Abstract
Objective
The serum triglyceride-glucose (TyG) index is a marker of inflammation. However, the relationship between TyG index and peritoneal dialysis-related peritonitis (PDRP) is unclear. This study aimed to investigate the potential relationship between the baseline TyG index and the initial episode of PDRP.
Methods
A total of 208 peritoneal dialysis (PD) patients were enrolled from January 1, 2012, to December 31, 2019 and followed up until December 31, 2022. They were divided into 2 groups according to the median TyG. The primary outcome was the occurrence of the initial episode of PDRP while on PD therapy. Kaplan-Meier curves and Cox regression analyses were used to examine the association between them.
Results
Eighty-five initial episodes of PDRP were identified. The risk of PDRP was higher in the high-TyG index group (p = 0.030). Multivariate Cox regression analysis showed a higher risk of PDRP in patients with a high TyG index (HR = 1.800, 95% CI 1.511–2.815, p = 0.010).
Conclusion
The baseline serum TyG index was an independent risk factor for the initial episode of PDRP in chronic PD patients.
Keywords: Triglyceride-glucose index, peritoneal dialysis, peritonitis
Introduction
Chronic kidney disease (CKD) has become a serious health problem [1], causing a huge burden to society and individuals. Peritoneal dialysis (PD) is an effective option for renal replacement therapy, and has been widely accepted because of its advantages of low cost, simple operation, and stable hemodynamics [2]. However, peritoneal dialysis-related peritonitis (PDRP) remains a common complication in patients undergoing PD, which can lead to hospitalization, technique failure, and mortality [3]. Therefore, identifying the risk factors for PDRP is of great significance for improving the prognosis of patients with PD.
PD causes inflammation in the peritoneal space and inflammation is a risk factor for the development of PDRP [4, 5]. The triglyceride-glucose (TyG) index has become a new option for predicting insulin resistance (IR) and is a risk factor for cardiovascular disease (CVD) [6, 7]. Recent studies have shown that the serum TyG index was a marker of inflammation [8, 9], so we hypothesize that higher TyG might be a risk factor for PDRP. However, the relationship between them was unclear, and no relevant studies have been conducted to date. This study aimed to explore the potential relationship between baseline serum TyG index and initial episode of PDRP to identify risk factors for adverse outcomes in patients with PD as early as possible and to provide new ideas for the clinical management of patients with PD.
Materials and methods
Study population
Patients aged ≥ 18 years who underwent peritoneal dialysis catheterization at our center from January 1, 2012, to December 31, 2019, were included and followed up until December 31, 2022. All patients received continuous ambulatory peritoneal dialysis (CAPD) or daytime ambulatory peritoneal dialysis (DAPD). No patients used the PD cyclers. The exclusion criteria were as follows: (1) PD treatment for < 3 months, (2) transfer from permanent hemodialysis, and (3) lack of follow-up data. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (authorization number: KY201302). All participants signed an informed consent form.
Study outcomes
The primary outcome was initial episode of PDRP. The subsequent episodes of PDRP were not included in this study. PDRP data were obtained from electronic medical records and telephone follow-up, which were completed by the PD follow-up group composed of professional nurses and doctors. All patients were followed up until initial episode of PDRP, death, transfer to hemodialysis therapy, transfer to renal transplantation, transfer to other centers, loss to follow-up, or censoring on December 31, 2022.
Data collection
We collected baseline demographic data and average laboratory indicators within 90 days of PD treatment initiation, including age, sex, primary cause of ESRD, comorbid diseases, and laboratory characteristics including hemoglobin, albumin, creatinine, urea nitrogen, cholesterol, triglycerides (TG), fasting blood glucose (FBG), corrected calcium, phosphorus, uric acid, ferritin, and high-sensitivity C-reactive protein (hs-CRP). The serum TyG index was calculated as ln (fasting TG [mg/dL] ×FBG [mg/dL]/2) [10]. Patients were classified into 2 groups according to the median baseline serum TyG index [11]: low TyG group (TyG < 8.39) and high TyG group (TyG ≥ 8.39). For the diagnostic criteria of PDRP, we followed 2022 ISPD [12], that is, as long as at least 2 of the following were met. (1) cloudy PD effluent or abdominal pain; (2) PD effluent total leukocyte count ≥100 × 106cells/L, and polymorphonuclear cells >50%; and (3) positive PD effluent culture. Patients were diagnosed with diabetes mellitus (DM) if their FBG was ≥7.0 mmol/L or their plasma glucose level at 2 h of oral glucose tolerance test was ≥11.1 mmol/L, or if they had a history of DM [13].
