Abstract
Integration of insulin dosing data into the electronic health record (EHR), combined with other patient-generated health care data, would facilitate the use of wirelessly connected insulin delivery systems, including smart insulin pens, insulin pumps, and advanced hybrid closed-loop systems. In 2022, Diabetes Technology Society developed the Integration of Continuous Glucose Monitoring Data into the EHR (iCoDE) Project, which is the first consensus standard for integrating data from a wearable device into the EHR. The iCoDE Standard is a comprehensive guide for any health care delivery organization or hospital for automatically integrating continuous glucose monitoring data into the EHR. Diabetes Technology Society is following iCoDE with the Integration of Connected Diabetes Device Data into the EHR (iCoDE-2) Project, to similarly provide guidance for integrating insulin delivery data into the EHR alongside continuous glucose monitoring data.
Keywords: data integration, data standard, diabetes devices, electronic health record, insulin dosing
Introduction
Connected devices like smart insulin pens, insulin pumps, and advanced hybrid closed-loop systems (AHCLs) make it possible to automatically measure, save, share, and analyze insulin dosing data through smart devices and web-based platforms. Clinicians and people with diabetes (PWD) alike benefit from having access to insulin data. Timeline views allow users to see their insulin doses within the context of their meals, activities, times of day, and glucose concentrations (if available). Clinicians use this information to support clinical decision-making and dosing optimization. PWD benefit from having accurate records and reminders of their insulin doses, which help them with day-to-day disease self-management, such as avoiding skipped doses (undertreatment) or double doses (overtreatment).
Despite the benefits of having access to insulin dosing data in the management of diabetes, this data remains underutilized in health care because of lack of data access and integration. Currently, insulin dosing data is available through proprietary mobile applications or web portals developed either by device manufacturers or third-party data aggregators, such as Tidepool, Glooko, and DreaMed. For PWD, this may mean managing multiple applications or portals to view all their diabetes-related data. Clinicians, in turn, must access this information from clinical versions of these same data portals, requiring them to toggle between multiple data platforms, manage various logins, become familiar with different visual representations of the same data, and manually transcribe insulin data into their notes. Integration of insulin dosing data into the electronic health record (EHR) has the potential to increase the use of the data and applications in health management, as well as address many of the barriers mentioned. Structured insulin dosing data in the EHR can be searched, analyzed, trended, and used to trigger both patient safety and clinical decision support systems, all while decreasing the documentation and time burden of accessing the same data through third-party platforms.
Continuous glucose monitor (CGM) data is obtained through another critical connected diabetes technology. Continuous glucose monitoring has shown significant progress in data integration over the past seven years, most recently exemplified by the successful completion of the 2022 Integration of Continuous Glucose Monitor Data into the Electronic Health Record (iCoDE) Standards and Recommendations Report. 1 This report was a cross-sector effort organized by Diabetes Technology Society (DTS). 2 The report includes extensive technical and operational guidance for both industry and health care delivery organizations (HDOs) for integrating CGM data into the EHR. By comparison, insulin dosing data integration is still in its infancy, and its complexity has slowed adoption. The goal of the Integration of Connected Diabetes Device Data into the EHR (iCoDE-2) Project is to leverage the collaborative and consensus-driven methodology of iCoDE to develop a set of technical and operational standards and recommendations for industry partners and HDOs to successfully integrate insulin dosing data into the EHR.
Current State: Connected Insulin Delivery Devices and Data Presentations
As of May 18, 2023, there are six product families cleared by the United States Food and Drug Administration (FDA) and commercially available for insulin delivery. A product family refers to related devices from the same manufacturer that share the same or similar name and a core set of functions and often represent iterative improvements.
For the sake of simplicity, we will only discuss the most recent model in each product family. The currently available connected insulin devices include three insulin pumps/AHCLs (Medtronic MiniMed 780G system [Medtronic Diabetes, Northridge, CA, USA], Tandem t:slim X2 Insulin Pump with Control-IQ [Tandem Diabetes Care, San Diego, CA, USA] and Omnipod 5 Automated Insulin Delivery Systems [Insulet, Acton, MA, USA]) and five connected insulin pen or pen caps (Bigfoot Biomedical Unity smart pen cap [Bigfoot Biomedical, Milpitas, CA, USA], Medtronic InPen [Medtronic Diabetes, Northridge, CA, USA], Lilly Tempo Personalized Diabetes Management Platform [Eli Lilly and Company, Indianapolis, IN, USA], SoloSmart [BioCorp, Issoire, Auvergne, France], and NovoPen6/NovoPen Echo [Novo Nordisk, Bagsværd, Denmark). Each product family mentioned is either FDA-cleared or from a major diabetes product manufacturer. A brief description of each device and how they display insulin dosing data is presented below. Each insulin delivery manufacturer was asked to provide a figure of their insulin dosing data platform, and the submitted figures are presented in Figures 1 to 9. Two data aggregators, Glooko and Tidepool, provide visualizations for insulin dosing data, and their formats are displayed in Figures 10 and 11.
