospitalization or patients with sarcopenia, poor social support, comorbidity, or polypharmacy). 23 In these patients, more-conservative targets for glycemic control should be adopted and treatments with a lower risk of hypoglycemia should be selected. The main priority will be the reduction of TBR level 1 (<70 mg/dL) to lower than 1% (<15 minutes/day) (Figure 1b). 9
The need for supporting care in this subgroup of people makes CGM especially useful, due to the availability of low-glucose and high-glucose alarms and remote glucose monitoring by the caregiver and/or health care professional. However, more specific and targeted studies in this population are needed.
Glycemic Control Objectives in Pediatric Population
In children and adolescents, T1D has distinctive characteristics in each age range in relation both by specific physiological demands of each period and by psychosocial aspects.
Children under the age of 6 are characterized by a marked sensitivity to insulin. Generally, at this age, they have a very pronounced sunset phenomenon (with greater insulin needs in the late afternoon and early evening), a characteristic anti-dawn phenomenon (decreased insulin needs at dawn) and a greater sensitivity to insulin in the second part of the morning.24-26 The circadian profile of insulin requirements in children older than 6 years is typically similar to those of young children, although sensitivity and time variations are usually less pronounced. In contrast, adolescence is characterized by a very marked increase in insulin resistance and a pronounced dawn phenomenon (Figure 2).25,26
Figure 2.
Variable insulin needs throughout the day according to age range in pediatric population. The graph shows the daily insulin sensitivity profiles for children and adolescents of different age groups, as well as for adult subjects. Data are represented as % of total daily basal-rate insulin requirements per hour, across a 24-hour period.
In Spain, children, adolescents, and pregnant women with T1D were the first patient groups that received public funding for FLASH since 2018. In the pediatric population, currently both the International Society for Pediatric and Adolescent Diabetes (ISPAD) and the ADA recommend that the glycemic control in these patients should be similar to those for adults with T1D.27,28 Then, hyperglycemia at younger age may have a significant impact on the subsequent development of microvascular complications due to the metabolic memory. 29 Therefore, the recommended targets for TIR, TBR, and TAR in this age group are a TIR target (70-180 mg/dL) of higher than 70% and an overall TBR target of lower than 4% with lower than 4% of readings at TBR level 1 (<70 mg/dL) and lower than 1% of readings at TBR level 2 (<54 mg/dL) 9 (Figure 1c). For PWD under 25 years whose HbA1c goal is 7.5%, TIR target can be set to approximately 60%. 9
To date, few studies report on the achievement of TIR targets in pediatrics, suggesting that the degree of subjects meeting the TIR target higher than 70% is significantly low at this age. 30 An inverse correlation has been described between HbA1c and TIR and between HbA1c and TBR in children and adolescents. 31 There are no longitudinal studies analyzing higher TBR and potential cognitive dysfunction in the pediatric population.
Different observational, real-world studies have shown an association between the use of FLASH and an increase in TIR in the pediatric population. 32 In randomized controlled trials and real-life studies, the increase in the number of daily sensor scans has been correlated with a higher percentage of TIR in children and adolescents.33,34 In a nonrandomized study that evaluated the use of FLASH in children, adolescents, and adults, the group aged <19 years showed a higher percentage of TIR, lower glycemic variability, and greater device use than the older group. 35
An increase in TIR has also been described in adolescents and young adults with T1D with the use of rtCGM compared with SMBG,36,37 without increasing TBR 36 or with a reduction in TBR. 37 In very young children with T1D (2 to < 8 years of age), a randomized clinical trial that compared the use of rtCGM with SMBG has shown a reduction in TBR, without changes in TIR. 38
Objectives of Glycemic Control in Diabetes and Pregnancy
The relationship between ambient maternal glucose levels and obstetric or neonatal outcomes is well known. Thus, in pregnant women with T1D or T2D, the risk of maternal fetal complications is greater than in pregnant women with gestational diabetes (GDM). 39
The reliability of CGM systems is not altered by pregnancy.40,41 In pregnant women with diabetes, a target range of 63 to 140 mg/dL is recommended. For those women using the system for at least 14 days consecutive days and having captured more than 70 % of data, it is recommended a TIR (63-140 mg/dL) higher than 70%, a TBR < 63 mg/dL lower than 4% and TBR < 54 mg/dL lower than 1%. TAR > 140 mg/dL should be lower than 25% in pregnant women (Figure 1d and 1e). 9
T1D in pregnancy
Achieving goals in pregnant women with T1D is more difficult than in other types of diabetes, because they have greater fluctuations in glucose levels. Most studies with CGM have focused on pregnant women with pregestational T1D. In 2019, Kristensen et al showed that women who delivered children large for gestational age had lower TIR levels and higher TAR levels in second and third trimester of gestation, reflecting a potential relationship of TIR with perinatal outcomes. 42 The CONCEPTT study in pregnant women with T1D, performed using rtCGM, showed that a decrease in HbA1c and an increase in TIR was associated with a lower rate of neonatal events such as hypoglycemia, admission to the neonatal intensive care unit or higher birth weight. 43 However, in both studies,42,43 TIR target was only achieved by a small percentage of pregnant women, indicating that still much work remains to be done to improve glycemic control during pregnancy. However, it is important to explain to those women that even small improvements in TIR are important for fetal well-being, both in the preconception period and during pregnancy. 44 Therefore, it is important to incorporate the concept of TIR into educational diabetes programs to increase engagement of women in achieving pregnancy-related glycemic goals.