Statistical analysis
Quantitative data are expressed as mean ± standard deviation and median (interquartile range), respectively, depending on whether they follow a normal distribution, while qualitative data are expressed as frequencies and percentages (%). The normality of distribution was tested using the Shapiro-Wilk test. t test, Kruskal-Wallis H test, and Chi-square test were used for comparison. Spearman rank correlation analysis was used to clarify the relationship between TyG and other inflammatory parameters. Kaplan-Meier curves were used to examine the differences in cumulative peritonitis-free survival. Multivariate Cox regression models were employed to analyze variables with statistically significant differences in univariate analyses, as well as variables considered to be associated with initial episode of PDRP. To investigate the reliability of the baseline serum TyG index in predicting initial episode of PDRP, ROC curves were used. To determine whether there was an interaction between subgroup variables and the TyG index, we conducted a subgroup analysis and represented the results with forest plots. All above statistical analyses were performed using SPSS 26.0. The post-hoc power analysis was further conducted by R (version 4.1.0). We focus on comparing the occurrence of PDRP between 2 groups (Chi-square test). The input parameters were effect size of 0.3, alpha error probability of 0.05, degree of freedom of 1, and sample size of 208. The power was 0.991. p < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 208 patients with PD were enrolled in this study (Figure 1). Baseline characteristics of the cohort studies are presented in Table 1. Among the total patients, the age was 49.85 ± 14.95 years, and 109 (52.4%) of patients were male. There were 41 (19.7%) patients with DM and 22 (10.6%) with a history of cardiovascular disease. The high TyG group had higher levels of hs-CRP, cholesterol, TG, and ferritin, and a higher rate of peritonitis, but a lower rate in men.
Figure 1.
Flow chart of the participants in the study cohort. HD: hemodialysis; PD: peritoneal dialysis; PDRP: peritoneal dialysis-related peritonitis.
Table 1.
Baseline characteristics of 208 PD patients.
| Characteristics | Total (n = 208) | TyG < 8.39 (n = 104) | TyG ≥ 8.39 (n = 104) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (year) | 49.85 ± 14.95 | 50.91 ± 15.37 | 48.79 ± 14.52 | 0.306 |
| Men n (%) | 109 (52.4) | 62 (59.6) | 47 (45.2) | 0.037 |
| BMI (kg/m2) | 22.11 ± 3.32 | 21.87 ± 3.20 | 22.34 ± 3.40 | 0.308 |
| Comorbid | ||||
| Diabetes n (%) | 41 (19.7) | 18 (17.3) | 23 (22.1) | 0.383 |
| Cardiovascular disease n (%) | 22 (10.6) | 13 (12.5) | 9 (8.7) | 0.367 |
| Primary kidney disease | 0.763 | |||
| Chronic glomerulonephritis n (%) | 117 (56.3) | 59 (56.7) | 58 (55.8) | |
| Hypertensive nephropathy n (%) | 33 (15.9) | 17 (16.3) | 16 (15.4) | |
| Diabetic nephropathy n (%) | 33 (15.9) | 14 (13.5) | 19 (18.3) | |
| Other n (%) | 25 (12.0) | 14 (13.5) | 11 (10.6) | |
| Laboratory variables | ||||
| Hemoglobin (g/L) | 77.3 ± 19.4 | 75.2 ± 18.8 | 79.4 ± 19.9 | 0.118 |
| hs-CRP (mg/L) | 2.20 (1.02, 6.08) | 1.52 (0.77, 4.09) | 2.90 (1.16, 8.27) | 0.030 |
| Serum creatinine (μmol/L) | 883.3 ± 332.1 | 881.8 ± 337.6 | 884.7 ± 328.2 | 0.950 |