Figure 1.
Medtronic MiniMed 780G system CareLink report. The long vertical red line shows that the sensor glucose level is >120 mg/dL and that the maximum automatic basal delivery is occurring, so that auto-corrections can begin as shown by the little blue lines. Up to 12 auto-corrections per hour can be delivered.
Source: Figure courtesy of Medtronic Diabetes.
Figure 9.
Two views of FreeStyle LibreLink after connecting with NovoPen 6 or NovoPen Echo Plus.
Source: Figure courtesy of Novo Nordisk.
Figure 10.
Glooko insulin dosing data platform.
Abbreviation: AGP, ambulatory glucose profile; CGM, continuous glucose monitor; CV, coefficient of variation; GMI, glucose management indicator.
Source: Figure courtesy of Glooko.
Figure 11.
Tidepool insulin dosing data visualization.
Abbreviation: BGM, blood glucose monitor; CGM, continuous glucose monitor.
Source: Figure courtesy of Tidepool.
The Medtronic MiniMed 780G system (Figure 1) is an insulin pump that offers automated insulin delivery by adjusting the rate of basal insulin delivery every five minutes, as well as making autocorrections up to every five minutes using sensor glucose data from the Guardian 4 CGM (Medtronic Diabetes, Northridge, CA, USA), which requires no calibration and no fingersticks while the system is in automation (SmartGuard). In addition, a proprietary algorithm suggests bolus insulin doses for meals and correction of hyperglycemia based on the current glucose level and its trend, carbohydrates consumed, and insulin sensitivity. The glucose and insulin data are available in Medtronic’s CareLink software to visually display glucose trends and insulin delivery patterns.
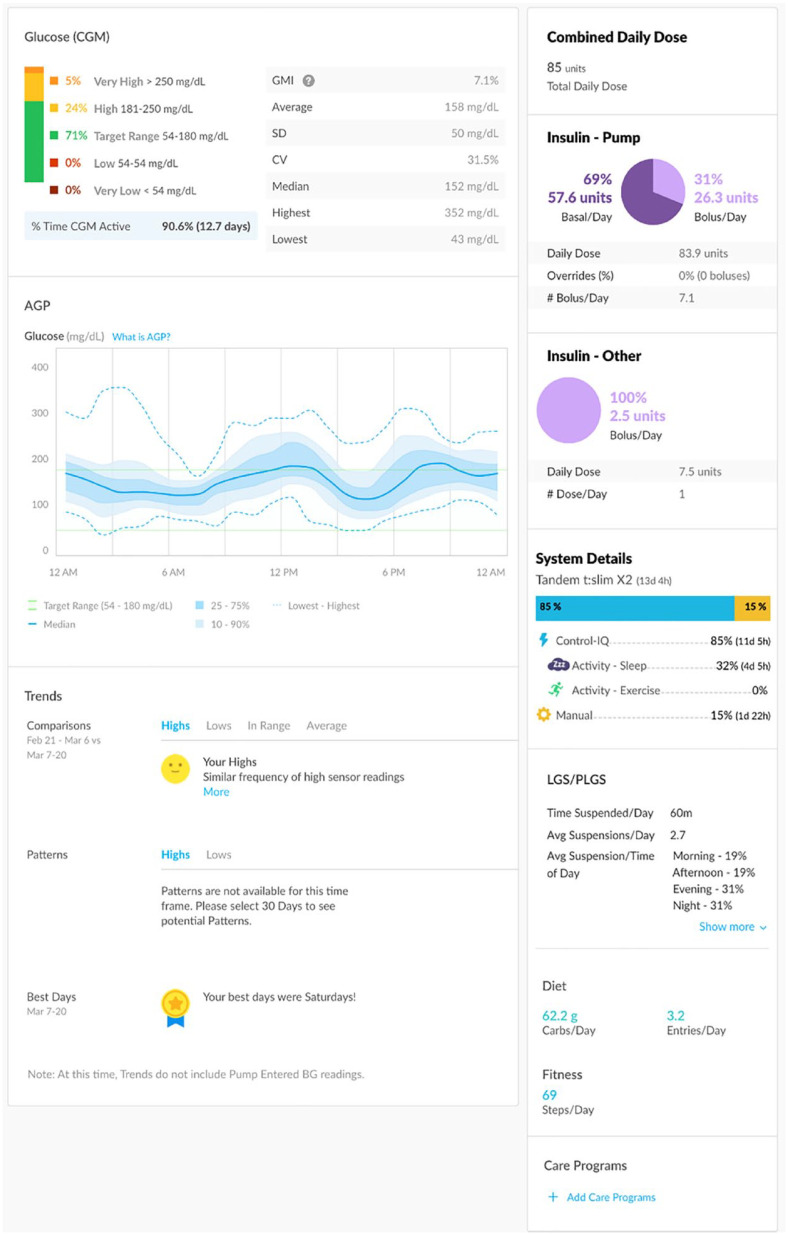
The t:slim X2 insulin pump with the Control-IQ technology (Figure 2) by Tandem Diabetes Care is an insulin pump that functions as part of an AHCL by adjusting both background insulin delivery to prevent hyperglycemia and hypoglycemia in real time and providing automatic correction boluses, using sensor glucose