T2D in pregnancy
To date, there are no specific randomized studies in pregnant women with T2D. Murphy 44 and Secher et al 45 included subgroups of PWT2D in their studies (25/71 and 31/155, respectively). It should be noted that these women use to be able to achieve TIR of up to 90% with less intensive therapies.
Gestational diabetes
A recent meta-analysis that evaluates the effect of CGM in GDM has shown lower HbA1c at the end of pregnancy with the use of CGM compared with SMBG. 46 In most of the studies, glucose monitoring in GDM has been performed using blind glucose monitoring.47-49 As pregnant women do not see their daily glucose readings, these results cannot be extrapolated to rtCGM or FLASH. It is important to note that, in these studies, TIR values for these patients are generally above 80%.
More data are needed to define the clinical CGM targets for pregnant women with GDM and T2D. There are several studies ongoing looking at CGM use in GDM. Because of the lack of evidence, percentages of time spent in range, below range, and above range for pregnant women with T2D or GDM were not included in the International Consensus on Time in Range. 9 However, until we have more data, considering that achieving a higher TIR may be relatively easy, we suggest a TIR (63-140 mg/dL) higher than 90%, which corresponds with a HbA1C ~ < 6%, for pregnant women with GDM or T2D with less intensive therapies. For pregnant women with T2D on multiple daily insulin injections, a TIR target (63-140 mg/dL) higher than 70% may be recommended (Figure 1e).
CGM in Hospitalized Patients
Hyperglycemia occurs in one-third of hospitalized patients, reaching up to 70% of patients in critical care units. 50 It is associated with higher mortality, greater in-hospital complications, longer stay, and increased frequency of readmissions. 50 However, the incidence of hypoglycemia has been reported to range from 0.5% to 32.8% of hospitalized patients. Hypoglycemic events are associated with prolonged hospital length of stay, as well as an increased risk for adverse events including mortality.51,52 Therefore, it is important to identify hyperglycemia in this setting and to treat it, as well as to avoid hypoglycemia. The current recommended target range for in-hospital treated patients is 140 to 180 mg/dL,53,54 but in some patients may be accepted a stricter target of 110 to 140 mg/dL provided that this does not significantly increase the risk of hypoglycemia.54-56
In hospitalized patients, point-of-care capillary glucose monitoring at the bedside is the reference for decision-making. Until 2020, the experience with CGM in hospitals was limited and was more-focused on ICU 57 or surgical patients. 58 However, the increased use of CGM systems during the COVID-19 pandemic has generated a greater understanding of the benefits in noncritical hospitalized patients.59,60 Studies show that CGM is safe for inpatients providing similar glycemic control with fewer capillary blood glucose tests 61 with a reduction in hypoglycemia.62,63 Currently, the incorporation of glucose alarms in more-recent CGM systems may potentially improve patient safety by minimizing and predicting hyperglycemia and hypoglycemia. The main limitations of CGM may be the potentially reduced accuracy in this setting, due to changes in tissue hydration (ischemia, edema, dehydration), compression of the glucose sensor by decubitus, possible drug interference, and the withdrawal need to some imaging tests (ie, magnetic resonance imaging) 64 or when using of diathermic therapies.
