Abstract
Background:
Strict monitoring of blood glucose during pregnancy is essential for ensuring optimal maternal and neonatal outcomes. Telemedicine could be a promising solution for supporting diabetes management; however, an updated meta-analysis is warranted. This study assesses the effects of telemedicine solutions for managing gestational and pregestational diabetes.
Methods:
PubMed, EMBASE, Cochrane Library Central Register of Controlled Trials, and CINAHL were searched up to October 14, 2020. All randomized trials assessing the effects of telemedicine in managing diabetes in pregnancy relative to any comparator without the use of telemedicine were included. The primary outcome was infant birth weight. A meta-analysis comparing the mean difference (MD) in birth weight across studies was applied, and subgroup and sensitivity analyses were performed. The revised Cochrane tool was applied to assess the risk of bias, and the certainty of evidence was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.
Results:
From a total of 18 studies, ten (totaling 899 participants) were used to calculate the effect on infant birth weight. The results nonsignificantly favored the control (MD of 19.34 g; [95% confidence interval, CI −47.8; 86.47]), with moderate effect certainty. Heterogeneity was moderate (I2 = 37.39%). Statistically significant secondary outcomes included differences in two-hour glucose tolerance postpartum (gestational diabetes; two studies: standardized mean difference 9.62 mg/dL [95% CI: 1.95; 17.28]) that favored the control (GRADE level, very low) and risk of shoulder dystocia (four studies: log odds −1.34 [95% CI: −2.61; −0.08]) that favored telemedicine (GRADE, low).
Conclusions:
No evidence was found to support telemedicine as an alternative to usual care when considering maternal and fetal outcomes. However, further research is needed, including economic evaluations.
Keywords: gestational diabetes mellitus, pregnancy, self-monitoring, telehealth, glycemic control, systematic review
Introduction
Diabetes during pregnancy is a major health problem worldwide. It is estimated that preexisting type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), or gestational diabetes mellitus (GDM) are present in approximately 16% of all pregnancies.1,2 If not sufficiently controlled, diabetes during pregnancy is associated with neonatal and maternal complications, 3 such as neonatal hypoglycemia, perinatal mortality, polyhydramnios, shoulder dystocia, stillbirths, large for gestational age (LGA), and increased cesarean section risk.4-7 Furthermore, both preexisting diabetes (T1DM and T2DM) and GDM have been shown to increase fetal macrosomia risk (birth weight >4000 g).7-9 Fetal macrosomia is associated with serious adverse maternal and neonatal adverse outcomes, including emergency cesarean section, severe postpartum hemorrhage, obstetric anal sphincter injury, shoulder dystocia, obstetric brachial plexus injury, birth fractures, and hypoxic-ischemic encephalopathy.10,11 Thus, interventions to manage diabetes during pregnancy to reduce the risk of fetal macrosomia are essential.7,10 Another reason to manage diabetes during pregnancy is to minimize the risk of developing T2DM later in life.11-13
Current management of preexisting T2DM and GDM typically includes nutrition therapy, exercise, and close glucose monitoring. Management of preexisting T1DM includes frequent titration of insulin to avoid hypoglycemia and hyperglycemia.13-15 However, some women with preexisting diabetes or GDM do not meet the recommendations and target levels of glycemic control in diabetic pregnancy. Poor glycemic control in pregnancy is not well characterized, although the prevalence of women with poor glycemic control in developing countries has been reported to be 6% to 58% in South Africa, 16 Sudan, 17 Nigeria, 18 and Pakistan. 19 Inadequate glycemic control seems to be related to barriers to adherence,20,21 which include lack of health information, teaching sessions, consultations, long waiting times, less tailored physical activity assessments, not knowing where to access appropriate information on the Internet, and unavailability of social media or support groups for women with GDM. 21 The barriers related to poor glycemic control call for new solutions in the management of GDM to avoid maternal and neonatal complications as well as to prevent pressure on maternal and diabetic health services.
Telemedicine has shown promise in regard to supporting people with diabetes in their disease management.22,23 Telemedicine is a broad term that includes telecommunication and information technology in the delivery of health care services. These services may include education, consultation, monitoring, and counseling tasks at a distance.23,24 Telemedicine services can be provided using various constellations, including text messaging, data transmission (eg, blood glucose and blood pressure), or video consultations. Individualized feedback from health care professionals is a key element.25,26 One may argue that telemedicine is an obvious solution for the management of diabetes in general since diabetes is mainly managed outside a hospital setting. In addition, telemedicine may provide value to patients who are unable to travel to health care facilities due to long distances and/or disabilities. 27
Previous research has shown that telemedicine is a promising method for improving glycemic control in people with diabetes in general.28-31 Such potential benefits of telemedicine may also extend to people with preexisting diabetes and GDM. 32 However, the approach still needs to be sufficiently evaluated with regard to its effectiveness in terms of patient-related outcomes within the diabetes context. In this context, a comparison and review of the effectiveness of different types of telemedicine interventions within preexisting diabetes in pregnancy and GDM is highly relevant. Other reviews have been performed within the field of preexisting diabetes and GDM.32-36 However, a more comprehensive review is needed because the existing reviews are limited to a specific diabetes type34,36 or narrow definitions of telemedicine.32,33 Moreover, due to the rapid development of telemedicine solutions, new reviews and meta-analyses should be performed on a regular basis to embrace studies published since previous reviews. Hence, this systematic review and meta-analysis aimed to evaluate the effectiveness of telemedicine solutions relative to any comparator without the use of telemedicine with regard to diabetes-related outcomes (maternal, fetal, and delivery) for patients with gestational or pregestational diabetes.
Methods
Literature Search
This systematic review and meta-analysis followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement. 37 A search protocol has been published elsewhere (PROSPERO number CRD42020123565). 38 The literature search included database searches in PubMed, EMBASE, the Cochrane Library Central Register of Controlled Trials, and CINAHL. The search histories covering T1DM, T2DM, and gestational diabetes are presented in Supplemental Appendix 1. All studies published prior to October 14, 2020 were considered. Two review authors (S.H.L. and L.B.) performed the database searches in collaboration with a research librarian. Various search terms (different synonyms, near-synonyms, spellings, and acronyms) were included and combined to perform an exhaustive search. The main search terms were “telemedicine,” “diabetes,” and “randomized controlled trials.” The pregnancy component was included in the selection process (screening of titles/abstracts and full text). Different search functions, such as thesauruses, Boolean operators, truncation, abstracts/titles/keywords, phrases, free text, and advanced searches, were applied to focus and structure the search. Additional searches included citation searches in SCOPUS and Web of Science, manual searches of reference lists of relevant systematic reviews, and searches of other relevant references.