| Serum urea nitrogen (mmol/L) | 31.0 ± 13.0 | 31.3 ± 12.4 | 30.8 ± 13.7 | 0.788 |
| Serum uric acid (μmol/L) | 508.0 ± 155.8 | 502.3 ± 154.4 | 513.8 ± 157.7 | 0.596 |
| Corrected calcium (mmol/L) | 2.01 ± 0.30 | 2.00 ± 0.28 | 2.03 ± 0.33 | 0.470 |
| Serum phosphorus (mmol/L) | 2.01 ± 0.71 | 2.00 ± 0.57 | 2.02 ± 0.83 | 0.821 |
| Serum albumin (g/L) | 32.4 ± 5.4 | 31.7 ± 5.1 | 33.0 ± 5.6 | 0.085 |
| Total cholesterol (mmol/L) | 4.15 ± 1.10 | 3.87 ± 1.06 | 4.43 ± 1.08 | <0.001 |
| Total triglycerides (mmol/L) | 1.32 ± 0.69 | 0.88 ± 0.38 | 1.75 ± 0.66 | <0.001 |
| Fasting blood glucose (mmol/L) | 5.10 ± 1.80 | 4.58 ± 0.96 | 5.63 ± 2.25 | <0.001 |
| TyG index | 8.42 ± 0.57 | 7.97 ± 0.35 | 8.86 ± 0.36 | <0.001 |
| Ferritin (μg/L) | 198.0 (76.9, 313.8) | 145.0 (63.5, 285.0) | 227.5 (90.8, 353.3) | 0.045 |
| Total Kt/V | 2.23 ± 0.71 | 2.15 ± 0.70 | 2.31 ± 0.71 | 0.138 |
| RRF (ml/min/1.73m2) | 2.62 (1.11, 4.44) | 2.37 (0.88, 3.87) | 2.83 (1.66, 5.63) | 0.086 |
| Outcomes | ||||
| Peritonitis | 85 (40.9) | 33 (31.7) | 52 (50.0) | 0.007 |
| Time until the first episode of peritonitis (months) | 32.0 (11.0, 55.8) | 31.5 (11.5, 54.8) | 32.0 (11.0, 56.0) | 0.820 |
TyG: triglyceride-glucose index; BMI: body mass index; hs-CRP: high-sensitivity C-reactive protein; RRF: residual renal function.
TyG and other parameters
TyG was positively correlated with inflammatory parameters, such as hs-CRP (r = 0.258, p = 0.006) and ferritin (r = 0.219, p = 0.002) (Table 2).
Table 2.
Correlations between TyG and various parameters of inflammation.
| TyG | hs-CRP | Ferritin | Albumin | WBC | Neutrophils | Creatinine | |
|---|---|---|---|---|---|---|---|
| hs-CRP | 0.258a | ||||||
| Ferritin | 0.219a | 0.163 | |||||
| Albumin | 0.115 | −0.256a | −0.062 | ||||
| WBC | 0.098 | 0.215b | 0.266a | −0.046 | |||
| Neutrophils | 0.076 | 0.241b | 0.234a | −0.064 | 0.953a | ||
| Creatinine | 0.016 | 0.023 | 0.010 | −0.065 | 0.202a | 0.245a | |
| Urea nitrogen | −0.015 | 0.026 | −0.001 | 0.029 | 0.106 | 0.183a | 0.562a |
TyG: triglyceride-glucose index; hs-CRP: high-sensitivity C-reactive protein; WBC: white blood cell.
Correlation is significant at the 0.01 level.
Correlation is significant at the 0.05 level. albumin, creatinine, urea nitrogen.
TyG and initial episode of PDRP
The median follow-up period was 32 months. 85 patients (40.9%) had initial episode of PDRP. Among the pathogenic bacteria, 36(42.4%) were gram-positive bacteria, 21(24.7%) were gram-negative bacteria, 3(3.5%) were fungi, 3(3.5%) were mixed infections, and 22 (25.9%) were culture-negative. The most common gram-positive bacteria were Staphylococcus epidermidis (36.1%) and the most common gram-negative bacteria were Escherichia coli (61.9%). The high TyG group had a lower cumulative peritonitis-free survival (p = 0.030) (Figure 2).
Figure 2.
Cumulative peritonitis-free survival curves. TyG: triglyceride-glucose index.
The hazard ratios of TyG associated with initial episode of PDRP from the adjusted Cox proportional hazards models are listed in Table 3. Regardless of the adjustment model used, TyG (per unit increase) and TyG ≥8.39 (vs. TyG < 8.39) maintained independent predictive values for the occurrence of PDRP.
Table 3.