Some observational CGM studies have suggested that there is a high satisfaction degree amongst HCPs regarding CGM reliability and time saving with these devices in hospitalized patients. However, training is required for insertion of glucose sensors, data downloading, and review of information. Other unresolved issues of CGM include connectivity problems, integration with electronic medical records, and its use to manage insulin dosing. 65 In addition, hospitals need to develop protocols for the use of CGM during hospitalization. 66
In conclusion, the use of CGM in the hospital seems to be safe and could be useful in glucose management for inpatients, although the data are preliminary and more studies are needed.
Some Practical Recommendations for The Interpretation of Flash Data
-
Check data reliability.
At least 70% of data should be captured over 14 consecutive days.
-
Check the number of scans:
At least once every 8 hours (Figure 3). Higher number of daily scans is associated with greater achievement of TIR targets, reduced time in hypoglycemia and lower glycemic variability. 34
ystematic rules for sensor scanning. Scan the glucose sensor regularly and in certain situations as when getting up, before meals, after meals (1-2 hours), when going to sleep, on sick days (scan every 2 hours), when exercising (before the activity, every 15-30 minutes during the exercise, at the end and 6-8 hours after the exercise to prevent late hypoglycemia).
Avoid compulsive scanning (eg, after correction of hypoglycemia). Glucose values may remain low in sensor readings for some time even after correction of hypoglycemia (Figure 3).
-
Review AGP for frequency, timing, and magnitude of hypoglycemia.
Avoiding hypoglycemia is a priority (Table 2).
Identify magnitude of hypoglycemia and patterns for when the hypoglycemia occurs (eg, nocturnal and postprandial). Search for potential causes.
In case of frequent hypoglycemia, check the individual hypoglycemia awareness by means of the Gold or Clarke methods.67,68
Review treatment and correction actions during hypoglycemic episodes.
Check current hypoglycemia alarms and modify if necessary.
-
Review AGP for frequency, timing, and magnitude of hyperglycemia.
Review AGP and the daily registries regarding when hyperglycemia occurs (if it is in preprandial or postprandial periods) (Table 2).
Search for potential causes (postprandial hyperglycemia, hyperglycemia after hypoglycemic correction, etc.) and implement necessary changes to avoid them.
Check current hyperglycemia alarms and modify if necessary.
-
Review AGP for glycemic variability (GV).
Coefficient of variation should be lower than 36%. 9 In cases of elevated GV, check insulin injection technique and injection zones, as well as carbohydrate counting accuracy, sensitivity factor and correction of hypoglycemia and hyperglycemia (Table 2).
-
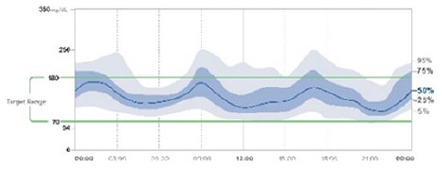
Look at the AGP chart: interquartile range (IQR 25th-75th) and fifth to 95th percentile band (Table 3).11,69
Increased width in IQR and low in fifth to 95th percentile band: usually factors related to treatment are responsible (insulin dose, insulin/carbohydrate ratio, sensitivity factor, etc.).
Increased width in fifth to 95th percentile band and low in IQR: usually related to patient irregular behaviors (insulin bolus omission, bolus after meals, excessive and/or disordered exercise, etc.).
Increased width in IQR and fifth to 95th percentile band: factors related to treatment and patient behavior.
-
Try to identify patterns in daily profiles.
Review insulin dosing, amount of carbohydrates, and level and timing of exercise with the patient. Keep records.
Review alarm usage and reprogramming when necessary.
Establish an action plan in agreement with the person with diabetes.
Figure 3.
Identifying some scanning patterns with CGM. The figure shows two examples of scanning behavior that can be discussed with a patient. (a) A loss of data because of insufficient scanning, failing to capture data at least every 8 hours: (b) indicates a period of compulsive scanning (for example, after correction of hypoglycemia). Abbreviation: CGM, continuous glucose monitoring.
Table 2.