Study Selection
We considered randomized controlled trials published as peer-reviewed full-text articles that compared telemedicine solutions with any comparator. Both pregestational and gestational diabetes (GDM) were considered for inclusion. There were no restrictions on publication date, although we only included articles published in English, Danish, Norwegian, and Swedish. Four reviewers (S.H.L., S.H., L.B., and J.D.A.) independently screened all titles and abstracts for eligibility and read the remaining full-text versions. Any disagreement was resolved by discussion among the four reviewers or by including other reviewers (P.V., F.W.U., P.H.S., or O.K.H.) in the decision-making process.
Outcomes
All diabetes-related outcomes (maternal, fetal, and delivery) for patients with gestational or pregestational diabetes were considered in the review. The primary outcome was neonatal birth weight. Secondary outcomes were as follows:
Neonatal outcomes: stillbirth, jaundice, LGA, hypoglycemia, admissions to neonatal intensive care, respiratory distress, and shoulder dystocia.
Maternal outcomes: hypoglycemic episodes, two-hour oral glucose intolerance (postpartum in women with GDM), fasting blood glucose, glycated hemoglobin (HbA1c%), and pregnancy-induced hypertension/preeclampsia.
Delivery outcomes: gestational age, cesarean section, labor-induced delivery, preterm delivery, premature membrane rupture, and placental abruption.
Outcomes were included if at least two of the included studies reported the same outcome.
Data Extraction
Four authors (S.H.L., S.H., P.H.S., J.D.A.) utilized a standardized worksheet in Microsoft Excel 2016 and resolved potential disagreements through discussion. Extracted data included trial characteristics (author, publication year, country, and sample size), average age, body mass index (BMI), and mean and standard deviation for outcomes in each treatment group. The following telemedicine characteristics were recorded: purpose (ie, general glycemic improvements, dietary support, exercise, weight loss, diabetes education, and prevention of hypoglycemia or hyperglycemic events), applied technology (eg, glucometers or weights), and setting (ie, primary care, hospital, specialized diabetes clinic, university, community, or cross-sectional). Data were transformed into means and standard deviations using traditional methods.39,40
Data Synthesis
Analyses were performed in Stata 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). Continuous outcomes were pooled using the mean difference (MD) (or standardized mean difference [SMD] when different scales could not be easily transformed) across studies using fixed- or random-effects meta-analysis depending on the amount of heterogeneity (random-effects model if I2 ≥50%). Binary outcomes were pooled based on the log odds ratio. Post hoc subgroup analysis for the primary outcome of all extracted study characteristics was applied, and sensitivity analyses investigated the impact of excluding study outliers on the results. Publication bias was assessed using funnel plot inspection and Egger’s test.
Assessment of Risk of Bias
Two reviewers (J.D.A. and F.W.U.) independently assessed the risk of bias for each included study. The revised Cochrane risk-of-bias tool was used. 41
Assessment of the Certainty of Evidence
Following the handbook for the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE), 42 the overall judgment of the risk of bias, 43 indirectness, 44 imprecision, 45 inconsistency, 46 and risk of publication bias 47 was used to create a summary of findings table in GRADEpro GDT 2015 (McMaster University, Hamilton, Ontario),48,49 which was used to rank the quality of evidence.
Results
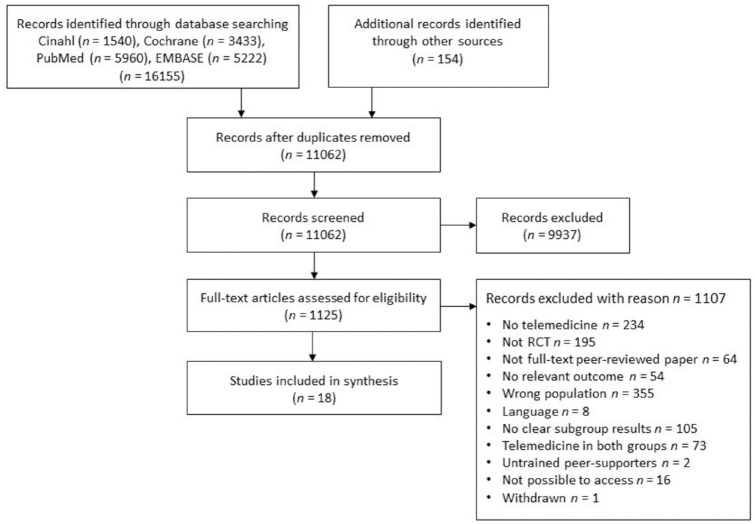
The selection process for studies is shown in Figure 1. Originally, 16 155 studies were found (11 062 after removal of duplicates). After screening, 1125 full-text articles were evaluated. Eventually, 18 articles were included.
Figure 1.
Selection of studies.
Abbreviation: RCT, randomized controlled trial.
Table 1 presents the study characteristics. One study was published prior to 2000 (6%), two were published in the 2000s (11%), and 15 were published in the 2010s (83%). Six studies were from Europe (33%), five were from North America (28%), four were from Australia/New Zealand (22%), and three were from Asia/Middle East (17%). The study size averaged 88 participants per study and ranged from 19 to 238. The mean age was 32 years (range 26-35 years), the mean baseline BMI was 29.8 kg/m2 (range 25.4-34.1 kg/m2), and the average baseline HbA1c% was 6.3% (range 5.0%-8.8%).
Table 1.
Study Characteristics.
| Study | Publication year | Country | Duration | Sample size | Mean age (years) | Baseline HbA1c% | Baseline BMI (kg/m2) |
|---|---|---|---|---|---|---|---|
| Downs et al 50 | 2017 | United States | Week 32 | 43 | 31.5 | N/A | 28.8 |
| Durnwald et al 51 | 2016 | United States | To birth | 101 | 32.0 | N/A | 31.5 |
| Fallucca et al 52 | 1996 | Italy | Week 37,6 | 19 | 28.5 | 6.3 | N/A |
| Given et al 53 | 2015 | Ireland | To birth | 50 | 31.7 | N/A | 33.1 |
| Homko et al 54 | 2007 | United States | To birth | 63 | 29.5 | N/A | 33.0 |
| Homko et al 55 | 2012 | United States | To birth | 80 | 30.2 | N/A | 34.1 |
| Mackillop et al 56 | 2018 | United Kingdom | To birth | 203 | 33.4 | 5.4 | 31.4 |
| Miremberg et al 57 | 2018 | Israel | To birth | 126 | 31.9 | 5.2 | 27.1 |
| Perez-Ferre et al 58 | 2010 | Spain | To birth | 100 | 33.8 | 5.1 | 28.5 |
| Rasekaba et al 59 | 2018 | Australia | To birth | 95 | 32.0 | N/A | N/A |
| Ładyzyński et al 60 | 2007 | Poland | Six months post partum | 32 | 26.0 | 8.0 | N/A |
| Al-Olfi et al 61 | 2018 | Saudi Arabia | Six weeks post partum | 57 | 32.5 | N/A | 30.6 |
| Borgen et al 62 | 2019 | Norway | To birth | 238 | N/A | N/A | N/A |
| Carolan-Olah and Sayakhot 63 | 2019 | Australia | To birth | 110 | 31.7 | 8.8 | 29.6 |
| Cheung et al 64 | 2019 | Australia | To birth | 60 | 34.0 | N/A | 28.4 |
| Guo et al 65 | 2019 | Canada | To birth | 124 | 30.9 | 6.0 | 25.7 |
| Jelsma et al 66 | 2018 | Australia | Six months post partum | 59 | 35.4 | N/A | 30.7 |
| Sung et al 67 | 2019 | South Korea | To birth | 21 | 33.4 | 5.4 | 25.4 |
Abbreviations: BMI, body mass index; N/A, not available.
