Abstract
Objective.
To determine whether clinical correlates of knee osteoarthritis (OA) affect the outcome of intraarticular steroid injections (IASI) in symptomatic knee OA.
Methods.
Men and women aged ≥ 40 years with painful knee OA who participated in an open-label trial of IASI completed questionnaires and clinical examination. The Outcome Measures in Rheumatology (OMERACT)–Osteoarthritis Research Society International (OARSI) criteria were used to assess response to therapy in the short term (within 2 weeks). Among those who initially responded, those whose pain had not returned to within 20% of the baseline Knee Injury and Osteoarthritis Outcome Score pain score at 6 months were characterized as longer-term responders. Log-binomial regression was used to examine factors associated with outcome.
Results.
One hundred ninety-nine participants were included, of whom 146 (73.4%) were short-term and 40 (20.1%) longer-term responders. Compared to short-term nonresponders, participants with these characteristics were more likely to be short-term responders: medial joint line tenderness [relative risk (RR) 1.42, 95% CI 1.10–1.82], medial and lateral joint line tenderness (RR 1.38, 95% CI 1.03–1.84), patellofemoral tenderness (RR 1.27, 95% CI 1.04–1.55), anserine tenderness (RR 1.27, 95% CI 1.06–1.52), and a belief that treatment would be effective [RR/unit increase (range 0–10) = 1.05 (1.01–1.09)]. Aspiration of joint fluid (RR 0.79, 95% CI 0.66–0.95) and previous ligament/meniscus injury (RR 0.63, 95% CI 0.44–0.91) were associated with a reduced risk of being a short-term responder. Compared to initial nonresponders and those whose pain recurred within 6 months, participants with a higher number of pain sites [RR/unit increase (range 0–10) = 0.83, 95% CI 0.72–0.97], chronic widespread pain (RR 0.32, 95% CI 0.10–0.98), perceived chronicity of disease [RR/unit increase (range 0–10) = 0.86, 95% CI 0.78–0.94], and a higher depression score [RR/unit increase (range 0–21) = 0.89, 95% CI 0.81–0.99] were less likely to be longer-term responders.
Conclusion.
Among patients with symptomatic knee OA, tenderness around the knee was associated with better short-term outcome of IASI. However, clinical-related factors did not predict longer-term response, while those with chronic widespread pain and depressive symptoms were less likely to obtain longer-term benefits.
Key Indexing Terms: PREDICTORS, KNEE OSTEOARTHRITIS, CLINICAL TESTS INTRAARTICULAR STEROID INJECTION, PSYCHOLOGICAL FACTORS
Intraarticular steroid injection (IASI) is an effective treatment for many individuals with symptomatic osteoarthritis (OA) of the knee with short-term pain relief lasting up to 4 weeks1,2,3,4,5 and longer-term response up to 24 weeks1,6. Previous systematic reviews and metaanalyses have shown there is variation in both the magnitude and duration of symptom relief following steroid injections1,3,7. Evidence from the previous systematic reviews suggests, however, that no factor consistently linked with response7,8. In more recent analyses, using an individual patient data metaanalysis of randomized controlled trials, patients with severe baseline pain were found to benefit more from a steroid injection than those with less-severe pain9. The presence of inflammatory signs did not appear to influence outcome9,10,11, while in a study of 174 women, increasing age, reduced knee range of movement (ROM), increased local knee tenderness and more severe radiographic disease were associated with a reduced response to IASI at 3 months12. In a more recent prospective study in individuals with knee OA, no clinical, radiographic, sonographic, and serological characteristics influenced response other than female sex, which was associated with response at 3 weeks (p = 0.045) and previous injection with nonresponse at 9 weeks (p = 0.021)11. In a different prospective cohort study in which repeated IASI were undertaken in predominantly knee OA of Kellgren-Lawrence arthritis grading scale (KL) 1–3, patients with persisting pain or ultrasound (US) effusion at 1 month after IASI showed a reduced probability of responding to additional injections and to treatment response at 1 year13.
There are few data concerning the effect of psychological factors on treatment response7. In our previous open-label study of IASI in knee OA14,15, not all participants responded to the therapy in the short term. Of those who responded, the majority had a recurrence of pain within 6 months. In previous work, we looked at the effect of disease severity on outcome following IASI and found that those with more severe disease [either magnetic resonance imaging (MRI) or radiographs] were less likely to be longer-term responders14,15. The aim of the current study was to determine the effect of a range of clinical correlates of disease including symptoms, clinical signs of knee OA, psychological factors, and quality of life, on both short-term (within 2 weeks) and longer-term (6 months) outcomes following IASI. Our IASI predictor of outcome study was larger in scale and longer in followup than prior studies, and was also designed to look at a more comprehensive list of predictor factors to IASI treatment.
MATERIALS AND METHODS
Participants.
Men and women aged 40 years and over were recruited from primary and secondary care for participation in an open-label study looking at efficacy of IASI in symptomatic knee OA (International Standard Randomised Controlled Trials Number: 07329370). Participants were included if they reported moderate knee pain for > 48 h in the previous 2 weeks or scored > 7 out of 32 on the Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire, questions P2–P9 (question P1 relates to frequency of knee pain, which is irrelevant given the inclusion criteria on pain frequency). Inclusion criteria were imaging confirmation of definite knee OA either radiologically (KL ≥ 2 on posteroanterior, lateral, or skyline view in any knee compartment in the past 2 yrs), or if no radiographs were obtained, evidence of OA on MRI or at arthroscopy. For MRI and arthroscopy, typical changes of OA with at least cartilage loss present were required. Exclusion criteria included gout, septic arthritis, inflammatory arthritis, hyaluronic acid or steroid injection within the previous 3 months, knee surgery within the previous 6 months, and concurrent life-threatening illnesses14,15. Participants were provided with study information sheets and subsequently gave written informed consent to participate. Ethics approval was received from the Leicestershire Multicentre Research Ethics Committee (reference 09/H0402/107).
Screening and baseline assessment.
Participants were assessed for eligibility at a screening visit14. Those who fulfilled the inclusion/exclusion criteria were invited to attend a baseline visit. Participants also completed questionnaires including the KOOS pain scale (relating to the index knee), in which higher score denotes lower severity of symptoms16, a global perception of change–Likert scale, a visual analog scale score for pain during an activity that a patient nominated as being most troublesome (VASNA), Medical Outcomes Study Short-Form 12 (SF-12)17, Hospital Anxiety Depression Scale (HADS)18, and Illness Perception Questionnaires-Brief (IPQ-B)19. The SF-12 is a validated survey designed to assess health status with both mental and physical health-related quality of life20,21. HADS is a 14-item scale, scored 0–3 with 7 items each, measuring anxiety and depression over the last week18. The IPQ-B provides a quantitative assessment of 5 components of cognitive and emotional representations of illness using Leventhal’s Self-Regulatory Model and includes 8 items scored 0–10, with a higher score representing stronger belief19. The occurrence of pain at other sites was assessed using a manikin for body pain (4 figures: front, back, left, and right side); participants were asked to complete this for aches and pains that lasted longer than 1 day that they experienced in the past month22. A further question asked about whether they had been aware of the pain for more than 3 months. Chronic widespread pain (CWP) was defined as pain experienced in contralateral quadrants of the body, above and below the waist and in the axial skeleton that had persisted for more than 3 months23,24. We also noted the number of the shaded regions on the manikin to reflect the number of pain sites23,24.