Clinical Situations and Practical Recommendations for Their Approach.
| Clinical case problem | Metrics | Recommended action | ||||
|---|---|---|---|---|---|---|
| TIR (%) | TBR (%) | TAR (%) | CV (%) | |||

|

|
69 | 28 | 3 | 41 |
Generalized hypoglycemia
Intervention on hypoglycemia is the first priority. Review type and dose of basal insulin (nocturnal hypoglycemia); adjustment of insulin/CH ratios and sensitivity factor; adjustments when exercising; review perception and correction of hypoglycemia. Possible candidate for PLGS or hybrid closed-loop system. |

|

|
57 | 10 | 33 | 41.9 |
Nocturnal hypoglycemia
Intervention on hypoglycemia is required before making other adjustments. Review type and dosing of basal insulin; evaluate physical exercise pattern (afternoons), if applicable; review perception and correction of hypoglycemia. |

|

|
68 | 2 | 30 | 44 |
Postprandial hyperglycemia
Check type of prandial insulin, insulin dosing and bolus time; evaluate food portion sizes, insulin/CHO ratio and ISF; analyze composition and quality of intake. |

|

|
11 | 0 | 89 | 25.6 |
Daytime and nighttime hyperglycemia
Review type of basal insulin, dosing and storage of insulin; evaluate prandial insulin type, dosing, and insulin adjustments; analyze insulin/CHO ratios and ISF; review zones and injection technique; check for omission of insulin; rule out hypoglycemia phobia. |

|

|
47 | 2 | 51 | 45.2 |
High glycemic variability
Hypoglycemia pattern after over-correction of hyperglycemia, and hyperglycemia after over-correction of hypoglycemia; review ISF and correction of hypoglycemia; review injection sites and injection technique; check for missed insulin injections. |

|

|
45 | 1 | 64 | 50 |
Fasting hyperglycemia
Increase basal insulin dose; decrease fat intake at dinner; rule out unscheduled nocturnal snacking, assess transfer to CSII therapy. |
|

|
81 | 2 | 17 | 32 |
Anti-dawn effect or hyperglycemia
At sunset, reduce fat intake in food; assess increase in prandial insulin at snack; suggest doing physical exercise in the afternoons; in case of noncorrection, assess transfer to therapy with continuous subcutaneous insulin infusion. |
Table 2 shows different common clinical situations, using reports that are automatically generated by LibreView®, with glucometry data, and practical recommendations for their resolution.
Abbreviations: TIR, time in range; TBR, time below range; TAR, time above range; CV, coefficient of variation; CHO, carbohydrate; PLGS, predictive low-glucose suspend; ISF, insulin sensitivity factor; CSII, continuous subcutaneous insulin infusion.
Table 3.
Assessing Glucose Variability Within an AGP.
| Variability profile | Interpretation | Possible causes |
|---|---|---|
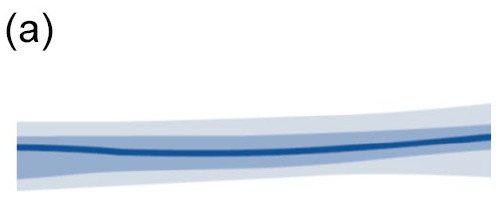
|
• Narrow blue IQR and outer gray bands • Low glucose variability • No treatment adjustment needed |
|
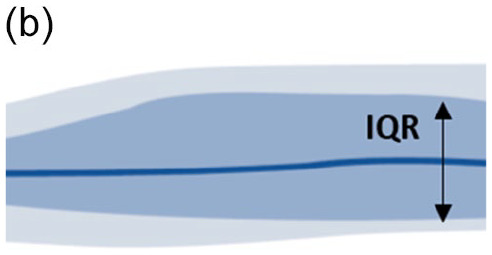
|
• Wide blue IQR band • Narrow gray outer band • High day-to-day glucose variability • Treatment adjustment needed |
Treatment-related causes
• Mealtime insulin dose and/or timing • Suboptimal correction dose calculation • Suboptimal insulin/CHO ratio |

|
• Narrow blue IQR band • Wide gray outer band • High occasional glucose variability • Lifestyle management needed |
Behavioral/social causes
• Unplanned exercise • Missed injections • Irregular mealtimes • Unplanned snacking • Alcohol |
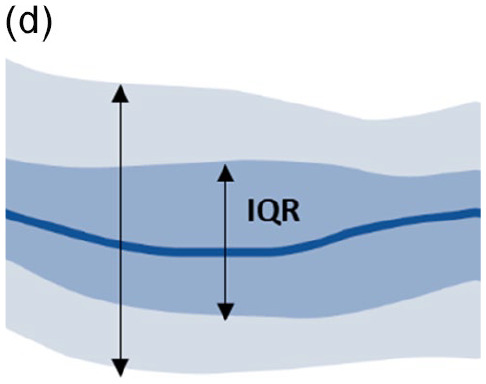
|
• Wide blue IQR band • Wide gray outer band • High day-to-day and occasional glucose variability • Adjustments to treatment and lifestyle management |
Treatment/behavioral causes
• All of the above causes |
Abbreviations: AGP, ambulatory glucose profile; IQR, interquartile range; CHO, carbohydrate.
