Abstract
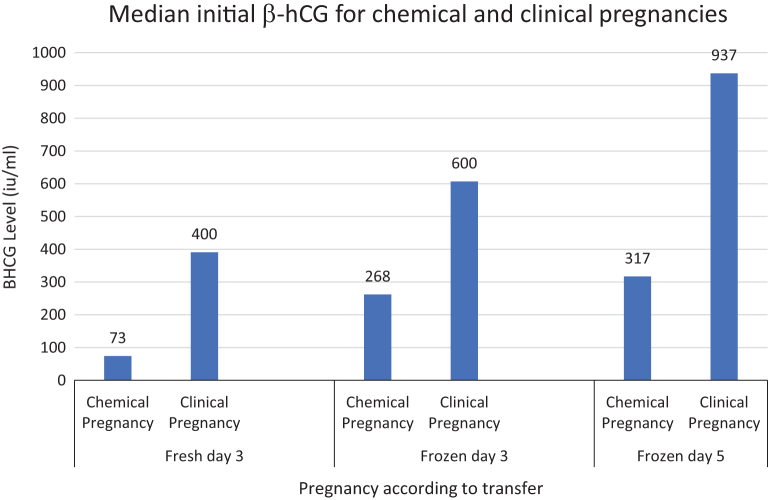
This study evaluated β-human chorionic gonadotropin (hCG) changes during the early period of pregnancy in an attempt to predict successful pregnancy outcomes in ART. It determined the median values of the β-hCG and the 2-day β-hCG increments of clinical vs biochemical pregnancies. The results of fresh day 3 embryo, frozen day 3 embryo, and frozen day 5 embryo transfers were evaluated. The cutoff values of β-hCG and the 2-day increments predicting clinical pregnancy and delivery were determined. All women who underwent embryo transfer and had a singleton pregnancy from January 2017 to December 2019 were included. As expected, clinical pregnancies had higher initial median β-hCG values compared to biochemical pregnancies (fresh day 3 (400 vs 73 mIU/mL), frozen day 3 (600 vs 268.5 mIU/mL) and frozen day 5 (937 vs 317 mIU/mL)). Nonetheless, the abortion rate was significantly lower in the group with β-hCG above the cutoff values in fresh (141 mIU/mL) and frozen (354.5 mIU/mL) cleavage stage transfers (17.2% vs 44%, P < 0.001 and 18.5% vs 38%, P = 0.003, respectively). Blastocyst transfers resulted in higher median initial β-hCG compared to cleavage embryo transfers (937 vs 600 mIU/mL), and the initial β-hCG values from frozen cleavage embryos were higher compared to fresh cleavage embryos (600 vs 400 mIU/mL). Earlier implantation in frozen cycles may be caused by freezing–thawing procedures. Moreover, in fresh cycles, negative effects of the hormonal milieu of fresh cycles may delay implantation. These results indicate that high initial β-hCG and high 2-day β-hCG increments demonstrated better outcomes, including more clinical pregnancies and fewer abortions.
Key Words: β-hCG, pregnancy outcome, IVF, ART, beta hCG increment
Introduction
A pregnancy test is performed 12–14 days after embryo transfer and a detectable human chorionic gonadotropin (β-hCG) level indicates a positive result.
β-hCG is a glycoprotein hormone, secreted by the syncytiotrophoblast cells of the embryo. This hormone is detected in the maternal circulation after implantation and as early as 9–10 days after ovulation (1) in spontaneous cycles. β-hCG is secreted from the cleaved embryo with 6–8 cells (2) and is expected to double every 1.4–2.1 days until 8–10 weeks of gestation (3, 4).
Although β-hCG values are a well-accepted marker for successful implantation, clear cutoff values are lacking, mainly in artificial reproductive technology (ART) treatments. Some studies correlated β-hCG levels to pregnancy outcomes (5) and others used repeated measurements (6). However, contradictory results were found.
This study aimed to determine the baseline β-hCG value and the increments that would predict pregnancy outcomes and obstetric complications in fresh and frozen IVF cycles in relation to the embryonic developmental stage.
Materials and methods
Patients
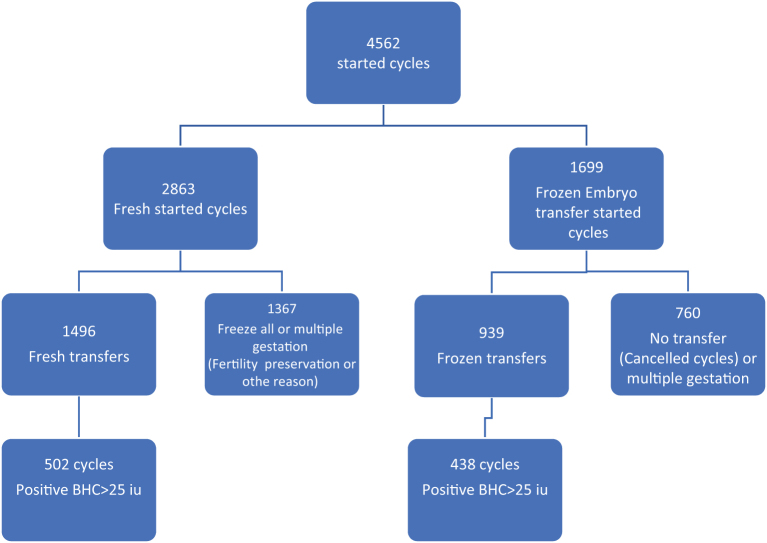
This retrospective study was conducted at a university-affiliated reproductive center from January 2017 through December 2019. All women undergoing fresh IVF or frozen transfers that resulted in singleton pregnancies were included in the study. Women undergoing fertility preservation, cycles in which embryo transfer was not performed, and women with multiple gestations were excluded. Cycles with missing data on the first β-hCG test and all cases in which β-hCG was <25 mIU/mL were also excluded from the analysis (Fig. 1). All data were collected from computerized patient’s files in our IVF unit.