data from the Dexcom G6 CGM (Dexcom, San Diego, CA, USA). In addition, this proprietary algorithm recommends bolus insulin doses based on carbohydrates entered by users and current glucose trends to target euglycemia. The t:slim X2 pump can also connect to a mobile app, which serves as a secondary pump display and allows users to administer a bolus of insulin from a compatible smartphone. The glucose and insulin data are made available in the t:connect web application to visually display glucose trends and insulin delivery patterns (Figure 3).
The Omnipod 5 Automated Insulin Delivery System (Figure 4) is a tubeless insulin pump that adjusts insulin in real time, using sensor glucose data from the Dexcom G6 CGM. In addition, its proprietary algorithm recommends bolus insulin doses based on carbohydrates entered by users and current glucose trends to user-defined target glucose levels. The glucose and insulin data are portrayed in the Glooko data-management platform to visually display glucose trends and insulin delivery patterns.
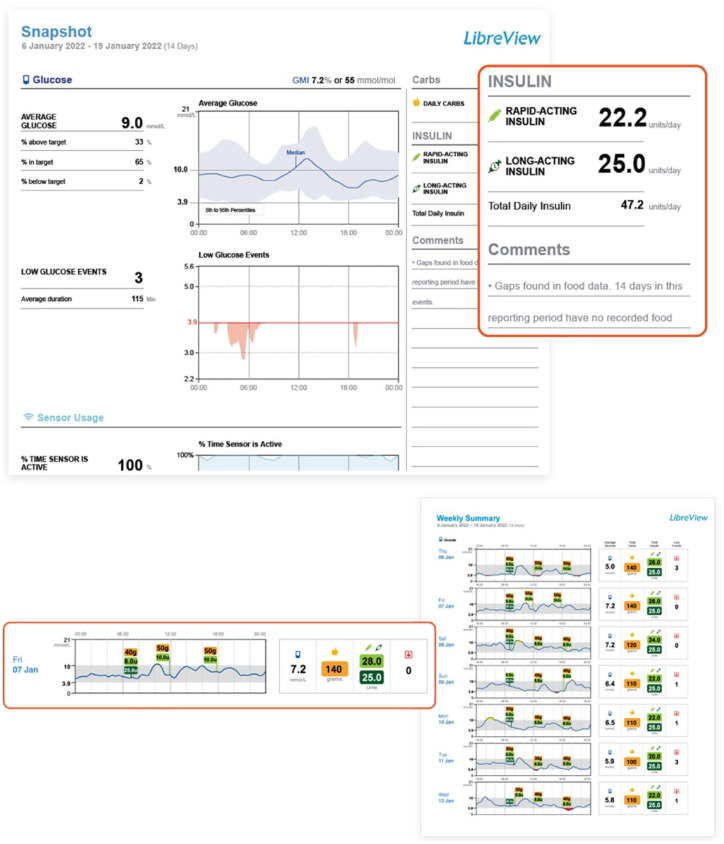
The Bigfoot Unity System is a smart pen cap system for most basal and bolus disposable insulin pens that integrates with the FreeStyle Libre 2 CGM (Abbott Diabetes Care, Alameda, CA, USA). The system also integrates with the Bigfoot blood glucose meter. The system includes a white cap for rapid-acting insulin and a black cap for long-acting insulin to ease identification of the insulin type. The white cap directly displays CGM glucose readings and trend arrows for the Libre 2 sensor and provides health care provider–based insulin dose recommendations directly. The Bigfoot Unity Report (Figure 5) displays dosing time data for each type of insulin, the current settings, standard ambulatory glucose profile metrics, and a history of system alerts. The application automatically collects and displays available data and provides real-time alerts for low glucose levels or when a long-acting insulin dose may have been missed.
