Abstract
World Health Organization (WHO) Risk Group-4 (RG-4) pathogens are among the most dangerous of the emergent and re-emergent viruses. International health agencies, working in concert, bridge the gaps in health care for populations at risk for RG-4 viral pathogen exposure. RG-4 virus research incorporates Biodefense Program and Biosafety Laboratory (BSL)-4 technologies. RG-4 viruses include Arena-viridae, Filo-viridae, Flavi-viridae, Herpes-viridae, Nairo-viridae, Paramyxo-viridae, and Pox-viridae.
Keywords: World Health Organization (WHO) Risk Group 4 (RG-4) virus pathogens, NIH, NIAID, Biosafety Laboratory (BSL)-4, Arena-viridae, Filo-viridae, Flavi-viridae, Herpes-viridae, Nairo-viridae, Paramyxo-viridae, Pox-viridae, emergent, global warming, vector, reservoir, human, mosquito, tick, bat, pig, mammal, bird, reptile, snake, healthcare setting, biodefense
Background:
Economic, social, and ecological instability, human migration, global warming, and vector and reservoir spread, all contribute to increased numbers of emergent and re-emergent viruses, including RG-4 viruses. Very few laboratories can work with RG-4 viruses since such research is restricted to Biosafety Level 4 (BSL4) laboratories. [1] The NIH reports new Biosafety Lab facility construction projects, five NIAID-funded BSL-4 laboratory suites: the C.W. Bill Young Center for Biodefense and Emerging Infectious Diseases at the NIH (Bethesda, MD), Fort Detrick (Frederick,Maryland, NIAID Rocky Mountain Laboratories (Hamilton, Montana), Boston University (Boston, MA), and University of Texas Medical Branch (Galveston, TX). This is part of the NIAID Biodefense Program, outlined in figure 1. [1] As of 2023 there are 69 BSL4 laboratories world-wide: 15 in North America, 1 in South America, 4 in Oceania, 3 in Africa, 20 in Asia, and 26 in Europe. [2]
Figure 1.
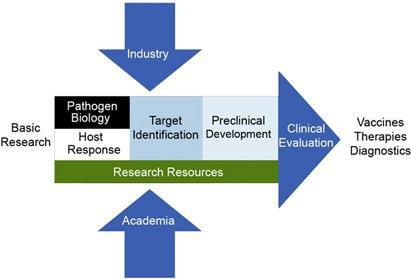
The inputs and stages of NIAID's biodefense research from basic research to the development of new diagnostics, drugs, and vaccines. Credit: NIAID, NIH. [1]
A few examples of emerging and re-emerging potential pandemic viruses include human immunodeficiency virus (HIV) in the1980s, severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV-2) in 2003, and re-emerging influenza virus pandemics in 1890, 1918, 1957, 1968, and 2010. Further, ZIKA virus in 2017, COVID-19 in 2019 and Monkey pox virus in 2022 were observed. The most dangerous viruses involve all the WHO RG-4 virus pathogens. These virus families include Arena-, Filo-, Flavi-, Herpes-, Nairo-, Paramyxo-, and Pox-viridae. [3, 4, 5]
RG-4 virus families:
Arena-viridae:
Arena-viridae includes Junin, Lassa Fever, Lujo Hemorrhagic Fever, Chapare, Guanarito, Machupo, Sabia, Argentinian Hemorrhagic Fever, Bolivian Hemorrhagic Fever, Venezuelan Hemorrhagic Fever, and Lymphocytic Choriomeningitis viruses. The host range and reservoirs of Arena viruses have expanded to include humans, rodents, bats, ixodid lone star ticks, fish, and reptiles (snakes). Lassa fever virus is endemic in West sub-Saharan Africa with occasional detection in the United States, Israel, the United Kingdom, Canada, Netherlands, Japan, and Germany. In endemic areas, the case fatality rate (CFR) is approximately 1-2% with an annual prevalence of 300,000 estimated infections. However, in hospitals in these areas, the CFR is closer to 69%, where infections are confirmed. Moreover, the high degree of virus genome sequence variability may contribute to variations in symptomatology. [3, 6]
Filo-viridae:
Filo-viridae includes Ebola, Marburg, Sudan, Bundibugyo, Ravn, and Tai forest viruses. Of these viruses, Ebola is well-known and there was a large Ebola epidemic in West Africa, 2013-2016. The Ebola CFR ranges from 25-90%. In addition to human virus reservoirs, bats are included as well. In humans, persistent virus infections occur. Subsequent sexual transmission is observed as well, and has been seen up to 500 days after initial transmission. The virus genome undergoes significant sequence variation; thus, quantitative real-time polymerase-chain-reaction assay (qRT-PCR) is limited. To increase the reliability of qRT-PCR and minimize false negatives, two separate genome target sequences are used. Clinical diagnostic methods require improvement and vaccine improvements are also in the pharmaceutical pipeline. [3, 7, 8]
Flavi-viridae:
Flavi-viridae: include Kyasanur Forest disease, Tick Borne Encephalitis, Omsk, Alkhurma, Yellow fever, Zika, Spondweni, West Nile, Yellow fever, St. Louis Encephalitis, Dengue, Japanese Encephalitis, Powassan viruses. These viruses have a wide range prevalence range, totaling 499 million, with Dengue topping the list at 390 million. Virus reservoirs include humans, mosquitoes, non-human primates, birds, pigs, rodents, lagomorphs (hares, rabbits, and pikas), and deer. The members of this virus family demonstrate a propensity for mutations in their genomes that are involved with spread among various vector mosquitoes and their hosts which likely increases their ability to infect humans. Global biomedical organizations attempt rapid tracking and containment of any viruses with the potential of becoming the next pandemic. [3, 9]
Herpes-viridae:
Herpes-viridae (Herpes B virus) recently caused deaths in China. Herpes B virus, although a benign herpes virus for macaque monkeys, is very pathogenic in humans with a CFR of 70-80%. At least 50 people were infected with Herpes B virus as of 2022. Herpes B virus is also known as Herpesvirus simiae, Macacine herpesvirus 1, Macacine Alphaherpesvirus 1, and Cercopithecine herpesvirus 1 (CeHV-1). In 1932, the first case of human Herpes B virus was reported. The individual died of progressive encephalomyelitis 15 days after a normal monkey bit him. The virus was named Herpes B virus by Sabin in 1934. It is observed that people who work with macaque monkeys or have them as pets are at risk for Herpes B virus infection. [3, 10, 11]
Nairo-viridae:
Nairo-viridae genus in the family of Bunyaviruses includes Crimea-Congo hemorrhagic fever, Ganjam, Finch creek, Nairobi sheep disease, Kupe, and Dugbe viruses. Additional sub-groups of Nairo viruses include Hughes, Der Ghazi Kahn, Qalyub, Sakhalin, and Thiafora. The geographic distribution of this virus genus ranges from sub-Saharan Africa to China and is entirely tick-borne. Crimea-Congo hemorrhagic fever, although uneventful in animal's infections, has a human CFR up to 30%. This virus is reported in the former Soviet Union, sub-Saharan Africa, Turkey, Bulgaria, Pakistan, India, China, the Arabian Peninsula, Iran, Iraq, and northern Greece. [3, 12, 13]
Paramyxo-viridae:
The Paramyxo-viridae genus includes Henipa-, Respiro-, Rubula-, and Morbilli-virus groups. Hendra and Nipah viruses (Henipa group) were the first dangerous paramyxoviruses that emerged and were identified in the last century: Hendra in August of 1995 in Australia and Nipah in September 1998 in Malaysia. The related Cedar virus is not as dangerous to humans. This virus genus is zoonotic, and Hendra and Nipah human infections stem from the Pteropus bat species. However, these and other members of the Paramyxo-viruses are spreading to other mammals, and their phylogenetically defined clades are diverging. They are considered at risk of becoming hazardous emergent viruses. Mammals in addition to humans that are being infected include goats, cats, horses, pigs, dogs, and cows. [3, 14, 15, 16]
Pox-viridae:
The Pox-viridae family includes the Orthopox genus, which includes Variola, cowpox, Vaccinia, and mpoxviruses. There are additional novel Orthopox viruses under investigation. Variola, as a human pathogen, emerged from East Africa approximately 3,000 - 4,000 years ago. Smallpox was eradicated by 1980; however, the global prevalence had reached 400 million people prior to its eradication. Mpox was first detected in primates in Denmark in 1959 and is endemic in the Democratic Republic of the Congo, Sudan, Cameroon, Central African Republic, Guinea, and Gabon. Mpox virus related disease recently emerged in clusters in non-endemic areas in what appears to be human to human transmission via close contact. Mpox virus shows neurological involvement and interacts with host immunologic response; in a way it may affect host circadian rhythms. Overall, caveats are manifest, since emergent pox viruses are most likely to continue, despite the eradication of smallpox. [3, 17, 18, 19, 20, 21]
Conclusions:
Emergent and re-emergent of RG-4 viruses are highly pathogenic and pose profound risks for increased spread among humans, vectors, and animals. Healthcare organizations, international databases, artificial intelligence, and improved Hazmat interventions are included in the arsenals used by healthcare workers in zones where these viruses emerge. Global health and security also require monitoring RG-4 pathogen potential weaponization threats as well as corresponding countermeasures. To produce molecular mechanisms that inhibit RG-4 viruses, specific gene pathogenic mechanisms need further development. The mechanisms of inhibitions, as well as vaccines and therapeutics, could then be used as countermeasures. Further risk-benefit analyses are needed to appraise laboratories as well as open public debate about RG-4 pathogens.