Table 2 presents an overview of the telemedicine characteristics. Eight studies (44%) were conducted in a hospital setting, seven were conducted in a specialized diabetes clinic (39%), and three were conducted at a university (17%). Two studies performed daily measurements (11%), three performed biweekly measurements (17%), five performed weekly measurements (28%), and three had a tailored approach (17%). The purpose of the intervention components was glycemic improvement (14 studies, 78%), dietary support (16 studies, 89%), exercise support (12 studies, 67%), education (eight studies, 44%), prevention of glycemic events (hypoglycemia or hyperglycemia, ten studies, 56%), and weight control (five studies, 28%). Seven studies used a web portal (61%), seven studies used a telephone (61%), seven studies applied an application (61%), six studies used Short Message System (SMS) technology (33%), and three studies used e-mail (17%). The applied peripherals were glucometers (14 studies, 78%), pedometers (four studies, 22%), blood pressure monitors (one study, 6%), and weights (one study, 6%).
Table 2.
Telemedicine Characteristics.
| Study | Year | Setting | Frequency of contact | Included technology | Purpose of intervention (components) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMS | Weight | Glucometer | Telephone | Web site | App | Blood pressure monitor | Pedometer | Glycemic improvement | Dietary support | Exercise support | Weight control | Prevention of glycemic events | Educational | |||||
| Downs et al 50 | 2017 | University | Biweekly | — | — | — | Yes | — | — | — | Yes | — | — | Yes | Yes | Yes | — | Yes |
| Durnwald et al 51 | 2016 | Hospital | Weekly | — | — | Yes | Yes | — | — | — | Yes | — | Yes | Yes | Yes | Yes | — | Yes |
| Fallucca et al 52 | 1996 | Specialized | Weekly | — | — | Yes | — | — | — | — | — | — | Yes | Yes | Yes | — | Yes | — |
| Given et al 53 | 2015 | Specialized | Weekly | — | Yes | Yes | Yes | Yes | — | Yes | — | Yes | Yes | — | — | Yes | Yes | — |
| Homko et al 54 | 2007 | University | N/A | — | — | Yes | — | Yes | — | — | — | — | Yes | Yes | Yes | — | Yes | Yes |
| Homko et al 55 | 2012 | University | Weekly | — | — | Yes | Yes | Yes | — | — | — | — | Yes | Yes | Yes | — | Yes | — |
| Mackillop et al 56 | 2018 | Hospital | N/A | Yes | — | Yes | — | Yes | Yes | — | — | — | Yes | Yes | — | — | Yes | — |
| Miremberg et al 57 | 2018 | Specialized | Daily | — | — | Yes | — | — | Yes | — | — | Yes | Yes | Yes | — | — | — | — |
| Perez-Ferre et al 58 | 2010 | Hospital | N/A | Yes | — | Yes | Yes | Yes | — | — | — | — | Yes | Yes | — | — | Yes | — |
| Rasekaba et al 59 | 2018 | Specialized | As needed | — | — | Yes | — | Yes | — | — | — | — | Yes | Yes | — | — | Yes | — |
| Ładyzyński et al 60 | 2007 | Specialized | As needed | — | — | Yes | Yes | — | — | — | — | — | Yes | Yes | Yes | — | Yes | — |
| Al-Olfi et al 61 | 2018 | Specialized | Weekly | Yes | — | Yes | — | — | Yes | — | — | — | Yes | Yes | Yes | Yes | Yes | — |
| Borgen et al 62 | 2019 | Specialized | N/A | — | — | Yes | — | — | Yes | — | — | — | Yes | Yes | Yes | — | — | Yes |
| Carolan-Olah and Sayakhot 63 | 2019 | Hospital | N/A | — | — | — | — | Yes | — | — | — | — | — | — | — | — | — | — |
| Cheung et al 64 | 2019 | Hospital | As needed | Yes | — | — | — | — | Yes | — | — | — | — | Yes | Yes | — | — | Yes |
| Guo et al 65 | 2019 | Hospital | Daily | — | — | Yes | — | — | Yes | — | — | — | Yes | Yes | Yes | Yes | Yes | Yes |
| Jelsma et al 66 | 2018 | Hospital | Biweekly | Yes | — | — | Yes | — | — | — | Yes | Yes | — | Yes | Yes | — | — | Yes |
| Sung et al 67 | 2019 | Hospital | Biweekly | Yes | — | Yes | — | — | Yes | — | Yes | — | Yes | Yes | Yes | — | — | Yes |
Abbreviation: SMS, Short Message System; N/A, not available.
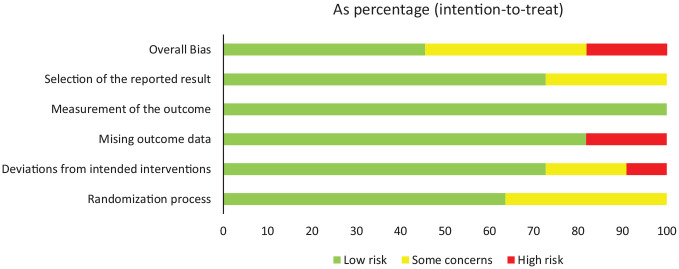
Supplemental Appendix 2 contains the risk of bias assessment for individual studies. Overall, there was a low risk of bias in 45.5% of the included studies, a moderate risk in 36.4% of the included studies, and a high risk in 18.2% of the included studies (Figure 2). Studies with a high risk of bias had a significant amount of missing outcome data at follow-up (18.2% of studies) without appropriately accounting for the potential impact of missing data or deviations from the intended interventions (9.1%).
Figure 2.
Risk of bias assessment.
Abbreviation: CI, confidence interval.