A subsample (n = 103) of participants had additional clinical tests performed by one of 2 assessors prior to having their steroid injections using standardized assessment procedures. These additional tests included assessment of bony enlargement (absent = 0, unsure = 1, present = 2), joint crepitus (absent = 0, unsure = 1, present palpable = 2, present audible = 3), quadriceps muscle wasting (absent = 0, possible = 1, present = 2), assessment of effusion using the bulge sign25, assessment of effusion using the ballottement test [absent = 0, present without click = 1, present with click (tap) = 2], patellofemoral joint tenderness (absent = 0, present = 1), pes anserine tenderness (absent = 0, present = 1), medial tibiofemoral joint tenderness (absent = 0, present = 1), lateral tibiofemoral joint tenderness (absent = 0, present = 1), and goniometric knee ROM, flexion and extension measured to the nearest degrees26. Maximal voluntary isometric strength of the quadriceps was measured by a strain gauge using a protocol developed for past studies27. Strength scores were measured as torque in Newton meters (Nm) and normalized for body size using the formula corrected strength = Nm/[weight in kg × (height in m divided by 2)]. The length of the distal lower limb was taken to allow calculation of torque. For the elements of the clinical examination, reliability evaluation intra- (κ = 0.60–0.98; ICC = 0.96–0.99) and interobserver (κ = 0.48–1.00; ICC = 0.87–0.97) showed moderate to excellent agreement28. While κ can be affected by the prevalence, in our study for most clinical signs the prevalence was not particularly low. We also asked participants, “Have you ever been told you have injured your ligaments or meniscus in your affected knee (yes, no, don’t know)?”
Following the assessments, arthrocentesis was performed with removal of synovial fluid (SF; if present) and injection of 80 mg methylprednisolone acetate (without local anesthetic). The majority of injections were nonguided using a medial approach to the knee joint by one of 2 experienced clinicians (TWO/NM). Following further ethics approval, during the course of the study we used US to guide localization of the injections for the remaining subjects, with a lateral approach to the suprapatellar bursa (NM). Any participant in whom the SF white cell count (WCC) was found to be > 1500/mm3 was excluded owing to concerns they might have a primary inflammatory arthritis. We treated and studied 1 knee per participant.
Followup.
We defined response to IASI using the Outcome Measures in Rheumatology (OMERACT)–Osteoarthritis Research Society International (OARSI) responder criteria based on the KOOS pain scale and global perception of change–Likert scale29. A responder was defined as having either (1) ≥ 20% change in KOOS pain and a “slightly” or “much better” score on the 5-point Likert scale for change in pain, or (2) ≥ 50% change in the KOOS pain; in both cases an absolute change of at least 3 units if the baseline KOOS was 15 or less. Participants were usually seen within 2 weeks after the injection and we characterized their response at that time as short-term response. Those who had not responded were not further followed. Those who responded were followed with regular telephone calls every 4 weeks during which the same KOOS pain questions and global Likert scale were administered. Those whose pain recurred to within 20% of the baseline KOOS pain were defined as having relapsed and were seen again for final followup. Those whose pain levels did not return to this level at 6 months of followup were classified as longer-term responders.
Analysis.
Means and SD for normally distributed variables, and medians and interquartile ranges (IQR) for variables with a skewed distribution, were used to summarize participant characteristics. Log-binomial regression was used to determine whether baseline factors were associated with both short-term response (i.e., those who responded within 2 weeks vs those who did not) and longer-term response to therapy (those who were responders at 6 mos vs those who did not respond initially, or who were initial responders and whose pain subsequently recurred within 6 mos). In all the analyses, the outcome was responder status (yes vs no). All categorical predictors were coded as dummy variables, thereby making no assumptions about the relationship between categories, regarding order (rank) or scale. This process was repeated for all categorical predictors, including those with ordinal categories (e.g., bulge sign). Because of low frequencies in subcategories, the crepitus and ballottement variables were collapsed into dichotomous variables, coded as absent = 0, present palpable and/or audible = 1; and absent = 0, present with/without click = 1; respectively. Any factors significantly associated with outcome were then included in a subsequent multivariable analysis [2 models: one for short-term (using Poisson regression with robust standard errors) and one for longer-term responders (using log-binomial regression)] to examine whether associations were retained in the presence of other predictors. Results were expressed as relative risks (RR) and 95% CI. No adjustment was undertaken for multiple comparisons30. Statistical analysis was undertaken using Stata version 14 (StataCorp).
RESULTS
Participants.
Two hundred nine participants were recruited. Two were withdrawn following recruitment because they received a steroid injection from their general practitioner (Figure 1). Following intervention with IASI, a further 8 were withdrawn for a number of reasons, as listed in Figure 1. Out of the remaining 199 participants, 103 had additional assessments performed. The mean age of the 199 remaining in the study was 62.8 (SD 10.3) years, and 105 (52.8%) were female (Table 1A). Median KOOS pain at baseline was 44.4 points (IQR 36.1–55.6), and median VASNA was 7.0 cm (IQR 5.6–8.1; Table 1A). The median time between baseline and first followup visit was 8 days (IQR 7–14). Median KOOS pain and VASNA at baseline, first followup, and followup at 6 months stratified by responder status is presented in Table 1A. Other participant characteristics including the psychological factors, quality of life, and clinical-related factors are presented in Table 1A and Table 1B. The baseline characteristics of subjects who received their injections unguided were broadly similar to those who received their injections guided (Table 2). There was no difference in the demographic characteristics or pain symptoms in those subjects who had additional clinical assessments performed and those who did not (Supplementary Table 1, available from the authors on request). Our findings regarding a subsample (n = 120) of participants who had an MRI of their knee performed have been published14,15.
Figure 1.