The authors report no conflicts of interest.
Edited by P Kangueane
Citation: Sinnott et al. Bioinformation 19(4):345-347(2023)
Declaration on Publication Ethics: The author's state that they adhere with COPE guidelines on publishing ethics as described elsewhere at https://publicationethics.org/. The authors also undertake that they are not associated with any other third party (governmental or non-governmental agencies) linking with any form of unethical issues connecting to this publication. The authors also declare that they are not withholding any information that is misleading to the publisher in regard to this article.
Declaration on official E-mail: The corresponding author declares that official e-mail from their institution is not available for all authors.
License statement: This is an Open Access article which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. This is distributed under the terms of the Creative Commons Attribution License
Comments from readers: Articles published in BIOINFORMATION are open for relevant post publication comments and criticisms, which will be published immediately linking to the original article without open access charges. Comments should be concise, coherent and critical in less than 1000 words.
Bioinformation Impact Factor:Impact Factor (Clarivate Inc 2023 release) for BIOINFORMATION is 1.9 with 2,198 citations from 2020 to 2022 taken for IF calculations.
Disclaimer:The views and opinions expressed are those of the author(s) and do not reflect the views or opinions of Bioinformation and (or) its publisher Biomedical Informatics. Biomedical Informatics remains neutral and allows authors to specify their address and affiliation details including territory where required. Bioinformation provides a platform for scholarly communication of data and information to create knowledge in the Biological/Biomedical domain.
References
- 1. https://swissmodel.expasy.org/
- 2. https://www.kcl.ac.uk/warstudies/assets/global-biolabs-report-2023.pdf .
- 3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7122670/
- 4. https://library1.chc.edu/
- 5.Bankar NJ, et al. Cureus. . 2022;14:e30062.. doi: 10.7759/cureus.30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7567487/
- 7.Feldmann H, et al. N Engl J Med. . 2020;382:1832. doi: 10.1056/NEJMra1901594. [DOI] [PubMed] [Google Scholar]
- 8. https://www.afro.who.int/health-topics/ebola-disease .
- 9.Pierson TC, Diamond MS. Nature Microbiology. . 2020;5:7962. doi: 10.1038/s41564-020-0714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu G, et al. Biosafety and Health. . 2022;4:213. doi: 10.1016/j.bsheal.2022.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabin AB, Wright AM. J. Exp. Med. . 1934;59:115. doi: 10.1084/jem.59.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crabtree MB, et al. Emerging Infectious Diseases. 2009;15:147. doi: 10.3201/eid1502.080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasecka L, Baron MD. Arch Virol. . 2014;159:1249. doi: 10.1007/s00705-013-1940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Witt E, Munster VJ. In Global Virology I - Identifying and Investigating Viral Diseases. NY, USA: Springer; 2015. 146 pp. [DOI] [Google Scholar]
- 15.Luby S, Gurley E. In Global Virology I - Identifying and Investigating Viral Diseases. NY, USA: Springer; 2015. 71 pp. [DOI] [Google Scholar]
- 16.Thibault PA, et al. Adv Virus Res. . 2017;98:1. doi: 10.1016/bs.aivir.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert Castro MD Beata Casanas DO FACP FIDSA. Orthopoxviruses and human disease. In Global Virology II-HIV and Neuro AIDS. NY,USA: Springer; 2017. 697 pp. [DOI] [Google Scholar]
- 18. https://en.m.wikipedia.org/wiki/List_of_human_disease_case_fatality_rates .
- 19.Shafaati M, Zandi M. Travel Med and Infect Dis . 2022;49:102414. doi: 10.1016/j.tmaid.2022.102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lum FM, et al. Nature reviews - Immunology. . 2022;22:597. doi: 10.1038/s41577-022-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zandi M, et al. J NeuroVirol. . 2023;29:1. doi: 10.1007/s13365-023-01118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