Effect on Infant Birth Weight
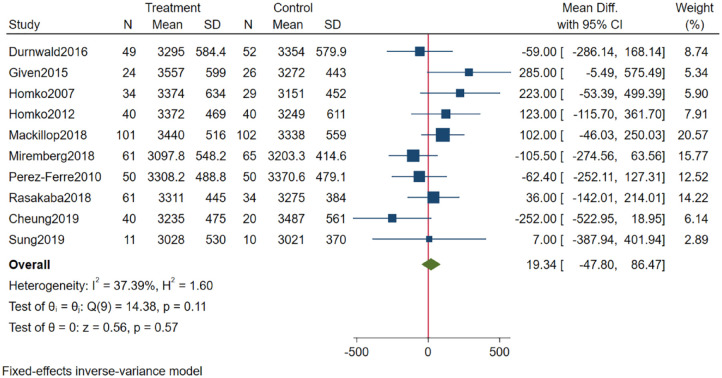
The forest plot in Figure 3 reveals that the MD across studies was 19.34 g (95% confidence interval [CI]: −47.80; 86.47). Although statistically insignificant, the results favored alternatives to telemedicine (the control alternative). Heterogeneity was moderate (I2 = 37.39%). Subgroups of studies with an average baseline BMI ≤30 kg/m2 achieved significantly higher effects with telemedicine than studies with average BMI values >30 kg/m2 (MD: −106.43 g [95% CI: −216.32; 3.47] vs MD: 94.22 g [95% CI: 9.42; 179.02]; see Supplemental Appendix 3). Furthermore, studies with no glycemic prevention component or no Web site component reported better outcomes on birth weight than studies including a glycemic or Web site component (MD: −110 g [95% CI: 226.45; 5.42] vs MD: 84.85 g [95% CI: 2.5; 167.19]; also, Supplemental Appendix 2). Removing the two largest differences in effect (Cheung et al and Given et al) did not alter the conclusion (MD: 22.12 [95% CI: −49.23; 93.48]; Supplemental Appendix 4).
Figure 3.
Results from meta-analysis on infant birth weight.
Abbreviation: CI, confidence interval.
Certainty of Findings for Infant Birth Weight
The GRADE profile and reasons for downgrading outcomes are presented in Table 3. The pooled estimate of the effect of telemedicine on infant birth weight was assessed as moderate due to serious problems with imprecision. No downgrades were performed due to risk of bias (a low proportion of studies with a high risk of bias, a low risk of bias among studies with high meta-analysis weights, and a nonsignificant relations between the risk of bias score and study weights or treatment effect) and indirectness (all studies fulfilled broad inclusion criteria). Evidence was not downgraded due to inconsistency. Although point estimates varied considerably, the test for equal treatment effects in studies was insignificant and heterogeneity was moderate. Publication bias was not strongly suspected (the funnel plot in Figure 4 is rather symmetrical), and Egger’s test was insignificant (P = .65). Industry sponsorship was undeclared or unknown in four of ten studies, although significant relationships were not observed among the presence of industry sponsorship, study size, or treatment effect. The evidence was not upgraded.
Table 3.
GRADE Profile.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Telemedicine | Usual care | Relative (95% CI) | Absolute (95% CI) | ||
| Infant birth weight | ||||||||||||
| 10 | Randomized trials | Not serious | Not serious | Not serious | Serious a | None | 471 | 428 | — | MD 19.34 g higher (47.8 lower to 86.47 higher) | ⨁⨁⨁◯ Moderate |
|
| Hypoglycemic events (mother) | ||||||||||||
| 2 | Randomized trials | Serious b | Not serious | Not serious | Very serious c | None | 26 | 25 | — | SMD 0.34 SD lower (0.9 lower to 0.22 higher) |
⨁◯◯◯ Very low |
|
| HbA1c% | ||||||||||||
| 5 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 148 | 140 | — | MD 0.14 lower (0.51 lower to 0.23 higher) |
⨁⨁◯◯ Low |
|
| Fasting blood glucose | ||||||||||||
| 4 | Randomized trials | Serious d | Not serious | Not serious | Serious a | None | 134 | 131 | — | MD 0.12 mg/dL lower (3.13 lower to 2.89 higher) |
⨁⨁◯◯ Low |
|
| Two-hour glucose tolerance | ||||||||||||
| 2 | Randomized trials | Not serious | Not serious | Serious e | Very serious a | None | 164 | 175 | — | MD 9.62 mg/dL higher (1.95 higher to 17.28 higher) |
⨁◯◯◯ Very low |
|
| Weight gain during pregnancy (mother) | ||||||||||||
| 2 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 150 | 154 | — | SMD 0 SD (0.22 lower to 0.23 higher) |
⨁⨁◯◯ Low |
|
| Pregnancy-induced hypertension/preeclampsia | ||||||||||||
| 7 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 29/352 (8.2%) | 26/350 (7.4%) | Log odds 0.21 (−0.37 to 0.79) | — per 1000 (from — to —) | ⨁⨁◯◯ Low |
|
| Gestational age at delivery | ||||||||||||
| 11 | Randomized trials | Not serious | Not serious | Not serious | Serious a | None | 504 | 478 | — | MD 0.05 weeks lower (0.24 lower to 0.14 higher) |
⨁⨁⨁◯Moderate | |
| Induced labor | ||||||||||||
| 3 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 104/221 (47.1%) | 110/233 (47.2%) | Log odds 0.00(−0.37 to 0.38) | — per 1000 (from — to —) |
⨁⨁◯◯ Low |
|
| Cesarean delivery | ||||||||||||
| 11 | Randomized trials | Not serious | Serious f | Not serious | Serious a | None | 191/595 (32.1%) | 209/575 (36.3%) | Log odds −0.11(−0.50 to 0.27) | — per 1000(from — to —) | ⨁⨁◯◯ Low |
|
| Preterm delivery | ||||||||||||
| 3 | Randomized trials | Serious d | Not serious | Not serious | Very serious a | None | 10/117 (8.5%) | 12/111 (10.8%) | Log odds −0.38(−1.31 to 0.55) | — per 1000 (from — to —) |
⨁◯◯◯ Very low |
|
| Premature membrane rupture | ||||||||||||
| 2 | Randomized trials | Serious d | Serious g | Not serious | Very serious a | None | 3/68 (4.4%) | 3/65 (4.6%) | Log odds 0.00(−3.13 to 3.13) | — per 1000(from — to —) | ⨁◯◯◯ Very low |
|
| Placental abruption | ||||||||||||
| 2 | Randomized trials | Serious d | Not serious | Not serious | Very serious a | None | 1/81 (1.2%) | 1/73 (1.4%) | Log odds −0.14(−2.14 to 1.87) | — per 1000 (from — to —) |
⨁◯◯◯ Very low |
|
| Stillbirth | ||||||||||||
| 2 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | Publication bias strongly suspected h | 0/81 (0.0%) | 1/86 (1.2%) | Log odds −0.58(−3.02 to 1.86) | — per 1000 (from — to —) |
⨁◯◯◯ Very low |
|
| Neonatal hypoglycemia | ||||||||||||