Relationship between TyG and initial episode of PDRP.
| TyG ≥ 8.39 (vs. TyG < 8.39) |
TyG (per unit increase) |
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Unadjusted | 1.610 (1.040–2.491) | 0.033 | 1.733 (1.198–2.508) | 0.004 |
| Model 1 | 1.609 (1.035–2.503) | 0.035 | 1.754 (1.197–2.571) | 0.004 |
| Model 2 | 1.689 (1.083–2.635) | 0.021 | 2.015 (1.335–3.041) | 0.001 |
| Model 3 | 1.789 (1.145–2.795) | 0.011 | 2.158 (1.427–3.261) | <0.001 |
TyG: triglycerides-glucose index; HR: hazard ratio; 95% CI: 95% confidence interval.
Model 1: age, sex, BMI.
Model 2: Model 1 plus history of diabetes, and history of CVD.
Model 3: Model 2 plus serum creatinine, serum uric acid, corrected calcium, serum phosphorus, and serum albumin.
ROC curves for TyG index on PDRP risk
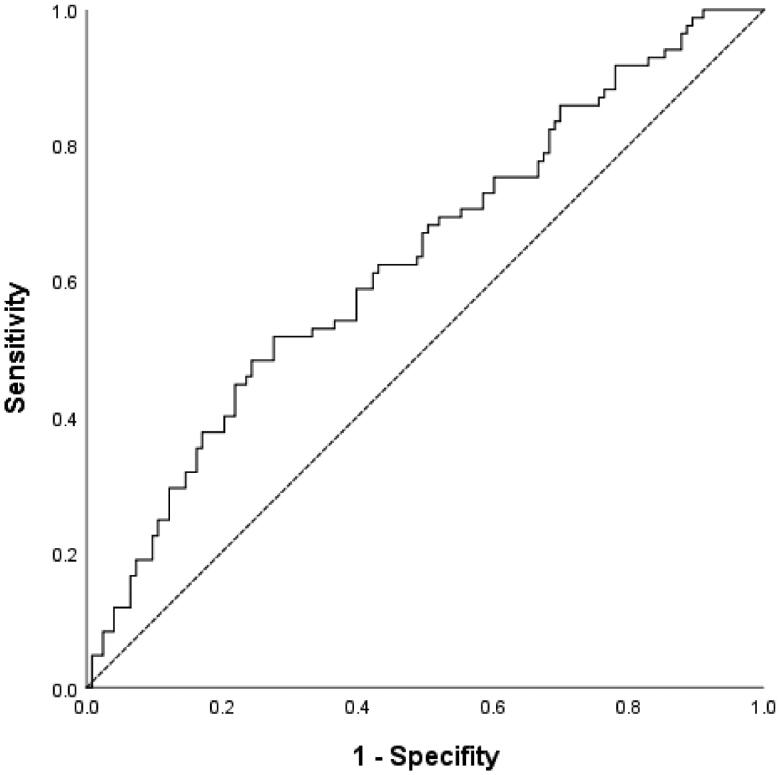
According to ROC analysis, the optimal cutoff value for the TyG index for predicting initial episode of PDRP was 8.57, with a Youden index of 0.241, sensitivity of 51.8%, and specificity of 72.4% (area under the curve [AUC], 0.633; 95% CI, 0.556–0.709) (Figure 3).
Figure 3.
ROC curves of TyG index. TyG: triglycerides-glucose index.
Subgroup analysis
We performed subgroup analysis in some subgroups including sex, age, BMI, and history of diabetes. Meanwhile, we explored the interaction between the subgroups and TyG. The forest plot showed that there was no interaction between them (Figure 4).
Figure 4.
The results of subgroup analysis with the forest plot. TyG: triglycerides-glucose index; DM: diabetic mellitus; BMI: body mass index; HR: hazard ratio; P1 value: P for each subgroup; P2 value: P for interaction.
Discussion
Through follow-up of 208 PD patients in our center, we found that PD patients with a higher baseline TyG index were more likely to develop initial episode of PDRP.
With the continuous development of medical technology, the incidence of PDRP has decreased steadily, but it is still a significant reason for hospitalization and mortality for PD patients with PD. A recent study of 4,245 PD patients in Taiwan found that the incidence of PDRP was 0.18 episodes per patient-year and was linked to increased mortality [14], and Nochaiwong et al. reported that the treatment failure rate caused by PDRP was as high as 25% [15]. Therefore, prevention and timely identification, diagnosis, and treatment of PDRP are essential for the prognosis of patients with PD. Some studies suggested that the systemic and intraperitoneal inflammation markers were associated with PDRP in PD patients and may be used to predict its occurrence [16, 17]. Moreover, as a special population, besides traditional risk factors for PDRP in patients with PD, careful consideration should also be given to glucose and lipid metabolism disorders.