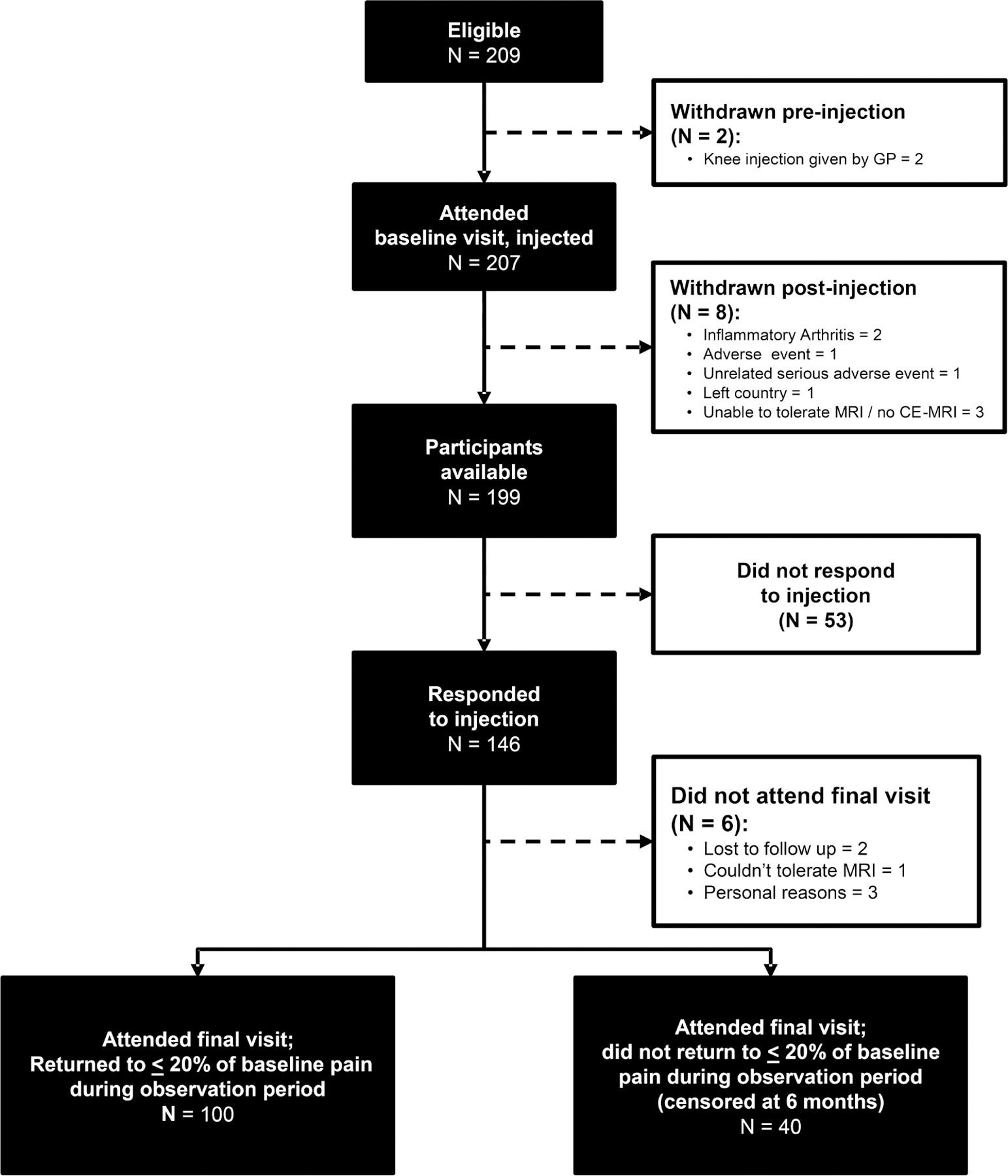
PRISMA flow chart of participants. PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses; GP: general practitioner; MRI: magnetic resonance imaging; CE-MRI: contrast-enhanced MRI.
Table 1A.
Baseline characteristics: subjects and treatment factors.
| Variables | Full Sample | First Followup Visit | 6-month Visit | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nonresponder | Responder | Nonresponder | Responder | |||||||
| N | Values | N | Values | N | Values | N | Values | N | Values | |
| Demographic/prior knee injury | ||||||||||
| Age, yrs, mean (SD) | 199 | 62.8 (10.3) | 53 | 62.6 (9.9) | 146 | 62.9 (10.5) | 159 | 63.4 (10.5) | 40 | 60.5 (9.5) |
| Females, frequency (%) | 199 | 105 (52.8) | 52 | 22 (41.5) | 146 | 83 (56.9) | 159 | 83 (52.2) | 40 | 22 (55.0) |
| Pain | ||||||||||
| KOOS pain subscale score (0–100)* | 199 | 44.4 (36.1–55.6) | 53 | 44.4 (38.9–61.1) | 146 | 44.4 (36.1–52.8) | 159 | 44.4 (36.1–55.6) | 40 | 44.4 (36.1–52.8) |
| Pain on nominated activity, VAS (0–10 cm)** | 190 | 7.0 (5.6–8.1)¤ | 49 | 7.0 (5.2–8.4) | 141 | 7.0 (5.8–8.0) | 152 | 7.0 (5.5–8.1) | 38 | 7.2 (6.4–8.1) |
| Pain in last week VAS (0–10 cm)** | 194 | 6.5 (5.0–8.0)¤ | 50 | 6.8 (3.3–8.0) | 144 | 6.5 (5.0–8.0) | 154 | 6.5 (4.8–8.0) | 40 | 6.5 (5.1–8.1) |
| No. pain sites | 177 | 4.0 (2.0–5.0) | 47 | 4.0 (2.0–6.0) | 130 | 3.0 (2.0–5.0) | 143 | 4.0 (2.0–5.0) | 34 | 2.0 (2.0–4.0) |
| Chronic widespread pain (ACR), frequency (%) | 150 | 39 (26.0) | 38 | 11 (29.0) | 112 | 28 (25.0) | 120 | 36 (30.0) | 30 | 3 (10.0) |
| Psychological factors | ||||||||||
| HADS anxiety | 170 | 6.5 (3.0–9.0) | 45 | 6.0 (3.0–9.0) | 125 | 7.0 (3.0–9.0) | 137 | 7.0 (3.0–9.0) | 33 | 5.0 (2.0–9.0) |
| HADS depression | 170 | 4.0 (2.0–8.0) | 45 | 5.0 (3.0–8.0) | 125 | 4.0 (2.0–7.0) | 137 | 5.0 (2.0–8.0) | 33 | 3.0 (2.0–6.0) |
| IPQ-B consequences score | 175 | 6.0 (4.0–8.0) | 46 | 7.0 (4.0–8.0) | 129 | 6.0 (4.0–8.0) | 141 | 7.0 (4.0–8.0) | 34 | 6.0 (4.0–7.0) |
| IPQ-B timeline score | 172 | 10.0 (8.0–10.0) | 46 | 10.0 (7.0–10.0) | 126 | 9.0 (8.0–10.0) | 139 | 10.0 (8.0–10.0) | 33 | 8.0 (6.0–10.0) |
| IPQ-B personal control score | 173 | 5.0 (2.0–7.0) | 45 | 5.0 (2.0–6.0) | 128 | 4.0 (2.0–7.0) | 140 | 5.0 (2.0–7.0) | 33 | 4.0 (3.0–7.0) |
| IPQ-B treatment score | 174 | 8.0 (5.0–10.0) | 46 | 7.0 (5.0–8.0) | 128 | 8.0 (6.0–10.0) | 141 | 8.0 (5.0–10.0) | 33 | 8.0 (7.0–9.0) |
| IPQ-B identity score | 175 | 7.0 (6.0–9.0) | 46 | 7.5 (5.0–8.0) | 129 | 7.0 (6.0–9.0) | 142 | 7.0 (6.0–9.0) | 33 | 7.0 (6.0–8.0) |
| IPQ-B illness concern score | 174 | 7.0 (5.0–9.0) | 46 | 7.5 (5.0–9.0) | 128 | 7.0 (5.0–9.0) | 141 | 7.0 (5.0–9.0) | 33 | 7.0 (5.0–8.0) |
| IPQ-B coherence score | 172 | 8.0 (7.0–10.0) | 46 | 8.0 (6.0–10.0) | 126 | 8.0 (7.0–10.0) | 139 | 8.0 (7.0–10.0) | 33 | 8.0 (7.0–10.0) |
| IPQ-B emotional representation score | 172 | 5.0 (2.0–7.0) | 46 | 5.0 (2.0–8.0) | 126 | 5.0 (2.0–7.0) | 139 | 5.0 (2.0–7.0) | 33 | 5.0 (1.0–7.0) |
| Quality of life | ||||||||||
| SF-12 physical component summary, mean (SD) | 184 | 32.3 (8.7) | 50 | 31.7 (8.8) | 134 | 32.5 (8.7) | 147 | 32.2 (8.9) | 37 | 32.6 (8.0) |
| SF-12 mental component summary, mean (SD) | 184 | 49.2 (11.7) | 50 | 47.2 (12.8) | 134 | 50.0 (11.2) | 147 | 48.8 (11.8) | 37 | 51.2 (10.9) |
| Treatment-related factors | ||||||||||
| Synovial fluid aspiration, frequency (%) | 199 | 89 (44.7) | 53 | 32 (60.4) | 146 | 57 (39.0) | 159 | 73 (45.9) | 40 | 16 (40.0) |
| Ultrasound-guided knee injection, frequency (%) | 199 | 79 (39.7) | 53 | 18 (34.0) | 146 | 61 (41.8) | 159 | 62 (39.0) | 40 | 17 (42.5) |
| Clinical-related factors (subset n = 101†) | ||||||||||
| Quadriceps muscle strength, Nm/kg | 98 | 0.6 (0.4–1.1) | 23 | 0.7 (0.3–1.5) | 75 | 0.6 (0.4–1.1) | 77 | 0.6 (0.3–1.0) | 21 | 1.0 (0.5–1.2) |
| Knee range of movement, degrees | ||||||||||
| Flexion | 101 | 120.0 (111.0–127.0) | 23 | 122.0 (106.0–127.0) | 78 | 120.0 (112.0–127.0) | 79 | 120.0 (111.0–126.0) | 22 | 121.0 (114.0–128.0) |
| Extension | 101 | 172.0 (170.0–176.0) | 23 | 172.0 (170.0–177.0) | 78 | 172.0 (170.0–176.0) | 79 | 172.0 (170.0–176.0) | 22 | 172.0 (170.0–177.0) |
Values are median (IQR) unless otherwise specified.