Figure 1.
Flowchart of study group inclusion analysis.
The Institutional Review Board of Hillel Yaffe Medical Center approved the study (number 0026-20-HYMC). As this was a retrospective study that used un-identified data, informed consent was not required.
Ovarian stimulation and oocyte retrieval
Stimulation protocols were performed using either gonadotropin-releasing hormone (GnRH) agonist (that are either flare protocols or long downregulation protocols) on one hand or antagonist protocol on the other hand, as described previously (14). Patients were treated with agonist protocol (decapeptyl 0.1 mg, FERRING, Israel) or antagonist protocol (cetrorelix 0.25 mg, Merck-Serono, Israel). The type of protocol and doses of gonadotropins were prescribed individually according to patient characteristics and clinician preferences and judgment. The initial dose of gonadotropin was determined for each patient according to age, basal follicle-stimulating hormone (FSH) concentration, antral follicle count, body mass index (BMI), and previous response to ovarian stimulation (7, 8, 9). Patients underwent controlled ovarian stimulation with one of the following methods: (i) recombinant FSH alone (Gonal-F, Merck Serono or Puregon, MSD, Israel); (ii) highly purified human menopausal gonadotropin alone (Menopur, Ferring, Israel); or (iii) a combination of both. Estrogen and progesterone concentrations were routinely measured at every follow-up visit, including the day of β-hCG (Ovitrelle Merck-Serono) administration. Oocyte retrieval was conducted under anesthesia approximately 36 h after β-hCG was administered.
On day 3, all embryos were evaluated according to known implantation data (KID) and Alfa ESHRE scores (10). Top-quality embryos on day 3 included KID scores of 5;3 or 5;2 and Alfa ESHRE scores of 4;3 or 4;2. A part of the top-quality embryos were vitrified for future use in FET (frozen embryo transfer) cycles and the rest were incubated until day 5. Based on our experience, day 3 transfers (fresh or frozen) yielded high pregnancy rates and thus day 3 embryo transfers were preferred mainly for older patients with few embryos. Most cases with more than 10 good-quality cleavage stage embryos were cultured until day 5.
Top-quality blastocysts were also vitrified. Cleavage stage day 3 embryos or blastocysts were transferred.
Frozen embryo cycles
Frozen cycles were conducted based on hormone therapy or ovulatory cycles. In both cases, on the third day of menstruation, transvaginal ultrasonography was performed to evaluate endometrial thickness and ovarian status. Hormonal treatment was 2 mg oral estradiol valerate TID (three times a day) (either Progynova, Bayer HealthCare, Germany, or Estrofem Novo Nordisk a/S, Denmark). Transvaginal ultrasonography was performed again 7 days later and the hormone dose was adjusted depending on the endometrial thickness. When the endometrial thickness was ≥7 mm, progesterone was added for luteal preparation. Embryos were transferred after progesterone supplementation, based on their developmental stage.
The ovulatory cycles followed the development of the dominant follicle, and the decision for either a natural cycle with spontaneous ovulation or ovulation triggering with Ovitrelle was based on the timing of the transfer (avoiding weekend transfers) and based on blood levels of luteinizing hormone and appropriate follicular diameter.
Embryo transfer and luteal support
According to our IVF unit protocol, one or two cleavage stage embryos were transferred. The decision regarding the number of embryos was based on the guidelines of the Israeli Fertility Association. Based on our good success rates with single transfers of either day 3 or 5 embryos, our policy is to transfer a single embryo in the first two cycles regardless of patient age to avoid multiple pregnancies. This restriction is stringent in the case of good-quality embryos for women after cesarean section or with any uterine scar.
Only one cleavage stage embryo was transferred in all fresh transfers. Similarly, only one blastocyst was transferred in frozen blastocyst cycles.
Luteal phase support included either oral dydrogesterone 10 mg TID (Duphastone Abbott, Chicago, IL, USA), vaginal micronized progesterone (MVP) 100 mg TID (Endometrin, Ferring, Caesarea, Israel), or MVP gel 90 mg BID (Crinone 8%, Merck Serono), daily.
β-hCG evaluation
Pregnancy was determined by serum β-hCG test 13 days post-transfer. β-hCG was measured again 2 days later. All serum β-hCG levels were evaluated at our hospital laboratory using electrochemiluminescence immunoassay (Roche Labs). Δ β-hCG increment was calculated as the ratio of the second β-hCG divided by the first. We evaluated the collinearity between the two measurements to rule out any correlation between the first and the second β-hCG values.