| 7 | Randomized trials | Not serious | Not serious | Not serious | Serious a | None | 51/358 (14.2%) | 44/354 (12.4%) | Log odds 0.19(−0.27 to 0.64) | — per 1000 (from — to —) |
⨁⨁⨁◯ Moderate |
|
| Jaundice | ||||||||||||
| 5 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 26/238 (10.9%) | 26/240 (10.8%) | Log odds 0.14(−0.47 to 0.76) | — per 1000 (from — to —) |
⨁⨁◯◯ Low |
|
| Shoulder dystocia | ||||||||||||
| 4 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 1/234 (0.4%) | 10/236 (4.2%) | Log odds −1.34 (−2.61 to −0.08) |
— per 1000 (from — to —) |
⨁⨁◯◯ Low |
|
| Infant respiratory distress | ||||||||||||
| 3 | Randomized trials | Serious d | Not serious | Not serious | Very serious a | None | 8/89 (9.0%) | 12/89 (13.5%) | Log odds −0.53(−1.49 to 0.44) | — per 1000(from — to —) | ⨁◯◯◯ Very low |
|
| Neonates large for gestational age | ||||||||||||
| 5 | Randomized trials | Serious d | Not serious | Not serious | Very serious a | None | 30/189 (15.9%) | 21/180 (11.7%) | Log odds 0.34 (−0.26 to 0.94) |
— per 1000(from — to —) | ⨁◯◯◯ Very low |
|
| Admissions to neonatal intensive care | ||||||||||||
| 7 | Randomized trials | Not serious | Not serious | Not serious | Serious a | None | 57/423 (13.5%) | 61/403 (15.1%) | Log odds −0.12 (−0.53 to 0.28) |
— per 1000(from — to —) | ⨁⨁⨁◯ Moderate |
|
Abbreviations: GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; CI, confidence interval; MD, mean difference; SMD, standardized mean difference; HbA1c, glycated hemoglobin A1c.
Recommendation would be different depending on whether or not the lower or the upper boundary of the confidence interval is the true underlying effect. Furthermore, evidence fails to meet the optimal information criteria.
One of the two studies had a high risk of bias.
Only two small studies, broad confidence intervals, not fulfilling optimal information size.
Studies with high weights in meta-analysis have serious or moderate problems with bias.
Assessed up to three months post partum.
Point estimates vary, test for similar effect size statistically significant, heterogeneity large.
Point estimates vary, heterogeneity large.
Studies assigned dominant weight in meta-analysis in favor of telemedicine is industry sponsored.
Figure 4.
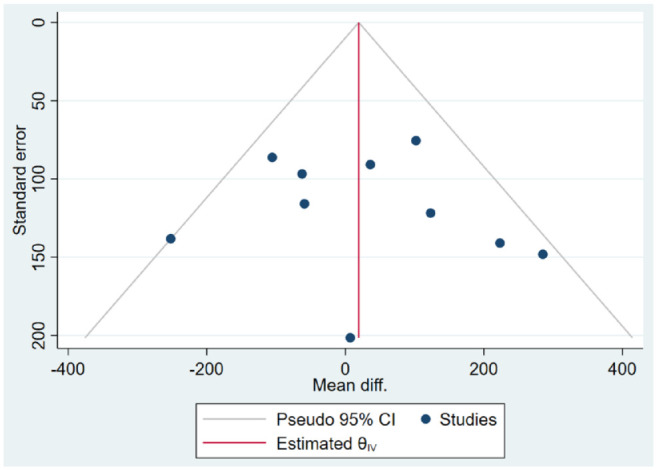
Funnel plot.
Abbreviation: CI, confidence interval.
Secondary Outcomes
Meta-analyses were conducted on maternal (Supplemental Appendix 5), delivery (Supplemental Appendix 6), and neonatal outcomes (Supplemental Appendix 7). Associated GRADE levels are presented in Table 3. For maternal outcomes, only the difference (SMD: 9.62 mg/dL [95% CI: 1.95; 17.28]) in two-hour glucose tolerance was statistically significant (5% level) and favored the comparator (GRADE level: very low). Nonsignificant differences favoring telemedicine were hypoglycemic events during pregnancy (GRADE: very low), HbA1c% (GRADE: low), and fasting blood glucose (GRADE: low). The nonsignificant risk of pregnancy-induced hypertension or preeclampsia favored the control group (GRADE: low). For neonatal outcomes, the risk of shoulder dystocia significantly favored telemedicine (log odds −1.34 [95% CI: −2.61; −0.08], GRADE level: low). The nonsignificant risks that favored telemedicine were risk of stillbirth (GRADE: very low), infant respiratory distress (GRADE: very low), and admission to neonatal intensive care (GRADE: moderate). Outcomes favoring the control were risk of jaundice (GRADE: low), neonatal hypoglycemia (GRADE: moderate), and receiving LGA (GRADE: very low). No differences in delivery outcomes were statistically significant, although outcomes favoring telemedicine included the risk of placental abruption (GRADE: very low) and preterm and cesarean delivery (GRADE: very low and low, respectively), while the outcome that favored the control was differences in gestational age at delivery (GRADE: moderate).
Discussion
The present systematic review and meta-analysis aimed to evaluate the effectiveness of telemedicine solutions versus any comparator without the use of telemedicine with regard to diabetes-related maternal and fetal outcomes for patients with gestational or pregestational diabetes. Eighteen studies were included in the final sample. With regard to the primary outcome (infant birth weight), the results nonsignificantly favored the control. For maternal outcomes, only the difference in two-hour glucose tolerance was statistically significant (favoring control). For neonatal outcomes, only the risk of shoulder dystocia was statistically significant (favoring telemedicine). The remaining outcomes were all insignificant and varied based on whether they favored telemedicine or control. Moreover, the GRADE value was low or very low for most of the outcomes.
A strength of the present review is that it was based on a very comprehensive literature search, although there are also several limitations to this study. First, the synthesis is based on a small number of studies in general and very few studies for some secondary outcomes. Second, the choice of unadjusted infant birth weight could ideally have been adjusted for both gestational age at delivery and fetal gender simultaneously to nuance the results. However, gestational age at delivery is underreported in the included studies (included in 9 of 18 studies for both groups) and fetal gender is lacking, making it hard to conduct a meaningful meta-regression. Third, despite a comprehensive search, certain articles may not have been identified because only studies published in English or Scandinavian languages were considered.
