Abstract
cobas® pulse is a point-of-care blood glucose (BG) measuring system for multiple-patient use in professional healthcare settings. The system provides advances in connectivity and BG measuring technology, and has multiple fail-safes to improve accuracy and reduce the risk of user error. Flavin adenine dinucleotide-dependent glucose dehydrogenase on the working electrode catalyzes oxidation of β-D-glucose in the blood sample. A redox mediator/electron acceptor, on both the working and the counter electrode, facilitates diffusion of electrons in proportion to the glucose concentration and compensates for the effects of potential interfering agents. During development, >1 million test strip measurements were performed using >8000 test scenarios to refine the algorithm model. No clinically relevant interference was identified with extreme variations in blood properties and drugs in whole blood samples.
Keywords: blood glucose, detection, interference, meter, test strip
Introduction
cobas® pulse (Roche Diagnostics GmbH, Mannheim, Germany) is a new point-of-care (POC) blood glucose (BG) measuring system intended for professional use. This system is designed to be accurate and safe, and to connect wirelessly with a hospital’s data management system (DMS). The system is the successor to the Accu-Chek® Inform II system (Roche Diagnostics GmbH). In this technology review, we describe the features of the system and the advances made in addressing challenges facing current strip-based BG measuring systems. Further accuracy data will be presented in a future paper.
System components include (1) the hand-held instrument, (2) the charging unit, (3) disposable single-use glucose test strips, (4) system-specific software, and (5) quality control (QC) solutions. The hand-held instrument is designed to be used on multiple patients, to withstand repeated disinfection, and to reduce blood-borne infection transmission risk. The instrument has been optimized for ease of use. The system minimizes handling steps for users in its default configuration; provides workflow guidance on display, displays color-changing strip port illumination, and uses a strip ejection process that avoids contact with contaminated test strips.
The instrument contains a radio frequency identification (RFID) reader that collects information from patient and operator identification badges. Test strip information is detected automatically and recorded by a second camera located inside the test strip port. An active strip push-out plus a sensor-based on/off switch facilitates efficient cleaning and disinfection. The instrument’s touch screen uses capacitive touch sensing, which, compared with resistive touch sensing, uses the electrical conducting properties of the body rather than mechanical pressure, requires less finger pressure, and provides better screen clarity. Power is transferred wirelessly between charging stations and the instrument’s battery. The instrument communicates by Wi-Fi and transmits test results wirelessly to a facility’s internal DMS.
Glucose Detection Principle
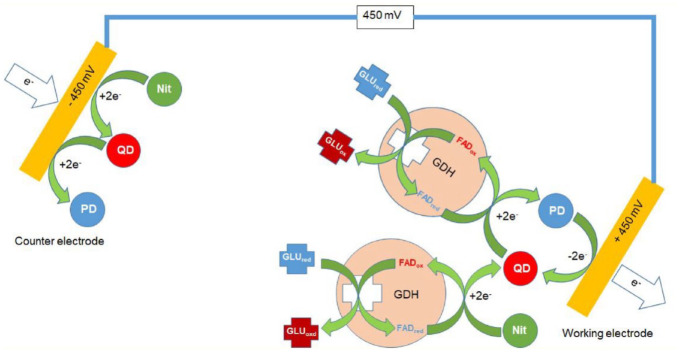
Glucose detection occurs on gold electrodes in the reagent-coated slot die (Figure 1). The working electrode is covered by a reagent layer (flavin adenine dinucleotide-dependent glucose dehydrogenase [FADGDH]) that catalyzes oxidation of β-D-glucose in the blood sample, and on both the working and the counter electrode, a redox mediator/electron acceptor (nitrosoaniline derivative) that facilitates diffusion of electrons from the co-factor to the electrode surface proportional to the concentration of glucose in the blood sample (Figure 2).
Figure 1.
An exploded view technical drawing of the system test strip architecture. Abbreviation: PET, polyethylenterephthalat.
Figure 2.