Patients were followed until a sonographic demonstration of an embryo with a heartbeat at 6 weeks of gestation. A clinical pregnancy was confirmed when a gestational sac with a fetal heartbeat was visible on ultrasound examination 6 weeks after embryo transfer. A biochemical pregnancy was defined as positive β-hCG with no confirmation of fetal heartbeat on ultrasound examination. Delivery was defined as a pregnancy resulting in the birth of a fetus beyond the viability limit of 24 weeks, while a pregnancy that ended before that limit was defined as no delivery. Only singleton pregnancies were evaluated in this study. Demographic data, treatment information and results, and pregnancy follow-up and outcomes were recorded and monitored until delivery.
Statistical analysis
Data were analyzed using the SPSS, version 25 (IBM Corp.). Continuous variables were expressed as mean ± s.d. and were compared using Student’s t-test. For normally distributed parameters, we used a t-test and for variables that were not normally distributed, we used Mann–Whitney U test. Categorical variables were expressed as percentage and were compared between groups with Pearson chi-square or Fisher exact tests. Normality of variables was assessed using the Shapiro–Wilk test of normality. All P-values were two-sided, and P-values <0.05 were considered statistically significant. A receiver operating characteristic (ROC) analysis was performed to determine the most efficient cutoff values for the β-hCG that would discriminate between successful and unsuccessful treatment outcomes.
To understand which parameters contribute to predicting delivery, we built a model that included the first β-HCG level, the Δ β-hCG increase after 2 days, maternal age and maternal BMI, type of treatment (fresh vs frozen), last measured estradiol level, endometrial thickness, and the number of embryos transferred.
We conducted a forward selection (conditional) stepwise selection method with entry testing based on the significance of the score statistic (0.05) and removal testing based on the probability of a likelihood-ratio statistic (0.10).
Results
Clinical pregnancy outcomes
Fresh cycle (cleavage stage embryo) with positive β-hCG test to predict clinical pregnancy
A total of 940 cycles with positive β-hCG test were evaluated, including 502 fresh cycles and 438 frozen transfers. Among the 502 fresh cycles with positive β-hCG, 19% were biochemical pregnancies and 81% were clinical pregnancies.
First β-hCG measurement
The median (IQR) of the first β-hCG test was significantly lower for pregnancies that culminated in a biochemical pregnancy compared to a clinical pregnancy (73 (42–131.5) vs 400 (228–648) mIU/mL, P < 0.0001 (Fig. 3)).
Figure 3.
Significant difference between delta β-hCG according to transfer protocol. Differences between biochemical and clinical pregnancies in all categories were significant (P < 0.001).
The optimal β-hCG cutoff value for predicting a clinical pregnancy by ROC was 141 mIU/mL, with AUC = 0.87, P < 0.0001, 95% confidence interval (CI) (0.812–0.92) (Table 1).
Table 1.
Cutoff values for predicting clinical pregnancy for each sub-group ROC–AUC.
| Fresh transfer cleavage stage | Frozen cycle cleavage stage | Frozen transfer blastocyst stage | ||||
|---|---|---|---|---|---|---|
| β-hCG, mIU/mL | 2-day Δ β-hCG | β-hCG, mIU/mL | 2-day Δ β-hCG | β-hCG, mIU/mL | 2-day Δ β-hCG | |
| 141 | 1.453 | 354.5 | 1.759 | 358.5 | 1.48 | |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Sensitivity | 0.892 | 0.995 | 0.714 | 0.953 | 0.895 | 0.982 |
| 1 – Specificity | 0.20 | 0.303 | 0.40 | 0.486 | 0.40 | 0.533 |
| Area under the curve | 0.87 | 0.823 | 0.674 | 0.709 | 0.82 | 0.68 |
| 95% AUC | 0.821–0.920 | 0.712–0.935 | 0.607–0.742 | 0.598–0.820 | 0.713–0.927 | 0.497–0.862 |
Δ β-hCG increment
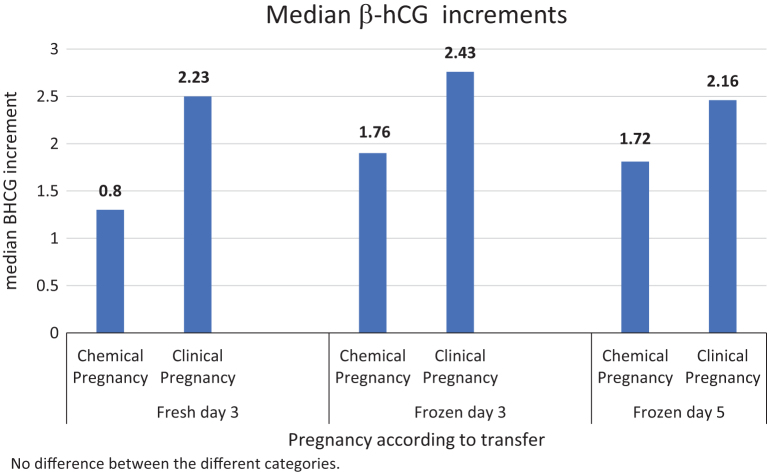
The mean 2-day increase in β-hCG was significantly higher in cycles that developed to clinical pregnancy as compared to a biochemical pregnancy (Δ = 2.5 vs Δ = 1.3, P < 0.001 respectively; Fig. 3). The optimal increase was evaluated in a ROC analysis and the cutoff value was Δ = 1.453; AUC = 0.823, P < 0.0001; 95% CI (0.71–0.935; Table 1).