KOOS pain subscale is scored from 100 (no pain) to 0 (extreme pain).
VAS are scored from 0 (no pain) to 10 (pain as bad as you can imagine).
N < 199 indicates patients not completing this particular element of the questionnaire.
Clinical tests were performed in a subset of 103 patients only; 2 patients did not complete KOOS. KOOS: Knee Injury and Osteoarthritis Outcome Score; VAS: visual analog scale; IQR: interquartile range; HADS: Hospital Anxiety and Depression Scale; IPQ-B: Illness Perception Questionnaire-Brief; SF-12: Medical Outcomes Study Short Form-12; ACR: American College of Rheumatology.
Table 1B.
Baseline characteristics: clinical examination.
| Clinical Factors (subset n = 101†) | First Followup Visit | 6-month Visit | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Full Sample | Nonresponder | Responder | Nonresponder | Responder | |||||
| Frequency (%) | N | Frequency (%) | N | Frequency (%) | N | Frequency (%) | N | Frequency (%) | |
| Previous ligament/meniscus injuries | |||||||||
| No | 75 (74.3) | 23 | 11 (47.8) | 78 | 64 (82.1) | 79 | 58 (73.4) | 22 | 17 (77.3) |
| Yes | 26 (25.7) | 23 | 12 (52.2) | 78 | 14 (18.0) | 79 | 21 (26.6) | 22 | 5 (22.7) |
| Crepitus | |||||||||
| Absent | 6 (5.9) | 23 | 2 (8.7) | 78 | 4 (5.1) | 79 | 5 (6.3) | 22 | 1 (4.6) |
| Audible and/or palpable | 95 (94.1) | 23 | 21 (91.3) | 78 | 74 (94.9) | 79 | 74 (93.7) | 22 | 21 (95.5) |
| Quadriceps muscle wasting | |||||||||
| Absent | 29 (28.7) | 23 | 9 (39.1) | 78 | 20 (25.6) | 79 | 25 (31.7) | 22 | 4 (18.2) |
| Possible | 18 (17.8) | 23 | 1 (4.4) | 78 | 17 (21.8) | 79 | 13 (16.5) | 22 | 5 (22.7) |
| Present | 54 (53.5) | 23 | 13 (56.5) | 78 | 41 (52.6) | 79 | 41 (51.9) | 22 | 13 (59.1) |
| Bony enlargement | |||||||||
| Absent | 54 (53.5) | 23 | 10 (43.5) | 78 | 44 (56.4) | 79 | 40 (50.6) | 22 | 14 (63.6) |
| Unsure | 7 (6.9) | 23 | 12 (52.2) | 78 | 28 (35.9) | 79 | 33 (41.8) | 22 | 7 (31.8) |
| Present | 40 (39.6) | 23 | 1 (4.4) | 78 | 6 (7.7) | 79 | 6 (7.6) | 22 | 1 (4.6) |
| Anserine tenderness | |||||||||
| Absent | 76 (75.3) | 23 | 21 (91.3) | 78 | 55 (70.5) | 79 | 60 (76.0) | 22 | 16 (72.7) |
| Present | 25 (24.8) | 23 | 2 (8.7) | 78 | 23 (29.5) | 79 | 19 (24.1) | 22 | 6 (27.3) |
| Patellofemoral tenderness | |||||||||
| Absent | 59 (58.4) | 23 | 18 (78.3) | 78 | 41 (52.6) | 79 | 46 (58.2) | 22 | 13 (59.1) |
| Present | 42 (41.6) | 23 | 5 (21.7) | 78 | 37 (47.4) | 79 | 33 (41.8) | 22 | 9 (40.9) |
| Tibiofemoral tenderness | |||||||||
| Absent | 44 (43.6) | 23 | 16 (69.6) | 78 | 28 (35.9) | 79 | 37 (46.8) | 22 | 7 (31.8) |
| Medial tibiofemoral joint | 10 (9.9) | 23 | 2 (8.7) | 78 | 8 (10.3) | 79 | 6 (7.6) | 22 | 4 (18.2) |
| Lateral tibiofemoral joint | 31 (30.7) | 23 | 3 (13.0) | 78 | 28 (35.9) | 79 | 25 (31.7) | 22 | 6 (27.3) |
| Medial and lateral tibiofemoral joint | 16 (15.8) | 23 | 2 (8.7) | 78 | 14 (18.0) | 79 | 11 (13.9) | 22 | 5 (22.7) |
| Ballottement* | |||||||||
| Absent | 77 (76.2) | 23 | 15 (65.2) | 78 | 62 (79.5) | 79 | 57 (72.2) | 22 | 20 (90.9) |
| Present with or without click | 24 (23.8) | 23 | 8 (34.8) | 78 | 16 (20.5) | 79 | 22 (27.9) | 22 | 2 (9.1) |
| Bulge sign¥ | |||||||||
| 0 | 35 (34.7) | 23 | 7 (30.4) | 78 | 28 (35.9) | 79 | 27 (34.2) | 22 | 8 (36.4) |
| Trace | 35 (34.7) | 23 | 7 (30.4) | 78 | 28 (35.9) | 79 | 27 (34.2) | 22 | 8 (36.4) |
| 1 | 16 (15.8) | 23 | 4 (17.4) | 78 | 12 (15.4) | 79 | 14 (17.7) | 22 | 2 (9.1) |
| 2 | 12 (11.9) | 23 | 4 (17.4) | 78 | 8 (10.3) | 79 | 9 (11.4) | 22 | 3 (13.6) |
| 3 | 3 (3.0) | 23 | 1 (4.4) | 78 | 2 (2.6) | 79 | 2 (2.5) | 22 | 1 (4.6) |
Values are frequency (%) unless otherwise specified.