The findings of the present review are in line with the findings from a previous systematic review and meta-analysis by Rasekaba et al, 34 who found similar glycemic control and cesarean section rates when comparing telemedicine for GDM to usual care. Similarly, a systematic review by Dennis and Kingston comparing telephone support to usual care found no significant improvement in the number of preterm births. In contrast, Dennis and Kingston found a statistically significant difference in the number of low-birth weight infants in favor of telephone support. 33 A systematic review and meta-analysis by Ming et al 35 found a modest significant improvement in HbA1c% in favor of telemedicine when comparing telemedicine for GDM to usual care. However, no significant differences were found in the mean glucose levels, fasting glucose, two-hour postprandial blood glucose, postprandial hypertension or preeclampsia rates, cesarean sections rates, birth weight, macrosomia, LGA, neonatal intensive care unit admissions, or neonatal hypoglycemia. 35 Hence, our findings mirror the findings of Ming et al, who did not find sufficient evidence to support the superiority of telemedicine for GDM over usual care. 35
One may assume that the lack of evidence supporting telemedicine for diabetes in pregnancy may partly be attributable to the fact that pregnant women are followed closely by relevant health care professionals throughout pregnancy. 68 Hence, adding telemedicine may not contribute to improved diabetes care since women may already be closely monitored as part of the usual care scenario.
Telemedicine studies often differ in terms of technology, intervention, organizational setup, and so on and are thus difficult to compare. However, for the present review, the heterogeneity was moderate (I2 = 37%), which is fairly low when compared with other reviews within the field of telemedicine.23,69 The included studies varied greatly in terms of duration, follow-up time, intervention components, and technologies used (Tables 1 and 2), which may explain the variations in the results of the meta-analysis. Another explanation for the variations could be the heterogeneity following diabetes subtypes. Thus, the differences in disease management, pathophysiology, and progress between preexisting diabetes and GDM may also be a possible explanation for the high variance reported in the study results.11,70,71
The focus of this review was solely on the effectiveness of telemedicine solutions for diabetes-related maternal, fetal, and delivery outcomes. Thus, the impact on personal resources and economics was not considered. However, such perspectives are highly relevant considering the growing diabetic population,1,12 lack of health care resources,72,73 and consequences of the COVID-19 pandemic. 74 With similar maternal, fetal, and delivery outcomes between telemedicine and usual care, the question remains as to whether telemedicine solutions may offer resource savings and cost advantages over usual care. Such a finding would be consistent with speculations in previous studies, where telemedicine has been stated to improve efficiency 55 and lead to net future cost savings in patients with gestational diabetes. 34 Moreover, a Danish study found an estimated net cost savings of approximately $9000 to $11 000 per 200 consultations per year when evaluating a telemedicine service for patients with T1DM and T2DM, which was mainly due to lower transportation costs. 75 Such perspectives would be highly relevant in future studies.
Conclusions
In conclusion, the present systematic review and meta-analysis did not find sufficient evidence to support telemedicine as an alternative to usual care when considering maternal and fetal outcomes for patients with pregestational or gestational diabetes. However, studies on telemedicine solutions for pregnant women with diabetes as well as economic evaluations in the field are still limited and should be prioritized in future research.
Supplemental Material
Supplemental material, sj-docx-1-dst-10.1177_19322968221094626 for Effectiveness of Telemedicine in Managing Diabetes in Pregnancy: A Systematic Review and Meta-Analysis by Sisse H. Laursen, Lise Boel, Flemming W. Udsen, Pernille H. Secher, Jonas D. Andersen, Peter Vestergaard, Ole K. Hejlesen and Stine Hangaard in Journal of Diabetes Science and Technology
Acknowledgments
The authors would like to thank the Research Librarian Connie Skrubbeltrang, who assisted in the literature search.
Footnotes
Abbreviations: GRADE, The Grading of Recommendations, Assessment, Development, and Evaluation; GDM, gestational diabetes mellitus; SMD, standardized mean difference; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; CENTRAL, the Cochrane Library Central Register of Controlled Trials; RCT, randomized controlled trial; BMI, body mass index; MD, mean difference; RoB tool, revised Cochrane risk-of-bias tool; HbA1c, glycated hemoglobin A1c.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This is an independent article commissioned and jointly funded by the Steno Diabetes Center North Denmark and Aalborg University.
ORCID iDs: Sisse H. Laursen  https://orcid.org/0000-0001-5884-8125
https://orcid.org/0000-0001-5884-8125
Flemming W. Udsen  https://orcid.org/0000-0003-2293-9169
https://orcid.org/0000-0003-2293-9169
Jonas D. Andersen  https://orcid.org/0000-0001-6619-9161
https://orcid.org/0000-0001-6619-9161
Stine Hangaard  https://orcid.org/0000-0003-0395-3563
https://orcid.org/0000-0003-0395-3563
Data Sharing: No additional data are available.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [DOI] [PubMed] [Google Scholar]
- 2. Iversen K, DeVoe M. Addressing hyperglycemia in pregnancy: the impact on maternal health and beyond. Diabetes Res Clin Pract. 2018;145:15-16. [DOI] [PubMed] [Google Scholar]
- 3. Bonora E, DeFronzo RA. Diabetes Complications, Comorbidities and Related Disorders. Cham, Switzerland: Springer; 2018. [Google Scholar]
- 4. Macintosh MCM, Fleming KM, Bailey JA, et al. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: population based study. Br Med J. 2006;333:177-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wahabi H. Maternal and perinatal outcomes of pregnancies complicated with pregestational and gestational diabetes mellitus in Saudi Arabia. J Diabetes Metab. 2014;5:7. [Google Scholar]
- 6. Buhary B, Almohareb O, Aljohani N, et al. Glycemic control and pregnancy outcomes in patients with diabetes in pregnancy: a retrospective study. Indian J Endochrinology Metab. 2016;20:481-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kong L, Nilsson IAK, Gissler M, Lavebratt C. Associations of maternal diabetes and body mass index with offspring birth weight and prematurity. JAMA Pediatr. 2019;173:371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim SY, Sharma AJ, Sappenfield W, Wilson HG, Salihu HM. Association of maternal body mass index, excessive weight gain, and gestational diabetes mellitus with large-for-gestational-age births. Obs Gynecol. 2015;123:737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yanga Y, Wang Z, Mo M, et al. The association of gestational diabetes mellitus with fetal birth weight. J Diabetes Complications. 2018;32:635-642. [DOI] [PubMed] [Google Scholar]