No collinearity was found between the two parameters of the first and the second β-hCG.
Frozen cycles with positive β-hCG test for predicting clinical pregnancy
A total of 438 frozen cycles with positive β-hCG test were evaluated, including 366 cleavage stage and 72 blastocyst stage. Overall, the frozen–thawed cycles resulted in 323 (73.7%) clinical pregnancies.
First β-hCG measurement
The median (IQR) β-hCG of the group with biochemical pregnancies was significantly lower compared with the clinical pregnancy group (271 (88–660) vs 651 (346–1130) mIU/mL, respectively; P < 0.0001, Fig. 2).
Figure 2.
Significant difference between median β-hCG in the different transfers. Differences between biochemical and clinical pregnancies were significant in all categories (P < 0.001). Among clinical pregnancies, significant differences between fresh day 3, frozen day 3, and frozen day 5 transfers, with adjustment for multiple comparisons were detected; P < 0.001. Among biochemical pregnancies, significant differences between groups with adjustment for multiple comparisons were detected, except frozen day 3 vs frozen day 5; P = 0.14.
The optimal β-hCG cutoff value for predicting clinical pregnancy based on the ROC was 354.5 mIU/mL, with AUC = 0.696, P < 0.0001; 95% CI (0.636–0.757) (Table 1).
Δ β-hCG
The median 2-day increase in β-hCG was significantly larger in the clinical pregnancy group as compared to the biochemical pregnancy group (Δ= 2.35 vs Δ = 1.75, respectively, P < 0.0001; Fig. 3). The optimal increase was evaluated in an ROC analysis and the cutoff value was Δ = 1.759; AUC = 0.704, P < 0.0001; 95% CI (0.611–0.798) (Table 1).
Frozen cycle (cleavage stage embryos) with positive β-hCG test for predicting clinical pregnancy
Among the 366 frozen–thawed cycles, cleavage stage transfers with positive β-hCG test resulted in 266 (72.7%) clinical pregnancies.
First β-hCG measurement
The median (IQR) β-hCG in the biochemical pregnancies was significantly less than the clinical pregnancy group 268.5 ((86–659) mIU/mL vs 600 mIU/mL (303–993), respectively; P < 0.0001; Fig. 2).
The optimal β-hCG cutoff value for predicting a clinical pregnancy using ROC was 354.5 mIU/mL, with AUC = 0.674, P < 0.0001, and 95% CI (0.607–0.742) (Table 1).
Δ β-hCG increment
The median 2-day increase in β-hCG was significantly greater in the clinical pregnancy group as compared to the biochemical pregnancy group (Δ = 2.43 vs Δ = 1.75, respectively; P < 0.001; Fig. 3). The optimal increase was evaluated in an ROC analysis and the cutoff value was Δ = 1.759, AUC = 0.709, P < 0.0001; 95% CI (0.598–0.820) (Table 1).
Frozen cycle (blastocyst stage embryos) with positive β-hCG test outcomes for predicting clinical pregnancy
The 72 frozen–thawed blastocyst stage cycles with positive β-hCG resulted in 57 (79.2%) clinical pregnancies.
First β-hCG measurement
The median (IQR) β-hCG in the biochemical pregnancy group was significantly lower than in the clinical pregnancy group (317 (121–672) vs 937 (558–1872) mIU/mL, respectively; P < 0.0001 (Fig. 3)). The optimal β-HCG cutoff value for clinical pregnancy prediction using ROC was 358.5 mIU/mL, with AUC = 0.820, P < 0.0001, and 95% CI (0.713–0.927) (Table 1).
Δ β-hCG
The median 2-day increase in β-hCG was significantly higher in the clinical pregnancy group as compared to the no clinical pregnancy group (Δ = 2.16 vs Δ = 1.72, P < 0.001; Fig. 3). The optimal increase was evaluated in an ROC analysis, and the cutoff value was Δ =1.48, AUC = 0.680, P < 0.0001, and 95% CI (0.497–0.862) (Table 1).
Women’s characteristics affecting treatment outcomes
We evaluated the effect of various women’s characteristics on treatment outcomes using a predictive model for clinical pregnancy as compared to biochemical pregnancy. Included in the model were age, BMI, fresh vs frozen embryo transfer, last measured estradiol, endometrial thickness, and first β-hCG level. We found that the first β-hCG, 2-day Δ β-hCG, and BMI of the women were significantly higher among cycles that resulted in clinical pregnancy as compared to biochemical pregnancy (OR 1.1, P = 0.023; 95% CI 1.01–1.271; Table 2).
Table 2.