Electrochemistry of glucose detection in the system. In a first step, glucose is oxidized by the FAD-GDH. The received electrons are transferred to the nitrosoaniline, which reduces the nitrosoaniline to quinonediimine. If the quinonediimine is produced, then the electrons from further glucose oxidations via the FAD-GDH can also be transferred to the quinonediimine via the FAD-GDH. Hereby, the quinonediimine gets reduced to phenylendiamine. At the polarized electrode, the phenylendiamine is oxidized back to quinonediimine, completing the redox cycle. Abbreviations: FAD, flavin adenine dinucleotide; GDH, glucose dehydrogenase; GLU, glucose; red, reduced; ox, oxidized; PD, phenylenediamine; QD, quinonediimine; Nit, nitrosoaniline.
The FADGDH enzyme system is specific for glucose, and does not react with other saccharides in blood, which greatly reduces the risk for false positives. The redox mediator/electron acceptor confers several benefits, including (1) preventing undesirable side reactions from occurring if test strips are exposed to moisture, (2) providing stability during storage, and (3) ensuring that the electrochemical potential at the working electrode is low enough to prevent oxidation of other substances in the sample, thereby avoiding false-positive results.
The reagent film (matrix) is designed to ensure efficient separation of blood cells, buffering of pH, stabilization and remobilization of the enzyme system, and rapid and homogeneous diffusion of the redox mediator. The composition of the matrix ensures rapid distribution of an applied blood sample over the reagent surface, followed by permeation of the plasma fraction into the reagent layer through swelling of the film. The swollen matrix provides a defined reaction space for the diffusion-controlled electrochemical detection reaction. Homogeneity of the matrix eliminates the need for lot-specific adaptation of the glucose calculation algorithm.
Glucose Prediction Algorithm
The instrument calculates the concentration of glucose in a whole blood sample based on a weighted combination of the voltametric and impedimetric current responses, and their products at selected time points. This algorithm model was derived from an extensive calibration program. During development, more than 1 million test strip measurements with blood samples and control solutions were performed using over 8000 test scenarios (i.e., combinations of blood properties, ambient conditions, sample dosing methods, sample volumes, test strip aging, test strip manufacturing tolerances, and drug interferences) to refine the calibration data and algorithm model.
Calibration
The system is calibrated at the factory. As part of the lot release process each test strip lot is tested against a plasma reference with venous blood samples containing low, medium, and high glucose concentrations. The plasma reference method is traceable to a National Institute of Standards and Technology (NIST) standard via isotope dilution gas chromatography/mass spectrometry. Two levels of QC solutions are provided with the instrument along with a dedicated QC workflow that can be configured to meet institutional requirements.
Test Strips and Instrument Self-Checks
The measuring cell has a 3-sided open capillary design (Figure 1) and is configured to aspirate a liquid sample between the electrodes. A 2-dimensional barcode (strip ID) on the test strip is read and matched with the lot information (e.g., expiration date) in the instrument before the BG measurement is performed. Lot-specific information is uploaded to the instrument via the site’s DMS. From the user’s point of view, this strip verification is automatic. This does not change the glucose calculation algorithm; the glucose calculation algorithm is not lot-specific. If the test strip lot data are not available the instrument (because of misconfiguration) or the test strip has expired, then no measurement is possible. The instrument only delivers glucose results if all self-tests are completed successfully.
Operating System
The system is an automated data collection and information management system that communicates by Wi-Fi to a hospital’s network. The system has Bluetooth connectivity to support third-party applications and near-field communication connectivity to read RFID operator and patient identification badges. The control software in the measuring unit runs on a dedicated processor distinct from the processor running the Android operating system, and communicates with this processor via a dedicated interface. For hospital glucose measurement, the system connects to the hospital DMS to receive operator and patient lists, and to transmit BG results.
A new error code system provides clear actionable recommendations to the user. The primary message informs the operator what action to perform next. For example, if a used test strip is inserted into the port, the user interface displays the message “Please retry with a new strip.”