Medtronic’s InPen system (Figure 6) is a reusable smart insulin pen that transmits short- or rapid-acting insulin dose data to a mobile application, which is paired with compatible CGM devices (e.g., Dexcom G6 and Medtronic Guardian 3). It permits the calculation of insulin doses for meals using carbohydrate counting, meal estimation, or fixed doses by incorporating insulin sensitivity, an insulin-to-carbohydrate ratio, the glucose target, and active insulin (insulin on board). Active insulin estimation reduces insulin stacking for both meal and correction dosing of insulin. By pairing the dose and time of insulin with rises in sensor glucose levels, it provides the health care professional with data about missed or late insulin boluses. The report also provides data on the dose and time of long-acting insulin (light blue circles above the glucose trend line).
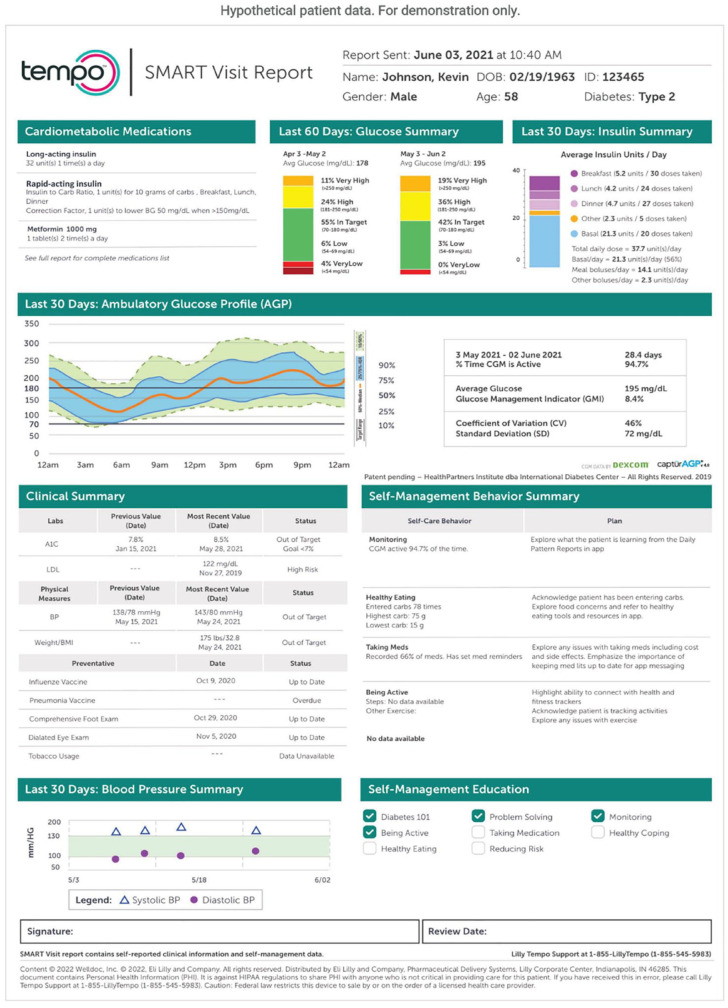
The Lilly Tempo Personalized Diabetes Management Platform (Figure 7) is comprised of three key components: the Tempo Smart Button, Tempo Pen, and TempoSmart app. The Tempo Smart Button is currently FDA-cleared but not commercially available. The TempoSmart app keeps track of insulin doses, glucose levels, and lifestyle data. The Tempo Pen is a prefilled disposable pen that is compatible with multiple Lilly-brand insulins. The Tempo Smart app offers medication reminders, insulin dose logging, and educational resources. The Tempo Personalized Diabetes Management Platform supports integration with the Dexcom G6 CGM system as a secondary display. The Dexcom data displayed within the Tempo Smart app on the user’s smartphone has a three-hour delay and is not intended to be used for real-time dosing decisions.