The TyG index is a biomarker of IR that has been widely used to predict cardiovascular events and diabetes complications [18]. Furthermore, the TyG index is related to inflammatory indicators [9]. A recent study demonstrated that the TyG index is correlated with cardiovascular events in patients [11]. Yan et al. pointed out that the TyG index is related to cardiovascular mortality in patients [19]. As an inflammatory indicator, hs-CRP can predict PDRP risk in PD patients [4], and higher ferritin levels indicated a higher risk of infection (including PDRP) in a study of 129 Japanese dialysis patients [5]. Using Spearman rank correlation analysis, we demonstrated that TyG was positively correlated with inflammatory indicators (hs-CRP and ferritin). This result is consistent with the findings mentioned above. Furthermore, we found that baseline TyG was an independent risk factor for initial episode of PDRP, regardless of whether it was used quantitatively and qualitatively. Although the reliable mechanisms between them have not been well established, it can be presumed that they mainly include the following aspects: First, IR may lead to excessive production of reactive oxygen species and reduced production of nitric oxide, thereby causing chronic inflammation and endothelial cell dysfunction induced by various oxidative stress markers and pro-inflammatory cytokines [20, 21]. In addition, IR can induce glucose metabolism imbalance, which further promotes inflammation and oxidative stress [22]. Second, numerous studies have indicated that IR can cause endothelial cell damage, leading to an increase in intestinal wall permeability, making it easier for intestinal bacteria to enter the abdominal cavity and ultimately increasing the incidence of PDRP. Third, a high TyG index is synonymous with disorders of glucose and lipid metabolism. Recently, it has been found that baseline serum triglyceride levels can predict early onset PDRP [23], and hypertriglyceridemia is an influencing factor of treatment failure in PDRP patients [24]. In addition, PD patients are more prone to metabolic abnormalities, partly due to the high glucose load absorbed from the PD effluent, which further leads to a state of metabolic syndrome and increases susceptibility to PDRP [25]. Fourth, the TyG index is linked to hyperuricemia [26], which induces IR [27], and hyperuricemia can increase the release of inflammatory cytokines and promotes oxidative stress [28, 29]. In addition, uric acid was associated with technique failure in patients undergoing PD due to peritonitis [30]. Further studies are needed to elucidate the precise mechanisms involved in this association. The TyG index is readily available in clinical practice, and no additional examinations are required to reduce the economic burden on patients. We sincerely hope that the TyG index can play a role in the early identification of PDRP, so that timely detection and intervention measures can be taken to improve the prognosis of PD patients.
We found that the high TyG group had a higher number of women, which is consistent with previous research results [19]. Moreover, the AUC value for the baseline TyG in predicting initial episode of PDRP was 0.633, which was close to that of hs-CRP (0.616) and ferritin (0.646). This finding suggests that the baseline TyG index has a potential predictive effect on initial episode of PDRP. Although the AUC value is not particularly ideal, this may be partly because this study only analyzed the relationship between baseline TyG and initial episode of PDRP, without considering the influence of TyG over time. DM patients are more likely to be complicated by abnormal glucose and lipid metabolism, and a recent study found a higher prevalence of comorbid diabetes among patients with higher TyG [31]. In this cohort, there was no statistical difference in the proportion of DM between the 2 groups, and subgroup analysis showed no interaction between DM and the TyG index. It is speculated that one of the reasons may be related to the effective medical treatment of patients with DM. In addition, the culture-negative rate of PDRP in this study was 25.9%. The reason for the high culture-negative rate may be that the PD patient had already received antibiotic treatment before collecting the PD effluent sample. When many PD patients developed PDRP, local physicians have already given antibiotics to ensure timely and effective treatment.
The present study had several advantages. First, this retrospective study had a long follow-up period and fully considered the relationship between glucose lipid metabolism disorders and initial episode of PDRP. Subgroup analysis showed that adverse outcomes were associated with glucose lipid metabolism disorders even in non-diabetic patients with PD. Third, we used the baseline TyG index quantitatively and qualitatively, and its influence on the occurrence of initial episode of PDRP in PD patients was consistent. Finally, the TyG index is easy to obtain, inexpensive, and has potential value for clinical application.