A model to predict clinical vs biochemical pregnancy outcome depending on women’s parameters.
| Variable | Odds ratio | 95% Confidence interval | P-value |
|---|---|---|---|
| Body mass index, kg/m2 | 1.1 | 1.01–1.271 | 0.023 |
| Last measured estradiol, pg/mL | 1 | 0.999–1.0 | 0.35 |
| Age, years | 0.996 | 0.908–1.093 | 0.93 |
| Endometrial thickness, mm (last measurement) | 1.07 | 0.803–1.401 | 0.64 |
| First β-hCG, mIU/mL | 1.02 | 1.006–1.018 | <0.001 |
| 2-day Δ β-hCG | 4.29 | 2.04–9.05 | <0.001 |
Delivery outcomes
We evaluated the entire group according to the outcomes of delivery vs no delivery. Initial β-hCG and the 2-day β-hCG increment in both categories were defined. The effect of various characteristics on the outcomes was evaluated (Table 3).
Table 3.
Characteristics of cycles resulting in delivery or no delivery.
| Characteristic | No delivery (n = 161) | Delivery (n = 507) | P-value |
|---|---|---|---|
| Age, years | 34.1 ± 6.3 | 32.6 ± 5.9 | 0.006 |
| Body mass index, kg/m2 | 26.3 ± 5.6 | 26.1 ± 5.6 | 0.74 |
| Baseline hormone profile | |||
| LH ≥ 20 mIU/mL | 7.62 (5.69–10.8) | 8.50 (6.58–10.43) | 0.35 |
| E2 (pg/mL) | 69.0 (50–185.5) | 73 (491–53) | 0.94 |
| Progesterone (ng/mL) | 0.23 (0.14–0.5) | 0.22 (0.11–0.38) | 0.26 |
| Previous deliveries | 0 (0–1) | 0 (0–1) | 0.99 |
| Previous cycles (mean ± s.d.) | 3.6 ± 2.6 | 3.0 ± 2.3 | 0.007 |
| Cause of infertility, n (%) | |||
| Advanced maternal age | 5 (3%) | 14 (3%) | 0.79 |
| Male factor | 63 (37%) | 217 (41%) | 0.37 |
| Anovulation | 23 (13%) | 47 (9%) | 0.10 |
| Mechanical factor | 19 (11%) | 69 (13%) | 0.59 |
| Unexplained | 60 (35%) | 170 (32%) | 0.51 |
| Endometriosis | 0 | 12 (2%) | 0.045 |
| Treatment parameters | |||
| Protocol | 0.034 | ||
| Fresh OPU (%) | 84 (52%) | 314 (62%) | |
| Frozen ET (%) | 77 (48%) | 193 (38%) | |
| Last E2 (pg/mL) | 355 (146–944) | 568 (208–1183) | 0.008 |
| Last progesterone <20 (ng/mL) | 0.44 (0.21–1.10) | 0.42 (0.18–0.95) | 0.54 |
| Last endometrial thickness (mm) | 7.01 ± 2.5 | 7.28 ± 2.72 | 0.32 |
| Embryos transferred, n (%) | |||
| 1 | 86 (53.4) | 320 (63.1) | 0.033 |
| 2 | 75 (46.6) | 187 (36.9) | |
| Treatment outcomes | |||
| β-hCG mIU/mL | 309 (146–632) | 518 (301–869) | <0.001 |
| Δ β-hCG mIU/mL | 2.28 (1.98–2.79) | 2.29 (1.99–2.76) | 0.96 |
ET, embryo transfer; LH, luteinizing hormone; OPU, ovum pick-up.
The median β-hCG level was significantly higher in the group that delivered compared to the no delivery group (518 (301–869) vs 309 (146–632) mIU/mL; P < 0.001). However, the incremental increase in β-hCG was comparable between groups. Maternal age, fresh vs frozen treatment, last measured estradiol, and the number of transferred embryos differed significantly between groups (Table 3).
A forward stepwise (conditional) calculation was performed based on the significant variables, including BMI, last measured estradiol, women’s age, endometrial thickness, first β-hCG, 2-day Δ β-hCG, and the type of treatment. In this multivariate analysis, only age, initial β-hCG, type of treatment, and fresh vs frozen transfer were significantly different. We found that the younger a women’s age (OR 0.95, 95% CI 0.92–0.99, P = 0.02) and the higher the initial β-hCG, the higher the likelihood of delivery. Moreover, the probability of delivery was greater in fresh cycles than in frozen cycles. When expanding the parameters and including embryonic age and previous deliveries in the model, in addition to the previously mentioned variables, the last estradiol measured before the transfer was significant. E2 above 221.9 predicted delivery with OR 3.72 (CI 1.881–7.355) compared to the last E2 below 221. 9 (P < 0.001). Embryonic age (day 3 vs day 5) was comparable between the groups (P = 0.091; Table 4).
Table 4.
A model to predict pregnancy outcome (delivery vs no delivery) depending on the women’s parameters.
| Variable | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Last measured estradiol cutoff 221.9 pg/mL | 3.72 | 1.881–7.355 | <0.001 |
| Age, years | 0.957 | 0.922–0.993 | 0.02 |
| First β-hCG, mIU/mL | 1.011 | 1.00–1.001 | 0.013 |
| Fresh vs frozen cycle | 1.760 | 1.052–2.946 | 0.031 |
Obstetrical outcomes according to β-hCG cutoff
Obstetrical outcomes, including delivery method and complications of preterm delivery, hypertension, and antepartum bleeding were evaluated in correlation with the β-hCG cutoff values.