Conclusions
In summary, CGM provides valuable data, including time-in-ranges and information regarding GV. A TIR of >70% is recommended for most individuals with T1D and T2D. However, targets should be individualized, and different targets should be considered according to time of diagnosis, age, the presence of comorbidities, as well as for pregnant women. CGM reports allow certain patterns of glucose levels to be identified, which can facilitate clinical decision-making to adjust treatments. Optimizing the interpretation of the reports generated by CGM, with special attention to time in range, time in hypoglycemia, and glycemic variability, may help to a more efficient use of diabetes technology, and finally, lead to an improved glycemic control.
Footnotes
Abbreviations: ADA: American Diabetes Association; AGP: Ambulatory Glucose Profile; CGM: Continuous Glucose Monitoring; CV: Coefficient of Variation; DCTT: Diabetes and Complications Trial; EASD: European Association for the Study of Diabetes; FLASH: Flash Glucose Monitoring; GDM: Gestational Diabetes Mellitus; GV: Glycemic Variability; HCP: Healthcare Professionals; isCGM: Intermittently Scanned CGM; ISPAD: International Society for Pediatric and Adolescent Diabetes; MDI: Multiple Daily Injections; PWD: People with Diabetes; PWT1D: People with Type 1 Diabetes; PWT2D: People with Type 2 Diabetes; rtCGM: Real-Time Continuous Glucose Monitoring; SMBG: Self-Monitored Blood Glucose; T1D: Type 1 Diabetes Mellitus; T2D: Type 2 Diabetes Mellitus; TAR: Time Above Range; TBR: Time Below Range; TIR: Time in Range.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: VB has received speaker/advisory honoraria from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Esteve, Janssen, Merck, Mundi- pharma, Novartis, Novo Nordisk, Roche, Sanofi. EAH has received honoraria from Abbott, Novo Nordisk, Sanofi, Lilly and Roche Diabetes Care. RCH has received speaker honoraria from Abbott, Lilly, Medtronic, Novo-Nordisk and Sanofi-Aventis and has consulted for Abbott, Lilly, Novo-Nordisk and Sanofi. GDS has received advisory honoraria/research support from NovoNordisk, Lilly, Medtronic and Abbott. NGPV has received speaker honorary from Sanofi, Novo Nordisk, Boehringer Ingelheim, Lilly Astra Zeneca, Abbott, and MSD. MJPC have received fees for collaborations from Abbott Diabetes Care, Ascensia Diabetes Care, Novo Nordisk and Sanofi. FJAB has received consultant/advisor honoraria from Abbott Diabetes Care, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, LifeScan, MannKind Co., Medtronic, Menarini, Merck, Novartis, Novo Nordisk, Sanofi; Speaker honoraria from Abbott Diabetes Care, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, LifeScan, Eli Lilly, Madaus, Medtronic, Menarini, Merck, Novartis, Novo Nordisk, Sanofi; Grant support from Novo Nordisk, Sanofi.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: All authors contribute to define scope and structure of the manuscript, as well to write the full content of the manuscript. Some support from Abbott Spain was given at the beginning of this initiative. However, position statement and expert opinions represent only those from authors and were not influenced anytime by Abbott Diabetes Spain.