The SoloSmart cap (Figure 8) is a single add-on connected device that is compatible with the SoloStar and DoubleStar insulin injection pens (Sanofi, Paris, France). The SoloSmart cap records information about the insulin dose, time, and date of each injection and transmits it via Bluetooth to a compatible mobile application, which may vary by country. One such example is the Health2Sync app, which is a health-management platform solution for PWD in Taiwan (and not available in the US market).
NovoPen6 and NovoPen Echo Plus from Novo Nordisk (Figure 9) automatically record insulin dosing information, including time, date, and number of units dosed, about each injection. The dose memory display shows the latest injected insulin units and time since last injection. All insulin dosing information can be viewed alongside glucose information from blood glucose monitors or CGMs on compatible apps. NovoPen 6 and NovoPen Echo Plus connect with, among others, Glooko, FreeStyle LibreLink, mySugr (Roche Diabetes Care, Indianapolis, IN, USA), and Health2Sync (Health2Sync, Taipei, Taiwan) mobile apps. NovoPen 6 and NovoPen Echo Plus are not available in the United States at this time.
Glooko (Figure 10) provides a personalized connected care platform that displays insulin dosing data. On its mobile app, insulin data is grouped by basal, bolus, and premixed insulin; it is color-coded accordingly. Glooko displays data from both insulin pumps and smart pens and incorporates data from CGMs. Glooko’s application programming interface (API) currently delivers total daily dose, total bolus, and total basal through the API. Glooko offers these metrics in a “Glooko flowsheet” for EHR integration.
Tidepool (Figure 11) was the first fully interoperable data-logging app. Tidepool can accommodate insulin dosing data from all commercially available pumps and smart pens in the United States. Data from CGMs are integrated and displayed on the same page as insulin dosing data.
Figure 2.
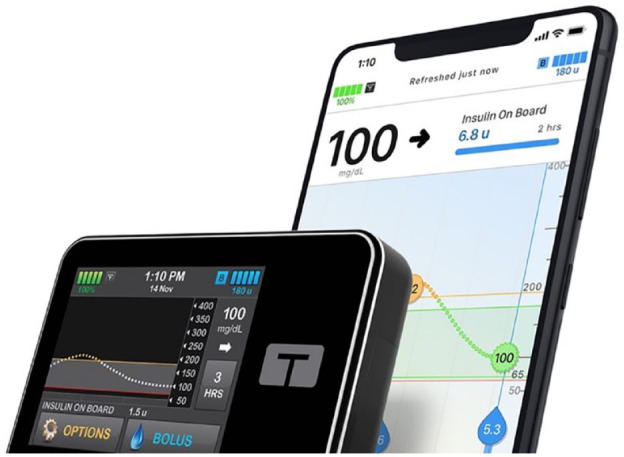
t:slim X2 insulin pump and t:connect mobile app with mobile bolus.
Source: Figure courtesy of Tandem Diabetes Care.
Figure 3.
t:connect Therapy timeline. Figure is a de-identified report.
Figure 4.
Glooko Week View report displaying information from two days of Omnipod 5 Automated Insulin Delivery System use in manual mode. Figure is a de-identified report.
Figure 5.
Bigfoot Biomedical insulin dosing data visualization.
Source: Figure courtesy of Bigfoot Biomedical.
Figure 6.
Medtronic InPen 14-day report.
Source: Figure courtesy of Medtronic Diabetes.
Figure 7.
“Summary page” from the Tempo Insights smart visit report in a hypothetical patient using a basal/bolus regimen and a Dexcom G6 continuous glucose monitor (CGM).
Source: Figure courtesy of Eli Lilly and Company.
Figure 8.

Data from SoloSmart pen cap displayed in the Health2Sync app.
Source: Figure courtesy of Sanofi.