The group with β-hCG above the cutoff value had significantly more vaginal deliveries as compared to the group with β-hCG below the cutoff value in all subgroups: fresh transfers (80% vs 50%, P = 0.001), frozen cleavage stage (53% vs 21.5%, P = 0.001) and frozen blastocyst stage transfers (49% vs 0; P = 0.009), respectively. No differences in pregnancy complications, including preterm delivery, hypertension, and antepartum bleeding were detected between subgroups below or above cutoff values. Fetal birth weights were comparable, as well.
Discussion
β-hCG values are well-accepted as a marker for successful implantation. However, their predictive value for pregnancy outcomes in ART may be influenced by factors such as fresh vs frozen ET cycles and embryonic age (11). This study used the first β-hCG test and the 2-day incremental difference to predict pregnancy outcomes. We analyzed fresh cleavage stage transfers and frozen transfers separately, based on cleavage vs blastocyst embryo transfers. We excluded multiple gestations to avoid possible confounding factors.
High initial β-hCG and high 2-day increase in β-hCG demonstrated better outcomes, including more clinical pregnancies (Figs. 2 and 3) and fewer missed abortions in all sub-groups. The cutoff values for initial β-hCG and the 2-day β-hCG increment for predicting clinical pregnancy were determined (cutoff values for fresh cleavage stage were 140.5 IU/mL and 1.45 mIU/mL, for frozen cleavage stage 354 IU/mL and 1.76 mIU/mL, and for frozen blastocysts 358.5 IU/mL and 1.48 mIU/mL, respectively). Moreover, our model also supports previous results (6), which reported that higher initial β-hCG values are predictive of favorable outcomes. In our model, the prediction of a clinical pregnancy based on the first β-hCG had an OR 1.02 (P < 0.001; 95% CI 1.006–1.018), and the 2-day Δ β-hCG had an OR of 4.29 (P < 0.001; 95% CI 2.24–9.25).
As expected, blastocysts resulted in higher initial β-hCG. Notably, all patients had β-hCG measured 13 days after transfer. Therefore, higher initial β-hCG for blastocysts is expected due to earlier implantation compared to cleavage stage embryos. However, it is possible that if β-hCG levels were measured earlier in blastocyst transfers, considering the difference in intervals of embryo implantation in correlation to embryonic age, the levels for blastocyst embryos would be comparable to those of cleavage embryos. Notably, the increase in β-hCG was 1.48-fold with frozen blastocysts compared to 1.76-fold with frozen cleavage embryos, raising the question of whether cleavage stage embryos grow faster and implant earlier post-fertilization (12). Perhaps β-hCG values increase more slowly in blastocysts, as the β-hCG profile is not linear. Previous studies reported earlier plateauing of β-hCG with IVF as compared to non-IVF pregnancies (13).
In addition to the high predictive value of β-hCG demonstrated in this study, other interesting findings were observed.
Day 3 transfers in fresh vs frozen cycles
The initial β-hCG and the 2-day increment for cleavage stage embryos were higher for frozen as compared to fresh embryos (354 vs 141 IU/mL; P < 0.0001 and Δ = 1.76 vs Δ = 1.45, respectively; P < 0.0001). Our results agree with those of other studies that demonstrated higher initial β-hCG values and larger increments following FET (14).
It is well-established that treatment outcomes are affected by the quality of the embryo and the endometrium. Frozen–thawed cycles demonstrated similar pregnancy rates as did fresh transfers; with some studies reporting better outcomes in FET cycles (15). This may be because only high-quality embryos are cryopreserved, the physiologic endometrial preparation in FET and by the advances in vitrification techniques over time.
Our results, in agreement with the literature, suggest that transfers of cryopreserved embryos resulted in higher β-hCG levels. This could be explained by the assumption that cryopreservation improves mitochondrial function and cell viability in embryos due to the beneficial effect of antioxidants in the medium. Consequently, they develop quicker, resulting in significantly higher initial β-hCG values (16, 17, 18).
On the other hand, in our model to predict pregnancies that ended with live birth, there were more fresh cycles in the delivery group compared to the no delivery group. A likely reason for this is the age difference between the frozen and fresh cycles.
Nonetheless, endometrial receptivity could be decreased in fresh cycles due to the supra-physiologic hormone level altering the gene expression that is fundamental for embryonal–endometrial interactions (16). Moreover, extremely high progesterone levels in fresh cycles would have a deleterious effect on the endometrial development (11).
In addition to the cycle type, improved endometrial receptivity resulting in more clinical pregnancies could be related to women’s parameters, such as BMI. Accordingly, we found that high BMI resulted in more biochemical pregnancies and fewer clinical pregnancies, indicating the negative effects of excess weight on treatment results (17).
As opposed to the beneficial effect of frozen cycles on the outcomes of implantation and clinical pregnancy, fresh cycles, as well as younger age of the woman, resulted in more deliveries. Perhaps, avoiding extra procedures on the embryo in fresh cycles has beneficial effects on the embryo. Nonetheless, it is well-known that embryo quality is often higher in younger women. Therefore, the outcome of more deliveries in fresh compared to frozen cycles suggests that higher quality embryos might compensate for the negative effect of hormones on the endometrium in fresh cycles.