ORCID iDs: Virginia Bellido  https://orcid.org/0000-0002-8384-043X
https://orcid.org/0000-0002-8384-043X
Francisco Javier Ampudia-Blasco  https://orcid.org/0000-0003-0221-0901
https://orcid.org/0000-0003-0221-0901
References
- 1. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 2. Campbell L, Pepper T, Shipman K. HbA1c: a review of non-glycaemic variables. J Clin Pathol. 2019;72:12-19. [DOI] [PubMed] [Google Scholar]
- 3. Bellido V, Pinés-Corrales PJ, Villar-Taibo R, Ampudia-Blasco FJ. Time-in-range for monitoring glucose control: Is it time for a change? Diabetes Res Clin Pract. 2021;177:108917. [DOI] [PubMed] [Google Scholar]
- 4. Wilmot EG, Lumb A, Hammond P, et al. Time in range: a best practice guide for UK diabetes healthcare professionals in the context of the COVID-19 global pandemic. Diabet Med. 2021;38:e14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13:614-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu J, Ma X, Zhou J, Zhang L, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41:2370-2376. [DOI] [PubMed] [Google Scholar]
- 8. Lu J, Wang C, Shen Y, Chen L, et al. Time in range in relation to all-cause and cardiovascular mortality in patients with type 2 diabetes: a prospective cohort study. Diabetes Care. 2020; dc201862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Dia Care. 2019;42:1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson ML, Martens TW, Criego AB, Carlson AL, Simonson GD, Bergenstal RM. Utilizing the ambulatory glucose profile to standardize and implement continuous glucose monitoring in clinical practice. Diabetes Technol Ther. 2019;21:S217-S225. [DOI] [PubMed] [Google Scholar]
- 11. American Diabetes Association Professional Practice Committee, American Diabetes Association Professional Practice Committee, Draznin B, et al. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S83-S96. [DOI] [PubMed] [Google Scholar]
- 12. Gibb FW, McKnight JA, Clarke C, Strachan MWJ. Preserved C-peptide secretion is associated with fewer low-glucose events and lower glucose variability on flash glucose monitoring in adults with type 1 diabetes. Diabetologia. 2020;63:906-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marren SM, Hammersley S, McDonald TJ, et al. Persistent C-peptide is associated with reduced hypoglycaemia but not HbA1c in adults with longstanding Type 1 diabetes: evidence for lack of intensive treatment in UK clinical practice? Diabet Med. 2019;36:1092-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1998;128:517-23. [DOI] [PubMed] [Google Scholar]
- 15. Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. [DOI] [PubMed] [Google Scholar]
- 16. Billings LK, Parkin CG, Price D. Baseline glycated hemoglobin values predict the magnitude of glycemic improvement in patients with type 1 and type 2 diabetes: subgroup analyses from the DIAMOND study program. Diabetes Technol Ther. 2018;20:561-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline J-P, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martens TW, Beck RW, Bergenstal RM. Continuous glucose monitoring and glycemic control in patients with type 2 diabetes treated with basal insulin-reply. JAMA. 2021;326:1330-1331. [DOI] [PubMed] [Google Scholar]
- 20. Elliott T, Beca S, Beharry R, Tsoukas MA, Zarruk A, Abitbol A. The impact of flash glucose monitoring on glycated hemoglobin in type 2 diabetes managed with basal insulin in Canada: A retrospective real-world chart review study. Diab Vasc Dis Res. 2021;18:14791641211021374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oyagüez I, Gómez-Peralta F, Artola S, et al. Cost analysis of freestyle Libre® 2 system in type 2 diabetes mellitus population. Diabetes Ther. 2021;12:2329-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rama Chandran S, Tay WL, Lye WK, et al. Beyond HbA1c: comparing glycemic variability and glycemic indices in predicting hypoglycemia in type 1 and type 2 diabetes. Diabetes Technol Ther. 2018;20:353-362. [DOI] [PubMed] [Google Scholar]
- 23. Bremer JP, Jauch-Chara K, Hallschmid M, Schmid S, Schultes B. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care. 2009;32:1513-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sundberg F, Barnard K, Cato A, et al. ISPAD Guidelines. Managing diabetes in preschool children. Pediatr Diabetes. 2017;18:499-517. [DOI] [PubMed] [Google Scholar]
- 25. Conrad SC, McGrath MT, Gitelman SE. Transition from multiple daily injections to continuous subcutaneous insulin infusion in type 1 diabetes mellitus. J Pediatr. 2002;140:235-240. [DOI] [PubMed] [Google Scholar]
- 26. Klinkert C, Bachran R, Heidtmann B, Grabert M, Holl RW. DPV-Initiative. Age-specific characteristics of the basal insulin-rate for pediatric patients on CSII. Exp Clin Endocrinol Diabetes. 2008;116:118-122. [DOI] [PubMed] [Google Scholar]