- 10. Beta J, Khan N, Fiolna M, Khalil A, Ramadan G, Akolekar R. Maternal and neonatal complications of fetal macrosomia : cohort study. Ultrasound Obs Gynecol. 2019;54:319-325. [DOI] [PubMed] [Google Scholar]
- 11. Ringholm L, Damm P, Mathiesen ER. Improving pregnancy outcomes in women with diabetes mellitus: modern management. Nat Rev Endocrinol. 2019;15:406-416. [DOI] [PubMed] [Google Scholar]
- 12. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Diabetes Association. 14. Management of diabetes in pregnancy: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):165-172. [DOI] [PubMed] [Google Scholar]
- 14. Ballas J, Moore TR, Ramos GA. Management of diabetes in pregnancy. Curr Diab Rep. 2012;12:33-42. [DOI] [PubMed] [Google Scholar]
- 15. Tion ADA. 13. Management of diabetes in pregnancy: standards of medical care in diabetesd—2018. Diabetes Care. 2018;41:137-143. [DOI] [PubMed] [Google Scholar]
- 16. Daponte A, Guidozzi F, Moisuc D, Marineanu A. Management of diabetic pregnant patients in a tertiary center in the developing world. Int J Gynecol Obstet. 1999;64:141-146. [DOI] [PubMed] [Google Scholar]
- 17. Mirghani OA, Saeed OK. A simplified management of diabetic pregnant women. Saudi Med J. 2000;21:335-339. [PubMed] [Google Scholar]
- 18. Ozumba BC, Obi SN, Oli JM. Diabetes mellitus in pregnancy in an African population. Int J Gynecol Obstet. 2004;84:114-119. [DOI] [PubMed] [Google Scholar]
- 19. Ahkter J, Qureshi R, Rahim F, et al. Diabetes in pregnancy in Pakistani women: prevalence and complications in an indigenous south Asian community. Diabet Med. 1996;13:189-191. [DOI] [PubMed] [Google Scholar]
- 20. Mukona D, Munjanja SP, Zvinavashe M, Stray-Pederson B. Barriers of adherence and possible solutions to nonadherence to antidiabetic therapy in women with diabetes in pregnancy: patients’ perspective. J Diabetes Res. 2017;1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martis R, Brown J, McAra-Couper J, Crowther CA. Enablers and barriers for women with gestational diabetes mellitus to achieve optimal glycaemic control—a qualitative study using the theoretical domains framework. BMC Pregnancy Childbirth. 2018;18:1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaufman N, Salahi A. Using digital health technology to prevent and treat diabetes. Diabetes Technol Ther. 2017;19(suppl 1):59-73. [DOI] [PubMed] [Google Scholar]
- 23. Faruque LI, Wiebe N, Ehteshami-Afshar A, et al. Effect of telemedicine on glycated hemoglobin in diabetes: a systematic review and meta-analysis of randomized trials. CMAJ. 2017;189(9):341-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aberer F, Hochfellner DA, Mader JK. Application of telemedicine in diabetes care: the time is now. Diabetes Ther. 2021;12:629-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crico C, Renzi C, Graf N, et al. mHealth and telemedicine apps : in search of a common regulation. Ecancermedicalscience. 2018;12:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahar JH, Rosencrance GJ, Rasmussen PA. Telemedicine: past, present, and future. Cleve Clin J Med. 2018;85(12):938-942. [DOI] [PubMed] [Google Scholar]
- 27. Shah TK, Tariq T, Phillips R, Davison S, Hoare A, Hasan SS. Health care for all: effective, community supported, healthcare with innovative use of telemedicine technology. J Pharm Policy Pr. 2018;11:10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanlon P, Daines L, Campbell C, McKinstry B, Weller D, Pinnock H. Telehealth interventions to support self-management of long-term conditions: a systematic metareview of diabetes, heart failure, asthma, chronic obstructive pulmonary disease, and cancer. J Med Internet Res. 2017;19(5):e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. So CF, Chung JWY. Telehealth for diabetes self-management in primary healthcare: a systematic review and meta-analysis. J Telemed Telecare. 2018;24:356-364. [DOI] [PubMed] [Google Scholar]
- 30. Borries TM, Dunbar A, Bhukhen A, et al. The impact of telemedicine on patient self-management processes and clinical outcomes for patients with Types I or II Diabetes Mellitus in the United States: a scoping review. Diabetes Metab Syndr Clin Res Rev. 2019;13:1353-1357. [DOI] [PubMed] [Google Scholar]
- 31. Von Storch K, Graaf E, Wunderlich M, Rietz C, Polidori MC, Woopen C. Telemedicine-assisted self-management program for type 2 diabetes patients. Diabetes Technol Ther. 2019;21:514-521. [DOI] [PubMed] [Google Scholar]
- 32. Sushko K, Menezes HT, Strachan P, Butt M, Sherifali D. Self-management education among women with pre-existing diabetes in pregnancy: a scoping review. Int J Nurs Stud. 2021;117:103883. [DOI] [PubMed] [Google Scholar]
- 33. Dennis CL, Kingston D. A systematic review of telephone support for women during pregnancy and the early postpartum period. J Obstet Gynecol Neonatal Nurs. 2008;37:301-314. [DOI] [PubMed] [Google Scholar]
- 34. Rasekaba TM, Furler J, Blackberry I, Tacey M, Gray K, Lim K. Telemedicine interventions for gestational diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2015;110:1-9. [DOI] [PubMed] [Google Scholar]
- 35. Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xie W, Dai P, Qin Y, Wu M, Yang B, Yu X. Effectiveness of telemedicine for pregnant women with gestational diabetes mellitus: an updated meta-analysis of 32 randomized controlled trials with trial sequential analysis. BMC Pregnancy Childbirth. 2020;20:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moher D, Liberati A, Tetzlaff J, Altman DG, The Prisma Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laursen S, Hangaard S, Udsen F, Vestergaard P, Hejlesen O. Effectiveness of telemedicine solutions for the management of patients with diabetes: protocol for a systematic review and meta-analysis. JMIR Res Protoc. 2020;9:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:1-8. [DOI] [PubMed] [Google Scholar]
- 42. Schünemann H, Brozek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. 2013. [Google Scholar]
- 43. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64:407-415. [DOI] [PubMed] [Google Scholar]
- 44. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64:1303-1310. [DOI] [PubMed] [Google Scholar]
- 45. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283-1293. [DOI] [PubMed] [Google Scholar]
- 46. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294-1302. [DOI] [PubMed] [Google Scholar]
- 47. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64:1277-1282. [DOI] [PubMed] [Google Scholar]