Frozen cycles
Frozen cycles had higher β-hCG levels on day 5 compared to day 3. Accordingly, there were more pregnancies in the blastocyst group. The ROC analysis demonstrated significantly higher β-hCG levels with frozen blastocyst transfers compared to fresh and frozen cleavage stage transfers. The initial β-hCG and the 2-day increment for frozen blastocysts were higher compared to cleavage stage embryos (358.5 vs 354.5; P < 0.0001). In contrast, the 2-day increment was smaller in the blastocyst compared to cleavage stage transfers (Δ = 1.48 vs Δ = 1.76, respectively; P < 0.0001).
Previous studies demonstrated that blastocyst transfers have higher pregnancy rates. This may be because blastocyst stage transfers result in the selection of higher quality embryos and mimic natural cycles; therefore, yielding higher implantation rates due to better embryo–endometrium synchrony (18).
Women’s BMI and the treatment outcomes
It is well-established that increased BMI may worsen implantation rates and pregnancy outcomes (19). We found that clinical pregnancy rates increased with increasing BMI. However, the group with increased BMI that delivered was significantly younger and we believe this had a significant impact on the delivery rate. In the multivariate analysis, BMI was not found as a significant parameter influencing cycle outcomes. We analyzed the impact of BMI in a model to predict delivery and the only parameter that showed a trend was the women’s age.
β-hCG values predict pregnancy outcomes
β-hCG values in fresh and frozen cycles were found to be significant clinical biomarkers for successful pregnancy results after IVF, enabling better clinical management of patient stress. As expected, the higher the initial β-hCG, the better the pregnancy outcomes, specifically, lower abortion rates and more vaginal deliveries. In univariate analysis, we found that the β-hCG was significantly higher in the delivery group than in the no delivery group. Women in the delivery group were significantly younger than those in the no delivery group. The higher β-hCG may be because oocyte quality is better in younger women and endometrial receptivity is higher, increasing the chances of achieving pregnancy and delivering. It is likely that the age of the embryo did not contribute to the difference between groups, as no difference in the prevalence of day 3 and day 5 embryos was found.
Due to the small number of pregnancy complications, such as preterm delivery, preeclampsia, and antepartum bleeding in our cohort, β-hCG could not predict pregnancy complications between subgroups according to cutoff values. Previous studies found β-hCG to be a valuable clinical indicator for predicting pregnancy complications such as preeclampsia but had little predictive value in the first trimester (20) (21).
High initial β-hCG and Δ β-hCG values are strongly predictive of clinical pregnancy. However, the live birth rate is associated with high β-hCG values solely while the Δ β-hCG increment is not predictive. Specifically, β-hCG above 518 strongly suggests a live birth, precluding the need for further measurements. Practically, adopting a policy of one adequate β-hCG measurement rather than repeated measurements has positive financial consequences in IVF procedures.
A limitation of this study included the difference in timing of the first β-hCG test compared to other studies. We checked after 13 days, while previous research reported measurements taken 12–14 days after transfer. Moreover, the retrospective nature of the study may limit the impact of the results. The lack of genetic data on the transferred embryos may also have biased the interpretation.
A strength of this study was the relatively large cohort of singleton pregnancies analyzed according to fresh and frozen groups. Importantly, the follow-up until delivery provided a predictive value of β-hCG to alert physicians for the need of earlier and repeated pregnancy follow-up to distinguish between normal and abnormal pregnancies.
In conclusion, high initial β-hCG values rather than Δ β-hCG increments were strongly predictive of live birth, precluding the need for repeated measurements. The higher β-hCG and the 2-day increment for frozen blastocysts compared to cleavage stage embryos are compatible with the superior results of blastocyst transfers (22).
Declaration of interest
The authors have no conflicts of interest to declare.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Wilcox AJ Baird DD & Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. New England Journal of Medicine 19993401796–1799. ( 10.1056/NEJM199906103402304) [DOI] [PubMed] [Google Scholar]
- 2.Bonduelle ML Dodd R Liebaers I Van Steirteghem A Williamson R & Akhurst R. Chorionic gonadotrophin-beta mRNA, a trophoblast marker, is expressed in human 8-cell embryos derived from tripronucleate zygotes. Human Reproduction 19883909–914. ( 10.1093/oxfordjournals.humrep.a136808) [DOI] [PubMed] [Google Scholar]
- 3.Fritz MA & Guo SM. Doubling time of human chorionic gonadotropin (hCG) in early normal pregnancy: relationship to hCG concentration and gestational age. Fertility and Sterility 198747584–589. ( 10.1016/S0015-0282(1659107-9) [DOI] [PubMed] [Google Scholar]