The operator is not confined to the workstation. The system has the potential to eliminate the need for healthcare workers to use other mobile devices, such as phones or tablets, to acquire laboratory data while on the ward.
The system has been designed to accommodate third-party apps, which can be downloaded from a Roche App Store; for example, apps that perform clinical applications used in routine workflows, such as reading body temperature badges, or calculating “use-by” dates. The system includes cybersecurity measures that comply with the 140 series of Federal Information Processing Standards (FIPS-140) security standards (Level 1) and General Data Protection Regulation (GDPR) requirements. Roche-specific Android adaptations ensure that only approved apps can be installed and run on the instrument.
Compensation for Potential Interfering Agents
A key goal during the development of the system was demonstrating the absence of clinically relevant interference. This is achieved by having the counter electrode generate a response current that closes the measurement circuit and by a change in polarity during the measurement sequence. This process compensates for the effects of electro-active substances in blood samples.
Venous blood samples were obtained from volunteers and then manipulated using established procedures to investigate the potential for endogenous and exogenous substances, and extreme variations in physicochemical properties to interfere with system performance (Table 1). Potential interfering substances were tested in combination with varying hematocrit levels, ambient temperatures, and fill levels. Figure 3 shows the low variation in normalized glucose error over extremes of BG, hematocrit, and sodium concentrations. No clinically relevant interference has been identified.
Table 1.
Examples of Properties and Substances Evaluated in Interference Experiments. a
| Substance or property | Parameters |
|---|---|
| Glucose concentration | Extreme concentrations (10-600 mg/dL) |
| Hematocrit | Extreme variation (5%-70%) |
| Sample temperature | Extreme variation (12°C-40°C) |
| Sodium concentration | Consistent with hypo- and hypernatremia (115-180 mmol/L) |
| Lipid levels | High cholesterol (≤500 mg/dL) and triglycerides (≤1500 mg/dL) |
| Dilution with plasma expanders | Hydroxy-ethyl-starch |
| Simulated changes in altitude | Altered oxygen concentration and atmospheric pressure |
| Drugs and other substances of interest b | Examples include acetaminophen, N-acetyl cysteine, acetylsalicylic acid, ascorbic acid, conjugated and unconjugated bilirubin, creatinine, dopamine, EDTA, galactose, gentisic acid, reduced glutathione, heparin, ibuprofen, levodopa, maltose, mannitol, methyldopa, salicylic acid, tolbutamide, tolazamide, uric acid, xylose, sugar alcohols, hydroxyurea, and many more |
Abbreviation: EDTA, ethylenediaminetetraacetic acid.
Interference testing procedures were derived from the Clinical and Laboratory Standards Institute (CLSI) EP07: Interference Testing in Clinical Chemistry, 3rd Edition guidance document.1
Several hundred commonly used drugs have been evaluated. Testing has been done where suggested test concentration (e.g., stipulations of EP37) exceeded a prespecified limit where a measurable effect could occur with the chemistry of the test strip. 1
Figure 3.
Normalized glucose error over extreme variations in blood glucose concentrations, hematocrit, and sodium concentration in remaining blood samples from patients treated in different intensive care units in Frankfurt (N = 2843). Anonymized intensive care unit samples (leftover samples) without information on medication were obtained from a clinic in Frankfurt (surgery and neurological intensive care) and, if possible (if sufficient volume), divided into 2 aliquots. Each sample was measured natively after receipt (first aliquot). The second aliquot was glycolyzed overnight and then measured. Alternatively, this aliquot was spiked with glucose and measured. Directly after the respective measurement, plasma was obtained for the reference analysis. This procedure covered the medically relevant measurement range (50-400 mg/dL). The glucose reference concentrations were obtained by the plasma hexokinase method on a cobas® lab analyzer (Roche Diagnostics GmbH). The hematocrit reference levels of the tested blood samples were obtained by the centrifuge tube method. The sodium reference concentrations were obtained by ion selective electrodes method on a cobas® C analyzer (Roche Diagnostics GmbH). Individual deviations of system measurement results versus the glucose reference method taken for the same sample as tested with the system are shown. Test results below 75 mg/dL glucose are shown as absolute bias and test results above 75 mg/dL glucose are shown as a relative bias (the “Normalized Error”). The horizontal bands represent specification boundaries for the normalized glucose error: 95% of all samples must be within −12 and 12, and 98% within −15 and 15. The deviations were plotted 3 times versus different x-axes, representing the reference glucose concentration, the hematocrit level, and the sodium concentration of the tested blood samples (measured with established reference methods). Blue lines are the regression lines calculated for the bias values. All received samples were tested as received (native). A certain number of samples (in cases of a sufficient available volume) was also tested with the glycolyzed blood sample (to achieve low glucose concentrations) and after spiking with glucose (to achieve higher glucose concentrations).