- 27. DiMeglio LA, Acerini CL, Codner E, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):105-114. [DOI] [PubMed] [Google Scholar]
- 28. American Diabetes Association Professional Practice Committee, American Diabetes Association Professional Practice Committee, Draznin B, et al. Diabetes technology: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S97-S112. [DOI] [PubMed] [Google Scholar]
- 29. Lachin JM, Bebu I, Nathan DM, DCCT/EDIC Research Group. The beneficial effects of earlier versus later implementation of intensive therapy in type 1 diabetes. Diabetes Care. 2021;44:2225-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Porcel-Chacón R, Antúnez-Fernández C, Mora Loro M, et al. Good metabolic control in children with type 1 diabetes mellitus: does glycated hemoglobin correlate with interstitial glucose monitoring using FreeStyle Libre? J Clin Med. 2021;10:4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Piona C, Marigliano M, Mozzillo E, et al. Relationships between HbA1c and continuous glucose monitoring metrics of glycaemic control and glucose variability in a large cohort of children and adolescents with type 1 diabetes. Diabetes Res Clin Pract. 2021;177:108933. [DOI] [PubMed] [Google Scholar]
- 32. Campbell FM, Murphy NP, Stewart C, Biester T, Kordonouri O. Outcomes of using flash glucose monitoring technology by children and young people with type 1 diabetes in a single arm study. Pediatr Diabetes. 2018;19:1294-1301. [DOI] [PubMed] [Google Scholar]
- 33. Biester T, Grimsmann JM, Heidtmann B, et al. intermittently scanned glucose values for continuous monitoring: cross-sectional analysis of glycemic control and hypoglycemia in 1809 children and adolescents with type 1 diabetes. Diabetes Technol Ther. 2021;23:160-167. [DOI] [PubMed] [Google Scholar]
- 34. Gomez-Peralta F, Dunn T, Landuyt K, Xu Y, Merino-Torres JF. Flash glucose monitoring reduces glycemic variability and hypoglycemia: real-world data from Spain. BMJ Open Diabetes Res Care. 2020;8:e001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bahíllo-Curieses MP, Díaz-Soto G, Vidueira-Martínez AM, Torres-Ballester I, Gómez-Hoyos E, de Luis-Román D. Assessment of metabolic control and use of flash glucose monitoring systems in a cohort of pediatric, adolescents, and adults patients with Type 1 diabetes. Endocrine. 2021;73:47-51. [DOI] [PubMed] [Google Scholar]
- 36. Thabit H, Prabhu JN, Mubita W, et al. Use of factory-calibrated real-time continuous glucose monitoring improves time in target and HbA1c in a multiethnic cohort of adolescents and young adults with type 1 diabetes: the MILLENNIALS study. Diabetes Care. 2020;43:2537-2543. [DOI] [PubMed] [Google Scholar]
- 37. Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323:2388-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Strategies to Enhance New CGM Use in Early Childhood (SENCE) Study Group. A randomized clinical trial assessing continuous glucose monitoring (CGM) use with standardized education with or without a family behavioral intervention compared with fingerstick blood glucose monitoring in very young children with type 1 diabetes. Diabetes Care. 2021;44:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Middleton P, Crowther CA, Simmonds L. Different intensities of glycaemic control for pregnant women with pre-existing diabetes. Cochrane Database Syst Rev. 2016; CD008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scott EM, Bilous RW, Kautzky-Willer A. Accuracy, user acceptability, and safety evaluation for the FreeStyle Libre flash glucose monitoring system when used by pregnant women with diabetes. Diabetes Technol Ther. 2018;20:180-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Castorino K, Polsky S, O’Malley G, et al. Performance of the Dexcom G6 continuous glucose monitoring system in pregnant women with diabetes. Diabetes Technol Ther. 2020;22:943-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kristensen K, Ögge LE, Sengpiel V, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia. 2019;62:1143-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390:2347-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murphy HR. Continuous glucose monitoring targets in type 1 diabetes pregnancy: every 5% time in range matters. Diabetologia. 2019;62:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care. 2013;36:1877-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. García-Moreno RM, Benítez-Valderrama P, Barquiel B, et al. Efficacy of continuous glucose monitoring on maternal and neonatal outcomes in gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials. Diabet Med. 2022;39:e14703. [DOI] [PubMed] [Google Scholar]