- 4.Kadar N & Romero R. Observations on the log human chorionic gonadotropin-time relationship in early pregnancy and its practical implications. American Journal of Obstetrics and Gynecology 198715773–78. ( 10.1016/s0002-9378(8780349-6) [DOI] [PubMed] [Google Scholar]
- 5.Doubilet PM Phillips CH Durfee SM & Benson CB. First-trimester prognosis when an early gestational sac is seen on ultrasound imaging: logistic regression prediction model. Journal of Ultrasound in Medicine 202140541–550. ( 10.1002/jum.15430) [DOI] [PubMed] [Google Scholar]
- 6.Yu H Liu J Guo H Chen C Han Y & Cui Y. Prognostic value of repeated serum kisspeptin measurements in early first trimester pregnancy: a preliminary study. Reproductive Biomedicine Online 201938465–471. ( 10.1016/j.rbmo.2018.11.014) [DOI] [PubMed] [Google Scholar]
- 7.Olivennes F Alvarez S Bouchard P Fanchin R Salat-Baroux J & Frydman R. The use of a GnRH antagonist (cetrorelix) in a single dose protocol in IVF-embryo transfer: a dose finding study of 3 versus 2 mg. Human Reproduction 1998132411–2414. ( 10.1093/humrep/13.9.2411) [DOI] [PubMed] [Google Scholar]
- 8.Huirne JAF, Lambalk CB, van Loenen ACD, Schats R, Hompes PGA, Fauser BCJM, Macklon NC. Contemporary pharmacological manipulation in assisted reproduction. Drugs 2004642, 97–322. [DOI] [PubMed] [Google Scholar]
- 9.Barbieri RL & Hornstein MD. Assisted reproduction-in vitro fertilization success is improved by ovarian stimulation with exogenous gonadotropins and pituitary suppression with gonadotropin-releasing hormone analogues. Endocrine Reviews 199920249–252. ( 10.1210/edrv.20.3.0363) [DOI] [PubMed] [Google Scholar]
- 10.Liu Y Chapple V Feenan K Roberts P & Matson P. Time-lapse deselection model for human day 3 in vitro fertilization embryos: the combination of qualitative and quantitative measures of embryo growth. Fertility and Sterility 2016105656–662.e1. ( 10.1016/j.fertnstert.2015.11.003) [DOI] [PubMed] [Google Scholar]
- 11.Vuong LN. Con: freeze-all for all? One size does not fit all. Human Reproduction 2022371388–1393. ( 10.1093/humrep/deac103) [DOI] [PubMed] [Google Scholar]
- 12.Dinnyés A, Lonergan P, Fair T, Boland MP, Yang X. Timing of the first cleavage post‐insemination affects cryosurvival of in vitro–produced bovine blastocysts blastocysts. Molecular Reproduction and Development 199953318–324. () [DOI] [PubMed] [Google Scholar]
- 13.Chung K Sammel MD Coutifaris C Chalian R Lin K Castelbaum AJ Freedman MF & Barnhart KT. Defining the rise of serum HCG in viable pregnancies achieved through use of IVF. Human Reproduction 200621823–828. ( 10.1093/humrep/dei389) [DOI] [PubMed] [Google Scholar]
- 14.Sung N Kwak-Kim J Koo HS & Yang KM. Serum hCG-β levels of postovulatory day 12 and 14 with the sequential application of hCG-β fold change significantly increased predictability of pregnancy outcome after IVF-ET cycle. Journal of Assisted Reproduction and Genetics 2016331185–1194. ( 10.1007/s10815-016-0744-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maheshwari A Pandey S Amalraj Raja E Shetty A Hamilton M & Bhattacharya S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Human Reproduction Update 20182435–58. ( 10.1093/humupd/dmx031) [DOI] [PubMed] [Google Scholar]
- 16.Shapiro BS Daneshmand ST Garner FC Aguirre M Hudson C & Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertility and Sterility 201196344–348. ( 10.1016/j.fertnstert.2011.05.050) [DOI] [PubMed] [Google Scholar]
- 17.Einarsson S, Bergh C, Friberg B, Pinborg A, Klajnbard A, Karlström PO, Kluge L, Larsson I, Loft A, Mikkelsen-Englund AL, et al. Weight reduction intervention for obese infertile women prior to IVF: a randomized controlled trial. Human Reproduction 2017321621–1630. ( 10.1093/humrep/dex235) [DOI] [PubMed] [Google Scholar]
- 18.Clua E Rodríguez I Arroyo G Racca A Martínez F & Polyzos NP. Blastocyst versus cleavage embryo transfer improves cumulative live birth rates, time and cost in oocyte recipients: a randomized controlled trial. Reproductive Biomedicine Online 202244995–1004. ( 10.1016/j.rbmo.2022.01.001) [DOI] [PubMed] [Google Scholar]
- 19.Petanovski Z Dimitrov G Ajdin B Hadzi-Lega M Sotirovska V Matevski V Stojkovska S Saltirovski S Suslevski D & Petanovska E. Impact of body mass index (BMI) and age on the outcome of the IVF process. Prilozi 201132155–171. [PubMed] [Google Scholar]
- 20.Zhang X Huangfu Z Shi F & Xiao Z. Predictive performance of serum β-hCG MoM levels for preeclampsia screening: a meta-analysis. Frontiers in Endocrinology (Lausanne) 202112619530. ( 10.3389/fendo.2021.619530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halscott TL Ramsey PS & Reddy UM. First trimester screening cannot predict adverse outcomes yet. Prenatal Diagnosis 201434668–676. ( 10.1002/pd.4407) [DOI] [PubMed] [Google Scholar]
- 22.Perrier d’Hauterive S Berndt S Tsampalas M Charlet-Renard C Dubois M Bourgain C Hazout A Foidart JM & Geenen V. Dialogue between blastocyst hCG and endometrial LH/hCG receptor: which role in implantation? Gynecologic and Obstetric Investigation 200764156–160. ( 10.1159/000101740) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a