This study had some limitations. First, we could only describe the phenomena and could not draw causal conclusions. Second, the sample size of this study was not large enough, and its findings have limited generalizability and need to be confirmed in a prospective and multicenter study. Third, we did not consider the potential effects of factors such as medication and exposure to glucose-based peritoneal dialysis solutions, nor were we able to remove all potential confounding factors. Finally, this study considered only the baseline serum TyG index, and the impact of the TyG index over time on PDRP was not included in the analysis. This is worth further research in the future.
Conclusion
In summary, we suggest that the baseline serum TyG index is an independent risk factor associated with initial episode of PDRP. Given that the TyG index is a convenient and economical laboratory parameter, it may have better clinical significance and prospects for clinical application.
Acknowledgments
We are grateful to all participants, including all patients and their family members, as well as to many colleagues and friends who provided support and assistance to us.
Funding Statement
This work was supported by grants from the Clinical Research and Cultivation Plan Project of the Second Affiliated Hospital of the Anhui Medical University (2021LCZD16).
Authors’ contributions
Guiling Liu designed the study, analyzed the data, and drafted the manuscript. Qiqi Yan, Ruifeng Wang, Dandan Li, Jingjing Cong, and Xiaoli Chen collected data. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethnic approval
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (authorization number: KY201302).
References
- 1.Sharma S, Sarnak MJ.. Epidemiology: the global burden of reduced GFR: ESRD, CVD and mortality [J]. Nat Rev Nephrol. 2017;13(8):1–7. doi: 10.1038/nrneph.2017.84. [DOI] [PubMed] [Google Scholar]
- 2.Himmelfarb J, Vanholder R, Mehrotra R, et al. The current and future landscape of dialysis [J]. Nat Rev Nephrol. 2020;16(10):573–585. doi: 10.1038/s41581-020-0315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szeto CC, Li PK.. Peritoneal dialysis-associated peritonitis [J]. Clin J Am Soc Nephrol. 2019;14(7):1100–1105. doi: 10.2215/CJN.14631218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su YJ, Liao SC, Cheng BC, et al. Increasing high-sensitive C-reactive protein level predicts peritonitis risk in chronic peritoneal dialysis patients [J]. BMC Nephrol. 2013;14(1):185. doi: 10.1186/1471-2369-14-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato S, Lindholm B, Yuzawa Y, et al. High ferritin level and malnutrition predict high risk of Infection-Related hospitalization in incident dialysis patients: a japanese prospective cohort study [J]. Blood Purif. 2016;42(1):56–63. doi: 10.1159/000445424. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: a systematic review [J]. Int J Endocrinol. 2020;2020:4678526–4678527. doi: 10.1155/2020/4678526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng G, Yang M, Xu L, et al. Combined effects of high sensitivity C-reactive protein and triglyceride-glucose index on risk of cardiovascular disease among Middle-aged and older chinese: Evidence from the China health and retirement longitudinal study [J]. Nutr Metabol Cardiovasc Dis. 2023;33(6):1245–1253. doi: 10.1016/j.numecd.2023.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Xiao S, Wang X, Zhang G, et al. Association of systemic immune inflammation index with estimated pulse wave velocity, atherogenic index of plasma, Triglyceride-Glucose index, and cardiovascular disease: a large Cross-Sectional study [J]. Mediators Inflamm. 2023:1966680–1966612. doi: 10.1155/2023/1966680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Lu Z, Wang X, et al. The chain mediating role of C-reactive protein and triglyceride-glucose index between lung function and cognitive function in a systemic low-grade inflammation state [J]. J Psychiatr Res. 2022;155:380–386. doi: 10.1016/j.jpsychires.2022.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp [J]. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 11.Quiroga B, Muñoz Ramos P, Sánchez Horrillo A, et al. Triglycerides-glucose index and the risk of cardiovascular events in persons with non-diabetic chronic kidney disease. Clin Kidney J. 2022;15(9):1705–1712. doi: 10.1093/ckj/sfac073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li PK, Chow KM, Cho Y, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment [J]. Perit Dial Int. 2022;42(2):110–153. doi: 10.1177/08968608221080586. [DOI] [PubMed] [Google Scholar]