- 48. Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles—continuous outcomes. J Clin Epidemiol. 2013;66:173-183. [DOI] [PubMed] [Google Scholar]
- 49. Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66:151-157. [DOI] [PubMed] [Google Scholar]
- 50. Downs DS, DiNallo JM, Birch LL, Paul IM, Ulbrecht JS. Randomized face-to-face vs. home exercise interventions in pregnant women with gestational diabetes. Psychol Sport Exerc. 2017;30:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Durnwald D, Kallan MJ, Allison KC, et al. A randomized clinical trial of an intensive behavior education program in gestational diabetes. Am J Perinatol. 2016;33:1145-1151. [DOI] [PubMed] [Google Scholar]
- 52. Fallucca F, Di Biase N, Sabbatini A, Borrello E, Sciullo E, Napoli A. Telemedicine in the treatment of diabetic pregnancy. Ann Ist Super Sanita. 1997;33:347-351. [PubMed] [Google Scholar]
- 53. Given JE, Bunting BP, O’Kane MJ, Dunne F, Coates VE. Tele-Mum: a feasibility study for a randomized controlled trial exploring the potential for telemedicine in the diabetes care of those with gestational diabetes. Diabetes Technol Ther. 2015;17:880-888. [DOI] [PubMed] [Google Scholar]
- 54. Homko CJ, Santamore WP, Bower M, et al. Use of an internet-based telemedicine system to manage underserved women with gestational diabetes mellitus. Diabetes Technol Ther. 2007;9:297-306. [DOI] [PubMed] [Google Scholar]
- 55. Homko CJ, Deeb LC, Rohrbacher K, et al. Impact of a telemedicine system with automated reminders on outcomes in women with gestational diabetes mellitus. Diabetes Technol Ther. 2012;14:624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mackillop L, Hirst JE, Bartlett KJ, et al. Comparing the efficacy of a mobile phone-based blood glucose management system with standard clinic care in women with gestational diabetes: randomized controlled trial. JMIR Mhealth Uhealth. 2018;6:e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miremberg H, Ben-Ari T, Betzer T, et al. The impact of a daily smartphone-based feedback system among women with gestational diabetes on compliance, glycemic control, satisfaction, and pregnancy outcome: a randomized controlled trial. Am J Obstet Gynecol. 2018;218:453e1-453e7. [DOI] [PubMed] [Google Scholar]
- 58. Perez-Ferre N, Galindo M, Fernandez M, et al. The outcomes of gestational diabetes mellitus after a telecare approach are not inferior to traditional outpatient clinic visits. Int J Endocrinol. 2010;2010:386941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rasekaba TM, Furler J, Young D, et al. Using technology to support care in gestational diabetes mellitus: quantitative outcomes of an exploratory randomised control trial of adjunct telemedicine for gestational diabetes mellitus (TeleGDM). Diabetes Res Clin Pract. 2018;142:276-285. [DOI] [PubMed] [Google Scholar]
- 60. Ładyzyński P, Wójcicki JM. Home telecare during intensive insulin treatment—Metabolic control does not improve as much as expected. J Telemed Telecare. 2007;13:44-47. [DOI] [PubMed] [Google Scholar]
- 61. Al-Olfi E, Mosli H, Ghramri K, Ghazali S. Management of postprandial hyperglycaemia and weight gain in women with gestational diabetes mellitus using a novel telemonitoring system. J Int Med Res. 2019;47(2):754-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Borgen I, Småstuen MC, Jacobsen AF, et al. Effect of the Pregnant+ smartphone application in women with gestational diabetes mellitus: a randomised controlled trial in Norway. BMJ Open. 2019;9:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Carolan-Olah M, Sayakhot P. A randomized controlled trial of a web-based education intervention for women with gestational diabetes mellitus. Midwifery. 2019;68:39-47. [DOI] [PubMed] [Google Scholar]
- 64. Cheung NW, Blumenthal C, Smith BJ, et al. A pilot randomised controlled trial of a text messaging intervention with customisation using linked data from wireless wearable activity monitors to improve risk factors following gestational diabetes. Nutrients. 2019;11(3):590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Guo H, Zhang Y, Li P, Zhou P, Chen LM, Li SY. Evaluating the effects of mobile health intervention on weight management, glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus. J Endocrinol Invest. 2019;42(6):709-714. [DOI] [PubMed] [Google Scholar]
- 66. Jelsma J, van Poppel M, Smith B, Cinnadaio N, Bauman A. Changing psychosocial determinants of physical activity and diet in women with a history of gestational diabetes mellitus. Diabetes/ Metab Res Rev. 2018;34(1):e2942-1-e2942-9. [DOI] [PubMed] [Google Scholar]
- 67. Sung J, Lee D, Min K, Park C. Peripartum management of gestational diabetes using a digital health care service: a pilot, randomized controlled study. Clin Ther. 2019;41(11):2426-2434. [DOI] [PubMed] [Google Scholar]
- 68. American Diabetes Association. 14. Management of diabetes in pregnancy : standards of medical care in diabetes—2021. Diabetes Care. 2021;44:200-210. [DOI] [PubMed] [Google Scholar]
- 69. Tchero H, Kangambega P, Briatte C, Brunet-Houdard S, Retali GR, Rusch E. Clinical effectiveness of telemedicine in diabetes mellitus: a meta-analysis of 42 randomized controlled trials. Telemed e-Health. 2019;25:569-583. [DOI] [PubMed] [Google Scholar]
- 70. Alexopoulos A, Blair R, Peters AL, et al. Management of preexisting diabetes in pregnancy: a Review. JAMA. 2019;321:1811-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang M, Zhou Y, Zhong J, Wang K, Ding Y, Li L. Current guidelines on the management of gestational diabetes mellitus : a content analysis and appraisal. 2019;2:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Blanchfield MA. Job stressors and burnout among immigrant nurses caring for the elderly in the United States of America: the role of working environment condition. J of Human Resource & Leadership. 2021;5(2):1-13. [Google Scholar]
- 73. Halter M, Boiko O, Pelone F, et al. The determinants and consequences of adult nursing staff turnover: a systematic review of systematic reviews. BMC Health Serv Res. 2017;17:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Manteghinejad A, Javanmard SH. Challenges and opportunities of digital health in a post—COVID19 world. J Res Med Sci. 2021;26(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Levin K, Madsen JR, Petersen I, Wanscher CE, Hangaard J. Telemedicine diabetes consultations are cost-effective, and effects on essential diabetes treatment parameters are similar to conventional treatment: 7-year results from the Svendborg telemedicine diabetes project. J Diabetes Sci Technol. 2013;7:587-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dst-10.1177_19322968221094626 for Effectiveness of Telemedicine in Managing Diabetes in Pregnancy: A Systematic Review and Meta-Analysis by Sisse H. Laursen, Lise Boel, Flemming W. Udsen, Pernille H. Secher, Jonas D. Andersen, Peter Vestergaard, Ole K. Hejlesen and Stine Hangaard in Journal of Diabetes Science and Technology