Conclusion
The described system is an advanced digital diagnostic tool. This system matches or exceeds current POC testing requirements (CLSI POC 12A 1 and “Blood Glucose Monitoring Test Systems for Prescription Point-of-Care Use Guidance for Industry and Food and Drug Administration Staff” 2 ), provides important advances in connectivity and BG measuring technology, has very low potential for interference with substances in patients’ blood, and contains multiple fail-safes to improve accuracy and reduce the risk of user error.
Acknowledgments
The authors would like to acknowledge the sustained collaborative effort and dedication of the entire Roche Point-of-Care Hospital Glucose Development Team in the realization of this project. Medical writing support for the development of the manuscript was furnished by Blair Jarvis, MSc, funded by Roche Diagnostics.
Footnotes
Abbreviations: BG, blood glucose; CLIA, Clinical Laboratory Improvement Amendments; CLSI, Clinical and Laboratory Standards Institute; DMS, data management system; EDTA, ethylenediaminetetraacetic acid; FADGDH, flavin adenine dinucleotide-dependent glucose-dehydrogenase; FIPS-140, 140 series of Federal Information Processing Standards; GDPR, General Data Protection Regulation; NIST, National Institute of Standards and Technology; POC, point-of-care; QC, quality control; RFID, radio frequency identification.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors received research funding in the form of medical writing support from Roche Diagnostics. Simon Gessler, Ulrich Porsch, and Michael Marquant are current employees of Roche Diagnostics GmbH. Simon Gessler holds shares in Roche. Michael Marquant declares that he has collaborated on patent applications that are issued or pending for the cobas® pulse system. Ulrich Porsch declares that he has collaborated on patent applications that are pending for the cobas® pulse system and that he holds participation certificates and common shares in F. Hoffmann-La Roche Ltd. David C. Klonoff is a consultant to Abbott, Dexcom, Eli Lilly, EOFlow, Integrity, Lifecare, Medtronic, Novo, Roche Diagnostics, and Thirdwayv.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or publication of this article: The development of the cobas® pulse system and the work described in this article was funded by Roche Diagnostics GmbH, Mannheim, Germany.
ORCID iDs: Simon Gessler  https://orcid.org/0000-0002-2369-4696
https://orcid.org/0000-0002-2369-4696
Ulrich Porsch  https://orcid.org/0000-0001-8555-3197
https://orcid.org/0000-0001-8555-3197
David C. Klonoff  https://orcid.org/0000-0001-6394-6862
https://orcid.org/0000-0001-6394-6862
Michael Marquant  https://orcid.org/0000-0001-5012-0447
https://orcid.org/0000-0001-5012-0447
References
- 1. Clinical and Laboratory Standards Institute. EP07 Interference Testing in Clinical Chemistry. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. https://clsi.org/standards/products/point-of-care-testing/documents/poct12/ [Google Scholar]
- 2. U.S. Food & Drug Administration. Blood Glucose Monitoring Test Systems for Prescription Point-of-Care Use: Guidance for Industry and Food and Drug Administration Staff. U.S. Food & Drug Administration. 2020. https://www.fda.gov/media/119829/download [Google Scholar]