The iCoDE Project: A Repeatable and Scalable Methodology
In 2021, DTS convened stakeholders from industry, academia, health care, and government, as well as patient representatives, to develop standard approaches to integrate CGM data into the EHR. The iCoDE Project (Figure 12) began in January of 2022, and over the course of the next ten months, more than 130 individuals from over 30 organizations met regularly in both large-format and small working group meetings to establish consensus on both the technical and operational aspects of CGM-EHR data integration. 3 The final output of this project was the publication of the 2022 iCoDE Report: CGM-EHR Integration Standards and Recommendations on November 7, 2022. 1 This 108-page report contains extensive documentation of data standards, interoperability standards, clinical implementation recommendations, and practical project management guidance. The final section of the report includes 54 formal recommendations adopted by the iCoDE Steering Committee to help guide future CGM-EHR integration projects.
Figure 12.

The iCoDE logo.
Abbreviation: iCoDE, Integration of Continuous Glucose Monitor Data into the Electronic Health Record.
Insulin Dosing Data and the EHR
An important place for insulin dosing data to be displayed is the EHR, where both clinicians and PWD can have immediate access to this important information. At least 18 types of information may be analyzed by a diabetes health care professional for each person with diabetes who uses a smart insulin delivery system (Table 1). In the future, site-specific information for dosing might also become available because site rotation can prevent lipohypertrophy and lead to more predictable insulin absorption and action. 6
Table 1.
Eighteen Types of Information That May Be Analyzed By a Diabetes Healthcare Professional for Each Person With Diabetes Who Uses a Smart Insulin Delivery System.4,5
| 1. Total daily insulin dose (units, U/kg). 2. Distribution of insulin administration by time of day. 3. Relationship between times of boluses and meals. 4. Number of missed boluses per day. 5. Number of delayed boluses per day. 6. Number of early boluses per day. 7. Number of episodes of bolus stacking per day. 8. Identification of correction boluses. 9. Distribution of episodes of hypoglycemia throughout the day. 10. Distribution of episodes of hyperglycemia throughout the day. 11. Distribution of mealtimes and snack times throughout the day. 12. Distribution of duration of time intervals between insulin bolus administration and onset of food ingestion for each type of meal. 13. Daily basal doses. 14. Time of day of basal insulin dosing. 15. Relationship between morning glucose concentration and basal dose. 16. Frequency of basal dose adjustment. 17. Magnitude of basal insulin dose adjustments. 18. Patterns of bolus and basal insulin according to the day of the week. |
Recommendations for the integration process will be captured in the iCoDE-2 standard. This standard will be developed in a similar way as the iCoDE Standard for integration of CGM data into the EHR. The iCoDE-2 standard will be built to present insulin dosing data either as a stand-alone datastream or on the same platform as the CGM datastream for both types of data to be visualized simultaneously on the same timelines.
ICoDE-2: Connected Insulin Delivery Devices
Building on the success of iCoDE and the continuing drive to integrate device data into the EHR to make data visible and actionable, simplify workflows, and decrease documentation burden, iCoDE-2 (Figure 13) will focus on developing standards for the integration of data from connected insulin delivery systems (pumps and pens) into the EHR. The first iCoDE project was divided into six working groups: (1) data standards; (2) account linkage; (3) integration and interoperability; (4) analytics and visualizations; (5) clinical workflows; and (6) partnerships and business models. Much of this work will be able to be reused, adapted, and leveraged to support iCoDE-2. For example, the use of message exchange standards, privacy and cybersecurity, and account linkage approaches are all concepts that will port over to insulin delivery devices with minimal adjustment. As such, iCoDE-2 will have two working groups, which will focus on the (1) technical and (2) clinical/operational aspects that are unique to pumps and pens. A steering committee with diverse representatives from various stakeholder groups will hear regular updates from the working groups and provide strategic direction and feedback. Eventually, iCoDE-2 will produce a set of standards and recommendations to guide both industry partners and health care delivery organizations through the process of EHR data integration.
Figure 13.

The iCoDE-2 logo.
Abbreviation: iCoDE-2, integration of Connected Diabetes Device Data into the Electronic Health Record.
One difference between the original iCoDE project and the iCoDE-2 project is that CGM manufacturers have made public data APIs available for several years, and so, several individuals and organizations participating in iCoDE had experience with EHR data integration. Also, public documentation of those APIs was available for review in preparation for iCoDE. As of this writing, insulin pump and pen manufacturers do not make their data available except through their proprietary platforms or approved third-party aggregators. As a result, iCoDE-2 will require openness and collaboration from industry partners to better understand the architecture and capabilities of their data systems to meaningfully exchange data. We are encouraged by our experience working with the industry during iCoDE, and we anticipate a similarly cooperative process with industry for iCoDE-2. An ideal outcome of iCoDE-2 will be the establishment of standardized data APIs for insulin pump and pen manufacturers.