Clinical tests were performed in a subset of 103 patients only; 2 patients did not complete KOOS.
Ballottement test defined as positive click/tap or downward movement of the patella on pressure and rebounding of patella upon removal of pressure.
0 = no wave produced on down stroke; trace = a small wave on medial side with down stroke; 1 = larger bulge on medial side with down stroke; 2 = spontaneously returned to medial side after upstroke; 3 = so much fluid that it was not possible to move the effusion out of the medial aspect of the knee. KOOS: Knee Injury and Osteoarthritis Outcome Score.
Table 2.
Baseline characteristics in those who had unguided and ultrasound-guided injections.
| Variables | Participants with Unguided Injection | Participants with Ultrasound-guided Injection |
|---|---|---|
| N | 120 | 79 |
| Age, yrs, mean (SD) | 62.3 (10.3) | 63.5 (10.4) |
| Females, frequency (%) | 62 (51.7) | 43 (54.4) |
| No. days to followup appointment | 8.0 (7.0–14.0) | 8.0 (7.0–14.0) |
| KOOS pain subscale score (0–100)* | 44.4 (36.1–55.6) | 41. 7 (36.1–52.8) |
| Pain on nominated activity VAS (0–10)** | 7.0 (5.5–7.7) ¤ | 7.2 (5.8–8.4) † |
| Pain in last week VAS (0–10)** | 6.5 (4.7–7.8) ¤ | 6.6 (5.3–8.3) † |
| No. responders to injection, at followup visit, frequency (%) | 85 (70.8) | 61 (77.2) |
Values are median (IQR) unless otherwise specified.
KOOS pain subscale is scored from 100 (no pain) to 0 (extreme pain).
VAS is scored from 0 (no pain) to 10 (pain as bad as you can imagine).
N = 5 and 3 participants neglected to complete their pain on nominated activity VAS and pain in last week VAS, respectively.
N = 4 and 2 participants neglected to complete their pain on nominated activity VAS and pain in last week VAS, respectively. IQR: interquartile range; KOOS: Knee Injury and Osteoarthritis Outcome Score; VAS: visual analog scale.
Predictors of short-term responder status.
Of those participants who had an IASI, 146 (73.4%) were defined as short-term responders. Participants were more likely to be responders if they had medial tibiofemoral joint tenderness (RR 1.42, 95% CI 1.10–1.82), medial and lateral tibiofemoral joint tenderness (RR 1.38, 95% CI 1.03–1.84), patellofemoral tenderness (RR 1.27, 95% CI 1.04–1.55), or anserine tenderness (RR 1.27, 95% CI 1.06–1.52); or if they had a positive belief about treatment with IASI (IPQ-B treatment score; RR per unit increase = 1.05, 95% CI 1.01–1.09). Aspiration of SF (RR 0.79, 95% CI 0.66–0.95) and previous ligament or meniscus injury (RR 0.63, 95% CI 0.44–0.91) were associated with a reduced likelihood of being a short-term responder (Table 3 and Table 4). None of the other patient-related factors including the use of guided injection, psychological factors, quality of life, or clinical signs of disease was linked with short-term responder status. In a multivariable analysis of the factors that were associated with short-term response, only 1 factor (previous ligament or meniscus injury) remained significant after adjustment (Supplementary Table 2, available from the authors on request).
Table 3.
Short-term and longer-term prediction of response to IASI: patient and treatment factors.
| Predictor Variable in Regression | Short-term Responder (Yes/No) | Longer-term Responder (Yes/No) | ||
|---|---|---|---|---|
| N | Relative Risk (95% CI) | N | Relative Risk (95% CI) | |
| Demographic/prior knee injury | ||||
| Age (per yr) | 199 | 1.00 (0.99–1.01) | 199 | 0.98 (0.95–1.01) |
| Sex, female vs male (ref.)* | 199 | 1.18 (0.99–1.40) | 199 | 1.09 (0.63–1.91) |
| Symptoms | ||||
| Pain on nominated activity VAS (0–10 cm) | 190 | 1.01 (0.96–1.06) | 190 | 1.08 (0.91–1.27) |
| Pain in the last week VAS (0–10 cm) | 194 | 1.01 (0.97–1.05) | 194 | 1.05 (0.92–1.21) |
| No. pain sites (range 0–10) | 177 | 0.98 (0.94–1.02) | 177 | 0.83 (0.72–0.97) |
| Chronic widespread pain (ACR) | 150 | 0.95 (0.76–1.19) | 150 | 0.32 (0.10–0.98) |
| Psychological factors | ||||
| HADS anxiety (0–21) | 170 | 0.99 (0.97–1.01) | 170 | 0.93 (0.86–1.01) |
| HADS depression (0–21) | 170 | 0.98 (0.95–1.01) | 170 | 0.89 (0.81–0.99) |
| IPQ-B consequences score (0–10) | 175 | 1.00 (0.97–1.04) | 175 | 0.93 (0.83–1.05) |
| IPQ-B timeline score (0–10) | 172 | 0.98 (0.95–1.02) | 172 | 0.86 (0.78–0.94) |
| IPQ-B personal control score (0–10) | 173 | 1.00 (0.97–1.04) | 173 | 0.99 (0.89–1.10) |
| IPQ-B treatment score (0–10) | 174 | 1.05 (1.01–1.09) | 174 | 1.08 (0.94–1.24) |
| IPQ-B identity score (0–10) | 175 | 1.01 (0.97–1.06) | 175 | 0.98 (0.85–1.13) |
| IPQ-B illness concern score (0–10) | 174 | 1.00 (0.97–1.04) | 174 | 0.95 (0.85–1.07) |
| IPQ-B coherence score (0–10) | 172 | 1.03 (0.98–1.08) | 172 | 1.12 (0.95–1.32) |
| IPQ-B emotional representation score (0–10) | 172 | 0.99 (0.96–1.02) | 172 | 0.97 (0.88–1.07) |
| Quality of life | ||||
| SF-12 physical component summary (0–100) | 184 | 1.00 (0.99–1.01) | 184 | 1.00 (0.97–1.04) |
| SF-12 mental component summary (0–100) | 184 | 1.00 (1.00–1.01) | 184 | 1.01 (0.99–1.04) |
| Treatment-related factors | ||||
| Synovial fluid aspiration, yes vs no (ref.)* | 199 | 0.79 (0.66–0.95) | 199 | 0.82 (0.47–1.45) |
| Ultrasound-guided knee injection vs unguided (ref.)* | 199 | 1.09 (0.92–1.29) | 199 | 1.16 (0.57–2.34) |
Ref. indicates the reference category in the log-binomial regression [e.g., yes vs no (ref.) indicates that “no” was the reference category]. IASI: intraarticular steroid injection; VAS: visual analog scale; HADS: Hospital Anxiety and Depression Scale; IPQ-B: Illness Perception Questionnaire-Brief; SF-12: Medical Outcomes Study Short Form-12; ACR: American College of Rheumatology.
Table 4.