- 47. Yu F, Lv L, Liang Z, et al. Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: a prospective cohort study. J Clin Endocrinol Metab. 2014;99:4674-4682. [DOI] [PubMed] [Google Scholar]
- 48. Wei Q, Sun Z, Yang Y, Yu H, Ding H, Wang S. Effect of a CGMS and SMBG on maternal and neonatal outcomes in gestational diabetes mellitus: a randomized controlled trial. Sci Rep. 2016;6:19920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paramasivam SS, Chinna K, Singh AKK, et al. Continuous glucose monitoring results in lower HbA1c in Malaysian women with insulin-treated gestational diabetes: a randomized controlled trial. Diabet Med. 2018;35:1118-1129. [DOI] [PubMed] [Google Scholar]
- 50. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978-982. [DOI] [PubMed] [Google Scholar]
- 51. Eiland L, Goldner W, Drincic A, Desouza C. Inpatient hypoglycemia: a challenge that must be addressed. Curr Diab Rep. 2014;14:445. [DOI] [PubMed] [Google Scholar]
- 52. Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32:1153-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fahy BG, Sheehy AM, Coursin DB. Glucose control in the intensive care unit. Crit Care Med. 2009;37:1769-1776. [DOI] [PubMed] [Google Scholar]
- 54. American Diabetes Association Professional Practice Committee, American Diabetes Association Professional Practice Committee, Draznin B, et al. diabetes care in the hospital: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S244-S253. [DOI] [PubMed] [Google Scholar]
- 55. Duncan AE, Abd-Elsayed A, Maheshwari A, Xu M, Soltesz E, Koch CG. Role of intraoperative and postoperative blood glucose concentrations in predicting outcomes after cardiac surgery. Anesthesiology. 2010;112:860-871. [DOI] [PubMed] [Google Scholar]
- 56. Price C, Ditton G, Russell GB, Aloi J. Reliability of inpatient CGM: comparison to standard of care. J Diabetes Sci Technol. 2021;19322968211062170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Salinas PD, Mendez CE. Glucose management technologies for the critically ill. J Diabetes Sci Technol. 2019;13:682-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Galindo RJ, Migdal AL, Davis GM, et al. Comparison of the FreeStyle Libre Pro Flash continuous glucose monitoring (CGM) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care. 2020;43:2730-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Longo RR, Elias H, Khan M, Seley JJ. Use and accuracy of inpatient CGM during the COVID-19 pandemic: an observational study of general medicine and ICU patients. J Diabetes Sci Technol. 2021;19322968211008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Faulds ER, Boutsicaris A, Sumner L, et al. Use of Continuous Glucose Monitor in critically ill COVID-19 patients requiring insulin infusion: an observational study. J Clin Endocrinol Metab. 2021; dgab409. [DOI] [PubMed] [Google Scholar]
- 61. Maiorino MI, Signoriello S, Maio A, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care. 2020;43:1146-1156. [DOI] [PubMed] [Google Scholar]
- 62. Spanakis EK, Levitt DL, Siddiqui T, et al. The effect of continuous glucose monitoring in preventing inpatient hypoglycemia in general wards: the glucose telemetry system. J Diabetes Sci Technol. 2018;12:20-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Singh LG, Levitt DL, Satyarengga M, et al. Continuous glucose monitoring in general wards for prevention of hypoglycemia: results from the glucose telemetry system pilot study. J Diabetes Sci Technol. 2020;14:783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Takatsu Y, Shiozaki T, Miyati T, Asahara M, Tani Y. Are the recorded data of flash glucose monitoring systems influenced by radiological examinations? Radiol Phys Technol. 2019;12:224-229. [DOI] [PubMed] [Google Scholar]
- 65. Faulds ER, Jones L, McNett M, et al. Facilitators and Barriers to Nursing Implementation of continuous glucose monitoring (CGM) in critically ill patients with COVID-19. Endocr Pract. 2021;27:354-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Galindo RJ, Umpierrez GE, Rushakoff RJ, et al. Continuous glucose monitors and automated insulin dosing systems in the hospital consensus guideline. J Diabetes Sci Technol. 2020;14:1035-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994;17:697-703. [DOI] [PubMed] [Google Scholar]
- 68. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18:517-522. [DOI] [PubMed] [Google Scholar]
- 69. Kröger J, Reichel A, Siegmund T, Ziegler R. Clinical recommendations for the use of the ambulatory glucose profile in diabetes care. J Diabetes Sci Technol. 2020;14:586-594. [DOI] [PMC free article] [PubMed] [Google Scholar]