Conclusions
Integrating medical device data into the EHR can be a complex process because of a number of technical, operational, and governance barriers that can be difficult to overcome. The iCoDE methodology was used to successfully address those complexities for CGMs through the creation of CGM-EHR integration standards and recommendations. Similar to CGM data, integration of insulin dosing data into the EHR will facilitate the management of diabetes. The integration process will require the establishment of data standards and implementation policies. This is an ideal time for key stakeholders from the health care, industry, academic, information technology, and regulatory sectors to come together and develop necessary standards and policies for this purpose. The iCoDE-2 project is the proposed approach of DTS to create consensus, advance the field, and make insulin dosing data accessible to all PWD and the health care professionals that care for them.
Acknowledgments
The authors thank Annamarie Sucher-Jones for her expert editorial assistance.
Footnotes
Abbreviations: AGP, ambulatory glucose profile; AHCL, advanced hybrid closed-loop system; API, application programming interface; CGM, continuous glucose monitor; BGM, blood glucose monitor; CV, coefficient of variation; DTS, Diabetes Technology Society; EHR, electronic health record; FDA, United States Food and Drug Administration; GMI, glucose management indicator; HDO, health care delivery organization; iCoDE, Integration of Continuous Glucose Monitor Data into the Electronic Health Record; iCoDE-2, integration of Connected Diabetes Device Data into the Electronic Health Record; PWD, people with diabetes.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.C.E. is a consultant for Sanofi, has received speaker fees from Glooko, and receives grant support from the FDA and NIH. J.J.S. is a consultant for Lifescan Diabetes Institute. D.C.K. is a consultant to Better Therapeutics, EOFlow, Integrity, Lifecare, Nevro, Novo Nordisk, Sanofi, and Thirdwayv. A.M.Y., J.H., and R.L. have nothing relevant to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Diabetes Technology Society, which received no external funding and paid all costs for conducting the study.
ORCID iDs: Juan C. Espinoza  https://orcid.org/0000-0003-0513-588X
https://orcid.org/0000-0003-0513-588X
Andrea M. Yeung  https://orcid.org/0000-0002-5592-453X
https://orcid.org/0000-0002-5592-453X
Jingtong Huang  https://orcid.org/0000-0002-3119-9361
https://orcid.org/0000-0002-3119-9361
Jane Jeffrie Seley  https://orcid.org/0000-0003-1582-4320
https://orcid.org/0000-0003-1582-4320
Rebecca Longo  https://orcid.org/0000-0003-0804-3913
https://orcid.org/0000-0003-0804-3913
David C. Klonoff  https://orcid.org/0000-0001-6394-6862
https://orcid.org/0000-0001-6394-6862
References
- 1. Espinoza J, Klonoff DC, Vidmar A, et al. iCoDE June 22, 2022, Steering Committee Meeting Summary Report. https://www.diabetestechnology.org/icode/. Published 2022. Accessed March 23, 2023. [DOI] [PMC free article] [PubMed]
- 2. Xu NY, Nguyen KT, DuBord AY, et al. The launch of the iCoDE standard project. J Diabetes Sci Technol. 2022;16(4):887-895. doi: 10.1177/19322968221093662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Espinoza J, Xu NY, Nguyen KT, Klonoff DC. The need for data standards and implementation policies to integrate CGM data into the electronic health record. J Diabetes Sci Technol. 2023;17:495-502. doi: 10.1177/19322968211058148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuritzky L, Reid TS, Wysham CH. Practical guidance on effective basal insulin titration for primary care providers. Clin Diabetes. 2019;37(4):368-376. doi: 10.2337/cd18-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolpert H, Polonsky WH, Rodbard D. Insulin metrics: need for development of consensus standards for reporting of insulin dosing data. Diabetes Technol Ther. 2021;23(7):522-526. doi: 10.1089/dia.2020.0631. [DOI] [PubMed] [Google Scholar]
- 6. Blanchard J, Ahmed S, Clark B, Sanchez Cotto L, Rangasamy S, Thompson B. Design and testing of a Smartphone Application for Real-Time Tracking of CSII and CGM Site Rotation Compliance in Patients With Type 1 Diabetes. [published online ahead of print December 20, 2022]. J Diabetes Sci Technol. doi: 10.1177/19322968221145178. [DOI] [PMC free article] [PubMed] [Google Scholar]