Short-term and longer-term prediction of response to IASI in subsample†.
| Predictor Variable in Regression | Short-term Responder (Yes/No) | Longer-term Responder (Yes/No) |
|---|---|---|
| Relative Risk (95% CI) | Relative Risk (95% CI) | |
| Previous ligament/meniscus injuries, frequency (%) | ||
| No | Reference category | Reference category |
| Yes | 0.63 (0.44–0.91) | 0.86 (0.35–2.10) |
| Crepitus¤ | ||
| Absent | Ref. | Ref. |
| Audible and/or palpable | 1.17 (0.66–2.08) | 1.33 (0.21–8.26) |
| Quadriceps muscle wasting¤ | ||
| Absent | Ref. | Ref. |
| Possible | 1.37 (1.05–1.79) | 2.01 (0.62–6.53) |
| Present | 1.10 (0.83–1.47) | 1.75 (0.63–4.87) |
| Bony enlargement¤ | ||
| Absent | Ref. | Ref. |
| Unsure | 1.05 (0.76–1.46) | 0.55 (0.08–3.57) |
| Present | 0.86 (0.68–1.09) | 0.68 (0.30–1.52) |
| Anserine tenderness | ||
| Absent | Ref. | Ref. |
| Present | 1.27 (1.06–1.52) | 1.14 (0.50–2.59) |
| Patellofemoral tenderness | ||
| Absent | Ref. | Ref. |
| Present | 1.27 (1.04–1.55) | 0.97 (0.46–2.06) |
| Tibiofemoral tenderness | ||
| Absent | Ref. | Ref. |
| Lateral tibiofemoral joint | 1.26 (0.86–1.84) | 2.51 (0.91–6.96) |
| Medial tibiofemoral joint* | 1.42 (1.10–1.82) | 1.22 (0.45–3.27) |
| Medial and lateral tibiofemoral joint | 1.38 (1.03–1.84) | 1.96 (0.73–5.31) |
| Ballottement | ||
| Absent | Ref. | Ref. |
| Present with or without click | 0.83 (0.61–1.12) | 0.32 (0.08–1.27) |
| Bulge sign¤ | ||
| 0 | Ref. | Ref. |
| Trace | 1.00 (0.79–1.26) | 1.00 (0.42–2.36) |
| 1 | 0.94 (0.68–1.30) | 0.55 (0.13–2.29) |
| 2 | 0.83 (0.54–1.28) | 1.09 (0.35–3.47) |
| 3 | 0.83 (0.37–1.89) | 1.46 (0.26–8.08) |
| Quadriceps muscle strength, Nm/kg | 0.92 (0.74–1.16) | 1.45 (0.73–2.85) |
| Knee range of movement, degrees | ||
| Flexion (0–180) | 1.00 (0.99–1.01) | 1.01 (0.98–1.04) |
| Extension (0–180) | 1.00 (0.98–1.02) | 1.01 (0.94–1.09) |
N = 101 in all variables apart from quadriceps muscle strength, where n = 98 was due to size of limb being too large to allow testing in 3 participants.
Further testing done using pairwise comparisons for equality by creating dummy variable coding confirms nonsignificance.
Further testing done using pairwise comparisons for equality by creating dummy variable coding confirms medial tibiofemoral joint tenderness improved response at short-term only. IASI: intraarticular steroid injection.
Predictors of longer-term responder status (6 mos).
Forty participants among those who were short-term responders (20.1% of the original cohort of 199 participants) were characterized as longer-term responders, in which at 6 months, their pain had not returned to within 20% of its baseline value. The presence of CWP (RR 0.32, 95% CI 0.10–0.98) was associated with a reduced likelihood of being a longer-term responder (Table 3). Also associated with a reduced likelihood of being a longer-term responder were an increased number of pain sites (RR 0.83/site, 95% CI 0.72–0.97), perceived chronicity of disease (IPQ-B timeline score; RR per unit increase = 0.86, 95% CI 0.78–0.94), and depressive symptoms (RR per unit increase = 0.89, 95% CI 0.81–0.99). Categorization of these variables suggests a linear relationship for both depressive symptoms and timeline score (Supplementary Table 3, available from the authors on request). None of the clinical signs of OA, the use of guided injection or aspiration, or other factors linked with short-term response were associated with longer-term response status (Table 3 and Table 4). In a multivariable analysis of the factors associated with longer-term response, only the IPQ-B timeline score remained significant after adjustment (Supplementary Table 4).
DISCUSSION
In this open-label study of IASI, using OMERACT-OARSI criteria as our definition of response, we found several factors associated with short-term response status. Knee tenderness and a stronger belief about the effectiveness of treatment were linked with a response to IASI, while aspiration of synovial joint fluid and having prior ligament or meniscus injury were linked with a reduced risk of response. But none of these factors were linked with longer-term response status. In contrast, depressive symptoms and the presence of CWP were associated with a reduced risk of being a longer-term responder.
Compared to those who did not respond to IASI, those who were short-term responders were more likely to have medial tibiofemoral joint tenderness, medial and lateral joint line tenderness, patellofemoral joint tenderness, and anserine tenderness. Our findings are in keeping with a study in which clinical assessment of local tenderness was linked with an improved response at 3 weeks (OR 1.80, 95% CI 1.03–1.67)31.
Previous studies do not support the impression that the presence of knee effusion is associated with response, with only 232,33 of 6 studies5,31,32,33,34,35 suggesting that response was better in those with effusion. In our study the presence of a clinical effusion (as determined by the bulge sign or ballottement) was not associated with treatment response, while aspiration of SF, if anything, was linked with a reduced response to IASI. However, we did not have information about clinical signs of effusion at followup. No other symptoms or clinical signs of OA were associated with response. We found in our previous analysis on structural predictors to IASI that MRI-effusion and MRI-synovitis were not linked with an improved response14,15. Interestingly, though, among a subsample of subjects in whom SF analysis was performed, compared to those with SF WCC in the lowest tertile (< 100 cells/mm3), those with WCC in the middle and upper tertiles had a greater reduction in knee pain following steroid injection36.
Compared to short-term nonresponders, a higher proportion of short-term responders received their injection using US-guided control (41.8% vs 34%). This difference, although not statistically significant, may be clinically relevant, and further large-scale studies are needed to confirm whether US guidance is linked with an improved outcome. Sibbitt Jr., et al37 reported that guided knee injections (compared with blinded injections) were associated with pain relief that lasted 1 month longer, although guided injections did not lead to better improvement of pain response in the longer term (6 mos). We did not have objective assessment of localization of the needle to within the joint and so were unable to determine whether accurate localization within the joint was linked with response. The results of a previous study, using air-arthrosonogram as an indicator of accuracy of localization, however, suggest that accurate localization of IASI to the knee did not result in superior outcome regarding pain compared to inaccurate injection11.
There are few studies that have looked at the influence of adverse psychological factors on treatment response. Our null findings for anxiety and depression are in keeping with the study by Jones and Doherty31 suggesting no effect on response in the short term. It is perhaps not surprising that those who had a stronger belief that treatment was going to be effective had a beneficial effect. Because we did not have detailed information about previous steroid injections to study whether it was prior experience of a successful outcome that may have driven their beliefs regarding treatment response, we cannot exclude this possibility. However, we note the findings of a study in which participants who had had a previous experience of injection were less likely to report response to treatment than those undergoing their first injection at 9 weeks but not 3 weeks11.
In contrast to our findings on “disease”-related factors predicting short-term response, we found no evidence that these were linked with longer-term response. We had anticipated that those with more marked clinical features of disease such as crepitus, bony enlargement, and muscle wasting may also have been less likely to be responders; however, this did not appear to be the case.