- 13.Diagnosis and classification of diabetes mellitus [J]. Diabetes Care. 2013;36(1):S67–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung MC, Yu TM, Wu MJ, et al. Impact of peritoneal dialysis-related peritonitis on PD discontinuation and mortality: a population-based national cohort study [J]. Perit Dial Int. 2022;42(2):194–203. doi: 10.1177/08968608211018949. [DOI] [PubMed] [Google Scholar]
- 15.Nochaiwong S, Ruengorn C, Koyratkoson K, et al. A clinical risk prediction tool for Peritonitis-Associated treatment failure in peritoneal dialysis patients [J]. Sci Rep. 2018;8(1):14797. doi: 10.1038/s41598-018-33196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Tong Y, Yan H, et al. High intraperitoneal interleukin-6 levels predict peritonitis in peritoneal dialysis patients: a prospective cohort study [J]. Am J Nephrol. 2018;47(5):317–324. doi: 10.1159/000489271. [DOI] [PubMed] [Google Scholar]
- 17.Keskin Gözmen Ş, Serdaroğlu E.. High C-reactive protein and number of previous episodes at diagnosis increase the risk of catheter removal in peritoneal dialysis-related peritonitis in children [J]. Ther Apher Dial. 2023;27(2):328–334. doi: 10.1111/1744-9987.13911. [DOI] [PubMed] [Google Scholar]
- 18.Pan Y, Zhong S, Zhou K, et al. Association between diabetes complications and the Triglyceride-Glucose index in hospitalized patients with type 2 diabetes [J]. J Diabetes Res. 2021;2021:1–6. doi: 10.1155/2021/8757996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan Z, Yu D, Cai Y, et al. Triglyceride glucose index predicting cardiovascular mortality in chinese initiating peritoneal dialysis: a cohort study [J]. Kidney Blood Press Res. 2019;44(4):669–678. [DOI] [PubMed] [Google Scholar]
- 20.Muniyappa R, Iantorno M, Quon MJ.. An integrated view of insulin resistance and endothelial dysfunction [J]. Endocrinol Metabol Clin N Am. 2008;37(3):685–711. doi: 10.1016/j.ecl.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paneni F, Beckman JA, Creager MA, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I [J]. Eur Heart J. 2013;34(31):2436–2443. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ormazabal V, Nair S, Elfeky O, et al. Association between insulin resistance and the development of cardiovascular disease [J]. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan S, Tian H, Cheng L, et al. Baseline serum triglyceride predicts early-onset peritonitis and prognosis in incident CAPD patients [J]. Medicine (Baltimore). 2021;100(2):e23673. doi: 10.1097/MD.0000000000023673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang YJ, Jiang ZP, Zhou JF, et al. Hypertriglyceridemia is a risk factor for treatment failure in patients with peritoneal dialysis-related peritonitis [J]. Int Urol Nephrol. 2022;54(7):1583–1589. [DOI] [PubMed] [Google Scholar]
- 25.Mcdonald SP, Collins JF, Rumpsfeld M, et al. Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations [J]. Perit Dial Int. 2004;24(4):340–346. doi: 10.1177/089686080402400408. [DOI] [PubMed] [Google Scholar]
- 26.Luo Y, Hao J, He X, et al. Association between Triglyceride-Glucose index and serum uric acid levels: a biochemical study on anthropometry in Non-Obese type 2 diabetes mellitus patients [J]. DMSO. 2022;ume 15:3447–3458. doi: 10.2147/DMSO.S387961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu W, Xie D, Yamamoto T, et al. Mechanistic insights of soluble uric acid-induced insulin resistance: insulin signaling and beyond [J]. Rev Endocr Metab Disord. 2023;24(2):327–343. doi: 10.1007/s11154-023-09787-4. [DOI] [PubMed] [Google Scholar]
- 28.Song C, Zhao X.. Uric acid promotes oxidative stress and enhances vascular endothelial cell apoptosis in rats with Middle cerebral artery occlusion [J]. Biosci Rep. 2018;38(3):BSR20170939. doi: 10.1042/BSR20170939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rock KL, Kataoka H, Lai JJ.. Uric acid as a danger signal in gout and its comorbidities [J]. Nat Rev Rheumatol. 2013;9(1):13–23. doi: 10.1038/nrrheum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh YP, Chang CC, Kor CT, et al. Relationship between uric acid and technique failure in patients on continuous ambulatory peritoneal dialysis: a long-term observational cohort study [J]. BMJ Open. 2017;7(4):e010816. doi: 10.1136/bmjopen-2015-010816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Xu W, Song Q, et al. Association between the triglyceride-glucose index and severity of coronary artery disease [J]. Cardiovasc Diabetol. 2022;21(1):168. doi: 10.1186/s12933-022-01606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]