A number of factors including CWP, having multiple sites of bodily pain, perceived chronicity of disease, and depressive symptoms were linked with a reduced likelihood of being a longer-term responder. The observation is in keeping with studies suggesting chronic pain, negative attitude, and depression can be predictors of poorer treatment outcome in other clinical settings38,39,40,41. It is possible that altered pain sensitivity or awareness of pain as a consequence of the psychological symptoms may have influenced the likelihood of poorer longer-term response.
There were several limitations to the study. Although this was a comparatively large study, the high frequency of the (short-term) response and relatively low frequency of some predictors mean that this study was relatively underpowered to detect some predictors of outcome. Further larger studies are needed to determine the effect of the putative predictor variables on outcome. Characterization of the clinical predictors was based on clinical examination, which is subject to measurement errors. The effect of errors of classification of individual clinical signs due to poor reliability would tend to reduce the chance of finding real biological associations; however, formal testing of reliability in the study was good, suggesting that this is unlikely to have been important in explaining our findings28. Other putative predictor variables were obtained largely by self-report and therefore subject to errors of recall; these factors, however, were obtained prior to intervention and it seems unlikely that any such errors would have resulted in bias. They may have led to reduced precision in estimates of effect. There was no placebo group in the trial because the short-term efficacy of IASI in knee OA is already established1,2,3,5. While it is likely that some of the response may be due to a contextual/placebo effect, the trial reflects clinical practice in which injections are administered in an “open” setting, with the patient aware of the intervention and so the observed “predictor” variables are likely to reflect those that would be observed in the clinical setting. Another limitation was the possible effect of “multiplicity,” because in this study we looked at a range of putative determinants without correcting for testing, and therefore a risk existed that some of the predictors found could be circumstantial, and replication of the findings may be needed for them. We considered variables that in our judgment could plausibly affect the outcome. Further, it is possible that some real biological associations may have been missed (type 2 errors). As outlined earlier, we could not exclude the possibility that previous IASI and/or their response may have influenced some of the results. The study was performed in a predominantly white population and the results should be generalized beyond this setting with caution.
Our data suggest there may be a limited role for clinical phenotyping in relation to targeting IASI therapy in patients with joint disease, although owing to the exploratory features of our study, other studies are required to confirm our findings. While knee tenderness was linked with an improved response in the short term, the effect was relatively small and unlikely to be of clinical utility; short-term response for those with patellofemoral or medial tibiofemoral joint line tenderness was 86% and 87.5% compared with 70% and 67% for those without, respectively. The data also suggest that targeting therapy based on symptoms, including for example the presence or absence of a knee effusion, should not influence the decision about whether to undertake the steroid injection. As outlined, psychological factors, including depressive symptoms and presence of widespread pain, and greater number of pain sites, although not affecting short-term outcome, reduced the likelihood of longer-term response; this reinforces the importance of targeting these other symptoms in any overall management strategy to reduce knee pain due to OA. Based on our data, such factors should not influence the decision to treat patients with more widespread pain if the target is short-term improvement.
Among patients with symptomatic knee OA, those with knee tenderness are more likely to respond to IASI therapy. Clinical signs of knee OA did not, however, predict longer-term response. The presence of CWP, having multiple pain sites, and depressive symptoms attenuate longer-term treatment response.
Supplementary Material
Acknowledgments
This study was funded by Arthritis Research UK grant 20380, and special strategic award grant 18676. The funding agency had no role in any of the following: design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. This report includes independent research supported by (or funded by) the NIHR Biomedical Research Unit Funding Scheme. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. The Research in Osteoarthritis Manchester group is supported by the Manchester Academic Health Sciences Centre. Prof. D.T. Felson is supported by NIH AR4778. N. Maricar is supported by an NIHR Allied Health Profession Clinical Doctoral Fellowship.
ACKNOWLEDGMENT
We appreciate the expert assistance of Helen Williams, Laura Heathers, Laura Forsythe, Rosie Perry, and the rest of the ROAM team. The authors are thankful for the generous contributions of time and energy of study participants. The authors also acknowledge the equipment and facilities provided by the Salford Royal NHS Foundation Trust.
Footnotes
From the Arthritis Research UK Centre for Epidemiology, Division of Musculoskeletal and Dermatological Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, The University of Manchester; National Institute for Health Research (NIHR) Manchester Biomedical Research Centre, Manchester University National Health Service (NHS) Foundation Trust, Manchester Academic Health Sciences Centre; Department of Physiotherapy, Salford Royal NHS Foundation Trust; Department of Health Professions, Manchester Metropolitan University; Department of Rheumatology, Salford Royal NHS Foundation Trust, Manchester, UK; Clinical Epidemiology Unit, Boston University School of Medicine, Boston, Massachusetts, USA.
Contributor Information
Nasimah Maricar, Arthritis Research UK Centre for Epidemiology, Division of Musculoskeletal and Dermatological Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, The University of Manchester, and NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Sciences Centre, and Department of Physiotherapy, Salford Royal NHS Foundation Trust.
Matthew J. Parkes, Arthritis Research UK Centre for Epidemiology, Division of Musculoskeletal and Dermatological Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, The University of Manchester, and NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Sciences Centre
Michael J. Callaghan, Arthritis Research UK Centre for Epidemiology, Division of Musculoskeletal and Dermatological Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, The University of Manchester, and NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Sciences Centre, and Department of Health Professions, Manchester Metropolitan University
David T. Felson, Arthritis Research UK Centre for Epidemiology, Division of Musculoskeletal and Dermatological Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, The University of Manchester, and NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Sciences Centre, and Clinical Epidemiology Unit, Boston University School of Medicine
Terence W. O’Neill, Arthritis Research UK Centre for Epidemiology, Division of Musculoskeletal and Dermatological Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, The University of Manchester, and NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Sciences Centre, and Department of Rheumatology, Salford Royal NHS Foundation Trust
REFERENCES
- 1.Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ 2004;328:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellamy N, Campbell J, Welch V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2006:CD005328. [DOI] [PubMed]
- 3.Bjordal JM, Klovning A, Ljunggren AE, Slordal L. Short-term efficacy of pharmacotherapeutic interventions in osteoarthritic knee pain: a meta-analysis of randomised placebo-controlled trials. Eur J Pain 2007;11:125–38. [DOI] [PubMed] [Google Scholar]
- 4.Juni P, Hari R, Rutjes AW, Fischer R, Silletta MG, Reichenbach S, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev 2015;CD005328. [DOI] [PMC free article] [PubMed]
- 5.Chao J, Wu C, Sun B, Hose MK, Quan A, Hughes TH, et al. Inflammatory characteristics on ultrasound predict poorer longterm response to intraarticular corticosteroid injections in knee osteoarthritis. J Rheumatol 2010;37:650–5. [DOI] [PubMed] [Google Scholar]
- 6.Smith MD, Wetherall M, Darby T, Esterman A, Slavotinek J, Roberts-Thomson P, et al. A randomized placebo-controlled trial of arthroscopic lavage versus lavage plus intra-articular corticosteroids in the management of symptomatic osteoarthritis of the knee. Rheumatology 2003;42:1477–85. [DOI] [PubMed] [Google Scholar]
- 7.Maricar N, Callaghan MJ, Felson DT, O’Neill TW. Predictors of response to intra-articular steroid injections in knee osteoarthritis—a systematic review. Rheumatology 2013;52:1022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch G, Kitas G, Klocke R. Intra-articular corticosteroid injection in osteoarthritis of the knee and hip: Factors predicting pain relief—a systematic review. Semin Arthritis Rheum 2013;42:451–73. [DOI] [PubMed] [Google Scholar]
- 9.van Middelkoop M, Arden NK, Atchia I, Birrell F, Chao J, Rezende MU, et al. The OA Trial Bank: meta-analysis of individual patient data from knee and hip osteoarthritis trials show that patients with severe pain exhibit greater benefit from intra-articular glucocorticoids. Osteoarthritis Cartilage 2016;24:1143–52. [DOI] [PubMed] [Google Scholar]
- 10.Bevers K, Zweers MC, Vriezekolk JE, Bijlsma JW, den Broeder AA. Are ultrasonographic signs of inflammation predictors for response to intra-articular glucocorticoids in knee osteoarthritis? Clin Exp Rheumatol 2014;32:930–4. [PubMed] [Google Scholar]
- 11.Hirsch G, O’Neill TW, Kitas G, Sinha A, Klocke R. Accuracy of injection and short-term pain relief following intra-articular corticosteroid injection in knee osteoarthritis - an observational study. BMC Musculoskelet Disord 2017;18:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatimah N, Salim B, Raja EU, Nasim A. Predictors of response to intra-articular steroid injections in patients with osteoarthritis of the knee joint. Clin Rheumatol 2016;35:2541–7. [DOI] [PubMed] [Google Scholar]
- 13.Calvet J, Orellana C, Galisteo C, Garcia-Manrique M, Navarro N, Caixas A, et al. Clinical and ultrasonographic features associated to response to intraarticular corticosteroid injection. A one year follow up prospective cohort study in knee osteoarthritis patient with joint effusion. PLoS One 2018;13:e0191342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Neill TW, Parkes MJ, Maricar N, Marjanovic EJ, Hodgson R, Gait AD, et al. Synovial tissue volume: a treatment target in knee osteoarthritis (OA). Ann Rheum Dis 2016;75:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maricar N, Parkes MJ, Callaghan MJ, Hutchinson CE, Gait AD, Hodgson R, et al. Structural predictors of response to intra-articular steroid injection in symptomatic knee osteoarthritis. Arthritis Res Ther 2017;19:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther 1998;28:88–96. [DOI] [PubMed] [Google Scholar]
- 17.Hurst NP, McRorie ER. The short-term health outcome of out-patient rheumatology consultations in relation to rationing: a pilot study. Br J Rheumatol 1998;37:509–13. [DOI] [PubMed] [Google Scholar]
- 18.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 19.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60:631–7. [DOI] [PubMed] [Google Scholar]
- 20.Carmona L, Ballina J, Gabriel R, Laffon A; EPISER Study Group. The burden of musculoskeletal diseases in the general population of Spain: results from a national survey. Ann Rheum Dis 2001;60:1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagen KB, Smedstad LM, Uhlig T, Kvien TK. The responsiveness of health status measures in patients with rheumatoid arthritis: comparison of disease-specific and generic instruments. J Rheumatol 1999;26:1474–80. [PubMed] [Google Scholar]
- 22.Harkness EF, Macfarlane GJ, Silman AJ, McBeth J. Is musculoskeletal pain more common now than 40 years ago?: two population-based cross-sectional studies. Rheumatology 2005;44:890–5. [DOI] [PubMed] [Google Scholar]
- 23.Nicholl BI, Macfarlane GJ, Davies KA, Morriss R, Dickens C, McBeth J. Premorbid psychosocial factors are associated with poor health-related quality of life in subjects with new onset of chronic widespread pain - results from the EPIFUND study. Pain 2009;141:119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- 25.Sturgill LP, Snyder-Mackler L, Manal TJ, Axe MJ. Interrater reliability of a clinical scale to assess knee joint effusion. J Orthop Sports Phys Ther 2009;39:845–9. [DOI] [PubMed] [Google Scholar]
- 26.Clarkson HM. Musculoskeletal assessment: joint range of motion and manual muscle strength 2nd ed. Baltimore: Williams & Wilkins; 1999. [Google Scholar]
- 27.Lord SR, Allen GM, Williams P, Gandevia SC. Risk of falling: predictors based on reduced strength in persons previously affected by polio. Arch Phys Med Rehabil 2002;83:757–63. [DOI] [PubMed] [Google Scholar]
- 28.Maricar N, Callaghan MJ, Parkes MJ, Felson DT, O’Neill TW. Interobserver and intraobserver reliability of clinical assessments in knee osteoarthritis. J Rheumatol 2016;43:2171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pham T, van der Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage 2004;12:389–99. [DOI] [PubMed] [Google Scholar]
- 30.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. [PubMed] [Google Scholar]
- 31.Jones A, Doherty M. Intra-articular corticosteroids are effective in osteoarthritis but there are no clinical predictors of response. Ann Rheum Dis 1996;55:829–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arden NK, Reading IC, Jordan KM, Thomas L, Platten H, Hassan A, et al. A randomised controlled trial of tidal irrigation vs corticosteroid injection in knee osteoarthritis: the KIVIS Study. Osteoarthritis Cartilage 2008;16:733–9. [DOI] [PubMed] [Google Scholar]
- 33.Gaffney K, Ledingham J, Perry JD. Intra-articular triamcinolone hexacetonide in knee osteoarthritis: factors influencing the clinical response. Ann Rheum Dis 1995;54:379–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pendleton A, Millar A, O’Kane D, Wright GD, Taggart AJ. Can sonography be used to predict the response to intra-articular corticosteroid injection in primary osteoarthritis of the knee? Scand J Rheumatol 2008;37:395–7. [DOI] [PubMed] [Google Scholar]
- 35.Pyne D, Ioannou Y, Mootoo R, Bhanji A. Intra-articular steroids in knee osteoarthritis: a comparative study of triamcinolone hexacetonide and methylprednisolone acetate. Clin Rheumatol 2004;23:116–20. [DOI] [PubMed] [Google Scholar]
- 36.McCabe PS, Parkes MJ, Maricar N, Hutchinson CE, Freemont A, O’Neill TW, et al. Brief report: synovial fluid white blood cell count in knee osteoarthritis: association with structural findings and treatment response. Arthritis Rheumatol 2017;69:103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sibbitt WL Jr, Band PA, Kettwich LG, Chavez-Chiang NR, Delea SL, Bankhurst AD. A randomized controlled trial evaluating the cost-effectiveness of sonographic guidance for intra-articular injection of the osteoarthritic knee. J Clin Rheumatol 2011; 17:409–15. [DOI] [PubMed] [Google Scholar]
- 38.de Rooij A, van der Leeden M, Roorda LD, Steultjens MP, Dekker J. Predictors of outcome of multidisciplinary treatment in chronic widespread pain: an observational study. BMC Musculoskelet Disord 2013;14:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karjalainen K, Malmivaara A, Mutanen P, Pohjolainen T, Roine R, Hurri H. Outcome determinants of subacute low back pain. Spine 2003;28:2634–40. [DOI] [PubMed] [Google Scholar]
- 40.McCracken LM, Turk DC. Behavioral and cognitive-behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment process. Spine 2002;27:2564–73. [DOI] [PubMed] [Google Scholar]
- 41.Reimer M, Hullemann P, Hukauf M, Keller T, Binder A, Gierthmuhlen J, et al. Prediction of response to tapentadol in chronic low back pain. Eur J Pain 2016;21:322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


